Abstract
Background:
Vaccination coverage is typically measured as the proportion of individuals who have received recommended vaccine doses by the date of assessment. This approach does not provide information about receipt of vaccines by the recommended age, which is critical for ensuring optimal protection from vaccine-preventable diseases (VPDs).
Objective:
To assess vaccination timeliness in the Federated States of Micronesia (FSM), and the projected impact of suboptimal vaccination in the event of an outbreak.
Methods:
Timeliness of the 4th dose of diphtheria, tetanus, and acellular pertussis vaccine (DTaP) and 1st dose of measles, mumps, and rubella vaccine (MMR) among children 24–35 months was assessed in FSM. Both doses are defined as on time if administered from 361 through 395 days in age. Timeliness was calculated by one-way frequency analysis, and dose delays, measured in months after recommended age, were described using inverse Kaplan-Meier analysis. A time-series susceptible-exposed-infected-recov ery (TSEIR) model simulated measles outbreaks in populations with on time and late vaccination.
Results:
Total coverage for the 4th dose of DTaP ranged from 36.6% to 98.8%, and for the 1st dose of MMR ranged from 80.9% to 100.0% across FSM states. On time coverage for the 4th dose of DTaP ranged from 3.2% to 52.3%, and for the 1st dose of MMR ranged from 21.1% to 66.9%. Maximum and median dose delays beyond the recommended age varied by state. TSEIR models predicted 10.8–13.7% increases in measles cases during an outbreak based on these delays.
Conclusions:
In each of the FSM states, a substantial proportion of children received DTaP and MMR doses outside the recommended timeframe. Children who receive vaccinations later than recommended remain susceptible to VPDs during the period they remain unvaccinated, which may have a substantial impact on health systems during an outbreak. Immunization programs should consider vaccination timeliness in addition to coverage as a measure of susceptibility to VPDs in young children.
Keywords: Vaccination timeliness, Vaccination coverage, Federated States of Micronesia, Measles, Pertussis, Measles outbreak modeling
1. Introduction
Childhood vaccination improves life expectancy, decreases healthcare costs, and reduces the spread of preventable diseases [1–4]. Routine vaccination averts an estimated 2–3 million deaths globally due to diphtheria, tetanus, pertussis, and measles every year [5]. In the United States alone, routine vaccinations prevented an estimated 322 million illnesses, 21 million hospitalizations, and 732,000 deaths, at a net savings of $295 billion in direct costs and $1.38 trillion in total societal costs, for children born during the period 1994–2013 [6]. International organizations such as the World Health Organization (WHO) implement programmatic immunization programs to ensure high vaccination coverage around the world [7].
The Federated States of Micronesia (FSM) is an island nation spread nearly 2700 km across the Pacific Ocean just north of the equator. There are four island states, Chuuk, Kosrae, Pohnpei, and Yap, and all states except Kosrae are comprised of one main “high” island surrounded by lower lying outer islands. According to the 2010 census, there were 12,073 children under the age of 5 years throughout the FSM states [8]. Researchers from the Centers for Disease Control and Prevention (CDC) partner with local immunization programs to monitor vaccination coverage among children and adults in FSM and other U.S. Affiliated Pacific Island jurisdictions. Technical assistance provided by CDC based on vaccination coverage assessments aid the local programs in assessing the level of vulnerability to vaccine-preventable diseases (VPD) in their jurisdictions, evaluating their program interventions, and developing recommendations and technical support for key stakeholders [9].
Most childhood vaccines are administered in FSM according to the Advisory Committee on Immunization Practices (ACIP) recommendations, with a few exceptions based on the increased burden of some VPDs in the region. The ACIP recommendations include guidelines for vaccination timeliness, or adherence to the recommended timing and spacing of doses, ensuring protection from VPDs as early in life as possible. Late vaccine administration increases the length of time required to obtain adequate protection from VPDs [10]. Previous studies have shown low vaccination timeliness despite high vaccination coverage in many countries [11–17].
While prior CDC assessments found moderate to high vaccination coverage in FSM, they did not account for timing of vaccination in the assessment. In 2016, CDC researchers reassessed vaccination data for 1824 children born between 2007 and 2014. The objective of this study was to examine the traditional measure of total vaccine uptake compared to on time vaccination coverage for four doses of diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) and one dose of the measles, mumps, and rubella vaccine (MMR) among children 24–35 months. Additionally, a standard deterministic compartmental model was developed to represent transmission dynamics and estimate the potential impact of a hypothetical measles outbreak in each state based on measured levels of vaccine uptake and timeliness. The assessment of on time coverage in this study provides a more rigorous examination of the nuanced aspects of vaccination coverage than a traditional vaccination coverage assessment; typically vaccination coverage assessments only measure the number of vaccine doses administered by a certain age, regardless of timing and spacing [16]. This study highlights the importance of vaccination timeliness in the context of vaccination coverage assessments, local immunization program objectives, and community protection against the spread of VPDs, and provides information that can be used by the immunization program to improve planning and strategy of vaccination outreach activities.
2. Methods
2.1. Survey methods
Cross-sectional vaccination coverage assessments were conducted in the four FSM states from 2010–2016. Vaccination dates for children 24–35 months were collected from shot cards, hospital medical records, foreign medical records, public health immunization log books, or immunization information system records, where available.
In Pohnpei, a randomized household-based survey was conducted by the FSM Department of Health and the CDC from October 18, 2010 to December 30, 2010. On the Pohnpei main island, a population-based systematic random sampling survey was conducted. Starting from a randomly chosen point in each enumeration district on the main island, every 4th household was selected to determine eligibility in the survey. Eligibility was determined by the presence of at least one child age 19–35 months living in the household. Enumeration districts in this survey are the same used by the FSM Census. Because the population on the outer islands was small (353 total households), a full census was conducted in those locations. If a respondent from any household on a neighboring island or any randomly selected household on the main island was not available, two follow-up attempts were made for that household.
In Chuuk (2016), Kosrae (2013), and Yap (2015), a census of administrative data and public health records was conducted as a cost- and time-efficient alternative to a household survey to estimate vaccination coverage. Children 24–35 months were identified using birth records and public health records, then all available vaccination records were collected for each child. Data from each available source were combined, compared, and de-duplicated to provide the most accurate vaccination history for each child.
Informed consent was obtained during survey interviews and all data collection protocols were approved by the CDC Institutional Review Board and the FSM Department of Health & Social Affairs review board.
2.2. Outcome measures
Vaccination doses recommended for this age group by the ACIP or FSM Department of Health included: 1 dose of Bacillus Calmette-Guerin vaccine, 4 doses of diphtheria and tetanus toxoids and acellular pertussis vaccine, 3 doses of inactivated poliovirus vaccine, 2 doses of measles, mumps and rubella vaccine, 3 doses of Haemophilus influenzae type B vaccine and 3 doses of Hepatitis B vaccine. For the purpose of this study, timeliness was assessed for two vaccines, DTaP, and MMR. These vaccines were of particular interest because FSM experienced outbreaks of pertussis and measles in recent years [18,19]. Only the first dose of MMR was included in this analysis because the open-ended definition of the recommended age for the second dose of MMR in FSM (>13 months) was not suitable for these types of analyses.
In addition to total coverage, or percentage of children up-to-date with the recommended number of vaccine doses by age 24–35 months, we analyzed receipt of vaccine doses according to the schedule approved by FSM Department of Health (Table 1). Because number of days in a month varies, an average of 30.4 days was used to calculate age at vaccine dose. Recommended age for routine administration of the four doses of DTaP according to ACIP are 2 months, 4 months, 6 months, and 15–18 months, respectively; ACIP states the 4th dose can be administered as early as age 12 months, provided a minimum interval of at least 6 months has elapsed since the third dose. Recommended age for routine administration of the 1st dose of MMR according to ACIP is 12–15 months. FSM recommends the 4th dose of DTaP and the 1st dose of MMR at the minimum age of 12 months. Measures of on time coverage for these doses follow the FSM recommendation. The recommended vaccine schedule describes minimum age and minimum intervals between doses of a vaccine series to confer optimal immunity. However, to avoid re-administration of early doses, the ACIP defines a dose as acceptably early if the dose occurs during a 4 day grace period before the minimum age or interval. Doses received within the grace period were included in the measure of on time coverage [10].
Table 1.
Recommended age, minimum age, and minimum acceptable interval for selected early childhood vaccine doses,a Federated States of Micronesia, 2010–2015.
| Vaccine dose |
Recommended age for routine administration |
Minimum acceptable ageb |
Minimum acceptable intervalc |
|---|---|---|---|
| DTaP | |||
| 1 | 2 months | 6 weeks | |
| 2 | 4 months | 10 weeks | 4 weeks |
| 3 | 6 months | 14 weeks | 4 weeks |
| 4 | 12 monthsd | 12 months | 6 months |
| MMR | |||
| 1 | 12 months | 12 months | |
| 2 | ≥13 monthse | 13 months | 4 weeks |
Abbreviations: DTaP = Diphtheria, tetanus, and acellular pertussis vaccine; MMR = Measles, mumps, and rubella vaccine; ACIP = Advisory Committee on Immunization Practices.
Approved by the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians.
Doses given within 4 days before the minimum age for all vaccines are considered acceptable.
Minimum acceptable interval since previous dose in the series.
ACIP recommends routine administration with the 4th dose of DTaP between ages 15–18 months, but states the dose can be administered as early as 12 months provided at least 6 months have elapsed since the 3rd dose. FSM recommends routine administration of the dose at the minimum age of 12 months, provided the minimum interval has elapsed.
ACIP recommends routine administration with the 2nd dose of MMR between ages 4–6 years, but states the dose can be administered as early as age 13 months provided at least 4 weeks have elapsed since the 1st dose. FSM recommends routine administration of the dose as early as the minimum age of 13 months, provided the minimum interval has elapsed.
2.3. Statistical analysis
2.3.1. Vaccine uptake and timeliness
Total vaccination coverage and on time coverage were calculated by one-way frequency analysis. Household survey results were weighted to match the characteristics of the island’s most recent census, where applicable. Data were entered into Epi Info v. 7 databases and analyzed using SAS v. 9.3 statistical software.
2.3.2. Time-course to vaccine completion
The Kaplan-Meier time-to-event analysis method is an approach that can be used to illustrate time to vaccination and estimate immunization coverage at any given age within a population [20]. The classical survival function, s(t), represents the cumulative probability of being unvaccinated at any given age, t, described here in months. Taking the inverse of s(t), the cumulative probability of children being vaccinated at any given age, in months, can be represented by the function 1-s(t). By incorporating information on birth dates and age at vaccination, the Kaplan-Meier analysis takes into account different lengths of individual observation periods. Children who had not received a vaccination by the end of the data collection period were right-censored. The main assumption of this method is that censoring is independent of event; in this study, the age of a child missing a vaccine dose at the end of data collection is independent of the probability of being vaccinated after the data collection period.
Median age at vaccination was calculated for the 4th dose of DTaP and the 1st dose of MMR. Median delay is the difference in time between the median age at vaccination and the recommended age. Maximum delay is the difference in months from the recommended age to the age at which total coverage is reached, according to the Kaplan-Meier curves.
In a secondary analysis, results for total coverage, on time coverage, median age at vaccination, and maximum delay of the 4th dose of DTaP and 1st dose of MMR are stratified by place of residence (main island or outer island). This analysis is presented for two of the three states for which this stratification scheme is relevant and sample sizes were sufficient.
2.3.3. Predictive modeling
Mathematical models are frequently used in epidemiology to estimate the spread of communicable diseases in fixed populations [21–24]. In this study, a model illustrates the impact of vaccination timeliness on the spread of a vaccine-preventable disease during an outbreak. A standard deterministic compartmental model, referred to as a time-series susceptible-exposed-infectious-remov ed (TSEIR) model, was developed to represent the transmission dynamics and potential impact of a hypothetical measles outbreak in each state of FSM.
The model was parameterized to compare two scenarios. The first scenario represents a best-case situation where all targeted persons, aged 24–35 months, received vaccinations on time, as recommended. In the second scenario, which is called delay, the starting vaccination level was equal to the coverage level observed at the time when the median-aged child in the target cohort should have been vaccinated. Thereafter, the model assumed vaccination coverage increased at the levels that were estimated from observed data. The observed data were illustrated by the inverse Kaplan-Meier analyses. Detailed model parameters and a more thorough description of the model methods can be found in the Appendix.
3. Results
At least one public health or medical record was available for each child included in the analyses; seven children in the Pohnpei dataset had no written documentation of vaccination, but vaccination events were recalled through caregiver interview. These children were excluded from the analyses. Data were analyzed for 1226 children in Chuuk, 172 children in Kosrae, 188 children in Pohnpei, and 238 children in Yap.
3.1. Vaccine uptake and timeliness
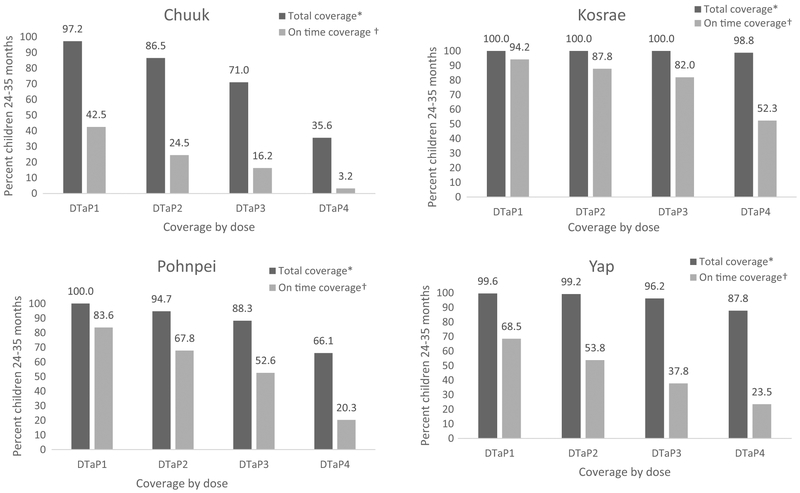
The proportion of children age 24–35 months up-to-date for each of the 4 doses of DTaP is presented in Fig. 1, along with proportion of children who completed each dose on time. Total coverage was highest in Kosrae (100% for dose 1–98.8% for dose 4), followed by Yap (99.6–87.8%), Pohnpei (100–66.1%), and Chuuk (97.2–35.6%). On time coverage was highest in Kosrae (94.2% for dose 1–52.3% for dose 4), followed by Pohnpei (83.6–20.3%), Yap (68.5–23.5%), and Chuuk (42.5–3.2%). Generally, as the DTaP schedule progressed, adherence to the recommendations declined, so later doses in the series were more likely to be administered late or not yet received at the end of follow-up; there was a significant (p < 0.05) correlation between children delayed for dose 3 and children delayed for dose 4 in all states. Among vaccinated children, the proportion who received the 4th dose of DTaP outside the recommended timeframe was significantly higher (p < 0.05) than the proportion who received the dose on time, in all states except Kosrae.
Fig. 1.
Estimated percentage of children 24–35 months who received recommended doses of diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP), by time frame*†, Federated States of Micronesia, 2010–2016§. *Total coverage is a measure of doses received by end of follow-up. †On time coverage is a measure of doses administered from 4 days before the age of 2 months (DTaP1), 4 months (DTaP2), 6 months (DTaP3), and 12 months (DTaP4) through the end of the respective month. §Data collected by census of medical records among children 24–35 months in Chuuk (2016; n = 1226), Kosrae (2013; n = 172) and Yap (2015; n = 238). Data collected by random sample of medical records among children 24–35 months in Pohnpei (2010; n=188). Estimates weighted to adjust for Pohnpei population characteristics based on most recent census.
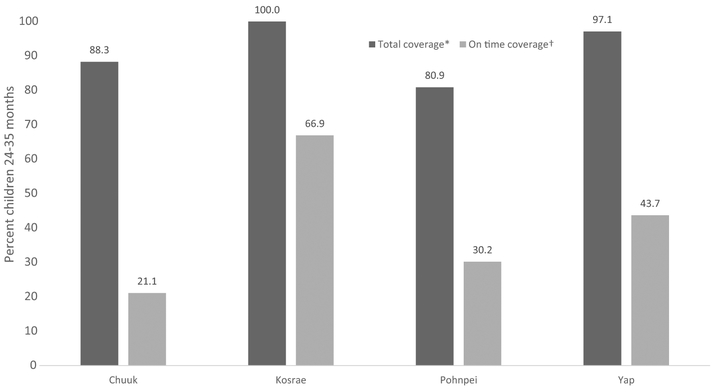
The proportion of children 24–35 months up-to-date for the first dose of MMR is presented in Fig. 2, along with the proportion of children who completed the dose on time. Total coverage for the recommended 1st dose of MMR was highest in Kosrae (100%), followed by Yap (97.1%), Chuuk (88.3%), and Pohnpei (80.9%). On time coverage was highest in Kosrae (66.9%), followed by Yap (43.7%), Pohnpei (30.2%), and Chuuk (21.1%). Among vaccinated children, the proportion of children who received the 1st dose of MMR outside the recommended timeframe was significantly higher (p < 0.05) than the proportion who received the dose on time, in all states except Kosrae.
Fig. 2.
Estimated percentage of children 24–35 months who received first dose of measles, mumps and rubella vaccine, by time frame*†, Federated States of Micronesia, 2010–2016§. *Total coverage is a measure of doses received by end of follow-up. †On time coverage is a measure of doses administered from 4 days before the age of 12 months through the end of the 12th month of age. §Data collected by census of medical records among children 24–35 months in Chuuk (2016; n =1226), Kosrae (2013; n = 172) and Yap (2015; n = 238). Data collected by random sample of medical records among children 24–35 months in Pohnpei (2010; n = 188). Estimates weighted to adjust for Pohnpei population characteristics based on most recent census.
3.2. Time-course to vaccine completion
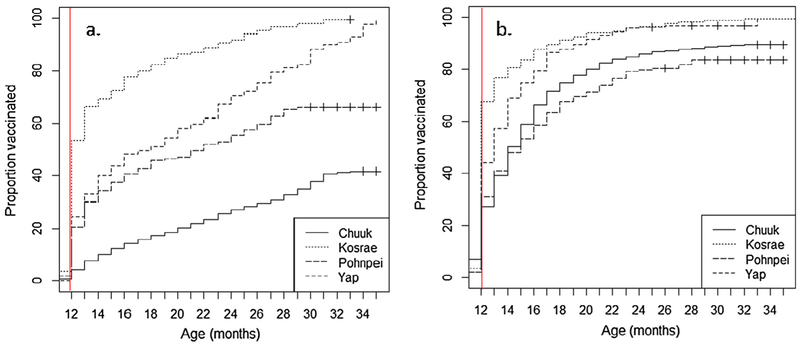
The time-course for completion of the 4 th dose of DTaP and the 1st dose of MMR in each of the three states are presented in Fig. 3. These figures depict the cumulative probability of age-specific vaccination. Reference lines are drawn at the age recommended for each vaccine dose. The proportion of children vaccinated for either vaccine at the recommended age varies across the states.
Fig. 3.
(a) Fourth dose of diphtheria and tetanus toxoids and acellular pertussis vaccination coverage and (b) first dose of measles, mumps and rubella vaccination coverage, presented with inverse and cumulative Kaplan-Meier curves (1-s(t))*. The vertical lines indicates recommended age of dose administration in the four states of the Federated States of Micronesia, 2010–2015†. *Figures depict the cumulative probability of age-specific vaccination. †Data collected by census of medical records among children 24–35 months in Chuuk (2016; n = 1226), Kosrae (2013; n = 172) and Yap (2015; n = 238). Data collected by random sample of medical records among children 24–35 months in Pohnpei (2010; n = 188). Estimates weighted to adjust for Pohnpei population characteristics based on most recent census.
Among those who were late with the 4th dose of DTaP, median age at vaccination was 20 months in Chuuk, 16 months in Kosrae, 17 months in Pohnpei, and 20 months in Yap. According to FSM’s recommended schedule, this is equivalent to a median delay of 8 months, 4 months, 5 months, and 8 months, respectively. Among children who received the 4th dose of DTaP by the end of followup, maximum delays up to 21 months in Chuuk, 19 months in Kosrae, 17 months in Pohnpei, and 23 months in Yap from the recommended age were apparent.
Among those who were late with the 1st dose of MMR, median age at vaccination was 15 months in Chuuk, 16 months in Kosrae, 16 months in Pohnpei, and 15 months in Yap. According to FSM’s recommended schedule, this is equivalent to a median delay of 3 months, 4 months, 4 months, and 3 months, respectively. Among children who received the 1st dose of MMR by the end of followup, maximum delays up to 20 months in Chuuk, 20 months in Kosrae, 16 months in Pohnpei, and 14 months in Yap from the recommended age were apparent.
Results for stratified analyses are presented in Table A-1. On time coverage was significantly (p < 0.05) lower on outer islands compared to the main island in both Chuuk (1.5% vs. 10.4% for DTaP4; 18.7% vs. 31.7% for MMR1) and Yap (10.7% vs. 37.5% for DTaP4; 28.7% vs. 60.7% for MMR1). Median age at vaccination, among all children who were vaccinated by the end of follow-up, is later in outer islands compared to the main island in Chuuk (21 months vs. 16 months for DTaP4; 14 months vs. 13 months for MMR1) and Yap (24 months vs. 14 months for DTaP4; 14 months vs. 12 months for MMR1). Despite significant differences in on time coverage, maximum delays on main islands are similar to, or exceed, maximum delays in the outer islands.
3.3. Predictive modeling
Table 2 presents the results of the TSEIR model and, in particular, the number of measles cases in the entire population that occurred during a hypothetical measles outbreak in each state of FSM. The delay scenario represents a situation where vaccinations occurred in the target cohort later than recommended. This delay scenario is compared to the ideal scenario, which is called the on time scenario, where the assumption is that all children are vaccinated on time. The on time scenario experiences fewer number of cases of measles (2025 in Chuuk, 249 in Kosrae, 1592 in Pohnpei, and 445 in Yap) compared to the delay scenario (2279 in Chuuk, 276 in Kosrae, 1789 in Pohnpei, and 506 in Yap). This represents an increase of 254 cases in Chuuk (12.5% increase), 27 cases in Kosrae (10.8% increase), 197 cases in Pohnpei (12.4% increase), and 61 cases in Yap (13.7% increase).
Table 2.
Number of cases in hypothetical, modeled outbreaks of measles in the Federated States of Micronesiaa for two simulations, one where all targeted children were vaccinated on timeb and one where delays in vaccination occur.c
| Vaccination Coverage (%) |
Cases by scenario |
Case count increased | Percent increase (%) | |||
|---|---|---|---|---|---|---|
| State | Startc | Finalb,c | Delay | On time | ||
| Pohnpei | 22 | 81 | 1789 | 1592 | 197 | 12.4 |
| Kosrae | 34 | 100 | 276 | 249 | 27 | 10.8 |
| Yap | 27 | 97 | 506 | 445 | 61 | 13.7 |
| Chuuk | 32 | 88 | 2279 | 2025 | 254 | 12.5 |
Data collected by census of medical records among children 24–35 months in Chuuk (2016; n = 1226), Kosrae (2013; n = 172) and Yap (2015; n = 238). Data collected by random sample of medical records among children 24–35 months in Pohnpei (2010; n = 188). Estimates weighted to adjust for Pohnpei population characteristics based on most recent census.
In the on time scenario, vaccination coverage in the target cohort has reached final coverage at the time the outbreak simulation begins.
In the delay scenario, vaccination coverage in the target cohort at the start of the outbreak equals what coverage would have been at the date when the median aged child in the target cohort should have been vaccinated, and steadily increases to final coverage by the end of the outbreak simulation.
Difference in measles case count (within the entire population) between delay scenario and on time scenario.
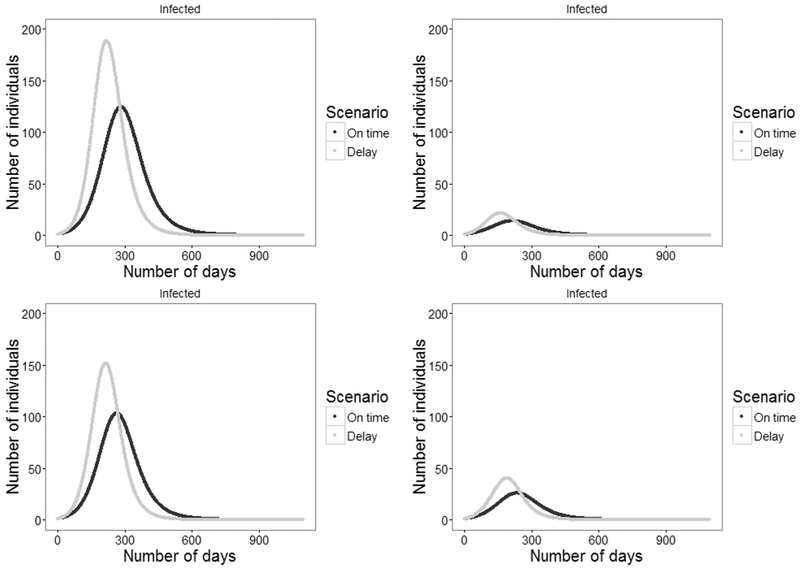
Fig. 4 presents the size of the infected population with respect to time during an outbreak for all four states, comparing the on time scenario with the delay scenario. Across all four states, outbreaks in the delay scenario have greater total case counts, experience peak case counts sooner, and are shorter in duration. The differences in case counts between the four islands is due largely to both the differences in population size as well as differences in vaccine coverage between the islands. Sensitivity analyses on contact rate and the starting level of vaccine coverage are available in the appendix.
Fig. 4.
Epidemic curve (number of infected) of a hypothetical model of a measles outbreak under two scenarios for vaccine timeliness (on time and delay) for four states in the Federated States of Micronesia (a) Chuuk, (b) Kosrae, (c) Pohnpei, and (d) Yap. Notes: Starting vaccine coverage in the delayed scenario equaled the coverage at the time when the median person in the target cohort should have been vaccinated. Total numbers of cases for each scenario are as follows: Chuuk, on time: 2025; Chuuk, delay 2279; Kosrae, on time: 249; Kosrae, delay: 276; Pohnpei, on time: 1592; Pohnpei, delay: 1789; Yap, on time: 445; Yap, delay: 506.
4. Discussion
Coverage for the fourth dose of DTaP and the first dose of MMR varied substantially across the four states of FSM. Even when coverage was high, on time coverage was substantially lower, meaning most children who were vaccinated did not receive doses at the recommended age of 12 months. Children in all states received these doses as late as ages 26–35 months; children who were not vaccinated by the end of the data collection period may have received doses even later or not at all. Models indicated increases greater than 10% in measles cases in scenarios where vaccinations were delayed, due to an increased pool of susceptible individuals in the population.
Children who receive vaccinations later than recommended remain susceptible to VPDs until they are vaccinated, which may have a substantial impact on health systems and local economies during an outbreak. Immunization programs should consider vaccination timeliness in addition to vaccine receipt as a measure of susceptibility to VPDs in young children, particularly in areas that have experienced recent outbreaks.
Measurement of up-to-date coverage alone may sometimes obscure substantial vaccination delays that occur during the first two years of life. Risk of disease due to delay in vaccination varies, but understanding vaccination timeliness is especially important for diseases that have the potential to cause large outbreaks, such as measles [25]. Recent VPD outbreaks in FSM highlighted a need for further investigation of vaccination outcomes, such as timeliness. Using methods such as Kaplan-Meier and TSEIR modeling are newer approaches in the context of vaccination coverage and timeliness, which can be utilized by immunization programs to better understand the level of protection against VPDs, and the risk of VPD outbreaks, in their communities.
Inverse Kaplan-Meier graphs provide a useful visual method to estimate the proportion of children vaccinated at any given age, and understand the nature of under-vaccination within a cohort over the first two years of life. Many children in this study were under-vaccinated for DTaP or unvaccinated for MMR for several months after the recommended age of vaccine administration, despite being vaccinated at the end of follow-up. It is important to note that doses were delayed up to two years from the recommendation for both vaccines, even in the states with highest total coverage.
In this study, the proportion of children receiving DTaP doses on time decreased as the schedule progressed, consistent with previous studies [12,15,26,27]. Because this vaccine requires a minimum interval of four weeks between the first three doses, and six months between the third and fourth dose, children late for those first doses will remain late for subsequent doses, and may be more likely to not receive the fourth dose by the age of 24 months, even with adequate time for catch-up [28]. Transplacental immunity wanes rapidly in the first three months of life [29]. The greater the delay during the early months in life, the longer the child remains under-protected against the commonly circulating VPD, pertussis. In studies of pertussis outbreaks, incidence tended to be highest among infants younger than six months, and many cases occurred in young children who were under-vaccinated for age [30,31].
In the case of the first dose of MMR vaccine, delays measured by the Kaplan-Meier method represent a period of complete lack of protection against measles. Investigations of measles epidemics in the United States have found a primary cause was a failure to provide vaccines at the recommended age [32]. Studies of measles outbreaks in England and Wales have shown a significant increase in the reproductive number, or number of secondary infections per infection, occurring immediately following years where there was a decrease in uptake of the MMR vaccine, as a result of a greater number of susceptible individuals in the population [33]. In our study, TSEIR models parameterized according to observed delays in FSM demonstrated the potential impact an increased pool of susceptible individuals in their populations may have in the event of an outbreak.
Since introduction of measles-containing vaccines in the late 1980s, outbreaks of measles cases have been identified in FSM during three different years [34]. From 1991–1994, FSM was part of a large measles outbreak across the region, in which a combined population of about 300,000 experienced more than 1300 cases of measles and 16 deaths due to measles [35]. In 1992, approximately 35 cases were identified. In 1994, approximately 930 cases were identified. More recently, in 2014, a measles outbreak in Pohnpei, Kosrae, and Chuuk resulted in 393 suspected cases, with 140 laboratory confirmed, 244 others linked through epidemiologic data, and 9 clinically compatible. The computed incidence for this outbreak was 2226.6 per million individuals. There was one death attributed to measles in 2014 [36].
An important caveat to consider is the FSM immunization program recommends a second dose of MMR as early as 13 months of age, due to increased risk of measles in the region. The results of this study demonstrated as many as 4 out of 5 children in some regions of the country were not receiving the first dose at the recommended age, and some children were not vaccinated until well into their second to third year of life. Further, recent studies have found increased risk of complications such as febrile seizures in children who received delayed MMR vaccinations [37,38]. This highlights the value of measuring timeliness of vaccination in conjunction with total coverage, as a means of better understanding both the level of VPD protection within a population, and the risk of complications in the population associated with delayed vaccines.
The data collection methods identified in this paper represent the most accurate and reliable methods for estimating vaccination coverage in the low resource setting of FSM, but are subject to limitations. First, data were collected across different years, so results may not be generalizable across states and may not reflect current trends. Second, data collected by administrative surveys in Chuuk, Kosrae, and Yap may underrepresent children who have not accessed the healthcare system and who may be more likely to be un- or under-vaccinated. Third, the Kaplan-Meier method produces slightly higher results than conventional coverage measures as a result of censoring; this method reduces the population “at risk” at the time point when censoring occurs. Conventional estimates of coverage are provided for comparison to Kaplan-Meier results. Fourth, in the delay scenario, the TSEIR model assumes a linear increase toward total coverage actually observed, which impacts the time to completion of the hypothetical outbreak. In the event of a real measles outbreak, it is likely a mass vaccination campaign would more rapidly reduce the number of susceptible individuals in the population and accelerate this timeline; the estimated difference in cases and difference in outbreak duration between the on time and delay scenarios may be overestimated.
5. Conclusion
This study provides new information about how long children in FSM remain under-protected from commonly circulating VPDs such as measles and pertussis, which may be useful for immunization programs in FSM to set priorities for immunization activities. More generally, this study underscores an important and potentially overlooked opportunity to improve public health and vaccination coverage. The success of an intervention that addresses vaccination timeliness benefits from targeting a portion of the population that is already predisposed and has the necessary healthcare access to obtain vaccinations. Caregivers of these children potentially need only a reminder to ensure vaccinations are occurring at the best time for the protection of the child’s health. As such, timeliness-based interventions may be an inexpensive way to improve vaccination coverage and public health protection from vaccine-preventable diseases. Some evidence-based interventions to consider to improve on time vaccination include: parental education on the importance of timely vaccinations, in conjunction with the use of the existing Immunization Information System which provides reminders to schedule visits at appropriate age and aid physicians in clinical decision support to reduce missed vaccination opportunities; recall of patients who have missed scheduled appointments, or who are otherwise at risk of delayed vaccination; increasing community awareness of vaccination activities; and increasing frequency of vaccination activities in alternate community settings [39]. Immunization programs in island nations with far-reaching outer islands may also benefit from planning regular community outreach to improve timing and spacing of doses for children in hard to reach locations, for whom access to public health clinics may be more difficult.
Supplementary Material
6.
Disclosure
This work was supported by the Centers for Disease Control and Prevention’s Immunization Services Division and the Federated States of Micronesia Department of Health & Social Affairs.
All authors have read and approved the manuscript, and there are no financial disclosures, conflicts of interests and/ or acknowledgements necessary.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
This work was supported by the Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
Abbreviations:
- WHO
World Health Organization
- VPD
vaccine-preventable disease
- ACIP
Advisory Committee on Immunization Practices
- FSM
Federated States of Micronesia
- DTaP
diphtheria and tetanus toxoids and acellular pertussis
- MMR
measles, mumps, and rubella
- TSEIR
time-series-susceptible-exposed-infected-removed.
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, athttps://doi.org/10.1016/j.vaccine.2017.10.001.
References
- [1].Centers for Disease Control and Prevention. Ten great public health achievements—worldwide, 2001–2010. Morbidity and mortality weekly report (MMWR) 2011;60(24):814–8. [PubMed] [Google Scholar]
- [2].Ehreth J The global value of vaccination. Vaccine 2003;21(7):596–600. [DOI] [PubMed] [Google Scholar]
- [3].Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol 2011;11(12):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou F, Santoli J, Messonnier ML, Yusuf HR, Shefer A, Chu SY, et al. Economic evaluation of the 7-vaccine routine childhood immunization schedule in the United States, 2001. Arch Pediat Adolesc Med 2005;159(12):1136–44. [DOI] [PubMed] [Google Scholar]
- [5].World Health Organization. Immunization Coverage Fact Sheet 2016. Available from: http://www.who.int/mediacentre/factsheets/fs378/en/.
- [6].Whitney CG, Zhou F, Singleton J, Schuchat A. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. MMWR Morb Mortal Wkly Rep 2014;63(16):352–5. [PMC free article] [PubMed] [Google Scholar]
- [7].Luman ET, Ryman TK, Sablan M. Estimating vaccination coverage: validity of household-retained vaccination cards and parental recall. Vaccine 2009;27 (19):2534–9. [DOI] [PubMed] [Google Scholar]
- [8].FSM 2010 Census of Population and Housing SBOC. Total Population by Age Group, FSM Office of Statistics, Budget and Economic Management, Overseas Development Assistance, and Compact Management; 2010. Available from: http://www.sboc.fm/index.php?id1=Vm0xMFlXRXlVWGhUYmtwT1ZrVTFVbFpyVWtKUFVUMDk.
- [9].Luman ET, Sablan M, Anaya G, Stokley S, McCauley MM, Shaw KM, et al. Vaccination coverage in the US Commonwealth of the Northern Mariana Islands, 2005. J Public Health Manage Pract 2007;13(6):595–604. [DOI] [PubMed] [Google Scholar]
- [10].Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports; 2011. p. 1–60. [PubMed] [Google Scholar]
- [11].Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. The Lancet 2009;373 (9674):1543–9. [DOI] [PubMed] [Google Scholar]
- [12].Fadnes LT, Nankabirwa V, Sommerfelt H, Tylleskär T,Tumwine JK, Engebretsen IM, et al. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine 2011;29(19):3564–70. [DOI] [PubMed] [Google Scholar]
- [13].Heininger U, Zuberbühler M. Immunization rates and timely administration in pre-school and school-aged children. Eur J Pediat 2006;165(2):124–9. [DOI] [PubMed] [Google Scholar]
- [14].Kurosky SK, Davis KL, Krishnarajah G. Completion and compliance of childhood vaccinations in the United States. Vaccine 2016;34(3):387–94. [DOI] [PubMed] [Google Scholar]
- [15].Suárez-Castaneda E, Pezzoli L, Elas M, Baltrons R, Crespin-Elías EO, Pleitez OAR, et al. Routine childhood vaccination programme coverage, El Salvador, 2011— In search of timeliness. Vaccine 2014;32(4):437–44. [DOI] [PubMed] [Google Scholar]
- [16].Luman ET, Barker LE, McCauley MM, Drews-Botsch C. Timeliness of childhood immunizations: a state-specific analysis. Am J Public Health 2005;95 (8):1367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dayan GH, Shaw KM, Baughman AL, Orellana LC, Forlenza R, Ellis A, et al. Assessment of delay in age-appropriate vaccination using survival analysis. Am J Epidemiol 2006;163(6):561–70. [DOI] [PubMed] [Google Scholar]
- [18].Hales C, Papania M, Larzelere M, Gopalani S, Lebo E, Wallace GS, et al. , editors. Effectiveness of Measles-Containing Vaccine During a Measles Outbreak, Pohnpei, Federated States of Micronesia—2014. Open Forum Infectious Diseases, Oxford University Press; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pertussis: Reported Cases by Country [Internet]; 2016. Available from: http://apps.who.int/gho/data/node.main-wpro.WHS3_43?lang=en.
- [20].Laubereau B, Hermann M, Schmitt H, Weil J, Von Kries R. Detection of delayed vaccinations: a new approach to visualize vaccine uptake. Epidemiol Infect 2002;128(02):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kathleen M. Modeling community containment for pandemic influenza: a letter report. Washington: DC: National Academy Press; 2006. [Google Scholar]
- [22].Longini IM, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science 2005;309 (5737):1083–7. [DOI] [PubMed] [Google Scholar]
- [23].Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005;437(7056):209–14. [DOI] [PubMed] [Google Scholar]
- [24].Lofgren ET, Halloran ME, Rivers CM, Drake JM, Porco TC, Lewis B, et al. Opinion: mathematical models: a key tool for outbreak response. Proc Nat Acad Sci 2014;111(51):18095–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States:days undervaccinated and number of vaccines delayed. JAMA 2005;293 (10):1204–11. [DOI] [PubMed] [Google Scholar]
- [26].Laryea DO, Parbie EA, Frimpong E. Timeliness of childhood vaccine uptake among children attending a tertiary health service facility-based immunisation clinic in Ghana. BMC Public Health 2014;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shrivastwa N, Gillespie BW, Lepkowski JM, Boulton ML. Vaccination timeliness in children under India’s universal immunization program. Pediat Infect Disease J 2016;35(9):955–60. [DOI] [PubMed] [Google Scholar]
- [28].Strine TW, Luman ET, Okoro CA, McCauley MM, Barker LE. Predictors of age-appropriate receipt of DTaP dose 4. Am J Prevent Med 2003;25(1):45–9. [DOI] [PubMed] [Google Scholar]
- [29].Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged< 12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morbidity and mortality weekly report 2011;60(41):1424. [PubMed] [Google Scholar]
- [30].Tanaka M, Vitek CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003;290 (22):2968–75. [DOI] [PubMed] [Google Scholar]
- [31].Black S. Epidemiology of pertussis. Pediat Infect Disease J 1997;16(4):S85–9. [DOI] [PubMed] [Google Scholar]
- [32].Henderson DA, Dunston FJ, Fedson DS, Fulginiti VA, Gerety RJ, Guerra FA, et al. The measles epidemic: the problems, barriers, and recommendations. JAMA 1991;266(11):1547–52. [PubMed] [Google Scholar]
- [33].Jansen VA, Stollenwerk N, Jensen HJ, Ramsay M, Edmunds W, Rhodes C. Measles outbreaks in a population with declining vaccine uptake. Science 2003;301(5634):804-. [DOI] [PubMed] [Google Scholar]
- [34].World Health Organization W. Federated States of Micronesia: Country Profile-Measles Elimination; 2015. [Google Scholar]
- [35].Gür D, Auerbach SB, Vitek C, Maes E, McCready J, Durand M, et al. Measles outbreaks in Micronesia, 1991 to 1994. Pediat Infect Disease J 1998;17 (1):33–9. [DOI] [PubMed] [Google Scholar]
- [36].Breakwell L, Moturi E, Helgenberger L, Gopalani SV, Hales C, Lam E, et al. Measles outbreak associated with vaccine failure in adults-federated states of Micronesia, February-August 2014. MMWR Morbid Mortal Weekly Report 2015;64(38):1088. [DOI] [PubMed] [Google Scholar]
- [37].Hambidge SJ, Newcomer SR, Narwaney KJ, Glanz JM, Daley MF, Xu S, et al. Timely versus delayed early childhood vaccination and seizures. Pediatrics 2014;133(6):e1492–9. [DOI] [PubMed] [Google Scholar]
- [38].Rowhani-Rahbar A, Fireman B, Lewis E, Nordin J, Naleway A, Jacobsen S, et al. Effect of age on the risk of Fever and seizures following immunization with measles-containing vaccines in children. JAMA Pediatr 2013;167:1111–7. PMID: . [DOI] [PubMed] [Google Scholar]
- [39].Force CPST. Guide to community preventive services: Increasing appropriate vaccination: Client reminder and recall systems; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.