Abstract
Aim
This study aims to establish the isolation method of stem cells from pulp tissue of carious deciduous teeth.
Methods
The teeth were soaked in 1% povidone–iodine solution for about 1 min followed by washing in PBS with 1% antibiotic–antimycotic thrice. Dental pulp tissue was removed by extirpation, and then cultivated in the culture medium. Characterization of mesenchymal stem cell (MSC) was carried out using human MSC analysis kit with positive markers CD90, CD73, and CD105, but negative for expressions of CD45, CD34, CD11b, CD19, and HLA-DR. Differentiation capacity of stem cells from human exfoliated deciduous (SHED) was determined by staining with Alizarin S, Alcian Blue, and Oil Red O.
Results
There is no contamination after 3 days of culture. SHED derived from dental pulp were expressions of 99.2% of positive marker and 0.3% of the negative marker. At passage 5, SHED was differentiated into osteocyte, chondrocyte, and adipocyte types of cells in the induction medium.
Conclusion
SHED derived from carious deciduous teeth can be used as a source of stem cell for regenerative medicine.
Keywords: stem cells from human exfoliated deciduous (SHED), Alizarin, Alcian Blue, Oil Red O, dental pulp stem cell (DPSC)
Introduction
Mesenchymal stem cell (MSC) is an adult stem cell that can be derived from many tissues of our body, such as bone marrow, adipose tissue, peripheral blood, Wharton’s Jelly, dental pulp, and pulp of exfoliated deciduous. MSCs from the pulp of exfoliated deciduous are called stem cells from human exfoliated deciduous (SHEDs). The proliferation rate of SHED is higher than other sources of stem cells, and the capabilities to differentiate into other cells are more than dental pulp stem cell (DPSC) and bone marrow mesenchymal stem cell (BMMSC). SHEDs have more than 140 of population double (PD), whereas 60–120 PD of DPS and 30–50 PD of BMMSCs. SHED can differentiate into other cells, such as odontoblast, osteoblast, chondroblast, adipocyte, and neuron. As the limited number of stem cell from adult tissue, it is necessary to develop an optimal method to get adult stem cell with good quantity and quality [1].
The bacteria and fungi contaminations are the major problems in the isolation of SHED. It happened because of resorption in the exfoliated deciduous roots, hence the pulp tissue would contact with the oral environment during tooth extraction. There are bacteria and fungi species in the oral environment. It was reported that among of 293 species of bacteria, streptococcus and candida are frequently found in oral cavity [2, 3]. Hence, exfoliated deciduous caries-free is a source of pulp tissue for SHED isolation.
In developing countries, education of oral health in the childhood has not gone well, so the rate of deciduous caries is very high. They need the development of SHED isolation techniques to be applied for exfoliated deciduous caries. This research aims to develop a method of stem cells isolation from exfoliated deciduous caries.
Materials and Methods
Culture and isolation of SHED
In this study, deep dentin caries human exfoliated deciduous incisor was with 2/3 physiologic root resorption. It was collected from a 7-year-old individual in the Usaha Kesehatan Gigi Sekolah program at elementary school, Indonesia. The sample must be washed on water flows to eliminate the blood. The inclusion criteria for a carious group were as follows: teeth with active caries lesions (internal half of dentin), no history of spontaneous pain, no sign suggesting irreversible pulpitis, or pulp necrosis.
Clean the entire surface of the teeth from plaque and calculus using a lesion. The caries is excavated until the hard tissue is reached and the teeth are washed in tap water for 5 min. Teeth were soaked in 1% povidone–iodine liquid for about a minute, then washed in phosphate-buffered saline (PBS) 7.4 (70011069 Gibco, Carlsbad, USA) with 1% antibiotic–antimycotic (Gibco 15240062) for three times. The pulp tissue was removed and cultivated in α-modified eagle medium (αMEM) (Sigma–Aldrich M0894, Saint Louis, USA) supplemented with NaHCO3 (Sigma–Aldrich S5761), 10% fetal bovine serum (FBS) (F4135 Sigma–Aldrich), 1% antibiotic–antimycotic (Gibco 15240062), and 1% non-essential amino acid (NEAA) (Sigma–Aldrich) in a 4-well plate (SPL 30004, Kowloon, Hong Kong). The next day, medium was replaced and every 2 days, the culture medium was changed for 13 days.
Flowcytometry analysis
In this study, the first passage was carried out after the stem cells are out of the explant (pulp tissue) approximately, 40% of confluent surface using 0.25% trypsin ethylenediaminetetraacetic acid (Sigma–Aldrich T3924). The next passage was done after 70%–80% confluent surface. The characterization was done using human MSC analysis kit (BD Biosciences, San Jose, USA), with positive markers CD90, CD73, and CD105, but negative for expressions of CD45, CD34, CD11b, CD19, and HLA-DR. Characterization was done on stem cells with the passage 5.
Osteogenic, chondrogenic, and adipogenic differentiation
For differentiation into osteocytes, adipocytes, and chondrocyte, cells were seeded in a 4-well plate at a density of 2 × 104 cells/cm2 and cultured in the culture medium. When the cell monolayer reached 80%–90% confluency, the medium was replaced with induction medium, which is osteogenesis (Gibco, A10072-01), adipogenesis (Gibco, A10070-01), and chondrogenesis differentiation medium (Gibco A10071-01), followed by incubation for 2 weeks with the induction medium replaced thrice a week. The staining procedure with Oil Red O, Alcian Blue and Alizarin Red S were done after successfully differentiated with marked by there droplets-lipid or vacuoles-lipid in the cytoplasm of the cell with adipogenesis medium, nodule–nodule of the cell monolayer in the chondrogenesis medium and produce calcium deposit of the cell with osteogenesis medium. The medium induction was removed and the cells were fixed with 4% paraformaldehyde (Sigma–Aldrich) for 15 min at room temperature. The plates were rinsed thrice with 1× PBS and stained with Oil Red O, Alcian Blue, and Alizarin Red S (Sigma–Aldrich) for 5–10 min at room temperature. Excess dye was removed in case of over-staining by washing thrice with 1× PBS. Cells were observed and images were captured using an inverted fluorescence microscope (Nikon Ti–E; Nikon Corporation, Tokyo, Japan).
Results
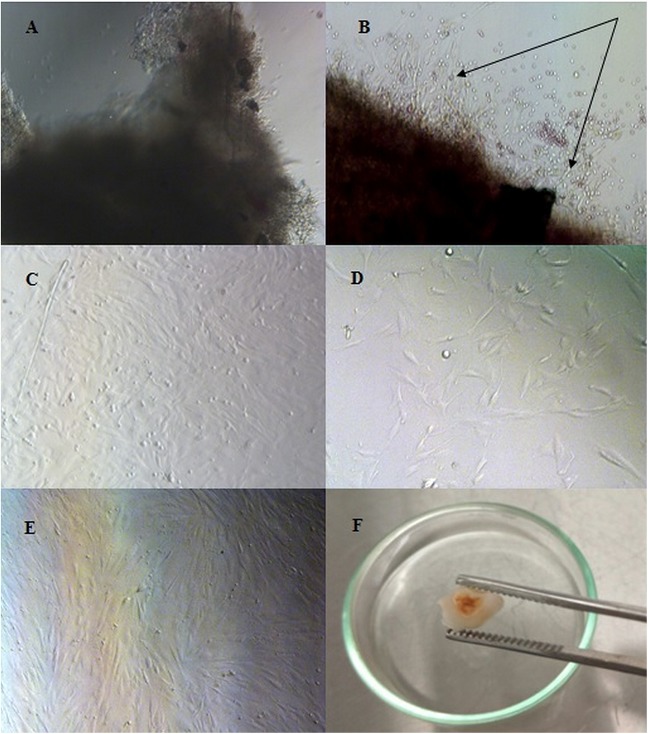
Explant (pulp tissue) has been attached on the well surface 2 h after culturing. SHED was observed to grow out from the pulp tissue within 4 days of culture. There is no contamination of bacteria and fungi in this culturing with this method. After 13 days of culture, passage 1 conducted when monolayer cells reached 40% confluence. Pulp tissue was removed and replanted in the new culture well. After passage, the cells were attached to the bottom of the culture well within 2 h and reached 70% confluence within 2 days. Most cells had a typical fibroblast-like appearance observed using an inverted microscope (Fig. 1).
Fig. 1.
Isolation and morphological observation of SHEDs. (A–E) Magnification 100× and (F) 40×. (A) Dental pulp tissue of caries human exfoliated deciduous teeth. (B) An outgrowth of stem cells from dental pulp tissue (arrow) of human exfoliated deciduous teeth (4 days of culture). (C) Phase-contrast images showing the fibroblast-like morphology of the in vitro expanded SHEDs within 13 days of culture (D) Passage 1 of SHEDs at 1 day of culture. (E) The cell monolayer reached 70% confluency within 4 days of culture. (F) Dentin caries exfoliated deciduous teeth
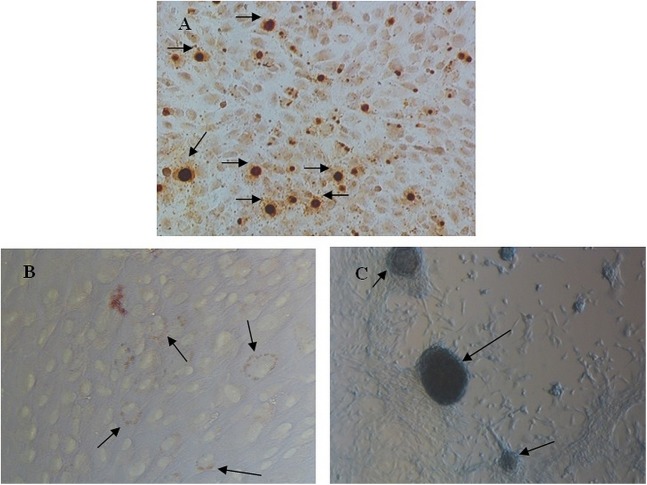
One of the characteristics of MSCs is its ability to differentiate into three types of cells, such as osteocytes, adipocytes, and chondrocytes in the induction medium. In this study, SHED that was isolated from dentin caries exfoliated deciduous could be differentiated into those cells after 2 weeks of induction. Alizarin Red S staining showed that the SHEDs formed a small number of calcified nodules. Oil Red O staining revealed the presence of numerous delicate lipid vacuoles, the morphology of certain cells changed from spindle-like to polygonal shapes, and cell hypertrophy was observed. Alcian Blue was used to detect the extracellular matrix proteoglycan and (nodule), and positive staining was observed after induction (Fig. 2). This result proves that MSCs can be isolated from dentin caries exfoliated deciduous incisors.
Fig. 2.
Characteristics of SHED. Phase-contrast images showing (A–C) SHEDs subjected to osteogenic, adipogenic, and chondrogenic inductions for 2 weeks. (A) Alizarin Red S staining to detect calcium deposits (arrows). (B) Oil Red O staining of SHEDs showing the lipid vacuoles inside SHEDs (arrow). (C) Alcian Blue staining of chondrogenic induced SHEDs showing proteoglycan (arrow). (A and C) Magnification 100× and (B) 200×
Characterization of SHEDs was analyzed by flowcytometry to determine the expression of cell surface markers. The result showed that SHEDs highly expressed CD90 and CD73 in every passage and optimal on 4th or 5th passage (Table I).
Table I.
Percentage surface marker expression of SHED
| Mesenchymal markers (%) | P2 | P3 | P4 | P5 | P7 | P8 |
|---|---|---|---|---|---|---|
| CD90+ | 99.8 | 98.8 | 99.8 | 99.9 | 96.9 | 100 |
| CD73+ | 99.8 | 98.7 | 99.7 | 99.8 | 97.1 | 100 |
| CD105+ | 20.8 | 79.6 | 98.1 | 99.1 | 82.2 | 40 |
| CD90+ and CD73+ | 99.7 | 98.7 | 99.4 | 97.5 | 98.5 | 100 |
| CD90+, CD73+, and CD105+ | 16.8 | 69.3 | 96.6 | 99.2 | 79.8 | 5.8 |
P denotes passage. SHED: stem cells from human exfoliated deciduous
Discussion
Conducting cell culture must avoid contamination of microorganisms, mainly bacteria and fungi. Contamination is obtainable from tools, human skills, and materials, which were used as a source of culture. In this study, dentin caries and resorption exfoliated deciduous were used as a source of SHED. Exfoliated deciduous was obtained from the elementary school, where the extraction process occurs in a school which was not integrated with the clinical laboratory. This distance allows the contamination of bacteria of the oral cavity to the pulp tissue after extraction.
Physiologically, there are at least 293 species of bacteria in the oral cavity. Most were included in the phylum Firmicutes, Bacteroides, Proteobacteria, Actinobacteria, Spirochaetes, Fusobacteria, Eeuryarchaeota, Chlamydia, Chloroflexi, Synergistetes, and Tenericutes. Streptococcus is a genus of bacteria mostly found in the oral cavity. Candida is a type of fungus that is most commonly found in the oral cavity, followed by Cladosporium, Aureobasidium, Saccharomycetales, Aspergillus, Fusarium, and Cryptococcus [2, 3]. It is important to use the right ingredients in the methods for decontamination of dentin caries exfoliated deciduous. One of the materials that can be used is povidone–iodine. Povidone–iodine is a broad spectrum antimicrobial agent and less potential for resistance. Povidone–iodine formed from mixing polyvinylpyrrolidone and iodine to produce bactericidal reagents is non-sensitizing and non-irritating to human cells or tissues [4]. Mechanisms of antimicrobial obtained by penetrating the cell wall of the microorganism and attack the protein (amino acids cysteine and methionine), nucleotides, and fatty acids that cause cell death [5]. An optimal concentration of povidone–iodine is needed to remove organism contaminants but safe for SHED. In this experiment, 1% povidone–iodine is the optimal concentration. This concentration was equal to the concentration of mouthwash [6].
Werle et al. [7] have successfully isolated the SHED from carious deciduous using enzymatic methods (0.2% Type I collagenase solution). In this research, explant technique was used to isolate SHED. This technique was easier and cheaper than enzymatic methods. Furthermore, it does not require a lot of samples. Using explant technique, we can harvest SHED from one carious deciduous. In this experiment, expression of CD90 showed more than 90% at every passage.
International Society for Cellular Therapy (ISCT) speculated that there are three requirements of MSC, such as plastic adherent, expressing the positive markers CD90, CD73 and CD105, expressing <2% marker negative CD45, CD34, CD11b, CD19, HLA-DR, and ability to differentiate into osteocytes, chondrocytes, and adipocytes. In this study, SHEDs were qualified as MSC that was isolated with 1% povidone–iodine. The culture medium used in this research was αMEM low glucose supplemented with NaHCO3, 10% FBS, and 1% NEAA. Using this culture medium, SHED can grow out from pulp tissue within 4 days of culture and passage 1 within 13 days culture. It is better than Zhang et al. [8] reported that the SHED grow out within 2–3 weeks culture.
In this research, passages 4 and 5 show expressions by ISCT requirements. The positive surface markers such as CD90, CD73, and CD95 are owned by fibroblast. Contamination cultures with fibroblast are very possible, but the number will be reduced because fibroblast undergoes senescence and die. In a morphological manner, surface marker cannot be distinguished between SHED and fibroblast. Kundrotas [9] studies suggest that the use of CD10, CD26, CD106, CD146, and ITGA11 could be helpful for the discrimination of human MSCs from human dermal fibroblasts. The low expression of a CD105 shows that SHED is more prone to differentiate into adipocytes and osteocytes [10].
Kerkis and Caplan [11] reported that the isolation of stem cells from baby teeth could be cultivated by explant method and cultured in embryonic stem cell medium (DMEM/F12, 10%–15% FBS, 1% antibiotics, and 1% NEAA). The population cells from baby teeth are called immature dental pulp stem cells and qualified as MSC which required ISCT. Huang et al. [12] reported that isolation of SHED with enzymatic was successfully qualified for MSC using embryonic stem cell medium supplemented with 20% FBS. Other studies reported the same results conducted by Miura, Silva, and Yamaza using normal exfoliated deciduous [13, 14]. This research showed that the dental caries could be a source of MSCs particularly at SHED. This opens up opportunities for countries with high levels of dental caries to be able to open stem cells banking derived from carious deciduous. Hence, this is an opportunity to use stem cell from carious teeth for clinical application in regenerative medicine.
Conclusions
SHEDs derived from the pulp of carious deciduous teeth expressed CD90, CD73, and CD105, but negative for 248 CD45, CD34, CD11b, CD19, and HLA-DR. This shows that SHED can be used as a source of stem cells for cell therapy.
Funding Statement
Funding sources: This study was funded by the Center for Research and Development of Biomedical and Basic Health Technology, National Institute Health Research and Development, Ministry of the Health Republic of Indonesia (2016).
Authors’ contribution
This work was carried out in collaboration between all authors. MZ designed the study, wrote the protocol, flow cytometry analysis, and wrote the first draft of the manuscript. RRP worked on cell culture, cells staining and EWB managed the analyses of the study and managed the literature searches.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S: SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 10, 5807–5812 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewhirst F, Chen T, Izard J, Paster B, Tanner A, Yu W, Laskshmanan A, Wade W: The human oral microbiome. Am Soc Microbiol 192, 5002–5017 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghannoum M, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM: Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 6, e1000713 [Internet]. 2010 Jan 8 [cited 2016 Oct 24]. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2795202&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Some S, Sohn J, Kim J, Lee S, Lee J, Shackery I, Kim S, Kim S, Choi N, Cho I, Jung H, Kang S, Jun S: Graphene–iodine nanocomposites: Highly potent bacterial inhibitors that are bio-compatible with human cells. Sci Rep 6, 1–12 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mcdonnell G, Russell A: Antiseptics and disinfectants activity, action and resistance. Clin Microbiol Rev 12, 147–227 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valderrama LS: Clinical application of povidone-iodine oral antiseptic 1% (Betadine® mouthwash) and povidone-iodine skin antiseptic 10% (Betadine® solution) for the management of odontogenic and deep fascial space infection. Dermatology 212, 112–114 (2006) [DOI] [PubMed] [Google Scholar]
- 7.Werle SB, Lindemann D, Steffens D, Demarco FF, de Araujo FB, Pranke P, Casagrande L: Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig 20, 75–81 (2016) [DOI] [PubMed] [Google Scholar]
- 8.Zhang NAN, Chen B, Wang WEI, Chen C, Kang JIE, Deng SQ, Zhang B, Liu S, Han F: Isolation, characterization and multi-lineage differentiation of stem cells from human exfoliated deciduous teeth. Mol Med Rep 14, 95–102 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundrotas G: Surface markers distinguishing mesenchymal stem cells from fibroblasts. Acta Med Litu 19, 75–79 (2012) [Google Scholar]
- 10.Anderson P, Carrillo-Gálvez AB, García-Pérez A, Cobo M, Martin F: CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS One 10, 1–13 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkis I, Caplan AI: Stem cells in dental pulp of deciduous teeth. Tissue Eng Part B Rev 18, 129–138 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang GTJ, Gronthos S, Shi S: Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res 88, 792–806 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva FDS, Ramos RN, De Almeida DC, Bassi EJ, De Sa F, Maranduba P, Sant’Anna OA, Marques MM, Barbuto JA, Câmara NO, da Costa Maranduba CM: Mesenchymal stem cells derived from human exfoliated deciduous teeth (SHEDs) induce immune modulatory profile in monocyte-derived dendritic cells. PLoS One 9, 1–13 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S: Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 1, 1–10 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]