Abstract
An 88-year-old man presented with a 1-month history of altered mental status and seizures. His electrographic and imaging findings were suggestive of herpes simplex encephalitis (HSE), for which he was empirically treated with acyclovir. He underwent two lumbar punctures 3 days apart; both cerebrospinal fluid analyses tested negative for herpes simplex virus (HSV) by polymerase chain reaction (PCR). These negative results and his continued deterioration after 9 days of acyclovir therapy prompted treatment with steroids for possible autoimmune encephalitis. Shortly after the change in management, the patient died from cardiac arrest. At autopsy, his brain showed both gross and microscopic evidence of encephalitis and was positive for HSV by immunohistochemistry. This fatal case of HSE emphasizes the limitations of HSV PCR and the importance of clinical suspicion in the diagnosis and management of this disease.
Keywords: Herpes simplex virus, Encephalitis, Polymerase chain reaction, False-negative results
Introduction
Herpes simplex encephalitis (HSE) is the most common cause of sporadic fatal viral encephalitis [1]. HSE has a mortality rate of approximately 70% in untreated patients and 19% in those treated with acyclovir. Of survivors, more than 50% have moderate to severe neuropsychiatric sequelae [2]. Currently, the diagnosis of herpes simplex virus (HSV) infection relies on cerebrospinal fluid (CSF) abnormalities and confirmation with CSF polymerase chain reaction (PCR) [3, 4]. This test has replaced invasive brain biopsy as the gold standard in the diagnosis of HSE [3-5]. HSV CSF PCR is 98% sensitive and 94% specific [6]. This high sensitivity should theoretically allow exclusion of an HSE diagnosis in patients who test negative [7]. Clinicians may presume that it is reasonable to discontinue acyclovir after a negative CSF PCR result, unaware of the test's limitations [3]. Herein, we present a case of autopsy-proven HSE with two false-negative CSF PCRs to emphasize the importance of clinical suspicion in the diagnosis and management of this disease.
Case Presentation
An 88-year-old Caucasian male presented to an outside hospital with a 1-month history of increasing forgetfulness, paranoia, and a 20-pound weight loss. In the prior week, he had progressed to having slurred speech, bizarre behavior, jerking leg movements, and intermittent lip smacking. On evaluation, he was found to have acute kidney injury with hyperglycemia. His mental status continued to decline, and he became agitated and unintelligible. Brain magnetic resonance imaging (MRI) with contrast demonstrated increased cortical signal intensity in the right temporal lobe with associated diffusion restriction, consistent with cytotoxic edema and highly suggestive of HSE. The patient was empirically started on intravenous acyclovir and transferred to our tertiary referral hospital for further management. Upon arrival, he was found to be unresponsive to verbal and tactile stimuli, with no withdrawal to pain. He also had intermittent lip smacking movements, suggesting seizure activity. Continuous video electroencephalography (EEG) showed diffuse slowing in the delta range (suggesting global cerebral dysfunction) and right-sided periodic discharges that correlated with the patient's rhythmic lip smacking movements. This ultimately progressed to epilepsia partialis continua. Multiple antiepileptic drugs, including levetiracetam, lacosamide, valproic acid, and lorazepam, were used without success. He was eventually intubated for seizure management with a propofol-induced coma in burst suppression. He had undergone a lumbar puncture on arrival and again 3 days later. CSF analyses included a meningitis/encephalitis panel by PCR, which tested negative for HSV-1/2 both times. A computed tomography (CT) scan of the chest, abdomen, and pelvis had already been obtained to evaluate for underlying malignancy, but was only remarkable for aspiration pneumonia, which was appropriately treated with antibiotics. The patient continued to deteriorate and there was talk of palliative care measures. Considering other possible etiologies, it was decided to empirically treat him for probable autoimmune encephalitis. He was administered 1 g of intravenous methylprednisolone daily. The results of the patient's panel of encephalitis autoantibodies only subsequently arrived and were found to be negative. He unfortunately died from cardiac arrest 2 days after having been switched to steroid therapy. At autopsy, his brain showed evidence of necrotizing viral encephalitis, and immunostaining was positive for HSV-1 (Fig. 1) and HSV-2.
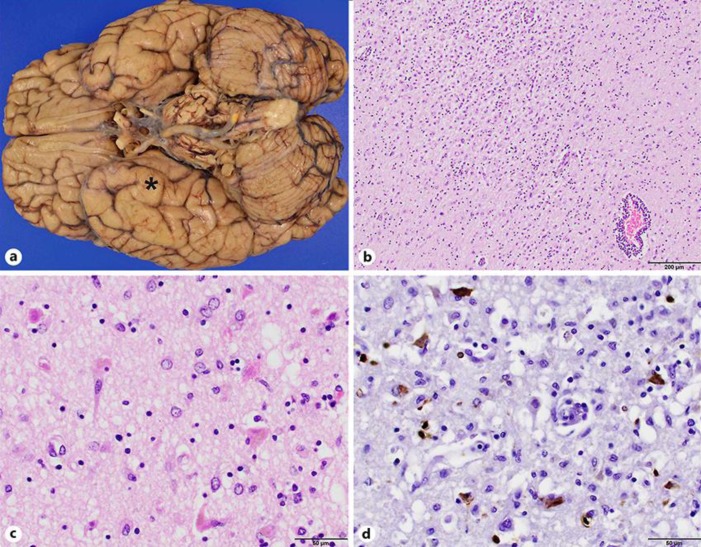
Fig. 1.
a The brain at autopsy was grossly unremarkable except for slight atrophy in the right temporal lobe cortex (asterisk). b, c Photomicrographs of hematoxylin and eosin-stained brain parenchyma from hippocampus and temporal lobe at ×100 (b) and ×400 (c) original magnification demonstrating subacute necrosis and mixed perivascular lymphocytic infiltrate. d Immunohistochemical staining for herpes simplex virus 1 at ×400 original magnification highlights neurons, glia, and macrophages positively. Overall, these findings are consistent with necrotizing viral encephalitis, more specifically herpes simplex encephalitis.
Discussion
HSV CSF PCR has become the gold standard in the diagnosis of HSE [3]. Despite its high sensitivity and specificity, there have been reports of HSE in patients with negative CSF PCR [3, 7-9]. The Infectious Disease Society of America guidelines suggest repeating an HSV PCR after 3–7 days if clinical suspicion is high [10], as the test is known to yield false-negative results early in the course of the disease [11]. Other causes of false-negative CSF PCR results include testing late in the disease [12], low viral levels in the CSF (due to most of the viral replication occurring within the brain with only a small quantity being released into the CSF) [3], the sensitivity of the assay (although high, it is not 100%) [13], and possible neutralization of HSV by antibodies [14]. In our case, the patient's clinical syndrome had already been present for approximately 1 month, so we suspect that the lateness of his testing may have been the cause for his negative CSF PCR results. He may also have had low viral levels in the CSF. This is supported by his autopsy findings, which showed that his infection appeared to be restricted to the interior brain parenchyma, without superficial cortical or leptomeningeal involvement.
Other tests can help support a diagnosis of HSE. Our patient's MRI showed the classic medial temporal lobe T2-weighted and fluid-attenuated inversion recovery hyperintensities (Fig. 2a). Such neuroimaging abnormalities are strongly associated with a positive CSF PCR assay [15]. MRI findings consistent with HSE have been reported in 86% of patients with HSV-positive PCR results obtained after 48 h from symptom onset [16]. Others have reported 100% sensitivity and specificity of HSE-associated MRI abnormalities within the 3–10 day period after symptom onset, with an overall sensitivity of approximately 80% for both CT and MRI [17].
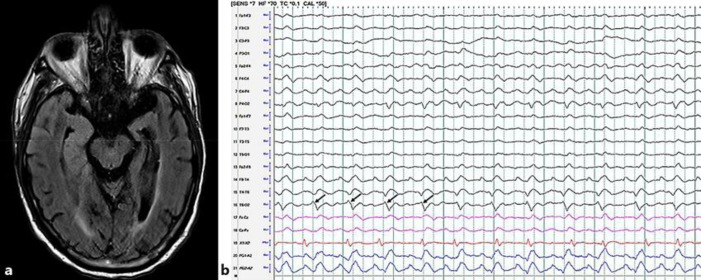
Fig. 2.
a Magnetic resonance imaging-fluid-attenuated inversion recovery, axial image; hyperintense signal involving the medial and anterior lateral right temporal lobe. b A 10-s interval of electroencephalography displayed using a longitudinal bipolar montage at a sensitivity of 7 mV with a time constant of 0.1 s and high-frequency filter of 70 Hz, demonstrating 40–50 mV 1.5 Hz right temporal lateralized periodic discharges (black arrows) with superimposed rhythmic delta activity.
EEG can also serve as an adjunct in diagnosing HSE. Periodic sharp waves are the most specific EEG finding for patients with HSE [18]. When used alone, the specificity of EEG remains low in the diagnosis of HSE; however, it can be helpful when the PCR is negative in patients with a high clinical probability [16]. In our case, the patient's EEG revealed right-sided periodic discharges (Fig. 2b) that correlated with his rhythmic lip smacking movements, which progressed into difficult-to-control epilepsia partialis continua. Electrographic seizures and periodic epileptiform discharges are associated with poor outcome (e.g., severe disability, vegetative state, or death) in patients with central nervous system infections [19].
Empiric treatment with acyclovir is recommended in all patients with suspected encephalitis [10]. Starting this antiviral treatment as early as possible is the most significant factor affecting the outcome of a patient with HSE [20]. We suspect that the lack of therapeutic response to acyclovir in our case was attributable to a delay in antiviral treatment due to the patient presenting so late in the course of the disease.
Conclusions
CSF PCR for HSV has revolutionized the way we diagnose HSE. With a high sensitivity and specificity, HSV PCR results guide the management of HSE. However, it is important to consider the limitations of this test when HSE is highly suspected. Clinical management in these cases should be conducted by clinical suspicion.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Croll BJ, Dillon ZM, Weaver KR, Greenberg MR. MRI diagnosis of herpes simplex encephalitis in an elderly man with nonspecific symptoms. Radiol Case Rep. 2016 Dec;12((1)):159–60. doi: 10.1016/j.radcr.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakob NJ, Lenhard T, Schnitzler P, Rohde S, Ringleb PA, Steiner T, et al. Herpes simplex virus encephalitis despite normal cell count in the cerebrospinal fluid. Crit Care Med. 2012 Apr;40((4)):1304–8. doi: 10.1097/CCM.0b013e3182374a34. [DOI] [PubMed] [Google Scholar]
- 3.Denes E, Labach C, Durox H, et al. Intrathecal synthesis of specific antibodies as a marker of herpes simplex encephalitis in patients with negative PCR. Swiss Med Wkly. 2010;140 doi: 10.4414/smw.2010.13107. w13107. [DOI] [PubMed] [Google Scholar]
- 4.Kaeley N, Bansal S, Bhatia R, Ahmad S. Herpes Simplex Encephalitis: An Uncommon Presentation. J Clin Diagn Res. 2016 May;10((5)):OD25–6. doi: 10.7860/JCDR/2016/19040.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBiasi RL, Kleinschmidt-DeMasters BK, Richardson-Burns S, Tyler KL. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J Infect Dis. 2002 Dec;186((11)):1547–57. doi: 10.1086/345375. [DOI] [PubMed] [Google Scholar]
- 6.Lakeman FD, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995 Apr;171((4)):857–63. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 7.Adler AC, Kadimi S, Apaloo C, Marcu C. Herpes simplex encephalitis with two false-negative cerebrospinal fluid PCR tests and review of negative PCR results in the clinical setting. Case Rep Neurol. 2011 May;3((2)):172–8. doi: 10.1159/000330298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puchhammer-Stöckl E, Presterl E, Croÿ C, Aberle S, Popow-Kraupp T, Kundi M, et al. Screening for possible failure of herpes simplex virus PCR in cerebrospinal fluid for the diagnosis of herpes simplex encephalitis. J Med Virol. 2001 Aug;64((4)):531–6. doi: 10.1002/jmv.1082. [DOI] [PubMed] [Google Scholar]
- 9.Aurelius E, Johansson B, Sköldenberg B, Forsgren M. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J Med Virol. 1993 Mar;39((3)):179–86. doi: 10.1002/jmv.1890390302. [DOI] [PubMed] [Google Scholar]
- 10.Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, et al. Infectious Diseases Society of America The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008 Aug;47((3)):303–27. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 11.Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002 Apr;34((8)):1154–7. doi: 10.1086/339550. [DOI] [PubMed] [Google Scholar]
- 12.McCabe K, Tyler K, Tanabe J. Diffusion-weighted MRI abnormalities as a clue to the diagnosis of herpes simplex encephalitis. Neurology. 2003 Oct;61((7)):1015–6. doi: 10.1212/01.wnl.0000082387.97051.f5. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraju SB, Wurst M, Horvat RT, Selvarangan R. Evaluation of three analyte-specific reagents for detection and typing of herpes simplex virus in cerebrospinal fluid. Diagn Microbiol Infect Dis. 2009 Mar;63((3)):286–91. doi: 10.1016/j.diagmicrobio.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Sauerbrei A, Wutzler P. Laboratory diagnosis of central nervous system infections caused by herpesviruses. J Clin Virol. 2002;25(Suppl 1):S45–51. doi: 10.1016/s1386-6532(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 15.Domingues RB, Tsanaclis AM, Pannuti CS, Mayo MS, Lakeman FD. Evaluation of the range of clinical presentations of herpes simplex encephalitis by using polymerase chain reaction assay of cerebrospinal fluid samples. Clin Infect Dis. 1997 Jul;25((1)):86–91. doi: 10.1086/514494. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shekhlee A, Kocharian N, Suarez JJ. Re-evaluating the diagnostic methods in herpes simplex encephalitis. Herpes. 2006 May;13((1)):17–9. [PubMed] [Google Scholar]
- 17.Granerod J, Davies NW, Mukonoweshuro W, Mehta A, Das K, Lim M, et al. UK Public Health England Aetiology of Encephalitis Study Group Neuroimaging in encephalitis: analysis of imaging findings and interobserver agreement. Clin Radiol. 2016 Oct;71((10)):1050–8. doi: 10.1016/j.crad.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illis LS, Taylor FM. The electroencephalogram in herpes-simplex encephalitis. Lancet. 1972 Apr;1((7753)):718–21. doi: 10.1016/s0140-6736(72)90232-2. [DOI] [PubMed] [Google Scholar]
- 19.Carrera E, Claassen J, Oddo M, Emerson RG, Mayer SA, Hirsch LJ. Continuous electroencephalographic monitoring in critically ill patients with central nervous system infections. Arch Neurol. 2008 Dec;65((12)):1612–8. doi: 10.1001/archneur.65.12.1612. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin DW. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes. 2007 Jun;14((1)):11–6. [PubMed] [Google Scholar]