Abstract
Aim
To describe and review the clinical, radiological, and histopathological characteristics of an orbital perivascular epithelioid cell tumor (PEComa).
Methods
A systematic review of clinical records, radiological investigations, microscopic features, and immunohistochemical characteristics was done.
Results
A 9-year-old female child presented with a year-long history of a large orbital mass associated with painless, progressive proptosis of the right eye. Radiologically, a well-defined orbital mass was seen with no intracranial extension. Excision was performed and histopathological examination showed uniform epithelioid cells in nests separated by thin fibrovascular septae. The tumor cells stained positively for Human Melanoma Black-45, but negatively for desmin, S-100, smooth muscle actin, MyoD1, microphthalmia-associated transcription factor, vimentin, CD10, CD31, and CD34 with a low proliferation index of 5–7%. Based on the tumor's morphological and immunohistochemical characteristics, a diagnosis of giant orbital PEComa was made. No recurrence was seen at the last follow-up.
Conclusions
PEComas are uncommon mesenchymal neoplasms that have typical histological features, with an immunohistochemical profile of negativity for epithelial markers and positivity for melanocytic markers. For benign PEComas, complete excision is advised. However, since PEComas elsewhere in the body have been known to be malignant, a close follow-up of such cases is recommended.
Keywords: Perivascular epithelioid cell tumor, Proptosis, Human Melanoma Black-45, Orbitotomy, Rhabdomyosarcoma, Eye, Tumor
Introduction
Perivascular epithelioid cell tumors (PEComas) are uncommon mesenchymal neoplasms that are believed to have originated from perivascular myoid cells [1, 2]. The term “PEComa” was first used by Bonetti et al. [3] to describe a cell type that was immunoreactive to melanocytic markers and had an epithelioid appearance, clear-acidophilic cytoplasm, and perivascular distribution. The PEComa “family of tumors” includes many neoplasms, namely angiomyolipoma (AML), clear-cell “sugar” tumor of the lung and extrapulmonary sites, lymphangioleiomyomatosis (LAM), clear-cell LAM, clear-cell myomelanocytic tumor of the falciform ligament/ligamentum teres, and abdominopelvic sarcoma of perivascular epithelioid cells [4, 5]. Other tumors with similar morphological and immunoreactivity features at other locations in the body are simply termed “PEComa” tumors or “PEComas” [4]. PEComas (excluding renal AML, LAM, and clear-cell “sugar” tumor of the lung) most commonly involve the retroperitoneal, visceral, abdominal, and pelvic sites. Multiple reports have described PEComas in the gastrointestinal tract, uterus, vulva, heart, breast, common bile duct, urinary bladder, and a variety of head and neck sites [6]. However, in the ophthalmic literature, there are only 9 cases of PEComas documented, of which 7 were located in the orbit and in 2 were located intraocularly [1, 2, 7-12].
Here we describe in detail the clinical and histopathological features of a giant orbital PEComa in a 9-year-old girl. We also review the available literature on PEComas of the eye and adnexa.
Case Report
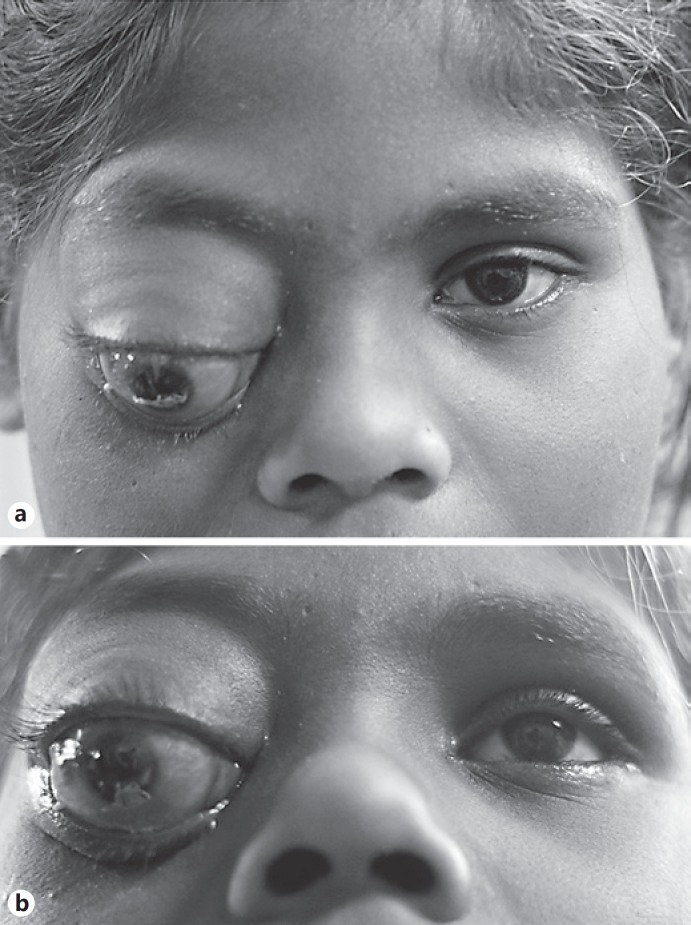
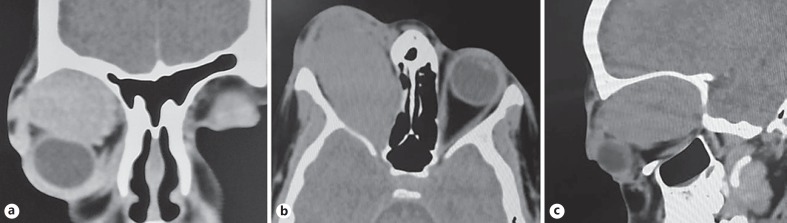
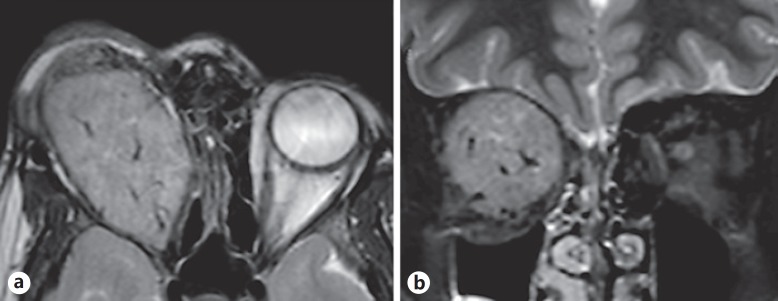
A 9-year-old girl presented to the ophthalmology department with a year-long history of a painless, progressively growing mass above the right eye. On examination, a large mass could be palpated between the superior orbital rim and the globe. The right eye was grossly proptotic (Fig. 1a) and inferiorly displaced (Fig. 1b). The cornea showed signs of exposure keratopathy. The vision recorded in the right eye was counting fingers at 1 m. The pupil was fixed and nonreactive to light, with a gross relative afferent pupillary defect present. On dilated examination the right eye demonstrated choroidal folds superiorly suggestive of indentation. The optic disc was pale. The left eye was unremarkable with normal vision, intraocular pressure, pupillary reflexes, and motility as well as a healthy fundus. No regional lymphadenopathy was observed. A computed tomography scan of the orbit showed a large, well-encapsulated, homogenously enhancing hypodense lesion in the right orbit situated superiorly (Fig. 2a), above the globe extending from beyond the anterior orbital rim to the orbital apex posteriorly (Fig. 2c). The lesion had caused obvious gross expansion of the right orbit and bowing of the medial orbital wall with resultant effacement of the right ethmoidal air cells (Fig. 2b). Laterally, there was scalloping of the greater wing of the sphenoid. Magnetic resonance imaging showed the lesion to be isointense on T1-weighted images and hyperintense on T2/STIR images. No evidence of restricted diffusion was seen. The lesion also showed patchy early arterial enhancement with intense, homogenous enhancement in delayed images. Flow voids were also seen, with no intracranial extension of the mass (Fig. 3a, b). Radiologically the mass measured 52 × 35 × 28 mm. Considering the age of the child and the location of the tumor, the differential diagnoses considered at that time included rhabdomyosarcoma (RMS), alveolar soft-part sarcoma (ASPS), Ewing's sarcoma/primitive neuroectodermal tumor, and orbital metastases. However, a whole-body scan failed to show any other metabolically active lesion elsewhere in the body, ruling out orbital metastases as a diagnosis. Therefore, an incisional biopsy was planned to ascertain the diagnosis. Intraoperatively, the tumor was well encapsulated with a smooth surface externally. A transseptal approach was taken through the upper lid, and multiple small bits of the tumor were obtained from the superficial and deep aspects of the tumor and sent for histopathological assessment.
Fig. 1.
External photographs of the child showing a large mass in the right orbit superior to the globe, causing proptosis (a) and gross inferior displacement of the globe (b).
Fig. 2.
Computed tomography scans of the orbit showing a large, well-encapsulated, homogenously enhancing hypodense lesion in the right orbit situated superiorly (a), above the globe extending from beyond the anterior orbital rim to the orbital apex posteriorly (c). Note the expansion of the right orbit and bowing of the medial orbital wall with resultant effacement of the ethmoidal air cells (b).
Fig. 3.
a T2-weighted magnetic resonance images showing the well-defined mass with small sinusoidal flow voids indicating its highly vascular nature. b The coronal image shows the large size of the tumor, which has completely compressed all apical structures.
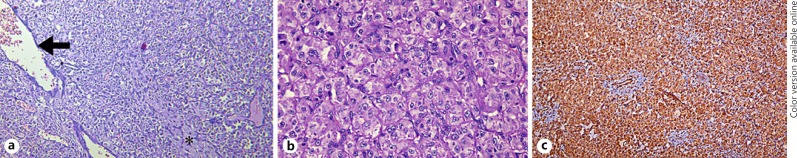
On microscopic examination, uniform epithelioid cells were seen, arranged in nests separated by thin fibrovascular septae (Fig. 4a). The tumor cells had eosinophilic to clear cytoplasm and vesicular nuclei with relatively small nucleoli. A prominent, thin-walled vascular meshwork was noted surrounding the tumor cells (Fig. 4b). At places, the tumor cells were arranged in a concentric pattern around the vessels and stromal hyalinization was also seen focally. There was no evidence of cytological atypia, increased mitotic activity, or necrosis. No spindle cells or giant cells were seen. A broad immunohistochemistry panel was used to narrow the diagnosis: the tumor cells stained negative for desmin, S-100, smooth muscle actin, MyoD1, microphthalmia-associated transcription factor, vimentin, CD10, CD31, and CD34. However, the cells in the vascular meshwork stained positive for CD34. The Ki-67 index was low with less than 5–7% of the cells staining. The tumor cells showed a strong immunopositivity for Human Melanoma Black-45 (HMB-45) (Fig. 4c). Therefore, going by the cellular structure and the immunohistochemistry staining patterns, a diagnosis of PEComa of the orbit was made and total excision of the mass was planned.
Fig. 4.
a Sheets of tumor cells. The figure also shows focal hyalinization (asterisk) in some areas alongside endothelium-lined vascular channels (arrow) with red blood cells within. Hematoxylin-eosin, ×100. b Classical appearance of the perivascular epithelioid cell: eosinophilic to clear cytoplasm and vesicular nuclei with relatively small nucleoli. A thin-walled vascular meshwork can be seen surrounding the tumor cells. Hematoxylin-eosin, ×400. c Strong immunoreactivity to Human Melanoma Black-45 (×100).
A large sub-brow incision was made and the superior orbital rim was visualized after dissecting through the orbicularis muscle. An incision was made 4 mm above the rim on the periosteum and a subperiosteal approach was advanced; the dissection was continued posteriorly. The structures passing through the superior orbital fissure were spared and the mass was removed in toto with the help of a cryoprobe. The mass was well encapsulated with multiple feeder vessels seen supplying the tumor; significant blood loss (>350 mL) was encountered. Pathological examination confirmed the earlier histological diagnosis. The patient had no signs or family history of tuberous sclerosis. At the last follow-up at 6 months, no recurrence was seen.
Discussion
Iyengar et al. [7] described the first case of an orbital PEComa in a 9-year-old female child who had a mass growing inferomedially in the right orbit. Subsequently, there were 6 other cases of orbital PEComas reported in the literature [2, 8, 9, 11, 12]. Additionally, there were 2 reported cases of PEComas arising from the ciliary body [1, 8]. The clinical features and immunohistochemical findings of these cases are summarized in Table 1.
Table 1.
Clinical features and immunohistochemical findings of all previously reported perivascular epithelioid cell tumors arising from the eye and orbit
| Reference | Age, years/sex | Clinical presentation | Location/dimensions | Treatment | Immunohistochemistry |
Status at last follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HMB-45 | melan-A | TFE3 | SMA | desmin | S-100 | vimentin | MITF | others | CD10 | CD31 | CD68 | ||||||
| Iyengar et al., 2005 [7] | 9/F | painless progressive swelling |
right inferomedial orbit/12×10×8 mm | excision | + | - | NA | + | - | - | - | NA | calponin +, tyrosinase + | NA | NA | NA | NR |
| Guthoff et al., 2008 [2] | 54/M | painless, progressive swelling |
right inferotemporal orbit/15×10×10 mm | excision | + | + | NA | NA | - | - | - | - | - | + | - | + | NR |
| Furusato et al., 2010 [8] (case 1) | 26/F | painless, progressive swelling |
left superolateral orbit/20×17×14 mm | excision | + | + | + | + | + | - | NA | - | - | NA | NA | NA | NR |
| Furusato et al., 2010 [8] (case 2) | 7/M | NA | left ciliary body/13×10 mm | excision | + | - | - | + | + | - | NA | - | - | NA | NA | NA | NR |
| Goto et al., 2015 [1] | 13/M | reduced vision, exotropia | right ciliary body/10×11×11 mm | excision | + | + | + | + | - | - | NA | NA | NSE –, GFAP– | NA | NA | NA | NR |
| Lubo et al., 2016 [9] | 47/M | progressive diplopia |
left inferomedial orbit/20×15 mm | excision | + | - | + | + | - | - | - | + | calponin + | - | - | NA | NR |
| Lin et al., 2016 [11] | 80/F | painless, progressive swelling |
left superomedial orbit/18×18×13 mm | excision | - | - | NA | NA | NA | NA | NA | NA | CD34 +, CK +, HHF-35 + | NA | NA | NA | NR |
| Paliogiannis et al., 2017 [10] | 46/M | painful swelling |
right medial orbit/15 mm (diameter) | excision | + | - | NA | + | NA | - | - | NA | cathepsin K + | NA | NA | NA | NR |
| Alam et al., 2017 [12] | 5/M | painless, progressive swelling |
right medial orbit/»50 mm along greatest dimension | excision plus CTx1 | + | NA | NA | + | NA | - | + | - | CD34 +, CK – synaptophysin – | NA | NA | NA | NR1 |
| Current case, 2017 | 9/F | painless, progressive swelling, reduced vision, proptosis | right superior orbit/52×35×28 mm | excision | + | - | NA | - | - | - | - | - | MyoD1 –, CD34 – | - | - | NA | NR |
CD, cluster of differentiation; CK, cytokeratin; CTx, chemotherapy; GFAP, glial fibrillary acidic protein; HHF-35, muscle actin antibody; HMB-45, Human Melanoma Black-45; MITF, microphthalmia-associated transcription factor; NR, no recurrence; NSE, neuron-specific enolase; SMA, smooth muscle actin; TFE3, transcription factor E3. 1 Recurrence was seen after 5 cycles of CTx following which re-excision was done. At last follow-up, the authors reported no recurrence.
Orbital PEComas presented as slow-growing, painless masses in most cases. Paliogiannis et al. [10] reported the lone case of an orbital PEComa where the patient presented with pain as a symptom. With increasing size, intraorbital masses can cause a pressure effect on the surrounding extraocular muscles, resulting in restricted ocular motility and diplopia [9]. In our case, the mass was extremely large, measuring over 50 mm anteroposteriorly, and therefore caused restricted ocular motility and optic neuropathy due to stretching of the nerve, as was evident on the scans.
Radiologically, orbital PEComas usually appear well circumscribed, with enhancement on administration of contrast on computed tomography imaging [2, 7, 8]. Magnetic resonance imaging also yields similar findings: PEComas usually are well-circumscribed lesions, and after infusion of contrast medium, the lesion shows a heterogeneous density reflecting hypervascularity, suggestive of a benign mass with a high vascular component, as was also seen in our case [9]. Alam et al. [12] reported a “fluid-fluid level” in the magnetic resonance imaging scans of an orbital PEComa. Occasionally, angiolipomatous tumors may exhibit heterogeneity owing to internal areas of fat attenuation [11]. While ultrasound imaging has been used to image orbital lesions, the findings tend to be nonspecific and noncontributory to the eventual management [2]. It may however be of more use while imaging intraocular PEComas [1]. Clinical findings and radiological characteristics remain ancillary in the diagnosis of PEComas, which essentially is based on histopathological and immunohistochemical features.
The histological features of PEComas are distinct: they predominantly have epithelioid cells, although in many cases spindle cells are also seen. The epithelioid cells tend to be round or oval, but the spindle cells appear elongated. The cells have a clear to eosinophilic cytoplasm that is rich in glycogen, described by some authors as “moth-eaten” cytoplasm [8]. The tumor cells are arranged in varying patterns: a combination of nested, fascicular, and sheet-like growth patterns are commonly observed. The nuclei are vesicular and have inconspicuous nucleoli [6]. While uniformity is the norm, PEComas may occasionally exhibit pleomorphism and atypia. Scattered giant cells may also be seen [8]. Other histological variations include myxoid change, stromal microcysts, and “spider” cells. One series reported that a rather large proportion of PEComas in females (19%) had extensive stromal hyalinization [13]. In our case, stromal hyalinization was only focally observed and not extensive. Mitotic figures are uncommon, although malignant variants of PEComas have been described where they might be encountered abundantly [12]. Mitotic figures and necrosis appear to be reliable predictors of clinical outcome [14].
On immunohistochemistry, the pathognomonic features of PEComas are HMB-45 and MART1 expression and no cytokeratin or S-100 protein expression [15]. In fact, HMB-45, melan-A, and microphthalmia-associated transcription factor are the most sensitive melanocytic markers for the diagnosis of PEComa [4, 16]. Folpe et al. [14] reviewed 26 PEComas of soft tissue and gynecologic origin and noted the expression of at least one melanocytic marker in all of their cases. Of these markers, HMB-45 was found to be the most sensitive marker, staining tumor cells in 92% of the reviewed cases, followed by melan-A (72%). Non melanocytic markers, namely smooth muscle actin and desmin, were expressed in 80 and 36% of the cases, respectively. Most of the reported orbital PEComas, including our case, showed strong positivity for HMB-45; however, immunopositivity for melan-A was seen in only 2 of the 7 orbital lesions [2, 8]. The sole exception was the orbital AML reported by Lin et al. [11] which was negative for HMB-45 and melan-A. The HMB-45 negativity may be explained by the rarity of the epithelioid cells in these cases, and the HMB-45 positivity is often weaker or absent in spindle cells, which were predominant in that case. Only one of the orbital PEComas showed positive results on staining with microphthalmia-associated transcription factor [9]. The muscle-specific markers showed variable positivity: immunopositivity for smooth muscle actin was seen in 5/8 of the orbital PEComas, whereas positivity for desmin was reported in only 1 case, and only 1 of the cases reported immunoreactivity for vimentin [12]. Immunopositivity for HHF-35 (muscle actin antibody) was seen in 1 case only [11]. While PEComas occasional express S-100 protein, desmin, and cytokeratin, none of the orbital cases reported any positivity for S-100. Calponin, an actin filament-associated regulatory protein, is essentially a muscle marker and typically stains smooth muscles, myoepithelial cells, myofibroblasts, and choroidal nonvascular smooth muscle cells. Although it is not a common- ly employed marker, the cases reported by Iyengar et al. [7] and Lubo et al. [9] stained positive for calponin. PEComas with predominantly spindle cells tend to express myoid markers more strongly than melanocytic markers [4]. Tyrosinase is yet another melanocytic marker that PEComas may express [7]. In renal PEComas, cathepsin K has been reported to be constantly and strongly expressed much more than other commonly used markers for their identification, although only one of the orbital PEComas in the literature was reported to have a positive result for this marker [10, 17].
RMS is the most common malignant orbital tumor of childhood. Clinically, a long-standing orbital RMS would have shown clinical and radiological signs associated with aggressive orbital malignancies such as bony erosion of the orbital walls, intracranial extension, or regional or distant metastases, which our case lacked. Patients with orbital RMS usually present with proptosis that develops rapidly over a matter of weeks [18]. Furthermore, radiologically, the tumor was extremely well circumscribed, which is not usually seen in advanced RMSs of this size. On histopathological examination, the classical round cells and spindle muscle cells seen in RMS were not found in our case. RMS stains positively for MyoD1, desmin, and less frequently for vimentin, all of which were not seen here, conclusively ruling out both RMS and rhabdomyoma, an exceedingly rare, benign, orbital tumor with immunohistochemical characteristics similar to those of RMS [19].
ASPS was also considered as one of the differential diagnoses [12]. Morphologically, ASPS (especially the solid type) may resemble PEComas in some ways. Furthermore, the orbit is a relatively common location for ASPS and is also seen in the pediatric age group [20, 21]. However, the cytoplasmic crystals encountered in >80% of ASPSs are absent in PEComas [8]. On immunohistochemistry, ASPSs often show TFE3 nuclear expression, and while they may show positivity for muscle markers, they classically lack expression of melanocytic markers, which in this case ruled out ASPS [6]. Furusato et al. [8] succinctly enumerated the various characteristics that help differentiate PEComas from ASPSs: the cellular structure and arrangement, the degree of atypia, the intratumoral vascular pattern, the cytoplasmic crystals, the expression of melanocytic markers, and the associated genetic mutations.
The possibilities of other rare tumors – such as hemangiopericytoma, a term which is now being replaced by “solitary fibrous tumor”, and orbital melanoma – were also entertained. However, the classical picture seen are solitary fibrous tumors: monotonous cellular proliferation without significant variability in cellularity and minimal collagenization was not seen in this case [22]. More importantly, hemangiopericytomas/solitary fibrous tumors are composed of ovoid to spindle-shaped cells separated by sinusoidal spaces, the so-called “staghorn” pattern, which was not observed [23]. Other factors that helped rule out an orbital melanoma were the lack of S-100 protein staining, significant cytological atypia, and mitotic activity [8].
With respect to orbital PEComas, the other differentials discussed in the literature include epithelioid hemangioma, paraganglioma, lymphangioma, and metastasis from tumors with low malignant potential, such as renal cell carcinoma [9, 12]. However, the differential diagnosis of clear-cell tumors, which include tumors of the lung, kidney, or female genital tract, was ruled out in the absence of epithelial markers. Angiomatous tumors were also ruled out owing to negativity to CD31 and CD34. Therefore, based on the cellular morphology, clinical history, radiological findings, and immunohistochemical staining pattern available, the diagnosis of a giant orbital PEComa was made.
The basic unit of a PEComa, the perivascular endothelial cell itself, has no normal counterpart, and the origin and function of this cell type remain unknown [3, 4, 7]. As stated earlier, while most PEComas are benign, malignant PEComas have also been described in the literature, and various anatomical sites have been reported to harbor malignant PEComas, such as the uterus, gastrointestinal tract, retroperitoneum, and bladder among other sites [4]. Metastases from such malignant tumors have also been reported [24]. It is considered that small tumors (<5 cm in diameter) with atypia but no mitoses appear to be benign. Furthermore, large size might also be an adverse prognostic factor, as a tumor >5 cm can be regarded as of uncertain malignant potential [13, 24]. In our case, in spite of the tumor size being >5 cm, microscopically no signs of atypia or malignancy were seen. Other parameters that may be indicative of a malignant potential in PEComas include infiltrative growth, high nuclear grade and hypercellularity, ≥1 mitoses per 50 high-power fields, necrosis, and vascular invasion [13, 25]. With only a handful of cases of orbital PEComas in the literature, there are no clear guidelines for their management. While excision followed by close follow-up is necessary in benign PEComas, additional chemotherapy may help in preventing recurrence in malignant cases [12]. Proliferation markers such as MIB-1 and Ki-67 are used to assess the malignancy potential of tumors. Iyengar et al. [7] reported that the MIB-1 was positive in <10% of the cells in their case. In our case, the Ki-67 index was also low, with only 5–7% of the cells staining for the protein, indicating that we were indeed dealing with a benign tumor.
PEComas are associated with tuberous sclerosis complex (TSC). Bonetti and colleagues [3, 26] postulated that PEComa may be a forme fruste of tuberous sclerosis. While there is an association of AML and LAM with TSC, this association has been documented in <10% of patients with PEComas of soft tissue and gynecologic origin [13]. In our case, neither the patient nor other family members exhibited any of the features of tuberous sclerosis.
The genes that undergo mutations in TSC are TSC1 (27%) and TSC2 (73%), which are present on chromosomes 9q34 and 16p13.3, respectively. These genes relate to enzymes involved in catecholamine metabolism and melanin formation [4, 27]. Rearrangements on the TFE3 gene have also been recently reported in PEComas [28]. However, these changes that eventually lead to cell growth and proliferation have been studied in TSC-associated renal tumors, sporadic AML, LAM, and also multifocal micronodular pneumocyte hyperplasia. The clinical implications of these genetic studies in sporadic, non-TSC-associated PEComas remain unclear.
In summary, we report a rare case of giant orbital PEComa in a 9-year-old female child. PEComas, although commonly seen elsewhere in the body, are extremely rare in the orbit. With no nonmalignant counterpart to the perivascular epithelioid cell itself, PEComas have distinct histological appearance with a typical immunohistochemical staining profile, on the basis of which they are diagnosed. It is important to identify other features of TSC, if present, in patients with PEComas. Adjunct studies such as ultrastructural assessment and molecular genetic studies may aid in the diagnosis and also be useful for ruling out other possible diagnostic entities [7]. It has been recommended by some authors that despite exceeding 5 cm in diameter, since the tumor cells lack significant mitotic activity and necrosis after thorough sampling, they can be regarded as of uncertain malignant potential. In such cases, close follow-up is advised, without the need for any further adjuvant therapy [4].
Statement of Ethics
The patient's photographs were taken after obtaining written informed consent from her parents, explicitly explaining that the patient would be identifiable through the images. The provisions of the Declaration of Helsinki for research involving human subjects were adhered to; no animal research was conducted as part of this work.
Disclosure Statement
None of the authors have any financial interests to disclose.
Acknowledgments
The authors wish to acknowledge the contribution of Dr. Divakant Misra, Sankaradeva Nethralaya, Guwahati, India; Dr. Neha Rathi, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, India; and Dr. Veena R. Iyer, Mayo Clinic, Rochester, MN, USA.
References
- 1.Goto H, Usui Y, Nagao T. Perivascular epithelioid cell tumor arising from ciliary body treated by local resection. Ocul Oncol Pathol. 2015;1:88–92. doi: 10.1159/000369330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthoff R, Guthoff T, Mueller-Hermelink HK, Sold-Darseff J, Geissinger E. Perivascular epithelioid cell tumor of the orbit. Arch Ophthalmol. 2008;126:1009–1011. doi: 10.1001/archopht.126.7.1009. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19:359–368. doi: 10.1016/j.anndiagpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119–132. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandhlish A, Leon Barnes E, Rabban JT, McHugh JB. Perivascular epithelioid cell tumors (PEComas) of the head and neck: report of three cases and review of the literature. Head Neck Pathol. 2011;5:233–240. doi: 10.1007/s12105-011-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar P, Deangelis DD, Greenberg M, Taylor G. Perivascular epithelioid cell tumor of the orbit: a case report and review of the literature. Pediatr Dev Pathol. 2005;8:98–104. doi: 10.1007/s10024-004-5055-0. [DOI] [PubMed] [Google Scholar]
- 8.Furusato E, Cameron JD, Newsom RW, Fujishiro T, Kojima T, Specht CS, Fetsch JF, Furusato B, Sesterhenn IA, Rushing EJ. Ocular perivascular epithelioid cell tumor: report of 2 cases with distinct clinical presentations. Hum Pathol. 2010;41:768–772. doi: 10.1016/j.humpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Lubo I, Fermín I, Massarelli O, Gobbi R, Cossu Rocca., P Perivascular epithelioid cell tumour with intraorbital location: report of a case and review of the literature. Case Rep Pathol. 2016;2016:1936421. doi: 10.1155/2016/1936421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paliogiannis P, Palmieri G, Tanda F, Cossu A. Perivascular epithelioid cell tumors (PEComas) of the orbit. J Pathol Transl Med. 2017;51:7–8. doi: 10.4132/jptm.2016.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CY, Tsai CC, Kau HC, Kao SC, Liu CJ. HMB-45 negative angiomyolipoma of the orbit: a case report and review of the literature. BMC Ophthalmol. 2016;16:8. doi: 10.1186/s12886-016-0185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam MS, Mukherjee B, Krishnakumar S, Biswas J. Malignant perivascular epithelioid cell tumor of the orbit: report of a case and review of literature. Indian J Ophthalmol. 2017;65:889–891. doi: 10.4103/ijo.IJO_331_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornick JL, Fletcher CD. Sclerosing PEComa: clinicopathologic analysis of a distinctive variant with a predilection for the retroperitoneum. Am J Surg Pathol. 2008;32:493–501. doi: 10.1097/PAS.0b013e318161dc34. [DOI] [PubMed] [Google Scholar]
- 14.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 15.Vang R, Kempson RL. Perivascular epithelioid cell tumor (“PEComa”) of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26:1–13. doi: 10.1097/00000478-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Chang KL, Folpe AL. Diagnostic utility of microphthalmia transcription factor in malignant melanoma and other tumors. Adv Anat Pathol. 2001;8:273–275. doi: 10.1097/00125480-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Martignoni G, Bonetti F, Chilosi M, Brunelli M, Segala D, Amin MB, Argani P, Eble JN, Gobbo S, Pea M. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod Pathol. 2012;25:100–111. doi: 10.1038/modpathol.2011.136. [DOI] [PubMed] [Google Scholar]
- 18.Jurdy L, Merks JH, Pieters BR, Mourits MP, Kloos RJ, Strackee SD, Saeed P. Orbital rhabdomyosarcomas: a review. Saudi J Ophthalmol. 2013;27:167–175. doi: 10.1016/j.sjopt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li YP, Nie L, Zang WX, Lin JX, Yang HS, Feng GG. Rhabdomyoma of the orbit. J Pediatr Ophthalmol Strabismus. 2008;45:113–115. doi: 10.3928/01913913-20080301-15. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Wojno T, Grossniklaus HE, Shehata BM. Alveolar soft-part sarcoma of the orbit: report of 2 cases with review of the literature. Ophthal Plast Reconstr Surg. 2013;29:e138–e142. doi: 10.1097/IOP.0b013e318281ecb9. [DOI] [PubMed] [Google Scholar]
- 21.Mulay K, Ali MJ, Honavar SG, Reddy VA. Orbital alveolar soft-part sarcoma: clinico-pathological profiles, management and outcomes. J Cancer Res Ther. 2014;10:294–298. doi: 10.4103/0973-1482.136570. [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith JD, van de Rijn M, Syed N. Orbital hemangiopericytoma and solitary fibrous tumor: a morphologic continuum. Int J Surg Pathol. 2001;9:295–302. doi: 10.1177/106689690100900406. [DOI] [PubMed] [Google Scholar]
- 23.Pacheco LF, Fernandes BF, Miyamoto C, Maloney SC, Arthurs B, Burnier MN., Jr Rapid growth of an orbital hemangiopericytoma with atypical histopathological findings. Clin Ophthalmol. 2014;8:31–33. doi: 10.2147/OPTH.S47901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanai H, Matsuura H, Sonobe H, Shiozaki S, Kawabata K. Perivascular epithelioid cell tumor of the jejunum. Pathol Res Pract. 2003;199:47–50. doi: 10.1078/0344-0338-00353. [DOI] [PubMed] [Google Scholar]
- 25.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Martignoni G, Pea M, Rocca PC, Bonetti F. Renal pathology in the tuberous sclerosis complex. Pathology. 2003;35:505–512. doi: 10.1080/00313020310001619136. [DOI] [PubMed] [Google Scholar]
- 27.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 28.Agaram NP, Sung YS, Zhang L, Chen CL, Chen HW, Singer S, Dickson MA, Berger MF, Antonescu CR. Dichotomy of genetic abnormalities in PEComas with therapeutic implications. Am J Surg Pathol. 2015;39:813–825. doi: 10.1097/PAS.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]