Abstract
Objective
We investigated whether the ultrasonographic measurement of maternal subcutaneous adipose tissue (SAT) thickness in the second trimester played a role in predicting gestational diabetes.
Materials and methods
This was a prospective cross-sectional study in which 223 women were classified as healthy (n = 177) or as gestational diabetes (n = 46) on the basis of a negative or positive two-step oral Glucose Challenge Test (GCT), respectively. The depth of the abdominal SAT was evaluated by two-dimensional ultrasonography. Body mass index (BMI), waist circumference (WC), and waist/hip ratio were determined.
Results
There was a positive strong significant correlation between a 50-g GCT level and BMI, WC, and SAT thickness (p < 0.001). Receiver-operating characteristic curve analysis showed SAT thickness above 16.75 mm predicted gestational diabetes mellitus (GDM) with a sensitivity of 71.7%, a specificity of 57.1%, a positive predictive value of 32.3%, and a negative predictive value of 87.6%. There was a good correlation between SAT, BMI, and WC.
Conclusion
Increased SAT, BMI, and WC measurements may be helpful in predicting the risk of the development of GDM in pregnant women.
Keywords: second trimester pregnancy, gestational diabetes, subcutaneous adipose tissue, waist circumference, body mass index
Introduction
Gestational diabetes mellitus (GDM) has been determined to consist of varying degrees of carbohydrate intolerance, with onset or first recognition occurring during pregnancy and affecting 1%–20% of pregnancies, depending on the population studies and criteria used for diagnoses [1]. Recent studies have shown that the risk of developing type 2 diabetes mellitus and metabolic syndrome (MetS) with the advanced age is increased in patients with a history of GDM. MetS is correlated with insulin resistance, abdominal obesity, hypertension, and atherogenic dyslipidemia. Abdominal obesity and insulin resistance are responsible for the central role in the pathogenesis of MetS [2–4]. Abdominal obesity seems to be more strongly linked to metabolic disease compared with body mass index (BMI) and anthropometric measures of abdominal obesity [e.g., waist circumference (WC) and waist-to-hip ratio (WHR)] [5].
Maternal obesity is a major public health problem that is linked to the increased morbidity of the mother and fetus. Pre-pregnancy obesity is also associated with the development of insulin resistance and GDM [6, 7]. An increase in maternal fat tissue is also a significant adaptive reaction to pregnancy, besides in the pathophysiology of preeclampsia, GDM, delivery of large-for-gestational-age (LGA) neonates, small-for-gestational-age infants, preterm labor, and stillbirth [8–11].
The storage of adipose tissue occurs in two distinct parts of body as subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). In non-pregnant women, increase in size of abdominal adipose tissue causes the increased risk of diabetes, expedited atherosclerosis, dyslipidemia, and MetS [12, 13]. But the relationship between which compartments increased adipose thickness and the development of GDM in pregnant women is clearly unknown. Some studies have reported links between increased VAT, SAT, and the development of GDM in early pregnancy [14, 15]. In addition, previous studies demonstrated that subcutaneous adiposity is associated with insulin resistance [16–18]. Therefore, determining a threshold value for SAT measurements may be beneficial in subsequently predicting GDM. In this study, we aimed to evaluate the association between SAT thickness in the second trimester and the presence of GDM.
Materials and Methods
Study population
This prospective cross-sectional study was conducted at Zekai Tahir Burak Women’s Health, Education and Research Hospital from December 2015 to June 2016. The study received from the hospital’s ethics committee (no: 2014-11-020). Written informed consent was collected from each participant.
The criteria for inclusion were singleton, low-risk healthy pregnant women, or those without routine gestational diabetes screening test at our hospital with a 1-h 50 g oral glucose challenge test (GCT) at 24–28 gestational ages, and willing to participate in a clinical trial. Pregnant subjects whose GCT was 140 mg/dl underwent 100 g oral glucose tolerance test (OGTT), administered between 8:00 and 9:00 a.m. after fasting for 8 h. Fasting blood glucose (FBG) and glucose levels 1, 2, and 3 h after the glucose challenge were recorded. Gestational diabetes is diagnosed if two or more plasma glucose measurements meet or exceed the following thresholds: fasting level ≥105 mg/dl, 1-h level ≥190 mg/dl, 2-h level ≥165 mg/dl, or 3-h level ≥145 mg/dl [19].
Women were excluded if they had pregnancies complicated by pre-gestational or gestational systemic diseases, evidence of fetal congenital malformations, multiple gestation, or polyhydramnios. BMI was calculated as weight (kg)/height (m)2. Height and weight were measured upon enrollment. WHR was calculated as waist (cm)/hip (cm).
Sonographic assessment
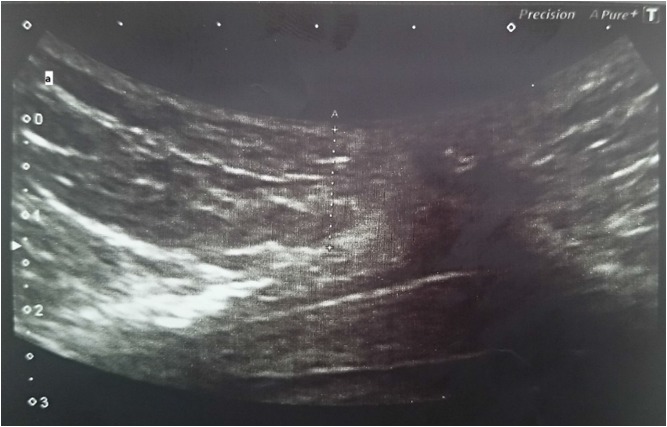
Measurements were obtained using a Toshiba Aplio 300 ultrasound machine with a 7–10 MHz probe and scans were done by one operator to provide good reliability and reproducibility. SAT thickness was measured in millimeters from the outer border of the rectus abdominis muscle to the skin surface, at the intersection of the linea alba, and the umbilicus umbilicus according to the method of Hamagawa et al. [20] (Fig. 1).
Fig. 1.
Image of an ultrasound scan showing abdominal subcutaneous adipose tissue thickness
Statistical analysis
Statistical analysis was carried out using the SPSS software version 17.0 (SPSS Inc., Chicago, IL). The normality of the variables was analyzed by the Kolmogorov–Smirnov test. Continuous variables with normal distribution were presented as mean ± standard deviation. An independent Sample t-test and Mann–Whitney U test evaluated associations between the categorical and continuous variables. Categorical variables were analyzed with a χ2 test. The receiver-operating characteristic (ROC) curves were constructed to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for different measures of BMI, WC, WHR, and SAT in predicting GDM. Correlations between ultrasonographic measurements/anthropometric measurements and 50 g GCT levels/SAT in second trimester were estimated using the Pearson’s correlation coefficient. Two-sided p values were considered statistically significant at p < 0.05.
Results
During the study period, 223 single pregnant women between 24 and 28 weeks of gestation underwent screening for GDM and were included in this study. Of these 223 women, 177 (79.3%) had normal OGTT, whereas 46 (20%) had GDM. All patients with GDM obtained a weekly fasting glucose level ≤92 mg/dl and second-hour postprandial glucose levels ≤120 mg/dl with dietary management alone.
The demographics of the groups are listed in Table I. There were no significant differences between the groups regarding parity, the gestational week at presentation, and history of GDM. Patients with GDM had higher rates of advanced age, increased BMIs, and positive family histories (p < 0.05).
Table I.
Demographics and medical history
| Variable [n (%)] | GDM groups (n = 46) | Control groups (n = 177) | p |
|---|---|---|---|
| Age (years)* | 30.52 ± 5.9 | 27.67 ± 5.45 | 0.002** |
| Multiparous [n (%)] | 17 (37) | 46 (26) | 0.141 |
| Gestational age (week)* | 26 (24–28) | 26 (24–28) | 0.164 |
| History of GDM [n (%)] | 5 (12.8) | 12 (9) | 0.475 |
| Positive family history [n (%)] | 29 (63) | 63 (35.6) | 0.001** |
Variables are median (min–max). GDM: gestational diabetes mellitus.
p < 0.05, significant
Table II shows BMI, measurements of WC, WHR, and maternal SAT thickness in the second trimester. The increased BMI, WC, WHR, and SAT thickness were significantly associated with GDM (p < 0.05). There was a good positive correlation between 50 g GCT level and BMI, WC, and SAT (p < 0.001), and WHR [correlation coefficient (CC) = 0.194, p = 0.004] (Table III). In addition, there was a strong positive correlation between SAT and BMI (CC = 0.716, p < 0.001), and WC (CC =0.647, p < 0.001) (Table IV).
Table II.
Anthropometric measures of body in second trimester
| Variable [n (%)] | GDM groups | Control groups | p |
|---|---|---|---|
| Maternal BMI | 29.45 (19–45) | 25.45 (17–34) | 0.001** |
| Waist circumference (cm) | 95 (72–111) | 91 (74–118) | 0.005** |
| Waist/hip ratio* | 0.89 ± 0.59 | 0.86 ± 0.62 | 0.001** |
| SAT thickness (mm) | 19 (11–28) | 15 (12–34) | 0.001** |
GDM: gestational diabetes mellitus; BMI: body mass index; SAT: abdominal subcutaneous adipose tissue.
Variables are median (min–max).
p < 0.05, significant
Table III.
Correlation between 50 g oral glucose challenge test levels and anthropometric measurements, BMI, and subcutaneous adiposity tissue thickness
| Variables | CC | p* |
|---|---|---|
| BMI (kg/m2) | 0.387 | <0.001 |
| Waist circumference (cm) | 0.258 | <0.001 |
| Waist/hip ratio | 0.194 | 0.004 |
| SAT thickness (mm) | 0.385 | <0.001 |
BMI: body mass index; SAT: subcutaneous adipose tissue.
p < 0.05, significant
Table IV.
Correlation between SAT thickness and BMI, WC, and WHR
| Variables | CC | p* |
|---|---|---|
| BMI (kg/m2) | 0.716 | <0.001 |
| Waist circumference (WC) (cm) | 0.647 | <0.001 |
| Waist/hip ratio (WHR) | 0.126 | 0.062 |
BMI: body mass index; SAT: abdominal subcutaneous adipose tissue.
p < 0.05, significant
ROC curve analysis showed that BMI above 25.75 kg/m2 predicted GDM with a sensitivity of 78.2%, a specificity of 40.9%, a PPV of 27.4%, and an NPV of 69.6%; WC measurement above 90.5 cm had a sensitivity of 78.2%, a specificity of 51.8%, a PPV of 28.0%, and an NPV of 83.8%; WHR above 0.85 had a sensitivity of 63.6%, a specificity of 28.2%, a PPV of 20.0%, and an NPV of 73.3%; SAT thickness above 16.75 mm had a sensitivity of 71.7%, a specificity of 57.1%, a PPV of 32.3%, and an NPV of 87.6% (Fig. 2).
Fig. 2.
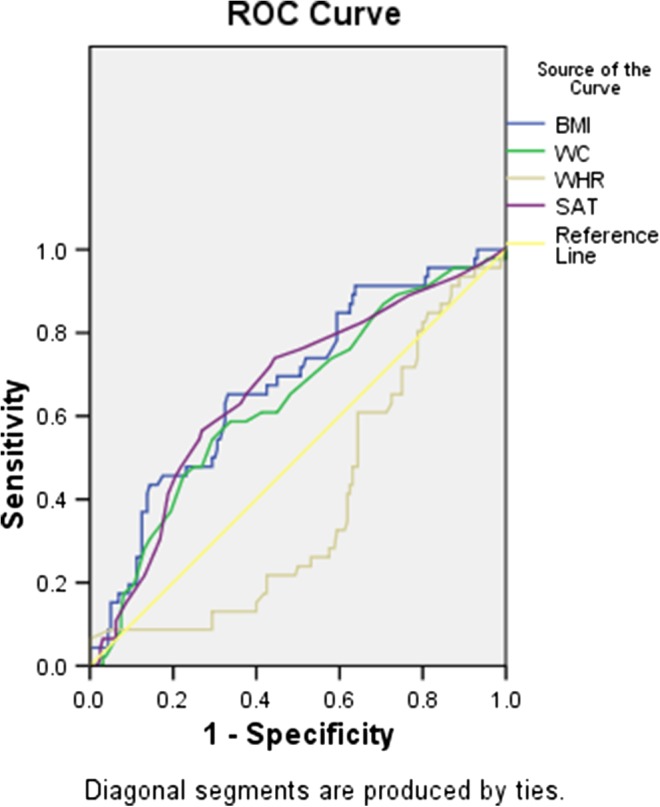
Receiver-operating characteristic (ROC) plot predicts the presence of GDM
Discussion
This prospective study showed that second-trimester sonographic measurement of maternal SAT thickness and increased BMI, and WC may independently predict the presence of GDM.
Normal pregnancy is a “diabetogenic state” because of the progressive increase in postprandial glucose and insulin response during the third trimester that is consistent with progressive insulin resistance [21]. According to the International Association of Diabetes and Pregnancy Study Groups and the American Diabetes Association all pregnant women should undergo the GCT at 24–28 of gestation, if the pregnant woman has no history of GDM in a previous pregnancy, obesity, glycosuria, or a strong family history of diabetes [22, 23].
Maternal obesity is related to some adverse pregnancy outcomes, including GDM, preeclampsia, preterm birth, and delivery of LGA [8–11, 24]. Traditionally, BMI has been the most widely used method by which to determine the prevalence of obesity. However, some recent studies showed that measures of central obesity, principally WC, are closely related to the development of GDM [5]. Because increased WC and WHR indicating fat accumulation in the abdominal region may result in insulin resistance [5]. In a previous study, Bergman et al. [25] demonstrated that a WC > 85 cm and a triglyceride level >1.7 mmol/L in early pregnancy were related a 6.1 times increase in GDM development risk. A pilot study on Asian and Indian patients conducted by Madhavan et al. demonstrated that the prevalence of GDM was seven times higher in those with WHR > 0.85 than in those with lower WHR (WHR > 0.85; OR: 12.05, 95% CI: 1.82–77.43, p = 0.007). They found that a WC of 85.5 cm with a sensitivity of 75%, a specificity of 81.4%, and a BMI of 24.3 kg/m2 with a sensitivity of 75% and a specificity of 86.5% had the best predictive value for GDM [26]. Comparable with previous studies, we found that GDM was predicted with 78.2% and 63.6% sensitivity; 51.8% and 28.2% specificity; 27.4% and 20% PPV; 83.8% and 73.3% NPV in pregnant women with WC > 90.5, WHR > 0.85 in the second trimester, respectively.
The role of SAT in the development of GDM is not exactly clear. A study of 1,106 patients demonstrated that SAT thickness was not effective for predicting type 2 diabetes mellitus when compared with VAT, especially in women [27]. In a study of 106 pregnant women in the first trimester, it was determined that there was a significant correlation between SAT and VAT and both of them were significantly higher in the MetS group [14]. Moreover, some recent studies demonstrated that subcutaneous adiposity is associated with insulin resistance [16–18]. A recent study revealed that increased biological activity in the SAT of pregnant women was associated with inflammation. The secretion of inflammatory agents, for example, leptin, adiponectin, and retinol-binding protein-4, was detected higher in subcutaneous tissue than in visceral adipocytes [28]. In addition, it was shown that increased inflammation and cytokines produced by fat tissue induce insulin resistance that leads to the development of diabetes mellitus [29, 30]. In a study by Kennedy et al. [31], maternal SAT was measured on routine ultrasounds at 11–14 and 18–22 weeks of gestation and they found mean SAT thickness was 21.2 mm in the first trimester and 20.3 mm in the second trimester. A retrospective cohort study by Suresh et al. compared maternal SAT and BMI as markers for adverse pregnancy outcomes at 18–22 weeks of gestation. They found that the median SAT was 18.2 mm and for every 5 mm increase in SAT, the odds ratio for developing GDM was 1.40 (95% CI: 1.22–1.61, p < 0.001) [32]. Another study by Kosus et al. evaluated the relationship between the maternal SAT and metabolic changes at 24–28 weeks of gestation. They found that presence of SAT ≥ 15 mm was very strong predictor of high CRP and HbA1c levels in pregnant women and suggested increased SAT during 24–28 weeks of gestation may be associated with pregnancy-related complications, such as GDM and preeclampsia [33]. Similar to this study, we demonstrated that GDM was predicted with 71.7% sensitivity, 57.1% specificity, 32.3% PPV, and 87.6% NPV in pregnant women with SAT thickness >16.75 mm during 24–28 weeks of gestation. This large variation for median SAT measurement in previous studies indicates that SAT thickness changes during pregnancy from women to women. In addition, it is likely depending on the differences in socioeconomic status, activity, and diets between nationalities [34].
Although this study had some limitations, we showed that GDM may be predicted using the second trimester measurements, such as BMI, WC, WHR, and SAT thickness. This study reports a relatively low sensitivity and specificity of WC, and SAT thickness predicting GDM, but an acceptable NPV of BMI, WC, WHR, and SAT. Another limitation of this study was that a predictive test for GDM in the second trimester is not useful in offering an early diagnosis of gestational diabetes and it cannot prevent the fetus from undergoing metabolic changes. Obstetricians may order ultrasonographic SAT measurement as a reliable, reproducible, accurate, fast, and safe test to the patients who are unwilling to attend oral GDM screening test or 75 g OGTT may be initially suggested for the patients with increased SAT, BMI, and WC measurements for GDM screening. In addition, based on test results, patients with increased risk of GDM can be alerted about this gestational complication and supported in terms of changes in diet, exercise, and the achievement of desirable gestational weight gain.
Conclusions
This study revealed that measurement of SAT thickness, WC, and WHR may be useful in predicting the risk of GDM. We determined that there was a positive correlation between 50 g GCT and BMI, WC, and SAT thickness. Increased SAT (SAT > 16.75 mm) and WC (>90.5 cm) in the second trimester can be used as a predictive factor for GDM development. Pregnant women with increased SAT and WC measurements may be tender to the development of GDM and determining the threshold point for SAT measurements may be helpful us to define risky pregnant women in early pregnancy. To offer clear recommendations, however, further study is needed.
Funding Statement
Funding sources: No financial support was received for this study.
Authors’ contribution
HK-C: project development, data management, and manuscript writing. BKK and SE: protocol development and data analysis. YT: project development and data analysis. NH and SY: data analysis and data management. SO: project development. NH: data Collection and manuscript writing.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P: Gestational diabetes: A clinical update. World J Diabetes 6, 1065–1072 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho NH, Ahn CH, Moon JH, Kwak SH, Choi SH, Lim S, Park KS, Metzger BE, Jang HC: Metabolic syndrome independently predicts future diabetes in women with a history of gestational diabetes mellitus. Medicine (Baltimore) 95, e4582 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valizadeh M, Alavi N, Mazloomzadeh S, Piri Z, Amirmoghadami H: The risk factors and incidence of type 2 diabetes mellitus and metabolic syndrome in women with previous gestational diabetes. Int J Endocrinol Metab 13, e21696 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakkarainen H, Huopio H, Cederberg H, Pääkkönen M, Voutilainen R, Heinonen S: The risk of metabolic syndrome in women with previous GDM in a long-term follow-up. Gynecol Endocrinol 32, 920–925 (2016) [DOI] [PubMed] [Google Scholar]
- 5. de Koning L, Merchant AT, Pogue J, Anand SS: Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur Heart J 28, 850–856 (2007) [DOI] [PubMed] [Google Scholar]
- 6. Scifres C, Feghali M, Althouse AD, Caritis S, Catov J: Adverse outcomes and potential targets for intervention in gestational diabetes and obesity. Obstet Gynecol 126, 316–325 (2015) [DOI] [PubMed] [Google Scholar]
- 7. Ferraro ZM, Contador F, Tawfiq A, Adamo KB, Gaudet L: Gestational weight gain and medical outcomes of pregnancy. Obstet Med 8, 133–137 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hod M: L14. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Preeclampsia. Pregnancy Hypertens 1, 246–247 (2011) [DOI] [PubMed] [Google Scholar]
- 9. Spaight C, Gross J, Horsch A, Puder JJ: Gestational diabetes mellitus. Endocr Dev 31, 163–178 (2016) [DOI] [PubMed] [Google Scholar]
- 10. Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, Dunne F, Lawlor DA: Hyperglycaemia and risk ofadverse perinatal outcomes: Systematic review and meta-analysis. BMJ 354, i4694 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazaki-Tovi S, Tarca AL, Vaisbuch E, Kusanovic JP, Than NG, Chaiworapongsa T, Dong Z, Hassan SS, Romero R: Characterization of visceral and subcutaneous adipose tissue transcriptome in pregnant women with and without spontaneous labor at term: Implication of alternative splicing in the metabolic adaptations of adipose tissue to parturition. J Perinat Med 44, 813–835 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibrahim MM: Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes Rev 11, 11–18 (2010) [DOI] [PubMed] [Google Scholar]
- 13. Bluher M: The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 21, 38–43 (2010) [DOI] [PubMed] [Google Scholar]
- 14. Gur EB, Ince O, Turan GA, Karadeniz M, Tatar S, Celik E, Yalcin M, Guclu S: Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine 47, 478–484 (2014) [DOI] [PubMed] [Google Scholar]
- 15. Gur EB, Genc M, Eskicioglu F, Kurtulmus S, Guclu S: Ultrasonographic visceral fat thickness measurement may be a good scan test for prediction of gestational diabetes mellitus. J Matern Fetal Neonatal Med 28, 893–894 (2015) [DOI] [PubMed] [Google Scholar]
- 16. Goel K, Misra A, Vikram NK, Poddar P, Gupta N: Subcutaneous abdominal adipose tissue is associated with the metabolic syndrome in Asian Indians independent of intra-abdominal and total body fat. Heart 96, 579–583 (2010) [DOI] [PubMed] [Google Scholar]
- 17. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH: Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278, 941–948 (2000) [DOI] [PubMed] [Google Scholar]
- 18. Tumurbaatar B, Poole AT, Olson G, Makhlouf M, Sallam HS, Thukuntla S, Kankanala S, Ekhaese O, Gomez G, Chandalia M, Abate N: Adipose tissue insulin resistance in gestational diabetes. Metab Syndr Relat Disord 15, 86–92 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coustan DR: Screening and testing for gestational diabetes mellitus. Obst Gynecol Clin North Am 23, 125–136 (1996) [DOI] [PubMed] [Google Scholar]
- 20. Hamagawa K, Matsumura Y, Kubo T, Hayato K, Okawa M, Tanioka K, Yamasaki N, Kitaoka H, Yabe T, Nishinaga M, Doi YL: Abdominal visceral fat thickness measured by ultrasonography predicts the presence and severity of coronary artery disease. Ultrasound Med Biol 36, 1769–1775 (2010) [DOI] [PubMed] [Google Scholar]
- 21. Newbern D, Freemark M: Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes 18, 409–416 (2011) [DOI] [PubMed] [Google Scholar]
- 22. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI: International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basevi V, Di Mario S, Morciano C, Nonino F, Magrini N: Comment on: American Diabetes Association. Standards of medical care in diabetes – 2011. Diabetes Care 34, e53 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gorkem U, Kucukler FK, Togrul C, Gungor T: Are adipokines associated with gestational diabetes mellitus? J Turk Ger Gynecol Assoc 17, 186–190 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M: Why visceral fat is bad: Mechanisms of the metabolic syndrome. Obesity (Silver Spring) 14, 16–19 (2006) [DOI] [PubMed] [Google Scholar]
- 26. Madhavan A, Beena Kumari R, Sanal MG: A pilot study on the usefulness of body mass index and waist hip ratio as a predictive tool for gestational diabetes in Asian Indians. Gynecol Endicrinol 24, 701–707 (2008) [DOI] [PubMed] [Google Scholar]
- 27. Forest JC, Girouard J, Masse J, Moutguin JM, Kharfi A, Ness RB, Roberts JM, Giguère Y: Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol 105, 1373–1380 (2005) [DOI] [PubMed] [Google Scholar]
- 28. Mazaki-Tovi S, Vaisbuch E, Tarca AL, Kusanovic JP, Than NG, Chaiworapongsa T, Dong Z, Hassan SS, Romero R: Characterizationof visceral and subcutaneous adipose tissue transcriptome and biological pathwaysin pregnant and non-pregnant women: Evidence for pregnancy-related regional-specific differencesin adipose tissue. PLoS One 10, e0143779 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baliutavičienė D, Buinauskienė JB, Petrenko V, Danytė E, Žalinkevičius R: Gestational diabetes, obesity, and metabolic syndrome diagnosed during pregnancy. Metab Syndr Relat Disord 10, 214–217 (2012) [DOI] [PubMed] [Google Scholar]
- 30. De Souza LR, Berger H, Retnakaran R, Vlachou PA, Maguire JL, Nathens AB, Connelly PW, Ray JG: Hepatic fat and abdominal adiposity in early pregnancy together predict impaired glucose homeostasis in mid-pregnancy. Nutr Diabetes 6, e229 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy NJ, Peek MJ, Quinton AE, Lanzarone V, Martin A, Benzie R, Nanan R: Maternal abdominal subcutaneous fat thickness as a predictor for adverse pregnancy outcome: A longitudinal cohort study. BJOG 123, 225–232 (2016) [DOI] [PubMed] [Google Scholar]
- 32. Suresh A, Liu A, Poulton A, Quinton A, Amer Z, Mongelli M, Martin A, Benzie R, Peek M, Nanan R: Comparison of maternal abdominal subcutaneous fat thickness and body mass index as markers for pregnancy outcomes: A stratified cohort study. Aust N Z J Obstet Gynaecol 52, 420–426 (2012) [DOI] [PubMed] [Google Scholar]
- 33. Köşüş N, Köşüş A, Turhan N: Relation between abdominal subcutaneous fat tissue thickness and inflammatory markers during pregnancy. Arch Med Sci 10, 739–745 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Widen EM, Gallagher D: Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 68, 643–652 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]