Abstract
Obesity, usually caused by high fat diets (HFD), is a major public health issue worldwide, causing obesity associated cardiomyopathy. Moreover, the environmental toxicant vinyl chloride (VC) can exacerbate HFD-induced fatty liver disease. However, whether VC serves to enhance obesity-associated cardiomyopathy remains unclear. This study aims to investigate the interaction of western diet (WD) containing relatively low fat (42%) with VC on cardiac remodeling and its underling mechanisms. Adult male C57BL/6J mice were exposed to WD coinhalation of low-dose VC (<1 ppm/d) for 12 weeks. Results showed that WD feeding for 12 weeks caused slight cardiac systolic dysfunction without significant hypertrophy or fibrosis, even with VC. Nevertheless, WD upregulated NF-κB function and expression of IL-1β and PAI-1, while VC showed no significant impact on these effects. In contrast, WD together with VC significantly increased the expression of CHOP and TGF-β1, key markers for endoplasmic reticulum stress and profibrotic cytokine, respectively. In summary, exposure to low-dose of environmental toxicant VC while a WD is consumed for a relatively short time does not have significant impact on cardiac remodeling except for a mild systolic dysfunction of the heart.
INTRODUCTION
With increasing prevalence, obesity [body mass index (BMI) ≥30] has become one of the most serious public health issues of the 21st century.1,2 Epidemiological studies have identified high BMI as a risk factor for an expanding set of chronic diseases including cardiovascular disease,3,4 diabetes mellitus, chronic kidney disease, and even many cancers.5
Obesity has been regarded as an independent risk factor and a direct cause of cardiovascular diseases.3,4,6 Our team found that 4-week-old C57BL/6J mice fed with a high fat diet (HFD) of 60% fat for 3 months developed significant cardiac inflammation and hypertrophy.7 Furthermore, Zeng et al.8 found that adult mice fed with HFD (60% fat) for 16 weeks developed apparent cardiac fibrosis. World Health Organization (WHO) recommends saturated fats intake should no less than 10% and not exceed 30% of total energy intake.9,10 Therefore, it is clear that 60% HFD is much higher than WHO recommendations and beyond the range of the current study. Therefore, this study aimed to investigate the cardiovascular effects of a “Western diet” (WD) containing 42% fat since this may be more representative of the current lifestyle.
Environmental toxicants can also cause cardiac injury, for instance, Pei et al.11 revealed that an environmental pollutant α,β-unsaturated aldehydes from cigarette smoke can trigger cardiomyocyte contractile dysfunction. Accumulated evidence indicates that obesity and environmental toxicants have synergistical effects to induce cardiovascular diseases.7,12,13 Turkcan et al.12 found that the combination of high cholesterol (after a 21.2% fat diet for 8 weeks) and cadmium increased the risk for heart failure through cardiac fibrosis in ApoE−/− mice. Furthermore, Dutta et al.13 revealed that HFD aggravates arsenic-induced oxidative stress in rat heart and liver.
As an environmental toxicant, vinyl chloride (VC) is a gaseous organochlorine chemical used in industry to produce polyvinyl chloride for commercial manufacturing of plastic pipes and so on. It is ranked fourth on the Centers for Disease Control and Protection’s Agency for Toxic Substances and Disease Registry (ATSDR) Substance Priority List. The United States currently remains the largest VC monomer manufacturing region, with China a large manufacturer, but also one of the largest consumers of VC monomer.14 VC that is released by industries or formed by the breakdown of other chlorinated chemicals can enter air, soil, and groundwater. People living around both manufacturing and Superfund sites are susceptible to VC migrating through soil into home foundations; then it can enter showers, basements, and living spaces in which these vapors recirculate and are inhaled by human beings. It is reported by ATSDR that VC is also present in the air surrounding production facilities at concentrations ranging from trace amounts to over 40 ppm.15 At high exposure levels (>5 ppm), VC’s effects on liver damage are well documented, which can cause hepatic steatosis (fat accumulation), inflammation (steatohepatitis), fibrosis, necrosis, and hepato cellular carcinoma.16 In our previous study, we have demonstrated that acute chloroethanol exposure, a metabolite of VC (equivalent to ~100 ppm VC inhalation for a short period of time) results in liver inflammation and damage. This effect is far more exacerbated by WD (42% fat).17,18 Since the Occupational Safety and Health Administration (OSHA) has decreased the occupational VC exposure criteria to a current standard of 1 ppm for an 8-h workday (OSHA VC Standard 29 CFR 1910.1017), the effects of VC occupational exposure have decreased dramatically. However, there are only few data (human or experimental) on the impact of chronic low-dose VC exposure. Thus, the biological effects of low-dose exposure (<1 ppm) of VC on various organs under normal and obese conditions suggested an interesting study, particularly in liver and heart.
Therefore, our research focused on two aspects: first, whether feeding a WD (42% fat) for 12 weeks can cause liver and cardiac remodeling; second, whether low-dose exposure of VC exacerbates WD-induced liver and cardiac remodeling and its potential mechanisms. The effects of WD with and without VC on the liver have been examined and reported recently;17 therefore, here we focused on examining the effect of WD with and without VC on the heart.
MATERIALS AND METHODS
Animals and Procedures.
Six-week-old male C57BL/6J mice from Jackson Laboratory (Bar Harbor, ME) were hosted in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and procedures were approved by the Institutional Animal Care and Use Committee of the University of Louisville (No. 12112). Animals were housed in shoebox cages with corncob bedding at 22 °C with a 12-h light/dark cycle and had free access to standard rodent chow and tap water.
Mice were randomly assigned to the following four groups (n = 5− 13 for each group): (1) LFD group (i.e., normal diet, 13% calories as fat), (2) LFD+VC group, (3) WD group (42% calories as fat), and (4) WD+VC group. Mice were exposed to VC (KIN-TEK, La Marque, TX) at ~0.85 ± 0.1 ppm, or room air, in inhalation chambers for 6 h per day, 5 days per week for 12 weeks, in bedding-free cages. Mice were fed LFD or WD (Envigo Teklad Diets, Madison, WI; for detailed description, please refer to our group’s previous study18). Upon sacrifice, fasted (4 h) animals were anesthetized with ketamine/xylazine (100:15 mg/kg, i.p.) and were exsanguinated via the vena cava. Citrated plasma was stored at −80 °C for further analysis. The chest cavity was opened; the heart was removed, weighted, and divided into three sections. One section was for protein and RNA analysis, a second for frozen sections, and the remainder was fixed in 10% neutral buffered formalin for histological analysis.
Echocardiography.
Transthoracic echocardiography (Echo) was performed on mice anesthetized with isoflurane (1%−3% isoflurane in 100% oxygen at a flow rate of 1 L/min), equipped with a high-frequency ultrasound probe (RMV-707B), designed for small animals (Vevo 770, Visual Sonics, Canada). Parasternal long-axis and short-axis views were acquired. LV dimensions and wall thicknesses were determined from parasternal short-axis M-mode images. The heart rates of the anesthetized animals were recorded. Ejection fraction (EF), fractional shortening (FS), and left ventricular (LV) mass were calculated by Vevo770 software simultaneously. Data represent average values of 10 cardiac cycles.
Oil Red O (ORO) Staining for Lipid Accumulation.
ORO staining was performed to examine lipid accumulation in the hearts. After cryostat-sectioning (7 μm), the slides were fixed in 10% neutral buffered formalin for 5−10 min, briefly washed with running tap water, immersed in 60% isopropanol for a few seconds, and then stained with ORO working solution (saturated ORO isopropanol solution was prepared by diluting as 4:6 with 60% isopropanol) for 20 min at room temperature. Slides were washed with 60% isopropanol and counter-stained with hematoxylin (DAKO, Carpinteria, CA) for 10 s. Images were captured at 400× magnification (Nikon Eclipse E600 microscope, Melville, NY). The proportion of area stained by ORO was analyzed in 5−10 fields of view for each heart by ImageJ (1.44p) software.
Histological Staining.
Excised heart tissue specimens were fixed in 10% formalin processed in graded alcohol, xylene, and then embedded in paraffin. Paraffin blocks were sliced into sections of 5 μm. After rehydration, the sections were stained with hematoxylin and eosin (HE). To detect fibrosis or collagen accumulation in tissues, sections were stained by Sirius Red. For the myocyte cross-sectional area measurement, after cryostat-sectioning (7 μm), frozen sections were fixed in the acetone for 5−10 min, then stained with fluoresceinconjugated wheat germ agglutinin (WGA) (Alexa Fluor-488, Invitrogen). Images were captured with fluorescent microscopy (Nikon ECLIPSE E600). The area of myocyte cross-section was analyzed in 5−10 fields of view for each heart by ImageJ (1.44p) software.
Western Blot Assay.
Heart proteins were extracted and separated on 10% SDS-PAGE gels followed by transferring to a nitrocellulose membrane (Bio-Rad, Hercules, CA). The membranes were blocked with a 5% nonfat dried milk for 1 h and then incubated overnight at 4 °C with the following antibodies at various dilutions of 1:1000 to 1:3000: atrial natriuretic peptide (ANP), connective tissue growth factor (CTGF), interleukin-1β (IL-1β), intercellular adhesion molecules-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), catalase (CAT), quinone oxidoreductase (NQO-1), and β-Actin (Santa Cruz Biotechnology, Santa, CA); 3-nitrotyrosine (3-NT) (Millipore, Billerica, CA); 4-hydroxynonenal (4-HNE) (Alpha Diagnostic International, San Antonio, TX); tumor necrosis factor-α (TNF-α), fibronectin (FN), activating transcription factor 4 (ATF4), 78 kDa glucose-regulated protein (GRP78), transforming growth factor beta 1 (TGF-β1) (Abcam, Cambridge, MA); heme oxygenase 1 (HO-1), total caspase 3, cleaved caspase 3, phosphorylated-P38 (p-P38), P38, CCAT-enhancer-binding protein homologous protein (CHOP), phosphorylated-P65 (p-P65), P65 (Cell signaling, Danvers, MA); activating transcription factor 6 (ATF6) (Novus Biologicals, Littleton, CO); plasminogen activator inhibitor-1 (PAI-1) (BD, Franklin Lakes, NJ); and metallothionein (MT) expression was detected with a modified Western blot protocol, as previously described19 using primary antibody against MT (1:1000 dilution; DakoCytomation, Carpinteria, CA). After three washes with Trisbuffered saline (pH 7.5) containing 0.05% Tween 20, the membranes were incubated with appropriate secondary antibodies for 1 h at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence kit (Bio-Rad). Photos were captured by ChemiDoc Touch Imaging System (Bio-Rad). Image lab software was used to analyze the grayscale value. Protein content was normalized to that of β-Actin.
RNA Purification, Reverse Transcription, and Quantitative PCR (RT-qPCR).
The hearts were frozen in liquid nitrogen and kept at −80 °C. Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA). RNA concentrations and purities were quantified using a Nano drop ND-1000 spectrophotometer. Before reverse transcription (RT), we use Thermos Scienctific RapidOut DNA Removal kit (K2981) to remove genetic DNA, following the manufacturer’s protocol. RT was performed using 1 μg of total RNA in 12.5 μL of the solution containing 4 μL of 25 mM MgCl2, 4 μL of AMV reverse transcriptase 5× buffer, 2 μL of dNTP, 0.5 μL of RNase inhibitor, 1 μL of AMV reverse transcriptase, and 1 μL of random primer, which were added with nuclease-free water to make a final volume of 20 μL. RT-qPCR was performed in duplicate with a 10 μL reaction system, which contained 5 μL TaqMan Universal PCR master mix, 4.2 μL of H2O,0.3 μL of cDNA, and 0.5 μL of appropriate primer, using the LightCycler 96 RT-PCR system (Roche Diagnostics Corporation, Indianapolis, IN). The comparative cycle time (Ct) method was used to determine fold differences between samples, and the amount of target genes was normalized to GAPDH as an endogenous reference (20−ΔCt).
The following primer sets were used to perform PCR: [glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Mm99999915_g1; ANP, Mm01255747_g1; myosin heavy chain beta (β-MHC), Mm00600555_m1; CTGF, Mm01192933_g1; FN, Mm01256744_m1; interleukin-6 (IL-6), Mm00446190_ml] for PCR were obtained from Thermo Fisher (Grand Island, NY, U.S.A.).
Data were expressed as means ± standard deviation (SD) (n = 5− 13 per group). Comparisons were performed by one-way ANOVA for the different groups, followed by posthoc pairwise repetitive comparisons with Turkey test. Statistical analysis was performed with Origin 8.6 software. P < 0.05 was considered to be statistically significant.
RESULTS
WD Induced Obesity, Insulin Resistance, and Hyper-lipidemia, with VC Slightly Exacerbating these Effects.
Systemic effects of WD with and without VC on mice were reported separately.17 Briefly the WD group showed a slight increase in bodyweight within 2 to 10 weeks but a significant increase at 12 weeks as compared to the LFD group. Consistently, DEXA scans analysis performed at 12 weeks showed that WD significantly increased fat mass, while VC did not alter body’s fat mass and composition. However, WD in combination with VC showed slight but significant increase in WD-induced time-dependent bodyweight gain, as summarized in Table 1.
Table 1.
Systemic Effect of WD and VC
| parameters | LFD | LFD+VC | WD | WD+VC |
|---|---|---|---|---|
| bodyweight | − | − | ±/+ | + |
| fat mass | − | − | ++ | ++ |
| OGTT | − | − | + | + |
| liver | ||||
| TG | − | − | ++ | +++ |
| cholesterol | − | − | ++ | ++ |
| FFA | − | − | + | + |
OGTT test at 6 weeks indicates postprandial blood glucose elevated in WD fed mice, with VC exacerbating this effect only at 15 min following glucose gavage (Table 1.), suggesting that WD elicits insulin resistance.
Because the blood sample is limited, we only measured the lipid profile in the liver. Triglycerides (TG), cholesterol, and free fatty acid (FFA) were all significantly elevated in WD group compared with LFD group. However, VC with WD effected only TG levels (Table 1).
WD Induced Mild Cardiac Systolic Dysfunction and Histological Abnormalities, with No Added Effects by VC.
Echo analysis (Table 2) showed a slight reduction of FS and decrease in tendency of EF (P = 0.066) in WD fed mice compared with LFD fed mice. VC did not change WD-induced effects on cardiac function.
Table 2.
WD Induced Mild Cardiac Dysfunction
| parameters | LFD | LFD+VC | WD | WD+VC |
|---|---|---|---|---|
| IVS;d | 0.62 ± 0.05 | 0.64 ± 0.05 | 0.57 ± 0.09 | 0.60 ± 0.04 |
| IVS;s | 0.95 ± 0.08 | 0.93 ± 0.06 | 0.81 ± 0.12 | 0.87 ± 0.09 |
| LVID;d | 4.14 ± 0.29 | 4.10 ± 0.18 | 4.01 ± 0.36 | 4.17 ± 0.22 |
| LVID;s | 2.58 ± 0.20 | 2.55 ± 0.14 | 2.64 ± 0.35 | 2.65 ± 0.20 |
| LVPW;d | 0.67 ± 0.05 | 0.67 ± 0.03 | 0.67 ± 0.06 | 0.66 ± 0.03 |
| LVPW;s | 1.01 ± 0.02 | 1.00 ± 0.04 | 1.01 ± 0.05 | 1.01 ± 0.05 |
| LV Vol;d | 76.63 ± 12.17 | 74.25 ± 7.92 | 71.12 ± 15.51 | 77.63 ± 9.65 |
| LV Vol;s | 24.32 ± 4.68 | 23.57 ± 3.02 | 26.32 ± 9.07 | 26.01 ± 4.78 |
| %EF | 68.37 ± 1.63 | 68.28 ± 1.76 | 63.71 ± 4.56 | 66.70 ± 2.59 |
| % FS | 37.82 ± 1.20 | 37.72 ± 1.36 | 34.26 ± 3.16* | 36.55 ± 1.89 |
| LV mass | 76.31 ± 14.30 | 75.57 ± 7.12 | 68.32 ± 15.06 | 74.07 ± 9.89 |
| HR | 546 ± 23 | 508 ± 28 | 537 ± 59 | 564 ± 53 |
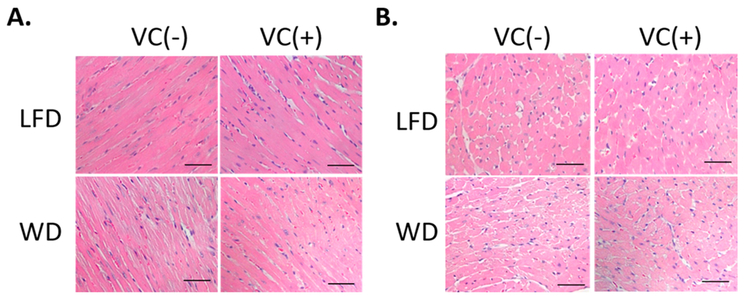
HE staining showed that WD-fed mice displayed cardiac structural abnormalities including broken fibers and deranged cellular structures. In contrast, VC exerted no added effects (Figure 1).
Figure 1.
WD induced mild myocytes histological abnormalities. Representative images from (A) longitudinal and (B) transverse HE staining (400×, scale bar = 50 μm).
In summary, feeding WD (42% fat) for 12 weeks induced marginal cardiac dysfunction and remodeling, which were not affected by simultaneous exposure to VC inhalation <1 ppm/d.
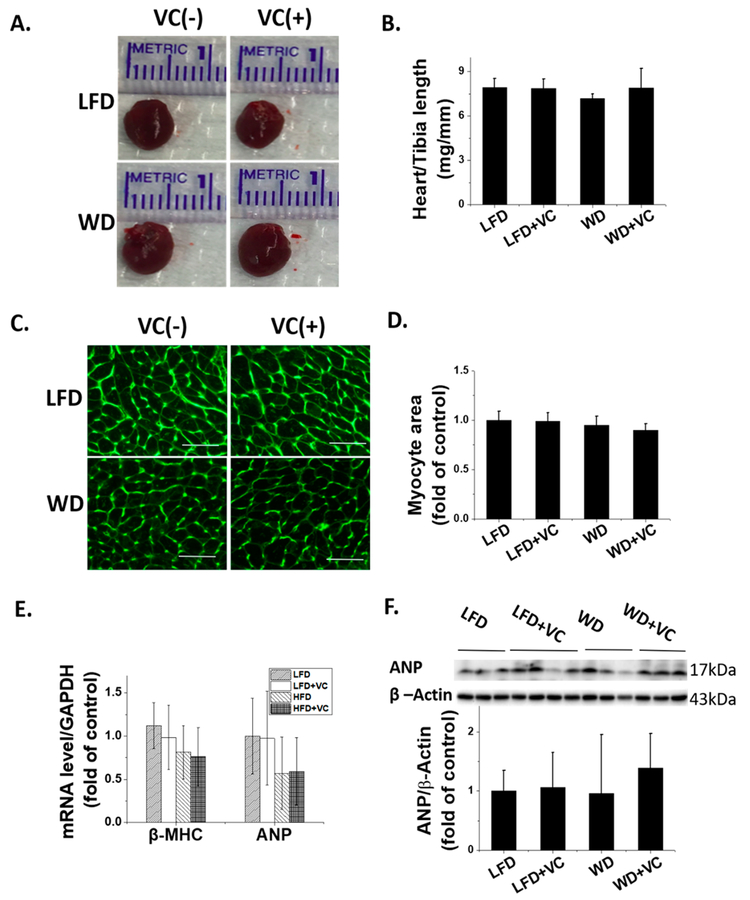
WD with and without VC Did Not Induce Overt Cardiac Hypertrophy.
The gross cardiac dimensions and the ratio of heart weight to tibia length showed no significant difference between these four groups (Figure 2A,B). Consistent with this, LV mass collected index, interventricular septal thickness (IVS), and LV posterior wall (LVPW) also showed no significant difference between these four groups (Table 2). Furthermore, cardiomyocyte cross-sectional area was comparable between these four groups determined by WGA staining (Figure 2C,D). These findings were further confirmed by molecular hyper-trophic markers of ANP protein expression by mRNA expression via RT-qPCR assay (Figure 2E) and protein expression by Western blot analysis (Figure 2F), and also β-MHC mRNA expression by RT-qPCR assay (Figure 2E). All of these hypertrophic markers demonstrated no significant difference between these four groups.
Figure 2.
WD and VC did not induce significant cardiac hypertrophy. (A) Heart size and (B) ratio of heart-weight to tibia length were presented as indicators of cardiac hypertrophy. (C) Cardiac tissue WGA staining and (D) quantification of myocyte cross-sectional area (400×, and scale bar = 50 μm). (E) RT-qPCR analysis of hypertrophic markers β-MHC and ANP to determine mRNA expression. (F) ANP protein expression was detected by Western blot. Data were presented as means ± SD (n = 5−13).
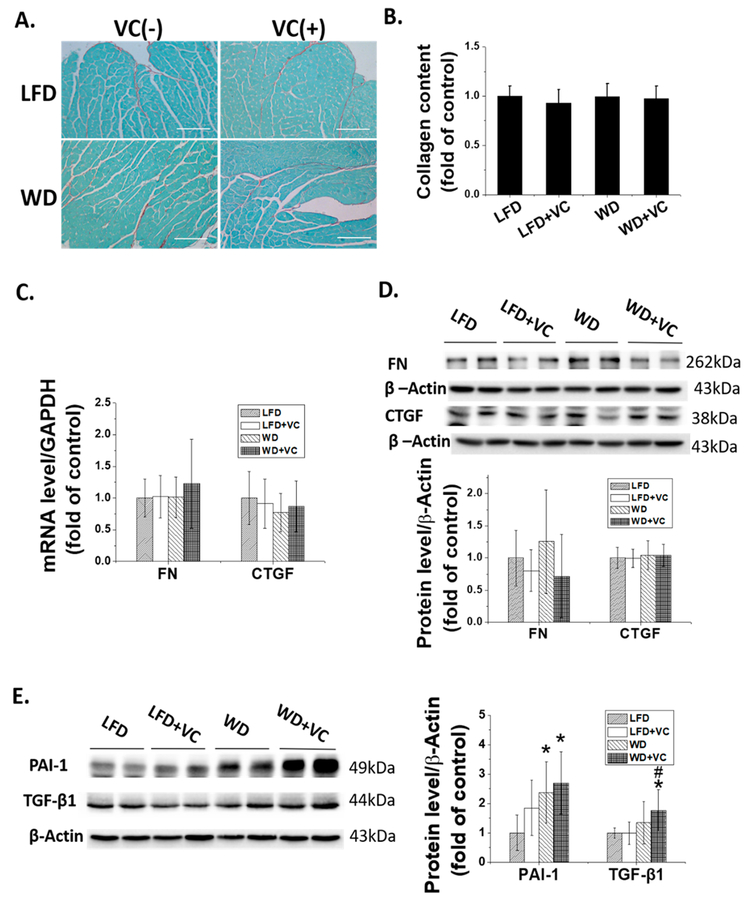
WD with and without VC Did Not Induce Apparent Cardiac Fibrosis, whereas WD Upregulates Some Profibrotic Markers, Particularly When Combined with VC.
Development of cardiac fibrosis has been extensively documented in obese patients.20 Thus, we next evaluated the fibrotic state of the heart. Data showed that WD did not induce a significant cardiac fibrotic response, defined by Sirius Red staining (Figure 3A,B). Consistent with this finding, mRNA (Figure 3C) and protein (Figure 3D) levels of fibrotic mediators, FN and CTGF, remained comparable between these four groups.
Figure 3.
WD and VC did not cause apparent cardiac fibrosis but increase the expression of some profibrotic cytokines. (A) Cardiac fibrosis, determined by Sirius Red staining of collagen accumulation (collagen is red; 200×, scale bar = 100 μm), and (B) quantitative analysis of Sirius Red staining for collagen accumulation. (C) mRNA expression of CTGF and FN was determined by RT-qPCR. Protein expression of (D) CTGF and Fibronectin, (E) PAI-1 and TGF-β1 were determined by Western blot. ∗, versus LFD group, P < 0.05; $, versus LFD+VC group, P < 0.05.
Although WD significantly upregulated the PAI-1 protein level, VC showed no added effect (Figure 3E). In contrast, neither WD nor VC affected TGF-β1 protein expression, but WD plus VC did significantly increase TGF-β1 protein expression (Figure 3E).
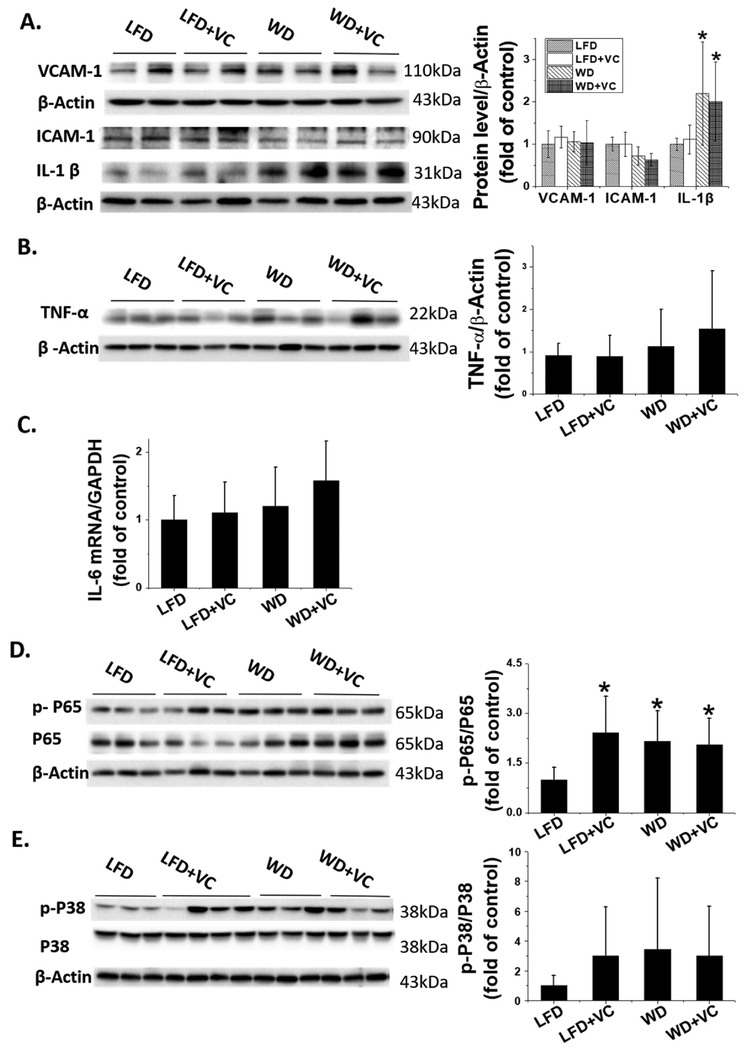
WD Activates Nuclear Factor-κB (NF-κB) and IL-1β Inflammatory Pathway.
Some studies revealed the NF-κB inflammatory pathway plays a pivotal role in obesity related cardiomyopathy.21,22 Therefore, we first investigated the downstream inflammatory markers of NF-κB; Western blot results revealed that WD significantly upregulated IL-1β protein expression, whereas VC showed no additional effect (Figure 4A). However, other proinflammatory cytokines, VCAM-1, ICAM-1, and TNF-α protein levels as well as IL-6 mRNA level showed no significant difference between WD fed mice and LFD fed mice, no matter with and without VC (Figure 4A–C).
Figure 4.
WD activates NF-κB and IL-1β pathway. (A) VCAM-1, ICAM-1, and IL-1β, and (B) TNF-α protein expression were determined by Western blot. (C) RT-qPCR analysis of IL-6 to determine mRNA expression. (D) Phosphorylated and total P65 (NF-κB) as well as (E) phosphorylated and total P38 (MAPK) protein expression were determined by Western blot. Data were presented as means ± SD; ∗, versus LFD group, P < 0.05.
Moreover, optimal induction of NF-κB target genes requires phosphorylation of NF-κB proteins such as the P65 subunit. Here Western blot assay exhibited that p-P65 to total P65 ratio was upregulated in either WD or VC treated group, but not synergistically increased in WD+VC group (Figure 4D), implicating NF-κB activation.
Additionally, our previous study indicated that P38, mitogen-activated protein kinase (MAPK) plays a critical role in obesity related cardiac hypertrophy.7 Therefore, we also examined the phosphorylation levels of P38 with Western blotting, which validated the p-P38 levels were comparable between these four groups (Figure 4E).
WD Has a Tendency To Increase Cardiac Lipid Accumulation.
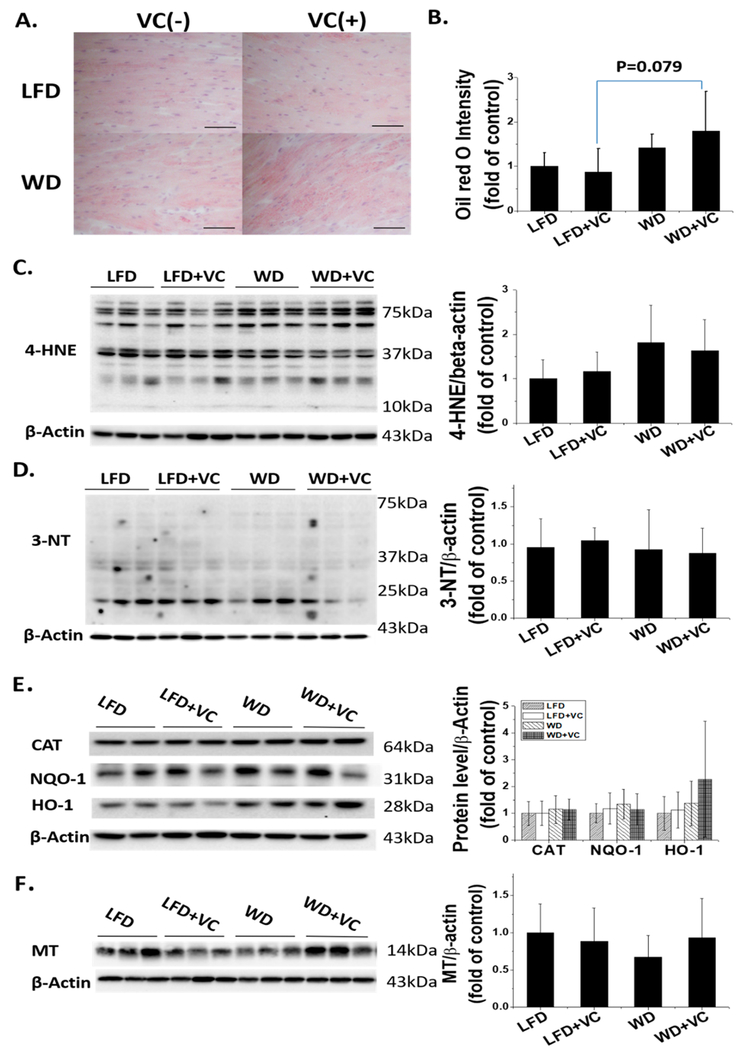
Accumulated evidence has shown that lipotoxicity and oxidative stress are major causal factors for obesity related cardiomyopathy.23–26 Additionally, enhanced fatty acid (FA) oxidation leads to an overproduction of reactive oxygen species.23,27,28 Therefore, next we assessed cardiac lipid accumulation levels by ORO staining. The results indicated WD had a tendency to increase the accumulation of cardiac lipids compared with LFD fed mice; in the presence of VC, this tendency was more obvious (Figure 5A,B, WD+VC group versus LFD+VC group, P = 0.079). These results demonstrated that VC may accelerate WD-induced cardiac lipid accumulation under obese conditions.
Figure 5.
Effects of WD and VC on cardiac lipid accumulation and oxidative stress. (A) Cardiac lipid accumulation was evaluated by ORO staining (400×, scale bar = 50 μm) and (B) quantification of ORO staining for lipid accumulation. Data were presented as means ± SD. Protein expression of(C) 4-HNE, (D) 3-NT, (E) CAT, NQO-1, and HO-1, and (F) MT were determined by Western blot. Data were presented as means ± SD.
Consistent with the data above, WD had a tendency to increase 4-HNE protein level compared with LFD fed mice, an indirect index of lipid peroxidation, but there was no significant difference. Another indicator of oxidative stress and damage markers, protein nitration was measured with 3-NT; the data showed that 3-NT protein levels were comparable between these four groups (Figure 5C,D). To validate the above findings, we further checked some well-known antioxidative markers’ protein levels by Western blot assay including CAT, NQO-1, HO-1, and MT; the results indicated all these antioxidative markers remain unchanged after exposing to either WD or VC, and even combined (Figure 5E,F).
Effect of WD and VC on Endoplasmic Reticulum (ER) Stress and the Apoptosis Pathway.
The ER stress response, also commonly known as the unfolded protein response (UPR), aims to clear unfolded proteins and restore ER homeostasis. It is activated in tissues under various conditions including obesity and type 2 diabetes.29 Using electron microscopy, our team found ER dilation with VC,17 which is well-known in activating ER stress.
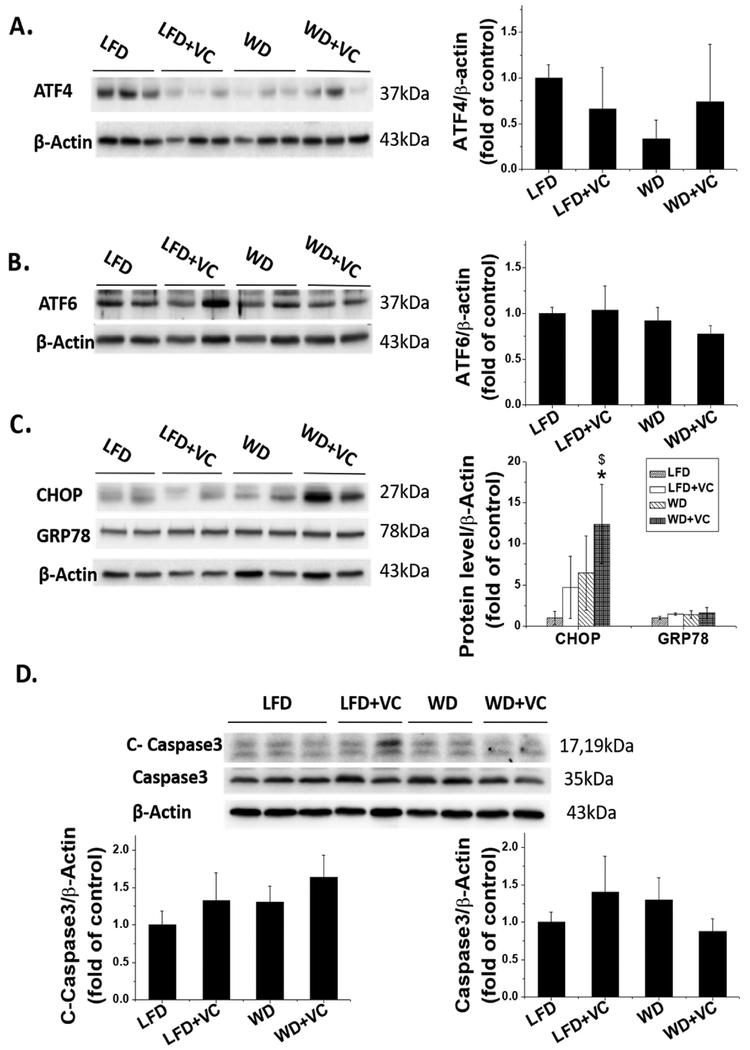
In addition, our group found that VC significantly increased mRNA expression of ER stress markers in livers of WD fed mice, for instance, ATF4, CHOP.17 Therefore, next we sought to verify whether the ER stress pathway was activated in the heart by examining protein levels of key ER stress markers. Interestingly, we found either WD or VC only had a trend to increase, but WD combined with VC significantly increased the CHOP protein levels (Figure 6C). In contrast, levels of ATF4, ATF6, and GRP78 were all comparable between these four groups (Figure 6A–C).
Figure 6.
Effects of WD and VC on ER stress and apoptosis pathway. (A−C) Protein expression of (A) ATF4, (B) ATF6, (C) CHOP, and GRP78 were determined by Western blot; (D) protein expression of cleaved caspase 3 and total caspase 3 was also determined by Western blot. Data were presented as means ± SD; ∗, versus LFD group, P < 0.05.
If the various UPR-induced mechanisms fail to alleviate ER stress, both the intrinsic and extrinsic pathways for apoptosis can become activated.30 Therefore, we further determined cleaved caspase 3 and total caspase 3 levels, which are the key markers of apoptosis by Western blot. Our results showed neither WD nor VC upregulated cleaved caspase 3 protein level, and there was also no synergistic effect of WD combined with VC on cleaved caspase 3 level (Figure 6D), suggesting WD and VC had no effects on apoptosis pathway.
DISCUSSION
In the present study, we observed that feeding male adult C57BL/6J mice with WD containing 42% fat for 12 weeks induced a mild obesity, and consistently increased fat mass. Furthermore, VC showed a tendency to increase the bodyweight gain in WD fed mice. The slightly obese mice also exhibited impaired glucose tolerance; VC partially aggravates this effect, implying that WD fed mice developed insulin resistance. More importantly, WD fed mice showed slightly lower FS compared with LFD fed mice; with EF showing only a tendency to decrease (P = 0.066). These parameters were not significantly affected by VC. The hearts of WD fed mice displayed structural abnormalities: broken fibers and deranged cellular structures. Sorop et al.31 established an obesity (hypercholesterolemia and hypertriglyceridemia), hypertension, and diabetes mellitus model, through following up for 6 months, they found that an increased left ventricular enddiastolic stiffness and a trend toward a reduced E/A ratio, while EF was maintained, which indicated that in obesity condition, diastolic dysfunction occurs before systolic dysfunction. In our present study, we did not measure the diastolic function, which is the limitation inherent to the design of the present study, and it must be considered when interpreting our results.
Feeding a WD with 42% fat for 12 weeks did not induce significant cardiac hypertrophy or fibrosis. Although feeding with the WD diet for 12 weeks did not cause remarkable cardiac oxidative stress, ER stress, oxidative damage, or remodeling, it did however significantly increase cardiac expression of PAI-1 protein in the WD group,17 while this effect was not altered by VC. Moreover, WD+VC upregulates the TGF-β1 protein level, which is a well-known profibrotic cytokine. Increased expression of cardiac TGF-β1 is consistently noted in experimental models of obesity and is associated with cardiac fibrosis.32−34
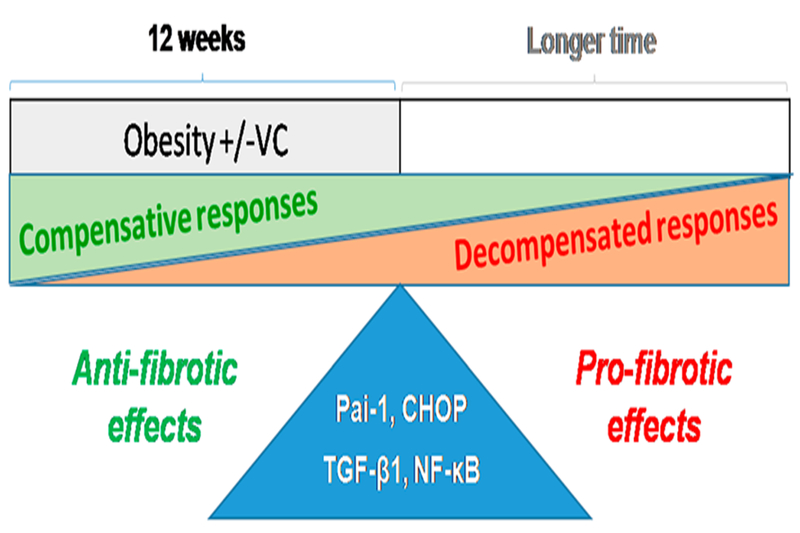
In previous studies, PAI-1 was found to be the downstream from TGF-β1,35,36 which acts as a profibrotic cytokine. However, during recent animal studies, evidence emerged indicating that PAI-1 could be the antifibrotic cytokine in the cardiac fibrotic process.37,38 Ghosh et al.13 demonstrated that aged PAI-1 deficient mice developed spontaneous myocardial fibrosis resulting from excessive accumulation of collagen, which accompanied by upregulated TGF-β 1/2 protein levels. Flevaris et al.38 further reported that treatment of young PAI-1−/− mice with Angiotensin II induced extensive hypertrophic and fibrotic cardiomyopathy, with increased cardiac apoptosis and fibrosis compared to the same treated wild type mice. These studies revealed a novel notion that PAI-1 is an essential repressor of cardiac fibrosis in mammals, acting as a molecular switch that controls the cardiac TGF-β1 axis. In the present study, we hypothesize the profibrotic effect of TGF-β1 reached a balance with the antifibrotic effect of PAI-1 along with other factors (see below and also Figure 7). Consequently, the downstream markers of the profibrotic pathway remained stable leading to no significant change of CTGF and FN mRNA and protein levels (Figure 3C,D). However, if the WD feeding is further prolonged, the pathogenic effects on the heart might be significantly manifested (Figure 7).
Figure 7.
Working hypothesis. Typical WD (42% fat) feeding with and without low-dose VC for 12 weeks does not induce significant cardiac effects but may do for longer such as 24 weeks. Our body, including organs such as heart, may exhibit compensative responses when exposed to subtoxic stresses, and this compensative response will last certain times dependent on the toxicity of exposed stress; meanwhile, the accumulated toxicities caused by exposed stress also gradually results in impairing the compensative mechanisms, leading to decompensation responses and eventually harmful effects.
A large body of evidence from experimental models and human patients suggests that obesity is associated with a systemic inflammatory response.39 NF-κB is a transcription factor that has crucial roles in inflammation, immunity, cell proliferation, and apoptosis. Accumulated studies indicate that using NF-κB as a therapeutic target in the treatment of obesity-related cardiomyopathy showed promising results.8,21 Therefore, in the current study, we focused on the NF-κB pathway. NF-κB is a heterodimeric activator of transcription, which normally locates in the cytoplasm, until it is activated by multiple signaling pathways; it then translocates to the nucleus of the cell. Optimal induction of NF-κB target genes also requires the phosphorylation of NF-κB proteins, such as canonical RelA/P65 subunit, within their transactivation domain by a variety of kinases in response to distinct stimuli.40 Thus, we evaluated the phosphorylated level of P65 to express the activation of NF-κB. We observed that NF-κB can be activated by either WD or VC, but WD and VC combined did not show any synergistic effect. Downstream of NF-κB, the protein level of IL-1β was significantly upregulated in WD fed mice but not in VC fed mice. Nevertheless, other proinflammatory cytokines, for example, VCAM-1, ICAM-1, and TNF-α protein as well as IL-6 mRNA, levels remained unchanged. We assumed that WD feeding for 12 weeks results in only a mild and chronic inflammation, but not a robust and acute inflammation, as illustrated in Figure 7. Indeed, similar results were observed in the liver.17 However, we cannot be certain whether the severe inflammation can be significantly manifested if the WD feeding time is further prolonged.
With increased obesity, plasma FA and TG concentrations are elevated. In addition, we also found elevated cholesterol, FFA, and TG levels in the liver of WD-treated mice compared to LFD.17 However, VC increased only liver TG levels in WD fed mice.17 It is known that with hyperlipidemia, FA uptake is dramatically enhanced in several organs,41,42 for instance, the heart. In addition, FA β-oxidation is also increased. Cardiomyocytes are metabolically flexible: normally it predominantly uses FA, but can also use glucose, lactate, and any other available substrates to produce ATP under unusual conditions. However, with increased myocardial FA intake, FA becomes almost the only substrate for cardiomyocytes; this may ultimately lead to cardiac metabolic inflexibility, lipotoxicity,43 oxidative stress,44 and subsequent development of cardiomyopathy at the very late stage.45 In our study, we showed that WD-treated mice had a tendency to increase lipid accumulation in the heart, particularly with the presence of VC. Enhanced FA oxidation leads to an overproduction of reactive oxygen species, consistent with our results of ORO staining, 4-HNE, a marker of lipid peroxidation, which showed an increase tendency in WD fed mice compared with LFD fed mice. Moreover, all of these markers were significantly increased in the liver, which is the first line of defense.17 To verify the above findings, we further determined some well-known antioxidative cytokines in the heart, for instance, CAT, NQO-1, HO-1, and MT; consistently, all of these antioxidative markers remained unchanged (Figure 5E,F).
ER is the central intracellular organelle in the secretory pathway. It is responsible for protein translocation, folding, and post-translational modifications that allow further transport of proteins to the Golgi apparatus and ultimately to vesicles for secretion or display on the plasma surface. Perturbations in ER function, a process known as “ER-stress”, trigger the UPR, a tightly orchestrated collection of intracellular signal transduction reaction designed to restore protein homeostasis. Our group found in the liver, that ER was significantly dilated when VC was present in LFD fed mice via electron microscopy, indicating the activation of the UPR.17 Moreover, VC enhances the mRNA expression of CHOP and ATF4 in WD fed mice in the liver, with CHOP protein levels also elevated in these mice, though VC did not amplify this effect.17 In the heart, unlike the liver, WD alone did not significantly elevated CHOP protein expression compared to LFD fed mice, but addition of VC to WD significantly increased CHOP protein level. It is known if the compensatory mechanisms fail to alleviate ER stress, apoptosis pathway can be activated; however, we did not find significant increase in cleaved caspase 3 protein level, confirming that neither WD nor VC elicits apparent cell apoptosis. We suppose although that elevating CHOP protein level indicates activation of ER stress, as illustrated in Figure 7. However, there are some underling compensatory mechanisms, which need to be further elucidated.
In summary, WD (42% fat) feeding for 12 weeks did not induce significant cardiac pathological changes similar to other study in which mice were fed with HFD (45% fat) for 14 weeks.46 This is possibly due to the relative short feeding time that may just reach the switch point of cardiac compensation to decompensation responses, as illustrated in Figure 7. Additionally, the effects and the potential mechanisms of HFD (45% fat) and equal caloric WD high in fat, sucrose, and cholesterol feeding for 8 weeks in male C57B1/6N mice are different.47 Inhalation of very low-dose VC neither significantly exacerbates nor attenuates WD-induced cardiac effects. For the lack of robust impact of VC on WD-induced cardiac effects except for synergistic increases in TGF-β1 (Figure 3E) and CHOP (Figure 6C), we assume the following: (1) the dose of VC may be too low; (2) the combined exposure time of VC and WD was relatively short; or (3) VC does not generate synergistic effects with WD. These possibilities will be further defined in our future studies; therefore, we conclude that combined exposures to typical WD and very low-dose exposure of VC for a relatively short time do not contribute to significant cardiac effects, and possibly longer-time exposure will cause harmful cardiac or synergistic effects. This will be investigated during future systemic studies with longer time and increased doses of VC.
ACKNOWLEDGMENTS
We appreciate Patricia Kralik for editing the manuscript and providing language assistance.
Funding
National Institutes of Health: K01 DK096042, R03 DK107912 to J.I.B. J.I.B. was also supported by Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant No. P20GM113226, P20 GM10349, the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award No. P50AA024337, and the National Institute of Environmental Health Sciences under Award No. P42 ES023716. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- HFD
high fat diet
- WD
western diet
- VC
vinyl chloride
- ORO
Oil Red O
- Echo
echocardiography
- ANP
atrial natriuretic peptide
- CTGF
connective tissue growth factor
- IL-1β
Interleukin-1β
- ICAM-1
intercellular adhesion molecules-1
- VCAM-1
vascular cell adhesion molecule 1
- CAT
catalase
- NQO-1
quinone oxidoreductase
- 4-HNE
4-hydroxynonenal
- 3-NT
3-nitrotyrosine
- TNF-α
tumor necrosis factor-α
- FN
fibronectin
- ATF4
activating transcription factor 4
- GRP78
78 kDa glucose-regulated protein
- TGF-β1
transforming growth factor beta 1
- HO-1
heme oxygenase 1
- MAPK
mitogen-activated protein kinase
- CHOP
CCAT-enhancer-binding protein homologous protein
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- ATF6
activating transcription factor 6
- PAI-1
plasminogen activator inhibitor-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- β-MHC
myosin heavy chain beta
- IL-6
Interleukin-6
- TG
triglycerides
- MT
metallothionein
- FA
fatty acid
- FFA
free fatty acid
- RT-qPCR
reverse transcription and quantitative PCR
- HR
heart rate
- EF
ejection fraction
- FS
fractional shortening
- IVS;d
interventricular septal thickness at diastole
- IVS;s
interventricular septal thickness at systole
- LV
left ventricular
- LVID;d
internal dimension of LV at diastole
- LVID;s
internal dimension of LV at systole
- LVPW;d
LV posterior wall at diastole
- LVPW;s
LV posterior wall at systole
- LV Vol;d
LV volume at diastole
- LV Vol;s
LV volume at systole
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- SD
standard deviation
- WHO
World Health Organization
- ATSDR
Agency for Toxic Substances and Disease Registry
- OSHA
Occupational Safety and Health Administration
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1). Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, et al. (2017) Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med 377, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2). Dibaise JK, and Foxx-Orenstein AE (2013) Role of the gastroenterologist in managing obesity. Expert Rev. Gastroenterol. Hepatol 7, 439–451. [DOI] [PubMed] [Google Scholar]
- (3). Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, and Ezzati M (2013) The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One 8, e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4). The Emerging Risk Factors, C., Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, and Danesh J (2011) Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377, 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5). Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, and Straif K (2016) Body Fatness and Cancer− Viewpoint of the IARC Working Group. N. Engl. J. Med 375, 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6). Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, and Eisenberg MJ (2010) The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol 56, 1113–1132. [DOI] [PubMed] [Google Scholar]
- (7). Wang S, Luo M, Zhang Z, Gu J, Chen J, Payne KM, Tan Y, Wang Y, Yin X, Zhang X, Liu GC, Wintergerst K, Liu Q, Zheng Y, and Cai L (2016) Zinc deficiency exacerbates while zinc supplement attenuates cardiac hypertrophy in high-fat diet-induced obese mice through modulating p38 MAPK-dependent signaling. Toxicol. Lett 258, 134–146. [DOI] [PubMed] [Google Scholar]
- (8). Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, Chakrabarti S, Wu L, Wang J, and Liang G (2015) Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-kappaB both in vitro and in vivo. J. Mol. Cell. Cardiol 79, 1–12. [DOI] [PubMed] [Google Scholar]
- (9). Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, and Summerbell CD (2012) Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ. 345, e7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10.) World Health Organization. (2003) Diet, Nutrition, and the Prevention of Chronic Diseases, World Health Organization, Geneva, Switzerland, www.who.int/dietphysicalactivity/publications/trs916/en/gsfao_overall.pdf?ua=1. [PubMed] [Google Scholar]
- (11). Pei Z, Zhuang Z, Sang H, Wu Z, Meng R, He EY, Scott GI, Maris JR, Li R, and Ren J (2014) alpha,beta-Unsaturated aldehyde crotonaldehyde triggers cardiomyocyte contractile dysfunction: role of TRPV1 and mitochondrial function. Pharmacol. Res 82, 40–50. [DOI] [PubMed] [Google Scholar]
- (12). Turkcan A, Scharinger B, Grabmann G, Keppler BK, Laufer G, Bernhard D, and Messner B (2015) Combination of cadmium and high cholesterol levels as a risk factor for heart fibrosis. Toxicol. Sci 145, 360–371. [DOI] [PubMed] [Google Scholar]
- (13). Dutta M, Ghosh D, Ghosh AK, Bose G, Chattopadhyay A, Rudra S, Dey M, Bandyopadhyay A, Pattari SK, Mallick S, and Bandyopadhyay D (2014) High fat diet aggravates arsenic induced oxidative stress in rat heart and liver. Food Chem. Toxicol 66, 262–277. [DOI] [PubMed] [Google Scholar]
- (14). Kielhorn J, Melber C, Wahnschaffe U, Aitio A, and Mangelsdorf I (2000) Vinyl chloride: still a cause for concern. Environ. Health Perspect 108, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15.) U.S.Department of Health and Human Services PHS. Agency for Toxic Substances and Disease Registry (ATSDR): Toxicological profile for Vinyl Chloride; 2006. [PubMed]
- (16). Tamburro CH, Mark L, and Popper H (1984) Early hepatic histologic alterations among chemical (vinyl monomer) workers. Hepatology 4, 413–418. [DOI] [PubMed] [Google Scholar]
- (17). Lang AL, Chen L, Poff GD, Ding WX, Barnett RA, Arteel GE, and Beier JI (2018) Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice. Hepatology communications 2, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18). Anders LC, Yeo H, Kaelin BR, Lang AL, Bushau AM, Douglas AN, Cave M, Arteel GE, McClain CJ, and Beier JI (2016) Role of dietary fatty acids in liver injury caused by vinyl chloride metabolites in mice. Toxicol. Appl. Pharmacol 311, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19). Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, and Cai L (2006) Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 113, 544–554. [DOI] [PubMed] [Google Scholar]
- (20). Cavalera M, Wang J, and Frangogiannis NG (2014) Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysio-logical pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res 164, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21). Qian Y, Zhong P, Liang D, Xu Z, Skibba M, Zeng C, Li X, Wei T, Wu L, and Liang G (2015) A newly designed curcumin analog Y20 mitigates cardiac injury via anti-inflammatory and anti-oxidant actions in obese rats. PLoS One 10, e0120215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22). Qian Y, Zhang Y, Zhong P, Peng K, Xu Z, Chen X, Lu K, Chen G, Li X, and Liang G (2016) Inhibition of inflammation and oxidative stress by an imidazopyridine derivative X22 prevents heart injury from obesity. J. Cell. Mol. Med 20, 1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23). Carvajal K, Balderas-Villalobos J, Bello-Sanchez MD, Phillips-Farfan B, Molina-Munoz T, Aldana-Quintero H, and Gomez-Viquez NL (2014) Ca(2+) mishandling and cardiac dysfunction in obesity and insulin resistance: role of oxidative stress. Cell Calcium 56, 408–415. [DOI] [PubMed] [Google Scholar]
- (24). Glatz JF, Angin Y, Steinbusch LK, Schwenk RW, and Luiken JJ (2013) CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins, Leukotrienes Essent. Fatty Acids 88, 71–77. [DOI] [PubMed] [Google Scholar]
- (25). Turkieh A, Caubere C, Barutaut M, Desmoulin F, Harmancey R, Galinier M, Berry M, Dambrin C, Polidori C, Casteilla L, Koukoui F, Rouet P, and Smih F (2014) Apolipoprotein O is mitochondrial and promotes lipotoxicity in heart. J. Clin. Invest 124, 2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26). Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, and Goldberg IJ (2010) PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J. Clin. Invest 120, 3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27). Boudina S, and Abel ED (2010) Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord 11, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28). St-Pierre J, Buckingham JA, Roebuck SJ, and Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem 277, 44784–44790. [DOI] [PubMed] [Google Scholar]
- (29). Cnop M, Foufelle F, and Velloso LA (2012) Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med 18, 59–68. [DOI] [PubMed] [Google Scholar]
- (30). Sano R, and Reed JC (2013) ER stress-induced cell death mechanisms. Biochim. Biophys. Acta, Mol. Cell Res 1833, 3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31). Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen TN, Octavia Y, van Duin RWB, Stam K, van Geuns RJ, Wielopolski PA, Krestin GP, van den Meiracker AH, Verjans R, van Bilsen M, Danser AHJ, Paulus WJ, Cheng C, Linke WA, Joles JA, Verhaar MC, van der Velden J, Merkus D, and Duncker DJ (2018) (2018) Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress and myocardial stiffening. Cardiovasc. Res, DOI: 10.1093/cvr/cvy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32). Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, and Sanders P (2013) Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 10, 90–100. [DOI] [PubMed] [Google Scholar]
- (33). Toblli JE, Cao G, DeRosa G, and Forcada P (2005) Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor beta1 in obese Zucker rats by perindopril. Heart 91, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34). Carroll JF, and Tyagi SC (2005) Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am. J. Hypertens 18, 692–698. [DOI] [PubMed] [Google Scholar]
- (35). Samarakoon R, Overstreet JM, and Higgins PJ (2013) TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell. Signalling 25, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36). van den Akker GG, van Beuningen HM, Vitters EL, Koenders MI, van de Loo FA, van Lent PL, Blaney Davidson EN, and van der Kraan PM (2017) Interleukin 1 beta-induced SMAD2/3 linker modifications are TAK1 dependent and delay TGFbeta signaling in primary human mesenchymal stem cells. Cell. Signalling 40, 190–199. [DOI] [PubMed] [Google Scholar]
- (37). Ghosh AK, Bradham WS, Gleaves LA, De Taeye B, Murphy SB, Covington JW, and Vaughan DE (2010) Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation 122, 1200–1209. [DOI] [PubMed] [Google Scholar]
- (38). Flevaris P, Khan SS, Eren M, Schuldt AJT, Shah SJ, Lee DC, Gupta S, Shapiro AD, Burridge PW, Ghosh AK, and Vaughan DE (2017) Plasminogen Activator Inhibitor Type I Controls Cardiomyocyte Transforming Growth Factor-beta and Cardiac Fibrosis. Circulation 136, 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39). Lumeng CN, and Saltiel AR (2011) Inflammatory links between obesity and metabolic disease. J. Clin. Invest 121, 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40). Viatour P, Merville MP, Bours V, and Chariot A (2005) Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem. Sci 30, 43–52. [DOI] [PubMed] [Google Scholar]
- (41). Aguer C, Mercier J, Man CY, Metz L, Bordenave S, Lambert K, Jean E, Lantier L, Bounoua L, Brun JF, Raynaud de Mauverger E, Andreelli F, Foretz M, and Kitzmann M (2010) Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia 53, 1151–1163. [DOI] [PubMed] [Google Scholar]
- (42). Coort SL, Bonen A, van der Vusse GJ, Glatz JF, and Luiken JJ (2007) Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: role of sarcolemmal substrate transporters. Mol. Cell. Biochem. 299, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43). Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, and Kumar SA (2014) Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl. Res 164, 345–356. [DOI] [PubMed] [Google Scholar]
- (44). Ilkun O, and Boudina S (2013) Cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Curr. Pharm. Des 19, 4806–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45). Harmancey R, Wilson CR, and Taegtmeyer H (2008) Adaptation and maladaptation of the heart in obesity. Hypertension 52, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46). Chen F, Chen D, Zhao X, Yang S, Li Z, Sanchis D, Jin L, Qiang X, Wang K, Xu Y, Zhang Y, and Ye J (2017) Interleukin-6 deficiency facilitates myocardial dysfunction during high fat diet-induced obesity by promoting lipotoxicity and inflammation. Biochim. Biophys. Acta, Mol. Basis Dis 1863, 3128–3141. [DOI] [PubMed] [Google Scholar]
- (47). Heinonen I, Rinne P, Ruohonen ST, Ruohonen S, Ahotupa M, and Savontaus E (2014) The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice. Acta Physiol 211, 515–527. [DOI] [PubMed] [Google Scholar]