Abstract
Background: The aims of this study were to 1. define the responses of glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), glucagon, and peptide YY (PYY) to an oral meal and to intravenous L-arginine; and 2. examine correlation of enteroendocrine hormones with insulin secretion. We hypothesized a relationship between circulating incretin concentrations and insulin secretion.
Methods: Subjects with normal glucose tolerance (NGT, n = 23), prediabetes (PDM, n = 17), or with type 2 diabetes (T2DM, n = 22) were studied twice, following a mixed test meal (470 kCal) (mixed meal tolerance test [MMTT]) or intravenous L-arginine (arginine maximal stimulation test [AST], 5 g). GLP-1 (total and active), PYY, GIP, glucagon, and β cell function were measured before and following each stimulus.
Results: Baseline enteroendocrine hormones differed across the glucose tolerance (GT) spectrum, T2DM generally >NGT and PDM. In response to MMTT, total and active GLP-1, GIP, glucagon, and PYY increased in all populations. The incremental area-under-the-curve (0–120 min) of analytes like total GLP-1 were often higher in T2DM compared with NGT and PDM (35–51%; P < 0.05). At baseline glucose, L-arginine increased total and active GLP-1 and glucagon concentrations in all GT populations (all P < 0.05). As expected, the MMTT and AST provoked differential glucose, insulin, and C-peptide responses across GT populations. Baseline or stimulated enteroendocrine hormone concentrations did not consistently correlate with either measure of β cell function.
Conclusions/interpretation: Both MMTT and AST resulted in insulin and enteroendocrine hormone responses across GT populations without consistent correlation between release of incretins and insulin, which is in line with other published research. If a defect is in the enteroendocrine/β cell axis, it is probably reduced response to rather than diminished secretion of enteroendocrine hormones.
Keywords: meal tolerance test, incretin, arginine, insulin secretion, GLP-1, type 2 diabetes
Introduction
The incretin effect is a term used to describe the observation that ingested glucose elicits a greater insulin secretory response when compared with an “isoglycemic” intravenous glucose challenge. This has been attributed to multiple gastrointestinal hormones secreted by enteroendocrine cells upon meal ingestion. Of these hormones, glucagon-like peptide-1 (GLP-1) has been developed as a therapy for type 2 diabetes (T2DM) based upon its abilities to stimulate insulin secretion and suppress glucagon secretion in a glucose-dependent manner. Another hormone, glucose-dependent insulinotropic polypeptide (GIP), also contributes to the stimulation of postprandial insulin secretion. In addition to GLP-1 and GIP, meal or oral glucose ingestion also promotes the release of peptide YY (PYY).1 PYY is an anorectic hormone that is synthesized and released by enteroendocrine L cells (as is GLP-1 and GLP-2)2 and is activated by dipeptidyl peptidase-4.3
In T2DM, incretin effect is impaired but it remains controversial whether it is caused by a reduced secretion of incretin hormones or a reduced response to incretins with multiple conflicting reports in the literature. This inconsistency may be explained by different study populations as diabetes duration, severity of diabetes, age, obesity, and study medication, all of which alter GLP-1 response. On the other hand, there is good evidence that the action of GLP-1 and GIP is impaired in T2DM. The insulinotropic effect of GLP-1 is retained in patients with T2DM but its potency is reduced.4,5 In contrast to GLP-1, GIP almost completely loses its ability to amplify the second phase insulin response in T2DM.5–7
At present, there is limited information on incretin secretion in response to intravenous secretagogues. L-arginine is a potent insulin secretagogue and has been used to evaluate β cell dysfunction in both type 28 and type 1 diabetes.9 Intravenous L-arginine has been shown to increase total GLP-1 in normoglycemic subjects and noninsulin-dependent T2DM patients.10
In a previously published study, we quantified the reproducibility of beta cell function across the glucose tolerance (GT) spectrum in response to a standardized mixed meal tolerance test (MMTT) and to intravenous L-arginine.11 The present analysis seeks to define the GLP-1, GIP, PYY, and glucagon responses to a standardized oral meal and to intravenous L-arginine in those participants.
Materials and Methods
Subjects
Three groups of subjects were studied, classified by their fasting and postchallenge GT status 2 hr post 75 g oral glucose tolerance test (OGTT) as previously described.11 Those with normal glucose tolerance (NGT) had a fasting glucose <100 mg/dL and postchallenge <140 mg/dL; those with prediabetes (PDM) had impaired fasting glucose (≥100 and <126 mg/dL) and impaired GT (≥140 and <200 mg/dL); and those with type 2 diabetes mellitus (T2DM) had fasting glucose 126–270 mg/dL and HbA1c 6.5%–10.0% on a stable dose of metformin (500–2000 mg/day).
Study design
After obtaining Institutional Review Board approval, this cross-sectional study was conducted at two study sites (ICON Development Solutions in San Antonio, Texas, and Omaha, Nebraska). After written informed consent, and following screening, all subjects in the original study underwent an MMTT and arginine maximal stimulation test (AST) on two consecutive days with MMTT on day 1 and AST on day 2. Although part of a larger study,11 none of the data have been previously reported.
Procedures
Subjects were admitted to the research unit the evening before the procedures. In T2DM subjects, metformin was withheld on the morning of the procedure. After a 10 hr overnight fast, a single indwelling intravenous catheter was placed in the forearm for the MMTT, while for the AST indwelling catheters were placed in both upper extremities for infusion and sample acquisition respectively. The procedures are described below.
Mixed meal tolerance test
Following baseline sampling (−30, −15 and 0 min), a test meal (470 Kcal,—66% CHO, 18% fat, and 16% protein) composed of one 8 fl oz Boost nutrition supplement drink (Nestlè Health Science) and one PowerBar (Nestlè Nutrition) was administered. The meal was consumed within 10 min, with the bar consumed first, and serial sampling for glucose and insulin was performed at 10, 15, 20, 30, 60, 90, 120, 180, and 240 min postmeal. After pilot analyses to determine time course of response, total and active GLP-1, GIP, PYY, and glucagon were measured in the entire cohort before and at 30, 60, and 120 min after MMTT. Blood samples for incretin analyses were collected into vacutainers containing protease inhibitors (P800 tubes; Becton-Dickinson).
Arginine maximal stimulation test
Following baseline sampling (−10, −5 and 0 min), an intravenous injection of 5 g of L-arginine (10% L-arginine HCl (as R-Gene [Pfizer]) was administered followed by serial sampling for glucose and insulin at 2, 3, 4, 5, 7, and 10 min at basal glucose. Subsequently, glucose levels were raised by a continuous infusion of glucose (900 mg/min) over 60 min with baseline sampling at 50, 55, and 60 min followed by a second dose of 5 g of arginine at 60 min with sampling at 62, 64, 65, 67, and 70 min.11
After pilot analyses to determine time course of incretin response, total and active GLP-1, PYY, and glucagon were measured immediately before (time 0) and at 3, 5, and 10 min after arginine. Repeat measures were also done before (time 60) and at 3, 5, and 10 min after arginine during hyperglycemic phase (63, 65, and 70 min from beginning of experiment, see Fig. 1). GIP was not assessed in the full cohort due to no evidence of response to arginine. Blood samples for incretin analyses were collected into vacutainers containing protease inhibitors (P800 tubes, Becton-Dickinson).
FIG. 1.
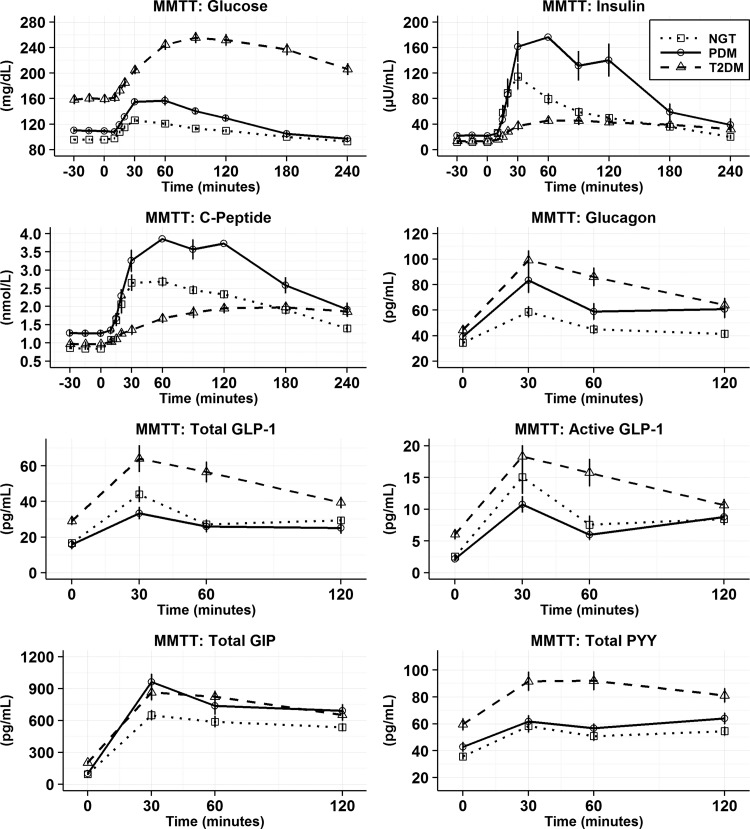
Mean (SEM) for MMTT analyte time profiles by GT population. After baseline samples, the meal was administered at time 0. Glucose, insulin and c-peptide measurements were made out until 240 min postmeal consumption. GLP-1, glucagon, PYY, and GIP were assayed for all subjects until 120 min postmeal after it was determined in a subset of subjects that values stabilized or returned to baseline by 120 min postmeal. GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; GT, glucose tolerance; MMTT, mixed meal tolerance test; PYY; peptide YY; SEM, standard error of mean.
Analyte assays
Total GLP-1 (RIA): Human total GLP-1 was quantified in duplicate using glucagon-like peptide-1 (total) RIA (#GLP-1T-36HK) from Merck Millipore (St. Charles). Assay range was 3–500 pM for total GLP-1.
Active GLP-1 and PYY
Active GLP-1 (7–36 amide) and total human PYY (3–36) levels were measured in duplicate using the Mesoscale Discovery (MSD) active GLP-1 (version 2, #K150JVC-1) and human total PYY assay kit (#K151MPD-1) (Gaithersburg). Assay range was 2–800 pg/mL for active GLP-1 and 25–1000 pg/mL for total PYY. The reactivity of the active GLP-1 assay with GLP-1 (1–36) or with glucagon is <0.3%.
GIP and glucagon
Human total GIP (3–42) was quantified in duplicate using the human GIP (Total) ELISA (Merck Millipore) and human glucagon using the Glucagon ELISA (Mercodia, Uppsala, Sweden). Assay range was 30–1800 pg/mL for total GIP and 10–400 pg/mL for glucagon. The cross-reactivity of the glucagon assay for GLP-1 is <0.30%.
Insulin, C-peptide, and glucose
Glucose was measured on the Roche Cobas c311 (Roche Diagnostics, Indianapolis, IN) utilizing a hexokinase reagent. C-peptide was measured by a two-site immunometric assay on the Roche Cobas e411 (Roche Diagnostics). Insulin (plasma) was measured by a two-site immunometric (sandwich) assay using electrochemiluminescence detection on the Roche Cobas e411 (Roche Diagnostics). All intra-assay CVs were <3% and inter-assay CVs <6%.
Data and statistical analyses
MMTT: β cell function
Glucose, insulin, and C-peptide responses were first plotted for each GT group as means (standard error of mean [SEM]). Insulin sensitivity (SI), was estimated using the oral glucose minimal model.12 β cell responsivity index (Φtot), a measure of insulin secretion, was estimated from the individual subject plasma glucose and C-peptide concentrations using the oral C-peptide minimal model.13 Disposition index (DI) was calculated as DI = SI × Φtot. For this study, a standardized approach to modeling across GT populations was used and no data were excluded. MMTT analyses were performed using Matlab US R2010B (MathWorks, MA) with code provided by Dr Cobelli. The three groups of subjects were analyzed separately.
AST: β cell function
Glucose, insulin, and C-peptide responses were first plotted for each GT group as means (SEM). The three groups of subjects were analyzed separately. The determination of insulin secretory response at basal glucose (AIRarg) and at elevated glucose (AIRargMAX) was performed as described previously.11 Insulin secretory reserve (ISR), represents the difference between insulin secretion at elevated and at basal glucose (AIRargMAX).
Incretin responses
For MMTT and AST incretin excursions were plotted as means (SEM). Incretin values were reported for the MMTT (0, 30, 60, and 120 min postmeal) and AST (0 3, 5, and 10 min postarginine injection at both the basal and elevated glucose state). Baseline subtracted incremental areas-under-the-curve from 0 to 120 min were derived for MMTT responses. Absolute changes from baseline to 5 min postarginine injection were derived for incretin values from the AST at both the basal and elevated glucose state. Incremental AUCs for MMTT and absolute changes for AST along with respective baseline values are summarized as arithmetic means (SD).
Missing data
Values less than the lower limit of quantification (LLQ) were assigned a value of 0.5 LLQ. Missing analyte values were interpolated and built into the standardized algorithms. No user intervention or imputation techniques were employed. All subjects with both β cell parameters and incretin values were included in the analysis.
Statistical analyses
B cell function parameters from both the MMTT and AST were summarized as geometric means (geometric CV). Analysis of variance (ANOVA) was used to estimate model predicted geometric means and respective 95% confidence intervals and to test for GT population differences at the two-sided, 0.05 level of significance. Geometric means (95% CI) were plotted along with results from the statistical significance tests. Additionally, baseline glucose, insulin, and C-peptide values were summarized by means (SD) and ANOVA was used to test for differences in GT population at the two-sided, 0.05 level of significance. Sample size of ∼20 subjects per group was derived to allow for detection of differences across GT groups for MMTT minimal modeling. Bias was addressed by performing identical procedures across the GT groups and standardizing all derivation algorithms without exclusion of any individual data points.
ANOVA was used to estimate model predicted means and respective 95% confidence intervals for the MMTT incremental AUCs and AST absolute changes in analyte concentrations, and to test for GT population differences at the two-sided, P < 0.05 level. Additionally, baseline incretin, and glucose, insulin, and C-peptide were summarized as means (SD). ANOVA was used to test for differences in GT population at the two-sided, P < 0.05 level.
Association Between β Cell Function and Incretin Responses: As assessment of association of the β cell parameters and incretin values was performed with Spearman's rank correlations because of the log-normal nature of the β cell parameters. Correlations were performed on MMTT baseline and incremental AUCs (total and active GLP-1, and total GIP) and on AST baseline and 5 min postarginine injection incretin values (total and active GLP-1). Correlations are presented across populations, and within populations.
Results
A total of 62 subjects were included in the studies of whom 23 (12 men/11 women) were NGT, 17 (6 men/11 women) PDM, and 22 (11 men/11 women) T2DM. All groups including NGT were obese. Complete demographic characteristics are summarized in Table 1.
Table 1.
Summary Demographics and Anthropometrics for Evaluable Subjects by Glucose Tolerance Population
| Parameter/population | NGT | PDM | T2DM |
|---|---|---|---|
| Number of evaluable (paired) observations N (men/women) | 23 (12/11) | 17 (6/11) | 22 (11/11) |
| Age (years ± SD) | 41.9 ± 8.4 | 44.8 ± 9.8 | 54.7 ± 8.1 |
| Weight (kg ± SD) | 85.8 ± 14.3 | 97.4 ± 12.9 | 91.1 ± 14.1 |
| BMI (kg/m2 ± SD) | 31.5 ± 2.8 | 35.0 ± 3.8 | 32.8 ± 3.9 |
| HbA1c (%) | 5.7% ± 0.4% (39 mmol/mol) | 8.3% ± 0.8% (67 mmol/mol) |
NGT, normal glucose tolerance; PDM, prediabetes; T2DM, type 2 diabetes.
MMTT-analyte time curves
As expected, significant differences were seen in fasting (baseline) glucose, insulin, and C-peptide across NGT, PDM, and T2DM (Fig. 1 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/met). Following the meal challenge, glucose rose progressively across populations (Fig. 1). The incremental AUCs of insulin and C-peptide differed significantly between groups (Fig. 2).
FIG. 2.
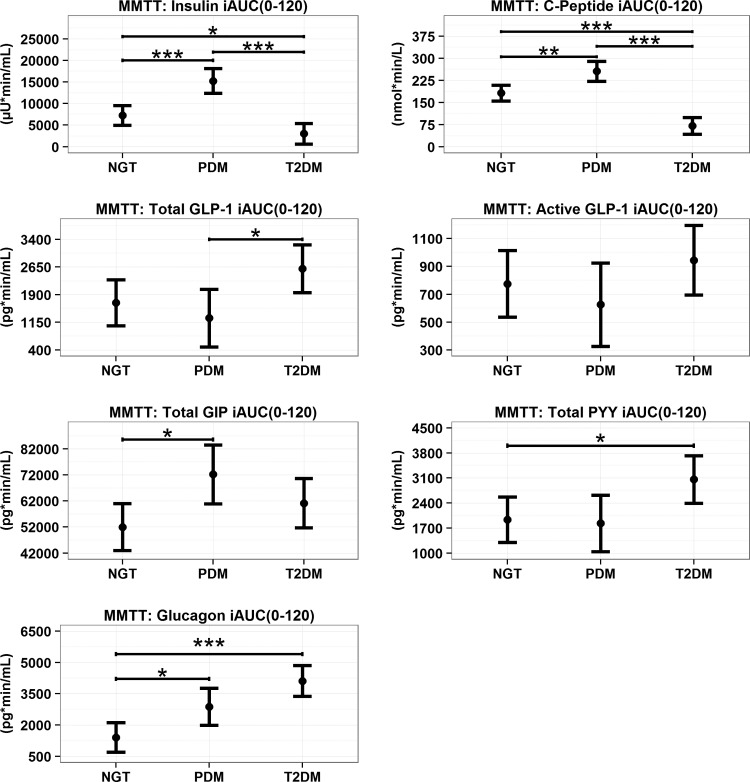
MMTT model predicted means (95% CI) for incretin incremental iAUC (0–120 min) Values across GT populations. *P < 0.05, **P < 0.01, ***P < 0.001.
Fasting values for, total and active GLP-1, total GIP, and total PYY were significantly higher in T2DM compared with NGT and PDM, whereas fasting plasma glucagon concentrations did not differ groups (Fig. 1 and Supplementary Table S1). The MMTT induced significant increases in all incretin hormones in all the GT populations (Figs. 1 and 3). Comparisons across GT populations showed several differences in total GLP-1, PYY, and GIP (Fig. 2). For example, the increase in AUC for total GLP-1 and PYY was significantly greater in those subjects with T2DM compared with PDM or NGT, respectively. In contrast, GIP increased the most in the PDM cohort. Meal ingestion increased glucagon concentrations across GT populations in a stepwise manner with NGT < PDM < T2DM (Fig. 2).
FIG. 3.
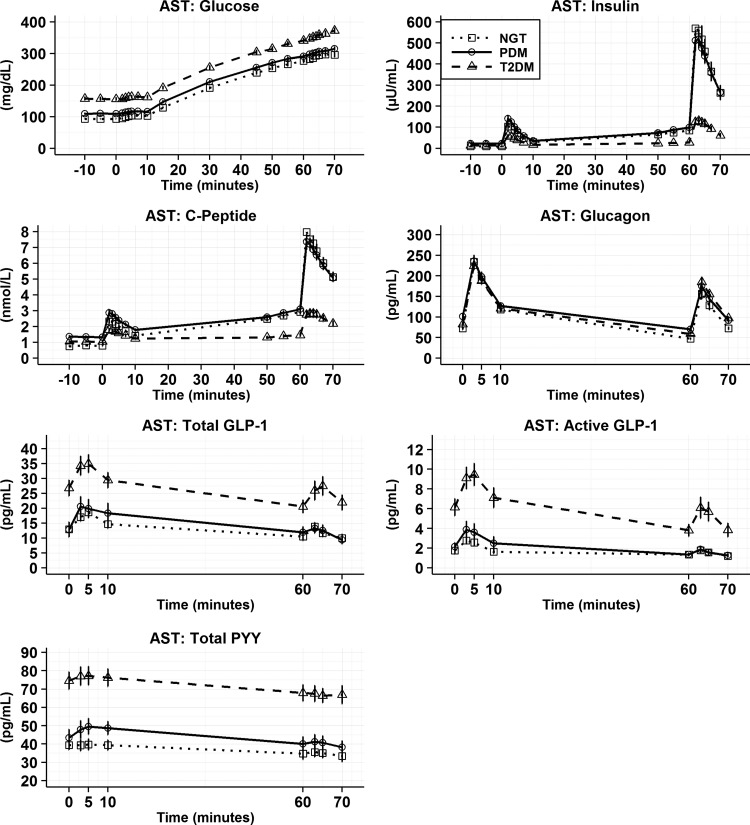
Mean (SEM) for AST analyte time profiles by GT population. After baseline samples, L-arginine was injected at time 0 and repeat samples for insulin, glucose, c-peptide, GLP-1, glucagon, PYY, and GIP were drawn. Glucose was then infused after the 10 min sample to raise circulating glucose concentrations. Repeat prearginine samples were drawn followed by an additional injection of L-arginine (5 gm). AST, arginine maximal stimulation test.
AST–analyte time curves
As observed for the MMTT, significant differences were seen in fasting (baseline) glucose, insulin, and C-peptide across NGT, PDM, and T2DM. Arginine caused significant increases in insulin and C-peptide under both basal and hyperglycemic conditions (Fig. 3). In contrast to NGT, the insulin secretory responses in PDM and T2DM were blunted.
In the basal glucose state before the bolus of L-arginine, individuals with T2DM had fasting total and active GLP-1 in addition to total PYY concentrations that were significantly higher (P < 0.001) than NGT and PDM. In response to L-arginine during the basal glucose state, consistent increases in total and active GLP-1 in all GT populations were observed (Fig. 4). However, only the T2DM population showed significantly higher absolute changes for active GLP-1 compared with PDM (P < 0.05) and NGT (P < 0.001). Total PYY showed no significant differences in absolute changes across the GT population at the basal glucose state (Fig. 4).
FIG. 4.
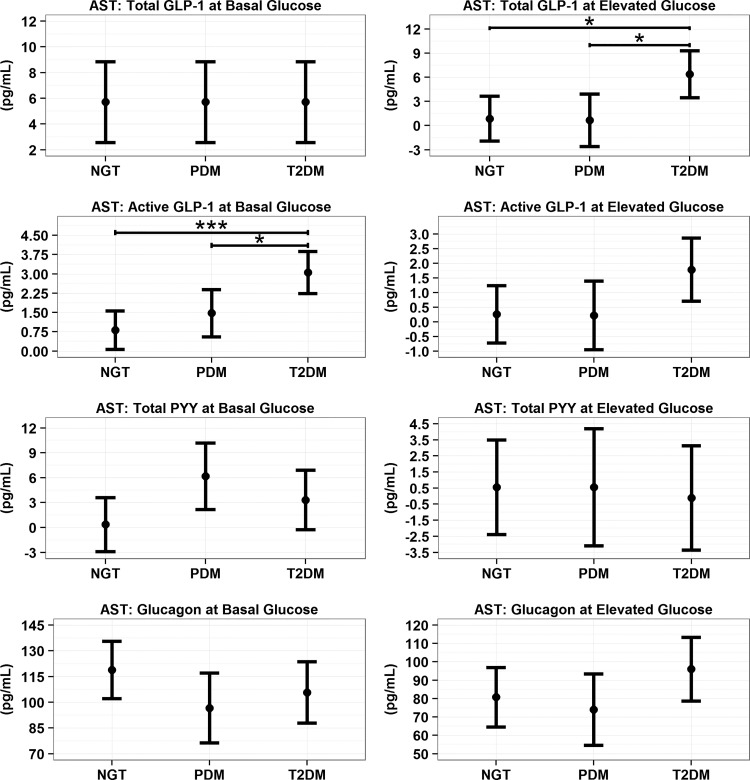
AST model predicted means (95% CI) for incretin absolute changes from baseline value. Within each GT population, the changes that do not include 0 within the confidence bound reflect a statistically significant change from baseline, at least P < 0.05. For comparisons across GT populations, notation of *P < 0.05 and ***P < 0.001.
In the hyperglycemic state, prearginine baseline total, active GLP-1, and total PYY were significantly higher in subjects with T2DM compared with NGT and PDM (Fig. 3 and Supplementary Table S2). In response to IV L-arginine, all GT groups showed increases in total and active GLP-1 during hyperglycemia. Absolute changes for total GLP-1 were significantly higher for T2DM versus NGT and PDM (Fig. 4, P < 0.05). In contrast, for active GLP-1 the response to L-arginine was not statistically significant and different across GT groups. Arginine did not induce a release of PYY under the elevated glucose state (Fig. 4).
Under basal glucose conditions, baseline glucagon concentrations were found to be significantly higher in the PDM population compared to NGT (Supplementary Table S2, P < 0.001) and T2DM (P < 0.05). Arginine elicited large increases in glucagon during basal and hyperglycemic conditions (Fig. 4). These increases did not differ across GT populations.
Tests of GT population differences for β cell function parameters
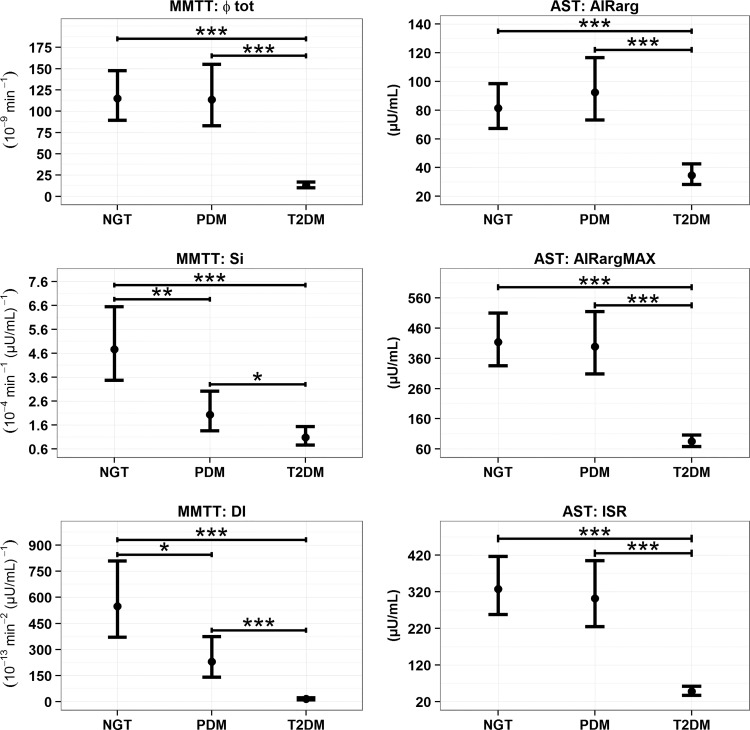
Figure 5 summarizes β cell parameters following MMTT. β cell responsivity (Φtot) in NGT and PDM were comparable and both were significantly higher compared with the T2DM group (P < 0.001). SI decreased progressively across GT populations with T2DM <PDM (P < 0.05) <NGT (P < 0.01). SI comparison of NGT versus T2DM was significant at P < 0.001. Correspondingly, DI showed progressive decreases across the GT glucose populations with NGT and PDM significantly higher (P < 0.001) compared with T2DM, and NGT significantly higher (P < 0.05) compared with PDM.
FIG. 5.
Model predicted geometric means (asymptotic 95% CI) for MMTT and AST beta cell health parameters. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5 also summarizes β cell function from the AST (AIRarg, AIRargMAX, and ISR). For all three parameters NGT and PDM were significantly higher than T2DM (P < 0.001). NGT and PDM were not found to be significantly different from one another for any of the parameters.
Correlations between baseline incretin and incretin responses compared to β cell function parameter
Given the responses of insulin and C-peptide, and the changes in circulating incretin concentrations, the relationship between these two groups of responses were explored for both the MMTT and AST. Table 2 summarizes the observed correlations between β cell function parameters and baseline incretin values and incremental AUCs in the MMTT. In general, although there are correlations that demonstrate statistical significance, there was no consistent relationship between either the baseline incretin measures or respective incremental AUCs and the measures of β-cell function.
Table 2.
Spearman's Correlations Between Mixed Meal Tolerance Test Beta Cell Parameters and Mixed Meal Tolerance Test Incretin Baseline Values and Incremental AUC (0–120 Min)
| Incretin value | Glucose tolerance population | Φtot (10–9 min−1) | SI [10–4 min−1 × (μU/mL)−1] | DI [10–13 min−2 × (μU/mL)−1] |
|---|---|---|---|---|
| Baseline premeal total GLP-1 (pg/mL) | Overall | −0.50*** | −0.30* | −0.47*** |
| NGT | 0.12 | −0.24 | −0.13 | |
| PDM | −0.22 | 0.00 | −0.07 | |
| T2DM | 0.11 | 0.28 | 0.13 | |
| Total GLP-1 iAUC (0–120 m) (pg × min/mL) | Overall | −0.25* | 0.18 | 0.00 |
| NGT | 0.33 | 0.41* | 0.54** | |
| PDM | −0.47 | 0.23 | −0.06 | |
| T2DM | −0.20 | 0.47* | 0.20 | |
| Baseline premeal active GLP-1 (pg/mL) | Overall | −0.45*** | −0.29* | −0.40** |
| NGT | 0.05 | 0.04 | 0.14 | |
| PDM | 0.04 | 0.00 | 0.01 | |
| T2DM | −0.01 | −0.12 | 0.14 | |
| Active GLP-1 iAUC (0–120 m) (pg × min/mL) | Overall | −0.15 | 0.01 | −0.06 |
| NGT | 0.37 | 0.25 | 0.39 | |
| PDM | −0.02 | −0.18 | −0.21 | |
| T2DM | −0.33 | 0.38 | −0.04 | |
| Baseline premeal total GIP (pg/mL) | Overall | −0.50*** | −0.23 | −0.38** |
| NGT | −0.11 | 0.08 | −0.02 | |
| PDM | 0.09 | 0.56* | 0.59* | |
| T2DM | 0.06 | 0.04 | 0.26 | |
| Total GIP iAUC (0–120 m) (pg × min/mL) | Overall | 0.05 | −0.11 | −0.07 |
| NGT | 0.09 | −0.04 | −0.11 | |
| PDM | 0.17 | −0.26 | −0.06 | |
| T2DM | 0.26 | 0.22 | 0.38 |
P < 0.05, **P < 0.01; ***P < 0.001.
AUC, area-under-the-curve; DI, disposition index; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; SI, insulin sensitivity.
Table 3 summarizes the correlational analyses of baseline total and active GLP-1 and measures of β cell function after arginine. As observed for the MMTT, under basal and elevated glucose conditions, AIRarg, AIRargMAX, and ISR negatively correlated with active and total GLP-1 at baseline and 5 min after IV arginine overall across GT populations. However, within each GT group, there were no consistent correlations of GLP-1 and β cell function.
Table 3.
Spearman's Correlations Between Arginine Maximal Stimulation Test Beta Cell Parameters and Baseline Incretin Values and 5 Min Postarginine Injection at the Basal and Elevated Glucose State
| Incretin value | Sample time and glucose state | Glucose tolerance population | AIRarg (μU/mL) | AIRargMAX (μU/mL) | ISR (μU/mL) |
|---|---|---|---|---|---|
| Total GLP-1 (pg/mL) | Baseline prearginine injection at the basal glucose state | Overall | −0.41** | −0.52*** | −0.52*** |
| NGT | 0.09 | 0.06 | 0.08 | ||
| PDM | −0.35 | −0.57* | −0.61** | ||
| T2DM | 0.03 | 0.12 | 0.15 | ||
| 5 Min postarginine injection at the basal glucose state | Overall | −0.22 | −0.37** | −0.38** | |
| NGT | 0.35 | 0.32 | 0.30 | ||
| PDM | 0.07 | −0.28 | −0.37 | ||
| T2DM | 0.16 | 0.25 | 0.26 | ||
| Baseline prearginine injection at the elevated glucose state | Overall | −0.22 | −0.44*** | −0.49*** | |
| NGT | 0.14 | −0.17 | −0.26 | ||
| PDM | −0.08 | −0.50* | −0.64** | ||
| T2DM | 0.18 | 0.17 | 0.16 | ||
| 5 Min postarginine injection at the elevated glucose state | Overall | −0.27* | −0.45*** | −0.49*** | |
| NGT | 0.50* | 0.28 | 0.23 | ||
| PDM | 0.04 | −0.35 | −0.50* | ||
| T2DM | 0.23 | 0.25 | 0.20 | ||
| Active GLP-1 (pg/mL) | Baseline prearginine injection at the basal glucose state | Overall | −0.46*** | −0.56*** | −0.57*** |
| NGT | 0.05 | −0.08 | −0.08 | ||
| PDM | −0.44 | −0.37 | −0.32 | ||
| T2DM | 0.01 | 0.06 | 0.06 | ||
| 5 Min postarginine injection at the basal glucose state | Overall | −0.40** | −0.53*** | −0.56*** | |
| NGT | 0.32 | 0.28 | 0.23 | ||
| PDM | −0.16 | −0.17 | 0.20 | ||
| T2DM | −0.03 | −0.12 | −0.16 | ||
| Baseline prearginine injection at the elevated glucose state | Overall | −0.45*** | −0.50*** | −0.50*** | |
| NGT | −0.16 | −0.09 | −0.09 | ||
| PDM | −0.20 | −0.45 | −0.50* | ||
| T2DM | −0.02 | −0.04 | −0.07 | ||
| 5 min postarginine injection at the elevated glucose state | Overall | −0.48*** | −0.53*** | −0.54*** | |
| NGT | 0.12 | 0.067 | 0.01 | ||
| PDM | −0.59* | −0.38 | −0.23 | ||
| T2DM | 0.11 | −0.03 | −0.09 |
P < 0.05, **P < 0.01, ***P < 0.001.
ISR, insulin secretory reserve.
Discussion
In this study, fasting total and active GLP-1, GIP, and PYY were found to be higher in T2DM compared with NGT and PDM. The mixed meal induced an increase in all incretin hormones across the GT populations. These responses tended to be higher in T2DM for total and active GLP-1 and PYY but not GIP compared with NGT and PDM. There is no evidence from this study that the circulating concentrations were deficient as GT worsens and are in good agreement with multiple other studies.14–21 Concurrent observation of stimulated insulin and incretin release demonstrated no clear association between these two systems. Of note, we cannot exclude that portal concentrations of GLP-1 may differ from those observed in the portal circulation and would therefore have a different relationship with beta cell response. L-arginine as an additional, provocative test of β cell function also increased total and active GLP-1 and again, there was no clear association between these systems.
Although metformin treatment was held from the day before the procedures, it may have contributed to the higher fasting GLP-1 concentrations in T2DM patient.22 In addition, an effect of metformin on fasting GIP in T2DM has not been reported but cannot be excluded. Nevertheless, the relationships between β cell function and plasma incretin concentrations are consistent.
The results of this study suggest that there is likely no deficiency of these incretins that can account for the altered insulin secretory responses. These data suggest that impairment in the incretin axis may be found in the responses to circulating incretins rather than the concentrations. Impairment in incretin action may be secondary to the development of impaired islet function as glucose intolerance develops (14). Importantly, the insulinotropic effect of GLP-1 is retained in patients with T2DM but its potency is reduced.4,5 In contrast, GIP almost completely loses its ability to amplify the second phase insulin response.4,6,7 As observed by Vilsboll et al. supra-physiological doses of GIP had no effect on insulin secretion in T2DM.7
There is very limited information on incretin secretion after intravenous secretagogues, and more specifically, few observations regarding L-arginine and incretin release in the literature. Recently, it has been shown that oral administration of L-arginine to mice significantly increased GLP-1 and insulin secretion and improved glucose clearance in wild type mice.23 In a clinical study from Orskov et al.,10 intravenous administration of L-arginine to normoglycemic subjects and noninsulin-dependent T2DM patients increased pancreas proglucagon, PG 33–61 amide and PG 78–107 amide, which is thought to be GLP-1.10 No direct comparisons to insulin secretory responses were made in these studies. Similarly, in this study we found that intravenous L-arginine caused an increase in total GLP-1 in all GT populations. These observations are extended in this study with the inclusion of active GLP-1, GIP, and PYY.
Unlike its effect on GLP-1, intravenous arginine did not elicit a consistent increase in PYY (only in PDM). A speculative question that these observations raise is discerning the cell type(s) from which GLP-1 is released. GLP-1 is predominantly secreted by enteroendocrine L cells in the small intestine and colon.21 L cells also secrete PYY. With no increase in PYY due to L-arginine, either the mechanism by which GLP-1 and PYY are released from L cells is different or GLP-1 is released by a different cell type. Although we cannot exclude that GLP-1 was also released at least in part by L cells, other cells have to be considered, such as pancreatic α cells, which have been shown to make GLP-1 under some circumstances.24
In conclusion, when used as stimuli for insulin secretion, a mixed meal and intravenous infusion of L-arginine resulted in a differential pattern of incretin release across GT populations. The release of GLP-1 was not impaired in T2DM patients but rather, if at all, even higher compared with NGT and PDM. Despite the robust response of GLP-1 of total and active GLP-1 to both challenges, there was no consistent correlation between release of these and other incretins by a mixed meal (or from L-arginine) and parameters of β cell function. Our data add to the building consensus that the secretion of incretins is not impaired in PDM and T2DM (compared to NGT) and suggest that if there is a defect in the incretin axis in type 2 diabetes mellitus, it probably lies in hormonal action rather than secretion. These results suggest that focusing on the mechanisms (hormonal and nonhormonal) driving tissue sensitivity to GLP-1 and other incretins might be a fruitful path to better understand the pathophysiology of type 2 diabetes and drive the development of new treatment approaches.
Supplementary Material
Acknowledgments
Current members of the β-Cell Project Team: Richard Bergman, PhD, Cedars-Sinai Diabetes and Obesity Research Institute. Roberto Calle, MD, Pfizer. Claudio Cobelli, PhD, University of Padova. Stephanie Cush, PhD-FNIH. Mark Farmen, PhD, Eli Lilly and Company. David Fryburg, MD–FNIH, ROI BioPharma Consulting. Atalanta Gosh, PhD, Janssen. Ilan Irony, MD, CDER/FDA. Douglas Lee, PhD, Pfizer. Frank Martin, PhD, JDRF. Malene Hersloev, MD, Novo Nordisk. Kolaczynski Jerzy, MD, Novo Nordisk. Stephanie Moran, MD, Takeda Development Center Americas. David Polidori, PhD, Janssen Ralph Raymond, MS, FNIH, R-Squared Solutions. R. Paul Robertson, MD, Pacific Northwest Research Institute and University Washington. Hartmut Ruetten, MD, PhD, Sanofi. Sudha Shankar, MD, Eli Lilly and Company. Myrlene Staten, MD, Kelly Government Solutions on contract to NIH/NIDDK. Darko Stefanovski, PhD, University of Pennsylvania. Lilit Vardanian, PhD, FNIH. Adrian Vella, MD, Mayo Clinic. Gordon Weir, MD, Joslin Diabetes Center. Marjorie Zakaria, MD, Novartis. Previous members of the B-Cell Project Team who contributed to this work: Mark Deeg, MD, PhD-formerly of Eli Lilly. David Kelley, MD, formerly of Merck. Peter Savage, MD, NIH/NIDDK. Nicole Spear, MS, formerly of FNIH. Maria Vassileva, PhD, formerly of FNIH. Sanya Whitaker, PhD, formerly of FNIH. Clinical trial no.: NCT01454973; NCT01663207; NCT01663220.
Contributor Information
Collaborators: Richard Bergman, Roberto Calle, Claudio Cobelli, Stephanie Cush, Mark Farmen, David Fryburg, Atalanta Gosh, Ilan Irony, Douglas Lee, Frank Martin, Malene Hersloev, Kolaczynski Jerzy, Stephanie Moran, David Polidori, Ralph Raymond, R. Paul Robertson, Hartmut Ruetten, Sudha Shankar, Myrlene Staten, Darko Stefanovski, Lilit Vardanian, Adrian Vella, Gordon Weir, Marjorie Zakaria, Mark Deeg, David Kelley, Peter Savage, Nicole Spear, Maria Vassileva, and Sanya Whitaker
Funding and Support
The methodological study described in this report was designed and implemented under the auspices of the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium. The Biomarkers Consortium is a public private partnership that develops and validates biological markers, which speed up the development of therapies for the detection, prevention, diagnosis, and treatment of disease, and are ultimately aimed at improving patient care. For this study, the Consortium brought together diabetes experts from leading academic institutions, the Food and Drug Administration, The National Institutes of Health, the nonprofit sector, and the pharmaceutical industry to develop the project. The results of the partnership, discussed here, are important because they address a critical unmet medical need, and involve key stakeholders in the diabetes treatment field. FNIH has acted as a neutral convener for the partners and provided the project management expertise needed for the execution of the overall project. In the future, this type of partnership can be used as a model for establishing common standards for testing in other therapeutic areas as well. Under the auspices of the FNIH, this project was jointly funded by the NIH (NIDDK) and FDA, and the following, participating pharmaceutical companies: Amylin (now AstraZeneca), Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Takeda. An in-kind donation of P800 tubes was made by Becton Dickinson (D. Craft). Additional support was also received from ADA and JDRF. The authors also thank the staff and leadership of the FNIH for their continuous support in planning, executing, and managing these studies and the overall project.
This work was performed through support to A.V. by the National Institutes of Health (DK78646 and DK116231).
Author Disclosure Statement
H.R., and M.G., employee and shareholder of Sanofi-Aventis Deutschland GmbH. R.A.C., employee and shareholder of Pfizer. A.G., employee and shareholder of Janssen Pharmaceuticals. S.S.S., employee and shareholder of Eli Lilly and Co. D.A.F., shareholder, Pfizer, Inc. For R.H.R., C.C., R.P.R., M.A.S., D.S., A.V., and K.W. no conflicting financial interests exist.
References
- 1. De Silva A, Bloom SR. Gut hormones and appetite control: A focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver 2012;6:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985;89:1070–1077 [DOI] [PubMed] [Google Scholar]
- 3. Grandt D, Schimiczek M, Struk K, et al. Characterization of two forms of peptide YY, PYY(1–36) and PYY(3–36), in the rabbit. Peptides 1994;15:815–820 [DOI] [PubMed] [Google Scholar]
- 4. Hojberg PV, Vilsboll T, Rabol R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009;52:199–207 [DOI] [PubMed] [Google Scholar]
- 5. Kjems LL, Holst JJ, Volund A, et al. The influence of GLP-1 on glucose-stimulated insulin secretion: Effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–386 [DOI] [PubMed] [Google Scholar]
- 6. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vilsboll T, Krarup T, Madsbad S, et al. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 2002;45:1111–1119 [DOI] [PubMed] [Google Scholar]
- 8. van Haeften TW, Veneman TF, van der Veen EA. Influence of lysine acetyl-salicylate on glucose and arginine stimulated insulin release in man. Horm Metab Res 1991;23:168–170 [DOI] [PubMed] [Google Scholar]
- 9. Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015;64:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orskov C, Jeppesen J, Madsbad S, et al. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 1991;87:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shankar SS, Vella A, Raymond RH, et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: Results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care 2016;39:1602–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cobelli C, Dalla Man C, Toffolo G, et al. The oral minimal model method. Diabetes 2014;63:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 14. Faerch K, Vaag A, Holst JJ, et al. Impaired fasting glycaemia vs impaired glucose tolerance: Similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 2008;51:853–861 [DOI] [PubMed] [Google Scholar]
- 15. Laakso M, Zilinskaite J, Hansen T, et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008;51:502–511 [DOI] [PubMed] [Google Scholar]
- 16. Nauck MA, Vardarli I, Deacon CF, et al. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011;54:10–18 [DOI] [PubMed] [Google Scholar]
- 17. Smushkin G, Sathananthan A, Man CD, et al. Defects in GLP-1 response to an oral challenge do not play a significant role in the pathogenesis of prediabetes. J Clin Endocrinol Metab 2012;97:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 19. Calanna S, Christensen M, Holst JJ, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: Systematic review and meta-analyses of clinical studies. Diabetologia 2013;56:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faerch K, Torekov SS, Vistisen D, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO Study. Diabetes 2015;64:2513–2525 [DOI] [PubMed] [Google Scholar]
- 21. Eissele R, Goke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 1992;22:283–291 [DOI] [PubMed] [Google Scholar]
- 22. Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin Pharmacol Ther 2010;88:801–808 [DOI] [PubMed] [Google Scholar]
- 23. Clemmensen C, Smajilovic S, Smith EP, et al. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology 2013;154:3978–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchetti P, Lupi R, Bugliani M, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 2012;55:3262–3272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.