Abstract
Background
Primary breast rhabdomyosarcoma (RMS) can occur in children. There is a lack of knowledge regarding radiologic findings and added diffusion-weighted magnetic resonance imaging (MRI) features of RMS in the literature.
Case Report
A 12-year-old girl was diagnosed with primary alveolar RMS of the breast. Gray scale ultrasound revealed posterior acoustic enhancement behind a well-circumscribed, multilobulated hypoechoic mass. Doppler ultrasound revealed increased peripheral and central vascularity. Hypointense septations on T2-weighted image exhibiting more enhancement than the stroma on late gadolinium-enhanced images were striking within a hyperintense mass. A hyperintense hemorrhagic focus on T1-weighted image was present in the absence of any necrosis. Avid enhancement on early postcontrast images proceeding from the periphery to the center was depicted.
Conclusion
A rapidly enlarging mass with an echogenic peripheral rim together with posterior acoustic enhancement on gray scale ultrasound, intense vascularity on Doppler ultrasound, axillary lymphadenopathy, and satellite nodules on MRI should raise suspicion. Enhancing central and peripheral septations are suggestive of RMS. Dynamic contrast-enhanced MRI in suspected cases can provide valuable data in the differential diagnosis.
Keywords: Primary rhabdomyosarcoma, Pediatric, Breast, Ultrasound imaging, Magnetic resonance imaging
Established Facts
Primary breast rhabdomyosarcoma (RMS) can occur in children. There is a lack of knowledge regarding radiologic findings and added diffusion-weighted magnetic resonance imaging (MRI) features of RMS, and no consensus with regard to management. We discuss specific properties distinguishing RMS from common pediatric breast masses against the background of a multidisciplinary therapeutic approach.
Novel Insights
A rapidly enlarging mass, central and peripheral septations, and axillary lymphadenopathy on ultrasound with diffusion restriction and satellite nodules on MRI would suggest RMS rather than cystosarcoma phyllodes.
Introduction
Rhabdomyosarcoma (RMS) is the most common solid extracranial soft tissue sarcoma, constituting 5% of all malignancies with an incidence of 4–7/million per year in childhood [1, 2]. The most common primary sites of RMS are the head and neck region (40%) [3], the genitourinary system (22%), the extremities (18%), the trunk, chest wall, perineum, and retroperitoneum [4, 5], and the biliary tract [6]. Breast involvement is very rare, both as a primary or as a metastatic site [7]. Only a few cases of primary RMS of the breast in children have been reported in the literature (table 1) [8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. Due to the extremely rare incidence of primary breast RMS, radiologic imaging features have yet to be clearly defined. Because fibroadenoma is the most common breast tumor in adolescence, distinguishing early-stage RMS from a fibroadenoma is of crucial importance. In this report, we discuss the distinguishing imaging features of primary breast RMS on gray scale ultrasound (US), Doppler US, and magnetic resonance imaging (MRI), and we review the literature on demographic features and clinical outcomes of primary RMS of the breast in childhood and adolescence.
Table 1.
Review of the literature regarding primary breast rhabdomyosarcoma in the pediatric population
| Author [ref.] | Year | Pathologic subtype | Age, years | Cases, n | Location | Lymph node status | Size, mm | Neoadjuvant therapy | Surgery | Adjuvant therapy | Follow-up duration | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Torres and Ferrer [21] | 1985 | 11 | 1 | bilateral | ||||||||
| Altomare et al. [20] | 1989 | 15 | 1 | ChT | died | |||||||
| Reale et al. [17] | 1994 | embryonal | 16 | 1 | ||||||||
| Rogers et al. [18] | 1994 | alveolar | 14 | 1 | right | wide excision | multiagent ChT | |||||
| Boothroyd and Carty [19] | 1994 | 12 | 1 | left | ChT + RT | |||||||
| Hays et al. [16] | 1997 | alveolar (5) embryonal (1) NOS (1) |
11–20 | 7 | 3.9–7 years | |||||||
| Herrera and Lugo-Vicente [9] | 1998 | embryonal | 13 | 1 | right | negative | 6×4 | simple mastectomy | VAC | 12 months | remission | |
| Binokay et al. [8] | 2003 | alveolar | 16 | 1 | right | positive | 6×10 | MRM | ||||
| Vishnevskaia et al. [14] | 2003 | alveolar | 14 | 3 | bilateral | 3–18 months | died | |||||
| Pandey et al. [15] | 2004 | embryonal | 12 | 1 | 15 | quadrantectomy | VAC + RT | 73 months | remission | |||
| Vranic et al. [38] | 2006 | 10 | 1 | |||||||||
| Nogi et al. [11] | 2007 | alveolar | 13 | 1 | left | positive | 13×8 | MRM | doxorubicin, ifosfamide, actinomycin D | 6 months | remission | |
| Kallianpur et al. [13] | 2015 | 19 | 1 | right | negative | 30×20 | simple mastectomy | ifosfamide + adriamycin + RT | 2 months | remission | ||
| Pareekutty et al. [12] | 2016 | alveolar | 12 | 1 | right | positive | 90×90 | ChT | MRM | IRS-IV protocol + RT | 35 months | remission |
| Sugar and Sapi [22] | 1988 | alveolar | 14 | 1 | ||||||||
| Da Silva et al. [10] | 2007 | embryonal | 15 | 1 | right | positive | ||||||
| Present case | 2017 | alveolar | 12 | 1 | left | positive | 40×45 | ChT | simple mastectomy | VAC+ RT | 34 months | remission |
NOS = Not specified; ChT = chemotherapy; MRM = modified radical mastectomy; RT = radiotherapy; IRS-IV = Intergroup Rhabdomyosarcoma Study-IV; VAC = vincristine, actinomycin D, cyclophosphamide.
Case Report
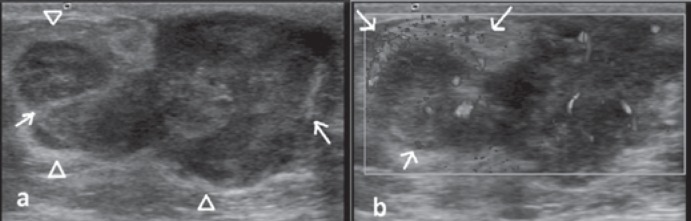
A 12-year-old girl presented with a firm mass in her left breast that had enlarged over the past 3 months. A round, mobile mass located in the left retroareolar region and palpable lymph nodes in the left axillary region were noticed on physical examination. The right breast and axillary region were examined and found to be normal. There was no history of trauma or nipple discharge. There was no edema nor any vascular engorgement of the skin. On US imaging, the mass had an irregular shape and well-circumscribed margins with a heterogeneous echotexture due to internal avascular and hyperechogenic areas considered to be hemorrhage. Acoustic enhancement was the predominant observation behind the tumor on gray scale US in addition to acoustic shadowing caused by fibrotic internal septations (fig. 1a). An echogenic peripheral rim encircling the mass was striking. The 45 × 20 × 40 mm mass demonstrated increased central and peripheral vascularity on color Doppler US evaluation, raising the suspicion of cystosarcoma phyllodes. The rapidly enlarging, multilobulated, hypoechogenic mass with increased vascularity and irregular shape was consistent with American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) category 4C (fig. 1b). Asymmetrical cortical thickening measuring 5 mm in diameter was found in a spherical lymph node by US examination.
Fig. 1.
Ultrasonography and Doppler images. a Gray scale ultrasonography image revealing a hypoechogenic mass with lobulated contour and echogenic linear structures within and encircling the mass. b Doppler ultrasound image demonstrating hypervascularity within the mass as well as increased peripheral vascularity (arrows).
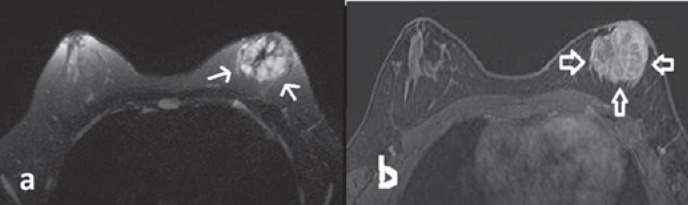
On MRI, the mass was predominantly hyperintense on T2-weighted image; it was multilobulated and had an irregular shape and central scar-like fibrotic hypointense septations without any peritumoral edema (fig. 2a). A hyperintense focus on T1-weighted image was observed within the predominantly hypointense mass, suggesting a focal hemorrhagic area. Dynamic contrast-enhanced breast MRI at 1.5 T revealed mild peripheral enhancement on early-phase images. Late-phase images demonstrated centripetal progressive enhancement with a plateau phase and heterogeneity due to diffusely enhancing internal fibrotic septations (fig. 2b). Type II enhancement kinetics were displayed within the tumor. In addition, multiple nodules around the mass presenting a similar enhancement pattern were compatible with satellite lesions. There was no necrotic area. The mass was adjacent to the nipple and areola complex without visible fat planes considered to be malignant involvement of the skin. Restricted diffusion was depicted on diffusion-weighted images at b = 1,000 s/mm2, and the apparent diffusion coefficient value was found to be 790 × 10-3 mm2/s. US-guided tru-cut biopsy with a 14-G needle was performed. Histopathologic evaluation revealed alveolar RMS (fig. 3a, b). On positron emission tomography imaging, the left retroareolar mass and the left axillary lymph node showed intense 18F-fluorodeoxyglucose uptake (maximum standardized uptake value = 6.3). The mass had no calcific foci on computed tomography. There was no distant metastasis.
Fig. 2.
Magnetic resonance imaging. a T2-weighted image showing a thin hypointense linear hypointensity encircling the mass and central scar-like hypointense internal septations. The lobulated mass was predominantly hyperintense, and no peripheral edema was present. b Dynamic contrast-enhanced subtraction image revealing marked contrast enhancement of the mass (arrows). Encircling and central internal septations exhibiting more enhancement than the stroma as well as a honeycomb-like pattern.
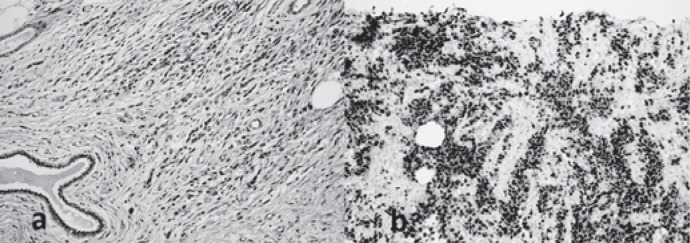
Fig. 3.
Histopathological photomicrographs. a Photomicrography of the mass revealing small cells with little cytoplasm. Alveolar-type spaces are present containing desquamated small, round, and poorly differentiated skeletal muscle cells (hematoxylin and eosin, ×40). b Photomicrograph of immunohistochemical evaluation of the primary mass showing diffusely positive staining for myogenin (×20).
The patient was treated with vincristine, actinomycin D, and cyclophosphamide (VAC) chemotherapy and showed a partial response after 3 courses of chemotherapy. After 7 courses of chemotherapy, the patient underwent breast-conserving mastectomy and an axillary lymph node dissection procedure. After mastectomy, the surgical margins were found to be free of tumor, and 1/16 lymph node was positive for tumor. Adjuvant radiotherapy was given to the left breast and axilla. Because of the large mass involving the whole retroareolar area, and in order to reduce aesthetic concerns in an adolescent, an expander was placed into the subcutaneous area and expanded progressively for 8 months followed by silicone breast implantation. Adjuvant chemotherapy was given for 42 weeks, and the patient has been followed-up without recurrence for 34 months.
Discussion
Primary breast malignancy in the pediatric population is extremely rare, with cystosarcoma phyllodes most commonly encountered [23]. Breast malignancies commonly diagnosed in children and adolescents have been reported to be metastases originating from neuroblastoma, RMS, or hematologic malignancies [23]. RMS presents mostly at around 5 years of age and in adolescence [5].
As well as presenting our current case, we review the demographic data and treatment of 24 cases of primary breast RMS in children and adolescents previously reported in the literature (table 1): All were adolescents aged between 10 and 19 years. Most cases (n = 14), including our case, had an unfavorable histology (alveolar subtype). All patients with breast metastasis from RMS also had an alveolar histology and were adolescent girls [7]. The primary breast RMS lesions were bilateral in 2 cases [14, 21], and regional lymph node involvement was present in 4 patients [8, 10, 11, 12]. RMS cases with bilateral breast metastasis have been reported to account for less than 25%, while metastatic lymph nodes have been encountered in 25–58% [24, 7].
The treatment procedure was reported in 10 cases, including our case. All patients had chemotherapy, 8 had surgery (2 simple mastectomies, 3 modified radical mastectomy, 1 wide excision, and 1 quadrantectomy), and 5 had radiotherapy. The range of duration of follow-up in the reported cases was 2 months to 7 years. Also, in patients without regional lymph node involvement, the outcomes were reported to be better; failure-free survival was reported to be 73 versus 43% [25, 26]. VAC has been reported as the gold standard combination chemotherapy in the treatment of RMS [9]. Our patient also received VAC as neoadjuvant chemotherapy and as adjuvant chemotherapy after surgery and radiotherapy. The degree of necrosis after chemotherapy and the enhancement ratio when compared to the initial examination have been found to be useful in assessing the response to chemotherapy [23]. Early and accurate diagnosis determines the treatment procedure, response to therapy, and prognosis.
Breast masses diagnosed as RMS commonly present as mobile, painless, well-circumscribed masses [9]. In order to avoid a delay in diagnosis or misdiagnosis, a radiologic examination should be done in all patients with enlarging breast masses. Because breast tissue is radiosensitive and exhibits higher density on mammography in childhood, US evaluation has been suggested as the first-choice diagnostic modality. Although most childhood breast masses are benign, being aware of ultrasonographic and Doppler characteristics of both common breast masses and less common pathologies is important [27]. Vascularity patterns of fibroadenomas as common benign breast lesions have been categorized as segmental, capsular, and feeding vessels; the average number of vessels was found to be 3.5 in mixed-type fibroadenomas (range 2–5) [28]. In the present case, more than 10 vascular codes were seen on color Doppler US. Obvious flame-like vessels have been demarcated as striking imaging findings in breast RMS, especially in metastatic foci. The increased number of vessels both in peripheral and central areas in the presented case was considered to be neovascularization, and histopathological examination was required. Increased breast vascularity in an adolescent should raise the suspicion of either a primary tumor or a metastasis. In all patients under follow-up with an initial diagnosis of a probably benign solid lesion or a fibroadenoma, color Doppler US examination in addition to patient history and physical examination of the breast and axillary regions should be considered.
Imaging findings of primary breast sarcomas were discussed in a recent study including 42 cases none of which were RMS [29]. Among the reported cases, the masses were mostly irregularly shaped, and margins were indistinct on US and mammography; 46% of the lesions were evaluated as BI-RADS category 3 on mammography, and most of the cases were either BI-RADS category 4 or 5 on MRI [29]. Most lesions were diagnosed as angiosarcoma, with secondary forms being encountered earlier and carrying a poor prognosis [30]. Typical features of breast carcinomas, which would be unexpected in primary breast sarcomas, are spiculated contours, angular margins, being longer than wide in shape, and posterior acoustic shadowing. In a previous study evaluating sonographic characteristics of primary breast sarcomas none of which were RMS cases, hypoechogenicity (82%) and ovoid shape (86%), indistinct margins (77%), posterior acoustic enhancement (59%), and internal (71%) and marginal (29%) vascularity have been reported as common features. Calcification (91%) and metastatic lymph nodes (100%) were not encountered in the majority of the cases [31]. An ovoid shaped mass with posterior acoustic enhancement in the pediatric population may be misdiagnosed as fibroadenoma. However, enlarged axillary lymph nodes and internal heterogeneity due to hemorrhage are suggestive of malignant processes such as cystosarcoma phyllodes or other malignancies, either primary or metastatic to the breast. RMS metastasis to the breast presents as either subcutaneous or parenchymal nodules [32]. Imaging features of RMS on US have been described as solitary lesions with a nodular appearance [27], bilateral diffuse involvement with a lobulated infiltrative hypoechoic pattern [33], lobulated well-circumscribed hypoechoic lesions with irregular margins, diffusely nodular infiltrations without a normal breast architecture [34], having a long axis perpendicular to the skin, and as heterogeneous. RMS may also present with posterior enhancement or indifferent shadowing [35]. Furthermore, breast RMS would resemble normal breast tissue with heterogeneous echo patterns and poor vascularity [36]. Absence of desmoplastic reaction around the mass besides any of the reported imaging features could facilitate the diagnosis of metastasis as well as primary breast RMS.
Imaging findings of metastatic breast RMS on conventional MRI have been reported as exhibiting intermediate or lower signal intensity with irregular margins and a dark rim around the mass on T1-weighted image [37], hyperintensity when compared to muscle, and non-enhancing hyperintense areas of necrosis on T2-weighted images [23]. In addition to the features described in the literature, we detected a central scar-like and circumscribing hypointensity both on T1- and on T2-weighted images due to fibrillar structures within the mass on T2-weighted image suggesting myofibroblastic accumulation as the striking diagnostic feature of spindle cell neoplasms. Although necrotic areas have been commonly reported, the presented mass included hyperintense areas on T1-weighted images, affirming intratumoral hemorrhage. On dynamic contrast-enhanced MRI images, breast RMS present as diffusely enhancing well-circumscribed lesions within the first 3 min, with fast annular contrast enhancement [37] and a washout phenomenon affirming their malignant nature [33]. Ring-like enhancement has been a typical imaging feature in metastatic foci of breast RMS. When a breast mass is identified as RMS, determining the presence of typical imaging features such as ring-like enhancement should be mandatory when investigating the primary site.
In conclusion, we aimed to emphasize that primary breast RMS can occur in children. A rapidly enlarging mass with an echogenic peripheral rim, posterior acoustic enhancement, intense vascularity on Doppler ultrasonography, axillary lymphadenopathy, and satellite nodules on MRI should be treated with suspicion. Dynamic contrast-enhanced MRI in suspected cases can provide valuable data in the differential diagnosis. Enhancing central and peripheral septations are suggestive of RMS. Although primary malignancies of the breast in the pediatric population occur less commonly, all breast masses should be evaluated carefully and a biopsy should be done if suspicious lesions are detected by radiologic evaluation.
Disclosure Statement
There is no conflict of interest.
Acknowledgement
We thank Carola Arndt, Pediatric Oncology, Mayo Clinic, USA, for valuable suggestions in the treatment of the case.
References
- 1.McCarville MB, Spunt SL, Pappo AS. Rhabdomyosarcoma in pediatric patients: the good, the bad, and the unusual. AJR Am J Roentgenol. 2001;176:1563–1569. doi: 10.2214/ajr.176.6.1761563. [DOI] [PubMed] [Google Scholar]
- 2.Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the United States. Cancer. 2009;115:1975–2005. doi: 10.1002/cncr.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzo PA, Poplack DG. ed 7. Philadelphia, PA: Wolters Kluwer; 2015. Principles and Practice of Pediatric Oncology. [Google Scholar]
- 4.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 5.Weigel BJ, Lyden E, Anderson JR, et al. Intensive multiagent therapy, including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation, in patients with high-risk rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2015;34:117–122. doi: 10.1200/JCO.2015.63.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebudi R, Görgün Ö, Ayan İ, Coşar R, Bilgiç B. Rhabdomyosarcoma of the biliary tree: case report and review of the literature. Pediatr Int. 2003;45:469–471. doi: 10.1046/j.1442-200x.2003.01763.x. [DOI] [PubMed] [Google Scholar]
- 7.Kebudi R, Koc BS, Gorgun O, Celik A, Kebudi A, Darendeliler E. Breast metastases in children and adolescents with rhabdomyosarcoma: a large single-institution experience and literature review. J Pediatr Hematol Oncol. 2017;39:67–71. doi: 10.1097/MPH.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 8.Binokay F, Soyupak SK, Inal M, Celiktas M, Akgül E, Aksungur E. Primary and metastatic rhabdomyosarcoma in the breast: report of two pediatric cases. Eur J Radiol. 2003;48:282–284. doi: 10.1016/s0720-048x(03)00041-x. [DOI] [PubMed] [Google Scholar]
- 9.Herrera LJ, Lugo-Vicente H. Primary embryonal rhabdomyosarcoma of the breast in an adolescent female: a case report. J Pediatr Surg. 1998;33:1582–1584. doi: 10.1016/s0022-3468(98)90506-1. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva BB, Lopes-Costa PV, dos Santos LG, et al. Primary embryonal rhabdomyosarcoma of the breast. South Med J. 2007;100:226–227. doi: 10.1097/SMJ.0b013e31802eaa6e. [DOI] [PubMed] [Google Scholar]
- 11.Nogi H, Kobayashi T, Kawase K, et al. Primary rhabdomyosarcoma of the breast in a 13-year-old girl: report of a case. Surg Today. 2007;37:38–42. doi: 10.1007/s00595-006-3326-2. [DOI] [PubMed] [Google Scholar]
- 12.Pareekutty NM, Bhagat M, Vora T, Qureshi SS. Rhabdomyosarcoma of the breast: report of two cases with the review of literature. J Indian Assoc Pediatr Surg. 2016;21:81. doi: 10.4103/0971-9261.176964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallianpur AA, Shukla NK, Deo SVS, Khanna P, Durgapal P. Primary mammary rhabdomyosarcoma in a nineteen year old female: a case report and review of literature. Indian J Cancer. 2015;52:295. doi: 10.4103/0019-509X.176702. [DOI] [PubMed] [Google Scholar]
- 14.Vishnevskaia I, Sharoev TA, Stepanova EV. Rhabdomyosarcoma of the breast in girls (Article in Russian) Arkh Patol. 2003;66:47–51. [PubMed] [Google Scholar]
- 15.Pandey M, Mathew A, Abraham EK, et al. Primary sarcoma of the breast. J Surg Oncol. 2004;87:121–125. doi: 10.1002/jso.20110. [DOI] [PubMed] [Google Scholar]
- 16.Hays DM, Donaldson SS, Shimada H, et al. Primary and metastatic rhabdomyosarcoma in the breast: neoplasms of adolescent females, a report from the Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997;29:181–189. doi: 10.1002/(sici)1096-911x(199709)29:3<181::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Reale D, Guarino M, Sgroi G, Castelli F, Bianchini E, Pascale M, Micoli G. Primary embryonal rhabdomyosarcoma of the breast. Description of a case (Article in Italian) Pathologica. 1994;86:98–101. [PubMed] [Google Scholar]
- 18.Rogers DA, Lobe TE, Rao BN, Fleming ID, Schropp K P, Pratt CB, Pappo AS. Breast malignancy in children. J Pediatr Surg. 1994;29:48–51. doi: 10.1016/0022-3468(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 19.Boothroyd A, Carty H. Breast masses in childhood and adolescence. Pediatr Radiol. 1994;24:81–84. doi: 10.1007/BF02020157. [DOI] [PubMed] [Google Scholar]
- 20.Altomare DF, Sbisa G, De Palo R, Gallo A, Loizzi P, Nappi R. Fatal breast rhabdomyosarcoma in a 15 year old primigravida. Eur J Gynaecol Oncol. 1989;11:149–151. [PubMed] [Google Scholar]
- 21.Torres V, Ferrer R. Cytology of fine needle aspiration biopsy of primary breast rhabdomyosarcoma in an adolescent girl. Acta Cytol. 1984;29:430–434. [PubMed] [Google Scholar]
- 22.Sugar J, Sapi Z. Alveolar rhabdomyosarcoma - a case report. Arch Geschwulstforsch. 1988;58:445–448. [PubMed] [Google Scholar]
- 23.Chung EM, Cube R, Hall GJ, Gonzalez C, Stocker JT, Glassman LM. From the archives of the AFIP: breast masses in children and adolescents: radiologic-pathologic correlation. Radiographics. 2009;29:907–931. doi: 10.1148/rg.293095010. [DOI] [PubMed] [Google Scholar]
- 24.Amichetti M, Perani B, Boi S. Metastases to the breast from extramammary malignancies. Oncology. 1990;47:257. doi: 10.1159/000226826. [DOI] [PubMed] [Google Scholar]
- 25.Meza JL, Anderson J, Pappo AS, Meyer WH. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 26.Rodeberg DA, Garcia-Henriquez N, Lyden ER, Davicioni E, Parham DM, Skapek SX, Meyer WH. Prognostic significance and tumor biology of regional lymph node disease in patients with rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2011;29:1304–1311. doi: 10.1200/JCO.2010.29.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang WT, Muttarak M, Ho LW. Nonmammary malignancies of the breast: ultrasound, CT, and MRI. Semin Ultrasound CT MR. 2000;21:375–394. doi: 10.1016/s0887-2171(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 28.Strano S, Gombos EC, Friedland O, Mozes M. Color Doppler imaging of fibroadenomas of the breast with histopathologic correlation. J Clin Ultrasound. 2004;32:317–322. doi: 10.1002/jcu.20041. [DOI] [PubMed] [Google Scholar]
- 29.Wienbeck S, Meyer HJ, Herzog A, Nemat S, Teifke A, Heindel W, Schafer F, Kinner S, Muller-Schimpfle M, Surov A. Imaging findings of primary breast sarcoma: results of a first multicenter study. Eur J Radiol. 2017;88:1–7. doi: 10.1016/j.ejrad.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Yin M, Wang W, Drabick JJ, Harold HA. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer. 2017;17:295. doi: 10.1186/s12885-017-3292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith TB, Gilcrease MZ, Santiago L, et al. Imaging features of primary breast sarcoma. AJR Am J Roentgenol. 2012;198:W386–W393. doi: 10.2214/AJR.11.7341. [DOI] [PubMed] [Google Scholar]
- 32.Howarth CB, Caces JN, Pratt CB. Breast metastases in children with rhabdomyosarcoma. Cancer. 1980;46:2520–2524. doi: 10.1002/1097-0142(19801201)46:11<2520::aid-cncr2820461134>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Perlet C, Sittek H, Forstpointner R, Kessler M, Reiser M. Metastases to the breast from rhabdomyosarcoma: appearances on MRI. Eur Radiol. 1999;9:1113–1116. doi: 10.1007/s003300050801. [DOI] [PubMed] [Google Scholar]
- 34.Ahn SJ, Kim SK, Kim EK. Metastatic breast cancer from rhabdomyosarcoma mimicking normal breast parenchyma on sonography. J Ultrasound Med. 2010;29:489. doi: 10.7863/jum.2010.29.3.489. [DOI] [PubMed] [Google Scholar]
- 35.Omranipour R, Hadi MR. Breast metastasis from multiple primary rhabdomyosarcoma in upper extremity. Clin Pract. 2012;2:e25. doi: 10.4081/cp.2012.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birjawi G, Haddad M, Tawil A, et al. Metastatic rhabdomyosarcoma to the breast. Eur Radiol. 2001;11:555. doi: 10.1007/s003300000632. [DOI] [PubMed] [Google Scholar]
- 37.Paulus DD, Libshitz HI. Metastasis to the breast. Radiol Clin North Am. 1982;20:561–568. [PubMed] [Google Scholar]
- 38.Vranic S, Kapur L, Foco F, Bilalovic N, Hainaut P. The first case of Li-Fraumeni syndrome in Bosnia and Herzegovina: case report. Pathologica. 2006;98:156–159. [PubMed] [Google Scholar]