Abstract
Due to high complexity, vitreoretinal surgery presents a higher number of patient safety incidents compared with other ophthalmic procedures. Intraocular gases are one of the most useful adjuncts to vitrectomy and surprisingly, surgeons commonly admit to having occasional problems with incorrect gas concentration. The aim of this study is to present a consecutive case series of patients with improper concentration of sulfur hexafluoride (SF6) applied during vitrectomy. Three patients underwent 27-gauge vitrectomy and at the end of surgery inappropriate dilution of 100% SF6 was administered. It was attributed to a calculation error, change in the gas supplier, or increased partial pressure of SF6 before dilution. Postoperatively, due to IOP increase, two eyes required intravitreal gas-air exchange. Subsequently, cataract surgery was performed in one eye with concomitant vitrectomy and silicone oil tamponade due to retinal detachment. To prevent such complications, we suggest using intraocular gases with great care, training of ophthalmic personnel, and prompting manufacturers to provide SF6 in a prepared concentration of 20%.
Keywords: Expandable gas, Intraocular pressure, Intraocular tamponade, Sulfur hexafluoride, Vitrectomy
Introduction
Intraocular gases are one of the most useful adjuncts in vitreoretinal surgery. Sulfur hexafluoride (SF6) has been used as a substitute for air in eyes requiring intraocular tamponade for over 40 years [1]. Complications related to the use of expandable gases include intraocular pressure (IOP) elevation, cataract development, and migration of the gas bubble to the subretinal space or subconjunctivally. The aim of this study was to present a consecutive case series of patients who underwent vitrectomy between November 2015 and December 2017 at the Department of Ophthalmology, Elbląg City Hospital, or the Department of Ophthalmology, Medical University of Gdańsk, Poland, with an inappropriate concentration of SF6 applied.
Case Reports
Patient data are summarized in Table 1. The first patient presented with an epiretinal membrane, and subsequently underwent vitrectomy with membrane peel in her left eye. As a result of creating an iatrogenic break in the retina intraoperatively, fluid-air exchange was performed. At the end of the surgery the eye was filled with 100% SF6 instead of the planned concentration of 20%. The reason was the change in gas supplier. The nurse was not properly trained that the new batch of gases was not 20 but 100%, and gave such information to the surgeon without double-checking. On the next day the IOP was 28 mm Hg, and as it was normalized with topical treatment the patient was discharged from the hospital with topical dorzolamide, timolol, and brimonidine prescribed. Regardless of the administered medications, in the following days the IOP was over 30 mm Hg. Six days after vitrectomy she underwent an exchange of 5 mL of intravitreal gas to sterile air. The IOP normalized, and the remaining gas lasted in the vitreous cavity for about 2 weeks. Due to the development of a cataract, the patient underwent phacoemulsification cataract surgery 2 months later.
Table 1.
Summary of patient data
| Patient | Age, years | Sex | Diagnosis | Vitrectomy gauge | Sclerotomies sutured | Planned SF6 concentration | Final SF6 concentration |
|---|---|---|---|---|---|---|---|
| 1 | 65 | female | epiretinal membrane, intraoperative retinal break | 27 | 2/3 | 20% | 100% |
| 2 | 60 | male | epiretinal membrane, macular hole | 27 | 1/3 | 20% | 40% |
| 3 | 67 | female | unclosed macular hole | 27 | 3/3 | 20% | unknown |
SF6, sulfur hexafluoride.
The second patient had an uneventful vitrectomy for epiretinal membrane and macular hole. However, he received 40% SF6 instead of the intended 20% concentration. A mistake in calculation took place, which was revealed by the assistant after the surgery; 20 mL of 100% SF6 was taken into a 50-mL syringe and subsequently diluted to 50 mL with sterile air, with a final concentration of 40%. Properly done, 10 mL of SF6 should be exhausted and the remaining 10 mL should be diluted to 50 mL with sterile air, achieving the concentration of 20%. Nevertheless, the IOP following vitrectomy was normal.
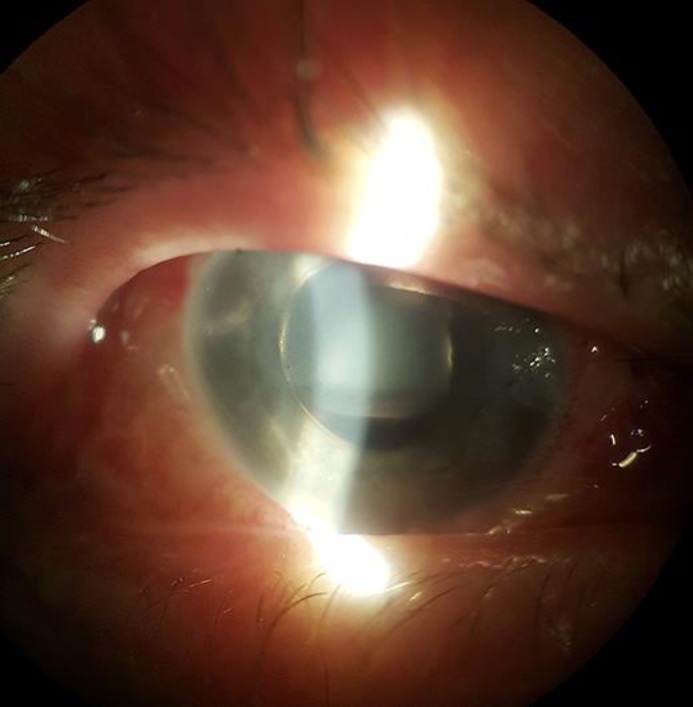
The third patient, a 67-year-old woman, presented with a stage IV full-thickness macular hole in her left eye. She underwent 23-gauge vitrectomy with internal limiting membrane peeling and 20% SF6 tamponade. As a result of nonclosure of the macular hole, she decided to undergo a second vitrectomy after 16 months. A 27-gauge vitrectomy was performed with removal of the remnants of the internal limiting membrane, draining of fluid from the macular hole, fluid air exchange, and administration of a 20% SF6 compound. After the surgery the patient complained of a severe headache and nausea, with the IOP reaching 45 mm Hg. With intravenous mannitol, topical dorzolamide, timolol, and brimonidine the IOP decreased to 38 mm Hg; however, the pain sustained. Two days after vitrectomy the surgeon in charge decided to perform a gas-air exchange. Although the IOP lowered, the eye fundus examination revealed retinal detachment with an iatrogenic retinal tear. Two days later the patient underwent phacovitrectomy and the eye was filled with 1,000 cSt of silicone oil. The reason for the unexpected gas expansion cannot be confirmed. The IOP was increased primarily in an open-angle mechanism. Pathology of the lens-iris diaphragm was excluded at all stages, and the patient had no complications in the primary vitrectomy with 20% SF6 tamponade. It is presumed that increased gas expansion was due to unanticipated SF6 concentration, when 10 mL of SF6 from a large tank of 125 g was taken into a 50-mL syringe through a reducer. Although SF6 was diluted to 50 mL with sterile air, the pressure of the SF6 in the syringe, and the final concentration of the gas could not be determined. Slit lamp examination of a patient with significant IOP increase after SF6 tamponade is demonstrated in Figure 1.
Fig. 1.
Slit lamp examination of a patient with significant intraocular pressure increase after SF6 tamponade. Ballooning of the bulbar conjunctiva, corneal edema, and diffuse shallowing of the anterior chamber is observed (photo by the author).
Discussion and Conclusions
Elevated IOP after vitrectomy may cause optic nerve damage, retinal ischemia, and subsequent visual loss. If a 30% SF6 tamponade is applied, up to 20.4% of patients might experience IOP over 30 mm Hg on postoperative day 1 [2]. The risk of increased IOP is associated with expansile gas concentration, concomitant circumferential scleral buckling, and patients' age [3]. SF6 is supplied within different concentrations ranging from 20 to 100%. Single- or multiple-use systems are available, in low- or high-pressure containers with reducers. In practice, the final concentration used during vitrectomy is at the surgeon's discretion, ranging from 18 to 30%; for example, in the UK a 20% compound is most commonly applied [4]. A 100% concentration of SF6 is used for retinal detachment pneumatic retinopexy [5], displacement of submacular hemorrhages secondary to choroidal neovascular membrane, and retinal arterial macroaneurysm [6]. In recent years the preference for retinal detachment repair shifted among surgeons toward vitrectomy with a decline in pneumatic retinopexy; thus 100% SF6 is used less frequently [7]. Although uncomplicated vitreoretinal procedures with normal preoperative IOP and no gas tamponade are unlikely to have uncontrolled IOP [2], one should not attribute postoperative IOP spikes solely to inappropriate gas concentration. Other common reasons include postoperative inflammation and angiogenic, steroid-induced, and blood-mediated mechanisms [8].
Due to high complexity, vitreoretinal surgery results in a higher number of patient safety incidents compared with other ophthalmic procedures [9]. Furthermore, a large percentage of diseases managed by vitreoretinal specialists have potential for severe visual impairment or blindness, thus presenting a risk for malpractice litigation [10]. The described cases highlight the danger associated with use of concentrated SF6 to the patients' visual function. A case of inappropriate percentage and volume of gas injected into an eye having a concurrent scleral buckling procedure was reported recently [9]. Surprisingly, vitreoretinal surgeons commonly admit to having occasional problems with incorrect gas concentration.
When faced with high IOP due to an enlarging gas bubble a treatment option is to tap the gas in the outpatient clinic. Intravenous mannitol is known to work suboptimally in vitrectomized eyes. The increase in IOP in the presented cases cannot be attributed to sclerotomy suturing, and an advantage of nonsuturing is allowing a free passage of air/gas if IOP is elevated. However, leaving leaking sclerotomies unsutured can result in hypotony and attendant complications. Another solution for preventing the aforementioned complications is using intraocular gases with great care and training of ophthalmic personnel in their proper application. This should be employed in every setting, although it is not possible to completely eliminate human errors. Thus, another concept would be prompting the manufacturers to provide SF6 solely in a prepared concentration of 20%.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that they have no competing interests. Dr. Kanclerz reports nonfinancial support from Visim. Dr. Grzybowski reports grants, personal fees, and nonfinancial support from Bayer, nonfinancial support from Novartis, Alcon, Thea, and Santen, and personal fees and nonfinancial support from Valeant, outside the submitted work. No funding has been received for this study.
References
- 1.Norton EW. Intraocular gas in the management of selected retinal detachments. Trans Am Acad Ophthalmol Otolaryngol. 1973 Mar-Apr;77((2)):OP85–98. [PubMed] [Google Scholar]
- 2.Wong R, Gupta B, Williamson TH, Laidlaw DA. Day 1 postoperative intraocular pressure spike in vitreoretinal surgery (VDOP1) Acta Ophthalmol. 2011 Jun;89((4)):365–8. doi: 10.1111/j.1755-3768.2009.01703.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen TC. Risk Factors for Intraocular Pressure Elevations After Pupillary Dilation in Patients With Open Angles. Ann Ophthalmol. 2005;37:069–076. [Google Scholar]
- 4.Kontos A, Tee J, Stuart A, Shalchi Z, Williamson TH. Duration of intraocular gases following vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2017 Feb;255((2)):231–6. doi: 10.1007/s00417-016-3438-3. [DOI] [PubMed] [Google Scholar]
- 5.Assi A, Gregor Z. The use of intravitreal gases in non-vitrectomised eyes. Eye (Lond) 2001 Aug;15((Pt 4)):497–506. doi: 10.1038/eye.2001.162. [DOI] [PubMed] [Google Scholar]
- 6.Abdelkader E, Yip KP, Cornish KS. Pneumatic displacement of submacular haemorrhage. Saudi J Ophthalmol. 2016 Oct-Dec;30((4)):221–6. doi: 10.1016/j.sjopt.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin MD, Hwang JC. Trends in Vitreoretinal Procedures for Medicare Beneficiaries, 2000 to 2014. Ophthalmology. 2017 May;124((5)):667–73. doi: 10.1016/j.ophtha.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Costarides AP, Alabata P, Bergstrom C. Elevated intraocular pressure following vitreoretinal surgery [v.] Ophthalmol Clin North Am. 2004 Dec;17((4)):507–12. doi: 10.1016/j.ohc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Wong SC, Kelly SP, Sullivan PM. Patient safety in vitreoretinal surgery: quality improvements following a patient safety reporting system. Br J Ophthalmol. 2013 Mar;97((3)):302–7. doi: 10.1136/bjophthalmol-2012-301988. [DOI] [PubMed] [Google Scholar]
- 10.Kraushar MF. Medical malpractice experiences of vitreoretinal specialists: risk prevention strategies. Retina. 2003 Aug;23((4)):523–9. doi: 10.1097/00006982-200308000-00013. [DOI] [PubMed] [Google Scholar]