Abstract
Purpose:
To determine the disease control rate and toxicity of treating patients with aggressive cutaneous squamous cell carcinoma (CSCC) with neoadjuvant gefitinib.
Experimental Design:
A prospective phase II clinical trial evaluating neoadjuvant gefitinib given prior to standard treatment with surgery and/or radiotherapy. Patients with stable disease after 1 cycle received escalated doses. Patients who responded were given gefitinib during radiation therapy, as well as maintenance therapy after definitive treatment. We analyzed the correlation between EGFR expression, mutation status, and gene copy number on available tissue samples and clinical response.
Results:
Twenty-three patients were accrued and 22 patients were evaluable for response prior to definitive local treatment; complete responses were attained by 18.2% of patients and partial responses by 27.3%. Grade 2–3 toxicities were observed in 59.1% of patients experiencing class-specific effects during induction therapy. After induction, 11.8% underwent surgery alone, 17.6% had definitive radiation, 11.8% were treated with radiation and concurrent gefitinib, and 47% had surgery with postoperative radiation and concurrent gefitinib. Median follow-up for the censored observations was 32 months. Two-year overall, disease-specific, and progression-free survival rates were 72.1%, 72.1%, and 63.6%, respectively. No EGFR-activating mutations were identified in tumors samples available from 10 patients. No associations between EGFR correlative studies and patient outcomes were identified.
Conclusions:
Gefitinib, in the neoadjuvant setting, was active and well-tolerated in patients with aggressive CSCC, and did not interfere with definitive treatment. In view of the 18% CR rate we observed, EGFR TKIs should be further explored in the treatment of aggressive CSCC.
Keywords: gefitinib, cutaneous squamous cell cancer, neoadjuvant therapy, EGFR
INTRODUCTION
The annual incidence of non-melanoma skin cancer is estimated to be considerably higher than 1 million cases, most of which are unreported(1). Of these, an estimated 20% are squamous cell carcinomas (2). The lifetime risk of developing cutaneous squamous cell carcinoma (CSCC) in the United States in 1994 was 4 to 9 percent among women and 9 to 14 percent among men (3). However, this rate has been increasing; between 1979 to 1980 and 1993 to 1994, the incidence of CSCC among New Hampshire residents increased 235% for men and 350% for women (4). While these numbers may reflect improved surveillance and reporting, establishing a true incidence would require serial standardized evaluations of a population; since the data of such a study are as of yet unavailable, the striking increase observed is believed to indicate at least a trend towards an increased incidence of CSCC.
More than 90% of CSCCs are cured by initial treatment; the 5-year recurrence rate is 8% and the 5-year rate of metastasis is 5% (2). Initial management strategies include electrodessication and curettage, excision, cryosurgery, or radiotherapy (2). Risk factors for aggressive behavior include size bigger than 2 cm, invasion beyond subcutaneous tissues, perineural invasion, an invasion depth of 7 mm or more, and recurrence (2, 5–7). Having 1 risk factor reduces 3-year disease-specific survival by 30% (5).
Recently, there have been several case reports describing the response of multiply recurrent and highly invasive CSCCs to epidermal growth factor receptor (EGFR) inhibitors (8–10). EGFR is overexpressed in most epithelium-derived neoplasms and high expression of EGFR has been associated with a worse prognosis in some tumors, such as mucosal squamous cell carcinomas (11). In preclinical studies, EGFR inhibitors have been demonstrated to increase radiosensitivity by enhancing apoptosis and delaying tumor growth (12) and suppressing skin tumorigenesis (13). Specifically, gefitinib has been shown to inhibit the proliferation and invasiveness of CSCC cell lines, as well as EGF-induced signaling and cell motility (14).
Gefitinib is an EGFR tyrosine kinase inhibitor (TKI) that was initially studied in chemotherapy-refractory non-small cell lung cancer, including the squamous subtype, and is currently marketed outside of the United States. More recently, it has been shown to improve progression-free survival (PFS) relative to carboplatin-paclitaxel when used as a frontline treatment for patients with adenocarcinoma of the lung with an activating mutation in the catalytic domain of EGFR (15). Gefitinib has also been evaluated as an agent in concurrent chemoradiation regimens for head and neck cancer (16, 17). In addition, EGFR TKIs have also been evaluated as single-agent treatments for recurrent or metastatic head and neck mucosal squamous cell carcinomas (HNSCC), with response rates ranging from 5% to10%. Longer overall survival (OS) and PFS has been correlated with older age, better performance status, and the development of a rash with EGFR TKI treatment (18).
Because gefitinib has shown clinical benefit in other squamous cell carcinomas and anti-tumorigenic and pro-apoptotic activity in CSCC in preclinical studies, we designed a phase II trial to evaluate the efficacy of gefitinib in aggressive CSCC. Herein, we report the results of gefitinib as neoadjuvant therapy prior to definitive treatment for patients with high-risk CSCC. Given that previous studies have suggested that EGFR expression and gene amplification are prognostic and further, that EGFR mutation status (19–21) is correlated with clinical benefit from gefitinib, we also assessed EGFR protein expression and evaluated tumors for EGFR mutations and ploidy/gene amplification to evaluate whether these parameters could be used as prognostic and/or predictive indicators.
PATIENTS AND METHODS
Patient eligibility
The primary endpoint was the disease control rate (CR + PR + SD) to neoadjuavnt gefitinib. All patients were required to have histologically or cytologically confirmed CSCC that was either aggressive or recurrent with measurable disease. Although biopsies were taken to confirm this diagnosis, these were small enough so as not to affect the size of the tumor being studied. Tumors were considered aggressive if they were ≥ 2 cm; invaded deep tissues, such as muscle, cartilage, or bone; demonstrated perineural invasion; and/or metastasized to lymph nodes. Patients may have had previous surgical interventions or been treated with investigational agents with residual or recurrent disease, but patients who had previously been irradiated at the lesion site evaluated for this study were ineligible; patients who had received radiation therapy to other lesion sites were considered candidates. Other eligibility criteria included an Eastern Cooperative Oncology Group performance status ≥ 2 and adequate bone marrow, renal, and hepatic function (as defined by absolute neutrophil count ≥ 1500/mm3, platelets ≥ 100,000/mm3, liver enzymes ≤ 2.5 times the institutional upper limit of normal, and total bilirubin and creatinine levels within normal institutional limits). Enrolled patients had potentially curable disease; resectability was determined before and after neoadjuvant gefitinib therapy and unresectable patients received definite radiation. Patients were excluded if they had distant metastases, other active cancers, interfering comorbidities, or if they were taking antiretroviral or liver cytochrome-inducing medications that would pharmacologically interact with gefitinib (22). The protocol was approved by the institutional review board at The University of Texas MD Anderson Cancer Center, and informed consent was obtained from each patient upon enrollment.
Treatment plan
On initial evaluation, a complete history was taken and all patients underwent a physical examination, which included measurement of local and regional tumor and photo-documentation of tumor dimensions, and axial imaging. Photo-documentation was particularly important in cases for which the tumor size was not satisfactorily or reliably demonstrated on axial imaging. Although serial cross-sectional images were obtained for each patient, tumor measurements were based on whichever modality (physical examination or axial imaging) was more feasible; 13 lesions were followed by physical examination and photography, 4 were followed by imaging, and 6 were followed by both modalities. Complete blood counts, serum chemistries, liver function tests, chest x-rays, and electrocardiograms were performed on all patients. Baseline contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was also obtained, and imaging was repeated at 15 and 60 days. Optional tumor tissue samples for correlative studies were collected at 15 and 60 days and at the time of surgery, when applicable. Every 2 weeks, tumor measurements were recorded, repeat laboratory studies were performed, and performance status was assessed.
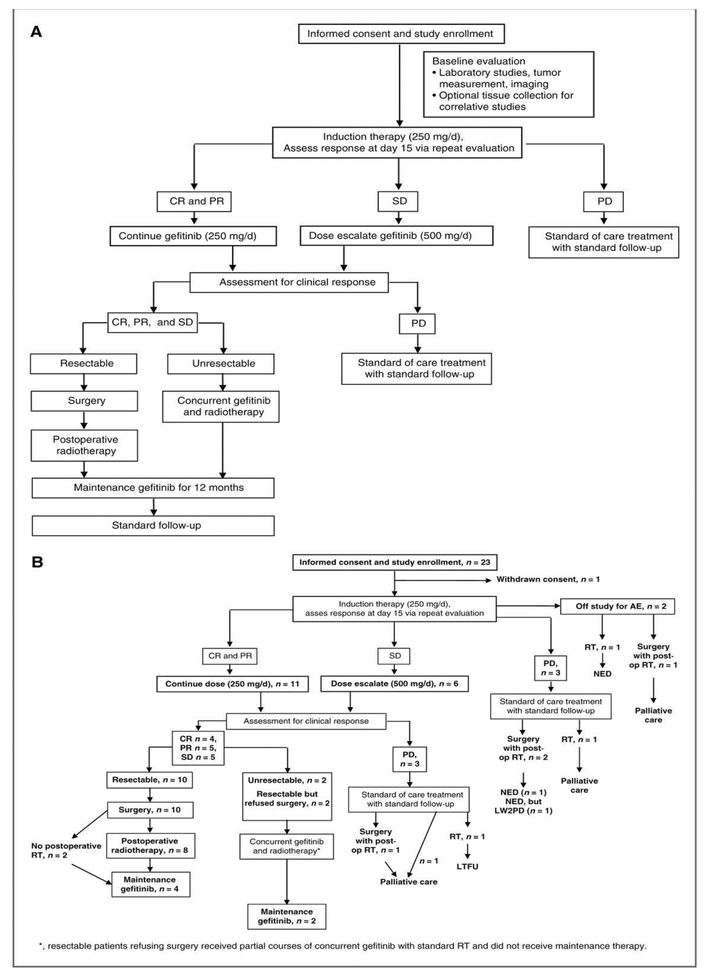
The neoadjuvant phase of the treatment regimen consisted of 2 30-day cycles of 250 mg gefitinib given by mouth daily (Fig. 1). Patients were restaged on days 15 and 60 of therapy. If a CR, PR or stable disease (SD) was observed on day 15, gefitinib was continued at the starting dose. If the patient had progression of disease (PD) at this point or any subsequent point, gefitinib was discontinued and the patient was reassigned to a standard of care treatment pathway. After 8 patients had been accrued, the protocol was changed to include a dose escalation: If a patient had SD on day 15, the gefitinib dose was escalated to 500 mg daily. After the second cycle (day 60), tumor resectability was determined. If a tumor was resectable, the patient proceeded to surgery with postoperative radiotherapy as indicated, followed by a maintenance phase of gefitinib. The maintenance phase consisted of continuation of the induction dose of gefitinib for 12 additional months or until a dose-limiting toxicity was encountered. If the patient’s tumor was deemed unresectable, the patient proceeded to concomitant gefitinib and definitive radiotherapy. If the patient was still not a surgical candidate after completing radiotherapy, the maintenance phase of gefitinib was started. Otherwise, the patient proceeded to surgery, followed by the maintenance phase of gefitinib.
Figure 1.
(a) Overview of the clinical trial design (b) CONSORT diagram of patient treatment. (CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease)
Evaluation of response, survival, and adverse events
Tumor response was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) (23). Clinical responses were measured by physical examination and, for tumors not well demonstrated on axial imaging, photography every 2 weeks, as well as by imaging (CT or MRI) on days 15 and 60. For patients undergoing surgery after completing neoadjuvant gefitinib, a pathologic CR was defined as the absence of tumor in the resection specimen. Overall response included patients achieving a clinical CR or PR. Survival was calculated from the time of enrollment to death or to the date of last contact. Progression-free survival (PFS) was calculated from the time of enrollment to the date of recurrence or death, whichever occurred first.
Toxicity was assessed every 2 weeks and graded using the National Cancer Institute Common Toxicity Criteria (24). Previously correlated morbidities were categorized as expected toxicities.
Correlative studies
Tissue for correlative studies was collected by optional punch biopsies obtained before the study (baseline), at the end of the second cycle of induction therapy, and at the time of surgical resection in eligible patients. These samples were analyzed for total EGFR and phosphorylated EGFR (pEGFR) by immunohistochemical (IHC) analysis, EGFR copy number by fluorescence in situ hybridization (FISH), and EGFR mutations by PCR-based sequencing. The pathologists performing these studies and evaluating the results were blinded to patient identity and clinical outcome. Although no specific measures were taken to assess inter-observer variability, the assessments of the IHC markers were performed jointly by two pathologists (XT and IW).
Immunohistochemical analysis.
IHC analysis was performed as previously described (25). Lung cancer and tissue specimens with known EGFR total and phosphorylated expression status by western blot and immunohistochemistry were used as controls. Samples were scored based on the fraction of cells with a given staining intensity multiplied by the degree of staining extension (0, no appreciable staining; 1, barely detectable staining; 2, readily appreciable staining; 3, moderate staining; and 4, very strong staining). A formalin-fixed and paraffin-embedded lung cancer tissue specimen with known EGFR total and phosphorylated-EGFR expression status determined by Western blot of the corresponding frozen tissue was used as positive and negative control in the IHC assay (Supplemental figure 1). These IHC assays have demonstrated a high level of specificity (EGFR 100%; p-EGFR 94%) and sensitivity (EGFR 100%; p-EGFR 94%) (I. Wistuba, personal communication).
EGFR mutation analysis.
Approximately 1000 cells per sample were microdissected and their DNA extracted as previously described (25). PCR-amplification was then used to evaluate EGFR exons 18 through 21 (the catalytic domain), using HotStarTaq Master Mix (Qiagen) for 40 cycles (94°C for 30 seconds, 63°C for 30 seconds, and 72°C for 30 seconds followed by a 7-minute extension at 72°C). PCR products were directly sequenced using the Applied Biosystems PRISM dye terminator cycle sequencing method (Perkin-Elmer Corporation).
FISH analysis.
FISH analysis was performed as previously described (25, 26). Briefly, after histology sections were deparaffinized and dehydrated, the EGFR Spectrum Orange/CEP 7/SpectrumGreen probe set (Abbott Molecular) was applied according to manufacturer’s instructions. The hybridization area was then covered with a coverslip and sealed. Slides were incubated first at 80 °C for 10 minutes and then placed in an incubation chamber for 20 to 24 hours at 37 °C. Post-hybridization washes were performed with 1.5 mol/L of urea and 0.1x SSC (pH 7.0–7.5) at 45 °C for 30 minutes and 2x SSC for 2 minutes at room temperature. Once the samples had been dehydrated with increasing concentrations of ethanol, chromatin counter-staining was performed with 4’,6’-diamidino-2-phenylindole (0.15 mg/ml in Vectashield Mounting Medium; Vector Laboratories). As it has been previously reported in EGFR FISH studies lung cancer correlating (26, 27), high polysomy and gene amplification categories were considered to have increased EGFR copy number and FISH-positive. As suggested in the guidelines for EGFR FISH testing(26), high polysomy was defined as ≥40% of cells displaying ≥4 copies of the EGFR signal; and gene amplification was defined according to one of the following criteria: (a) an EGFR to CEP7 ratio ≥2 over all scored nuclei and calculated using the sum of EGFR divided by the sum of CEP7 when mean CEP7 per cell is ≥2 copies; (b) the presence of gene cluster (≥4 spots) in ≥10% of tumor cells; (c) at least 15 copies of the EGFR signals in ≥10% of tumor cells. Samples that did not display gene amplification according to the criteria defined above and with <40% of cells displaying ≥4 copies of the EGFR signal were considered FISH-negative (26) (Supplemental figure 1). Lung cancer and tissue specimens with known EGFR FISH-positive and negative status were used as controls. Non-malignant cells present in each tumor tissue samples a NSCLC cell line with gene amplification were used as negative and control, respectively.
Statistical analyses
Definitive surgery or radiation therapy was delayed for up to 2 months while patients received neoadjuvant gefitinib. Thus, the regimen was to be considered unacceptable if a large fraction of patients progressed during gefitnib therapy. An early progression rate of >25% was deemed unacceptable, while a rate of 10% was deemed acceptable. These arbitrary rates were chosen to minimize risks to participants. Lack of early progression, defined as CR + PR + SD after 2 cycles of therapy, was employed in the 2-stage decision making, such that the lack of early progression null rate was (p0) = 0.75 and alternative rate was (p1) = 0.90.
Simon’s 2-stage optimal design was employed to evaluate the rate of early progression. In the first stage, 23 patients were to be enrolled. If 18 or fewer achieved CR, PR or SD, then the trial would be terminated. If 19 or more achieved CR, PR or SD, then an additional 40 patients would be enrolled. At the end of the trial, if 52 or fewer of the 63 patients achieved CR, PR or SD, then the regimen was to be rejected. If 53 or more achieved CR, PR or SD then the regimen was to be considered for further development. At 5% significance, this design has 90% power to determine a 25% early progression rate (unacceptable) from that of 10% (acceptable).
The clinical response rates were calculated with their respective exact 95% confidence intervals (CIs). The Kaplan-Meier method was used to analyze time-to-event outcomes, including OS, disease-specific survival (DSS), and PFS. Median time to event in months was calculated with a 95% CI. Statistical software SAS 9.1.3 (SAS, Cary, NC) and S-Plus 8.0 (TIBCO Software, Inc., Palo Alto, CA) were used for all analyses.
RESULTS
Patient characteristics
Twenty-three patients were enrolled between April 2005 and January 2009. Of these, 22 were evaluable for response and toxicity. The patient who was not evaluable for response withdrew consent prior to beginning treatment. Table 1 details patient demographics. Most lesions were located on the head or neck (Table 1). On presentation, 7 (30%) patients were newly diagnosed with CSCC, 7 (30%) had persistent disease, and 9 (39%) had recurrent disease. Five patients had disease that was unresectable at enrollment. No patient presenting with resectable disease had disease that progressed to an unresectable status while receiving induction treatment.
Table 1.
Patient characteristics
| N | |
|---|---|
| Total no. enrolled patients | 23 |
| Protocol Evaluable | 22 (96%) |
| Toxicities Evaluable | 22 (96%) |
| Median age in years (range) | 65 (27–93) |
| Gender | |
| Male | 17 (74%) |
| Female | 6 (26%) |
| Race | |
| African American | 1 (4%) |
| Native American | 1 (4%) |
| Latin American | 2 (9%) |
| Caucasian | 19 (83%) |
| Subsitea | |
| Peri-auricular | 7 (30%) |
| Nose | 5 (22%) |
| Face (including forehead) | 4 (17%) |
| Ear | 3 (13%) |
| Scalp | 2 (9%) |
| Chest | 1 (4%) |
| Lymph node metastases | 5 (22%) |
| Lesion characteristics | |
| Size ≥ 2cm | 13 (57%) |
| Deep tissue invasion | 11 (48%) |
| Perineural invasion | 8 (35%) |
| Regional metastases | 18 (78%) |
| Type of presentation | |
| New diagnosis | 7 (30%) |
| Persistent disease | 7 (30%) |
| Recurrent disease | 9 (39%) |
| Previous treatment | |
| None | 2 (9%) |
| Biopsy only | 8 (35%) |
| Surgery | 11 (48%) |
| Surgery and radiation | 2 (9%) |
Some patients had more than one involved subsite.
Response and survival
Among the 22 evaluable patients, the overall response rate to induction therapy was 45.5% (95% CI: 24.4–67.8%); there were 4 clinical CRs (18.2%; 95% CI: 5.2–40.3%), 6 PR (27.3%), 5 SDs (22.7%), and 7 PDs (31.8%) during the induction phase (Table 2). Six (27%) of the last 15 patients accrued received the escalated gefitinib dose (500 mg) during induction therapy; upon completion of induction therapy, 3 patients had SD, 2 patients had PD, and 1 patient achieved a CR. The median duration of induction therapy was 58 days (range, 14–76 days; mean, 47.9 +/− 20.5 days). Of the 4 patients with a clinical CR who underwent surgery after induction therapy, 3 were found to have achieved a pathologic CR and had no histological evidence of carcinoma in their surgical specimens. One patient who had a clinical CR was judged not to have a pathologic CR because focal carcinoma in situ was found in the research biopsy of his surgical specimen, which was otherwise free of tumor.
Table 2.
Response to and treatment after gefitinib induction therapy and current disease status
| Presentation status | ||||
|---|---|---|---|---|
| Response to gefitinib induction therapy | N (%) | Newly diagnosed | Recurrent | Persistent |
| CR | 4 (18.2) | 2 | 2 | |
| PR | 6 (27.3) | 2 | 3 | 1 |
| SD | 5 (22.7) | 4 | 1 | |
| PD | 7 (31.8) | 1 | 4 | 2 |
| Treatment after gefitinib induction therapy | ||||
| Surgery | 2 (10) | 2 | ||
| Definitive radiation | 3 (15) | 1 | 2 | |
| Definitive radiation and gefitinib | 3 (15) | 1 | 2 | |
| Surgery with postoperative radiation and gefitinib | 10 (50) | 2 | 6 | 2 |
| Surgery and chemoradiation | 1 (5) | 1 | ||
| Palliative care | 1 (5) | 1 | ||
| Current disease status | ||||
| No evidence of disease | 12 (54.5) | 5 | 5 | 2 |
| Dead of disease | 6 (27.3) | 2 | 3 | 1 |
| Dead of other causes | 2 (9.1) | 1 | 1 | |
| Living with disease | 2 (9.1) | 1 | 1 | |
Of the 17 patients who completed neoadjuvant gefitinib, 14 had a CR, PR, or SD; of these 14 patients, 2 patients (14.3%) were treated with surgery alone, 2 (14.3%) were treated with definitive radiation and concurrent gefitinib, and 8 (57.1%) were treated with surgery and postoperative radiation. Two patients (14.2%) who had resectable disease refused surgery and received definitive radiation off-protocol. Three patients had PD after neoadjuvant gefitinib, 2 of whom had unresectable disease at enrollment; 1 patient received palliative care, 1 received definitive radiation, and 1 underwent surgery with postoperative radiation but had disease progression during radiation treatment and received palliative care. One patient had a PR during neoadjuvant treatment, but was taken off-study for elevated liver enzymes. These results are summarized in Table 2 and Figure 1b. Twelve patients were eligible for maintenance therapy (Figure 1b), however, of these, 2 patients were taken off-study for adverse events after induction therapy and before surgery, 1 was lost to follow up upon completion of radiation, 1 had severe ocular toxicity from radiation so maintenance therapy was deferred, 1 pursued radiation elsewhere and was taken off-study, and 1 had regional recurrence immediately following radiation. This left 6 patients who received maintenance gefitinib after definitive treatment for a median period of 10 months; of these 6 patients, 3 completed 12 months of treatment, 2 were taken off-protocol for adverse events, and 1 was lost to follow up. Of the 5 patients who did not complete induction therapy, 2 were taken off-study for adverse events, and 3 had PD at the initial 2-week evaluation. Three of these patients were treated with surgery and postoperative radiation, and 2 received definitive radiation.
The median follow-up time for the censored observations was 32 months. Five patients were not rendered free of disease; 4 of these patients had unresectable disease at enrollment and 1 patient was lost to follow-up before tumor status could be documented. Of the 17 patients who attained no evidence of disease (NED) status, 12 (70.6%) did not have tumor recurrence. Of the 5 (29.4%) patients who did have recurrence, 2 developed local recurrence, 2 developed regional and distant metastases, and 1 developed dermal metastases; all 5 of these patients had recurrence within the first year after completion of treatment.
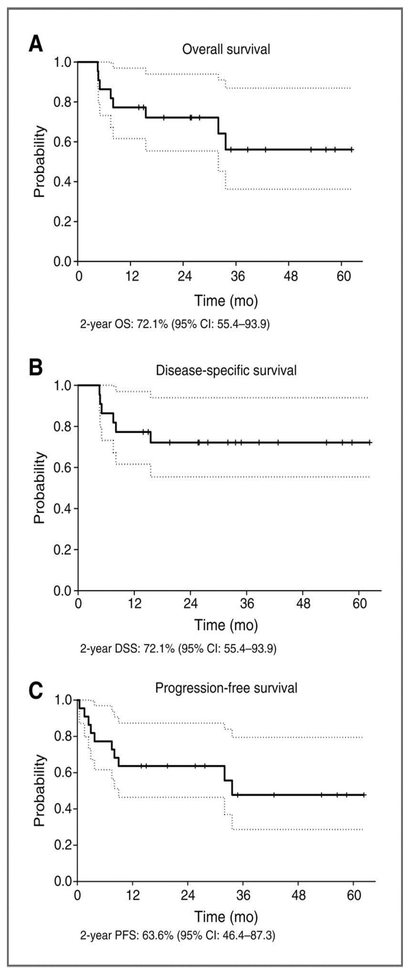
At the time of last contact, 12 of 22 evaluable patients (54.5%) remained NED, 6 (27.3%) died of their disease (DOD), 2 (9.1%) died from other causes, and 2 (9.1%) were living with disease (Table 2). The two-year OS, DSS, and PFS rates were 72.1% (95% CI: 55.4–93.9%), 72.1% (95% CI: 55.4–93.9%) and 63.6% (95% CI: 46.4–87.3%), respectively (Figure 2). When we stratified the data based on response to induction, patients who achieved a clinical CR after induction chemotherapy had durable control of disease with 100% OS and DSS at last follow-up.
Figure 2.
Kaplan-Meier plots of composite (a) overall, (b) disease-specific, and (c) progression-free survival.
Toxicity
There were no grade 4 or 5 toxicities encountered (Table 3), although 13 of 22 evaluable patients (59.1%) experienced grade 2 toxicities during gefitinib neoadjuvant therapy, with 4 of these 13 patients (30.8%) also experiencing a grade 3 toxicity. Two patients (9.1%) were taken off-study during neoadjuvant therapy for adverse events and 3 of 6 (50%) receiving maintenance gefitinib after treatment were taken off study because of toxicity. Gefitinib given concurrently with radiation did not appear to increase in-field toxicity beyond what is commonly seen with radiation alone. Among the expected toxicities from gefitinib, the most common grade 1 toxicity was diarrhea (10 patients) followed by fatigue (7 patients), acneiform rash (7 patients), and nausea (7 patients). The most common expected grade 2 toxicity was fatigue (5 patients), followed by acneiform rash (4 patients). There was no apparent association between developing a rash and clinical response. One patient experienced grade 3 fatigue and 2 patients experienced grade 3 alanine transaminase elevation. The most common unexpected grade 1 toxicity was anemia (4 patients). The most common unexpected grade 2 toxicities were allergic reaction (2 patients) and blurred vision (2 patients). Other unexpected grade 3 toxicities included infection, insomnia, depression, and tooth pain, each experienced by 1 patient. These results are further detailed in Table 3.
Table 3.
Summary of toxicities associated with gefitinib therapy
| Induction | Induction with dose escalation | Radiation with gefitinib | Maintenance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | Grade | Grade | |||||||||
| Expected Toxicities | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Fatigue | 7 | 4 | 3 | 2 | 1 | 1 | 1 | 2 | ||||
| Dry skin | 2 | 2 | 1 | 3 | ||||||||
| Pruritis | 3 | 1 | 1 | 1 | 1 | |||||||
| Rash/desquamation | 3 | 1 | 2 | 1 | 1 | |||||||
| Rash/acneiform | 6 | 1 | 2 | 2 | 1 | 1 | 1 | 3 | ||||
| Anorexia | 1 | 1 | 1 | 1 | 2 | 2 | 1 | |||||
| Diarrhea | 8 | 2 | 3 | 3 | 2 | 1 | ||||||
| Nausea | 6 | 1 | 2 | 1 | ||||||||
| Vomiting | 2 | 1 | 1 | |||||||||
| Elevation of ALT | 1 | 2 | 2 | |||||||||
| Elevation of AST | 2 | 2 | 1 | 1 | 1 | 1 | ||||||
| Elevation of creatinine | 1 | 1 | ||||||||||
| Abdominal pain | 2 | 1 | ||||||||||
| Dry eyes | 1 | 1 | ||||||||||
| Ocular/visual, other | 1 | |||||||||||
| Unexpected Toxicities | ||||||||||||
| Allergic reactiona | 1 | 1 | ||||||||||
| Anemiaa | 3 | 1 | 2 | 2 | 1 | |||||||
| Weight lossa | 1 | 1 | 1 | |||||||||
| Alopeciaa | 1 | 1 | ||||||||||
| Taste alterationa | 1 | |||||||||||
| Epistaxisa | 1 | |||||||||||
| Infection | 1 | |||||||||||
| Elevated BUN | 1 | 1 | ||||||||||
| Elevated WBC | 1 | |||||||||||
| Blurred vision | 1 | 1 | 1 | |||||||||
| Insomnia | 1 | |||||||||||
| Depressiona | 1 | |||||||||||
| Tooth pain | 1 | |||||||||||
NOTE. Each entry represents the number of patients affected. Each adverse event has been considered by investigators to be related to gefitinib treatment. Of the 22 patients receiving induction gefitinib, 6 received dose escalations.
These toxicities have been reported in other gefitinib trials, with the relationship to gefitinib still undetermined.
ALT: Alanine transferase; AST: Asparagine transferase; BUN: Blood urea nitrogen; WBC: White blood cell count
Correlative studies
Eleven tissue samples were available for 10 patients, and findings of the correlative studies are summarized in Table 4. Samples were not available from all subjects because the protocol specified that consenting to tissue biopsies was optional. In addition, some patients presented with regional recurrences, which were not superficially accessible. Two specimens (biopsies after induction chemotherapy from patient 10 and the surgical specimen from patient 19) displayed no EGFR or p-EGFR staining, despite being of reasonable quality and staining well for pan-cytokeratin. Although no baseline tissue was available for patient 10, patient 19 had p-EGFR present in his baseline biopsy. Patient 19’s baseline specimen revealed a total EGFR score of 0 despite a p-EGFR score of 180. In the analysis of several hundred lung cancers, this is not a usual phenomenom, possibly reflecting the presence of p-EGFR in the absence of EGFR overexpression. Four patients had samples that were FISH positive; of these, patient 19 had conflicting results from the baseline and surgical specimens, but of the remaining 3, 2 died of their disease. Of the 6 patients with FISH-negative samples (excluding patient 19 who had conflicting results from the 2 specimens), 2 died of their disease. FISH-positivity was not correlated with DSS (p = 0.524) by 2-tailed Fisher exact test, although interpretation is limited by the small sample size. In addition, although 2 cases of EGFR mutations were identified by sequencing, they were non-activating mutations in the EGFR catalytic domain. One was a sense mutation (AAC to AAT; asparagine) in exon 18 and the other a missense mutation (arginine to lysine; CGC to TGC) in exon 21. No statistically significant associations were identified between EGFR expression, mutation status, or FISH staining and response to gefitinib treatment, although analyses were limited by the small sample size.
Table 4.
Results of the EGFR correlative studies
| Patient #, Biopsy type | Responsea | Total EGFRb | p-EGFRb | FISH resultc | EGFR sequencing | Current disease status |
|---|---|---|---|---|---|---|
| 1 Baselined | CR | 100 | 80 | + | wild-type | NED |
| 2 Baseline | PD | 60 | 160 | + | wild-type | DOD |
| 3 Baseline | SD | 300 | 20 | − | wild-type | NED |
| 8 Surgerye | PR | 50 | 40 | − | wild-type | DOC |
| 10 EOIf | PD | 0 | 0 | − | wild-type | DOD |
| 15 Baseline | SD | 80 | 50 | − | wild-type | DOC |
| 17 Surgery | PR | 90 | 30 | − | wild-type | NED |
| 19 Baseline | SD | 0 | 180 | − | mutation | LWD |
| 19 Surgery | SD | 0 | 0 | + | wild-type | |
| 21 Surgery | PD | 100 | 10 | − | mutation | NED |
| 22 Surgery | SD | 60 | 90 | + | wild-type | DOD |
at the end of induction per RECIST criteria(23)
Final score calculated by staining intensity (%) multiplied by extent of staining
(+) indicates high polysomy or gene amplification
(−) indicates low trisomy or low polysomy
baseline biopsy
specimen from surgical resection
biopsy taken at the end of induction chemotherapy
Abbreviations: CR, complete response; PD, progressive disease; SD, stable disease; PR, partial response; NED, no evidence of disease; DOD, died of disease; DOC, died of other causes; LWMD, living with disease.
DISCUSSION
To our knowledge, this is the first prospective investigation of an EGFR TKI in the CSCC. Our trial with neoadjuvant gefitinib was terminated after the first stage of a Simon two-stage design given a 32% (7/22) rate of progression. However, gefitinib therapy was associated with clinical response in 10 of 22 patients (45.5%), 4 of whom had clinical CR; 3 of these 4 had pathologic CR. After completing treatment, 70.6% of patients who were rendered disease-free did not have disease recurrence; of the 5 patients who had disease recurrence, all had recurrence within 1 year after treatment. The two-year OS, DSS, and PFS rates were 72.1%, 72.1%, and 63.6%, respectively. Despite failure to meet the primary endpoint as specified in the original design, we believe the overall response rate and especially the complete clinical and pathologic responses observed with gefitinib in this setting support further investigation of EGFR TKIs in aggressive CSCC.
Chemotherapy for CSCC has not been investigated in reasonably-powered formal prospective trials. Previous case series have reported experience with chemotherapy in patients deemed incurable or who refused resection. These series report on small numbers of patients and long-term survival rates are generally low (28–31). Neoadjuvant chemotherapy has also been studied in small case series for patients with advanced non-melanoma skin and lip cancer. Cisplatin in combination with doxorubicin (32), bleomycin (33), or 5-fluorouracil (34), has been utilized. Chemotherapy administration in these reports (32–34) has been of variable duration and uniformity depending upon patient co-morbidities and/or age. Evaluation criteria for response are not detailed consistently and the use of axial imaging not commented upon with the exception of the report by Sadek et al (34). Of a total 17 patients with CSCC in these 3 reports, the overall response rate was 8/17 (47%), with 6 patients experiencing CR. Response was not uniformly confirmed with imaging and the reports do not discuss pathologic response. These data support the general chemosensitivity of aggressive CSCC; however, without formal investigation, the tangible benefit is unproven and chemotherapy in the neoadjuvant setting has no recognized standard role in patients with CSCC that can be cured with surgery or radiation.
Our group has performed serial phase II trials of the combination of retinoic acid and interferon alfa, with and without cisplatin, in patients with unresectable CSCC. Eligibility included patients whose disease was unresectable based on extent of disease or infeasiblity of resection due to the cosmetic or functional deformity that would ensue (35–37). Although response rates to both regimens were high in patients with only local tumor (93 and 67%), response and durability of response was modest in regional and/or metastatic disease (35, 37). Further, fatigue related to interferon, as well as myelosuppressive and neuropathic effects related to cisplatin, limited intensity and duration of therapy in this generally elderly population of patients. A randomized phase III trial of retinoic acid and interferon compared to no adjuvant therapy failed to prolong time to recurrence or second primary CSCC in patients status post resection or radiation for an index aggressive CSCC(36). Thus, this regimen was not further investigated.
Compared to historical data with chemotherapy, we believe that the efficacy of gefitinib is generally similar; however, this would only be established by a randomized trial and even then, may be problematic, given the heterogeneity in the patient population. Toxicity profiles differ, with gefitinib’s main toxic effects skin rash and diarrhea and chemotherapy toxicity greatly dependent upon the agents being studied.
Gefitinib has been used to treat locally advanced and recurrent HNSCC. When added to a chemoradiotherapy regimen for locally advanced HNSCC consisting of carboplatin/paclitaxel induction chemotherapy followed by radiotherapy and concurrent fluorouracil, hydroxyurea, and gefitinib and 2 years of gefitinib maintenance therapy, CR and survival rates were somewhat improved over historical controls (38). However, when Hainsworth et al. evaluated adding gefitinib to a neoadjuvant chemotherapy regimen followed by concurrent chemoradiation for locally advanced HNSCC they did not find a significant increase in survival (39). Because neither trial was randomized it is difficult to determine if there was any benefit from gefitinib . Further, those studies used gefitinib at 250 mg, which is the standard dose used to treat lung cancer, and the dose we were required to study initially by our study’s sponsor, the National Cancer Institute’s Cancer Therapy Evaluation Program. When compared with intravenous methotrexate for the treatment of recurrent HNSCC, gefitinib given at 500 mg induced a nearly 3-fold higher overall response rate than it did when given at 250 mg, although this was only a 5% increase (from 2.7% to 7.6%) (40). However, there was no difference in overall survival among patients treated with methotrexate or gefitinib, or between the patients treated with low and high dose gefitinib. In parallel with the trial reported herein, we have investigated gefitinib given at 250 mg for patients with incurable CSCC. Similar to findings with other systemic therapy in CSCC, gefitinib is much less active in this more advanced population with only an 11% overall response rate (41).
It is notable that of the 6 patients with SD who received a dose escalation from 250 mg to 500 mg at 2 weeks, 1 achieved a CR.. The dose of gefitinib in our trial was selected based on experience in patients with refractory metastatic non-small cell lung cancer. Although gefitinib at 250 mg/day was not the maximal tolerated dose (MTD) identified in phase I studies, this was chosen for subsequent trials due to better tolerability and pharmacodynamic studies showing EGFR inhibition in squamous cells of the skin (42). It has been hypothesized that a dose closer to the MTD could theoretically improve the clinical activity of the drug through more complete inhibition of the wild-type EGFR tyrosine kinase. Alternatively, erlotinib, another EGFR tyrosine kinase inhibitor approved and marketed in the U.S. at the MTD level of 150 mg/day, may also be more effective than gefitinib at 250 mg/day.
A recent report from Clayman et al. identified CSCC presenting as a local recurrence, with invasion deeper than subcutaneous tissues, perineural invasion, lesion size ≥ 4 cm, or depth of invasion ≥ 7 mm as having statistically significant associations with worse DSS (5). In that study, patients received standard treatment, including wide local excision, with or without local lymphadenectomy, and radiotherapy as primary or adjuvant treatment. The median follow-up time was 22 months. At 3 years, the estimated OS and DSS rates were 70% and 85%, respectively. Patients with none of these risk factors had a 100% 3-year DSS rate, as compared with 70% in patients with at least 1 risk factor (5). In this study, our 3-year OS and DSS were 56.1% and72.1%, respectively. However, our eligibility criteria specifically selected for patients with high risk lesions, with 65% of patients presenting with recurrent disease or lesions refractory to prior treatment (Table 1); this may explain the worse 3-year OS and DSS observed in our study population. Despite the advanced-disease status of our cohort, only 1 of 10 patients achieving CR or PR had disease recurrence, and all 10 had NED at their last clinic visit. This approaches the 100% DSS reported by Clayman et al. for patients with no risk factors, despite the fact that all of our patients had risk factors for aggressive disease (5). In our trial, patients who responded to gefitinib induction therapy had significantly better outcomes than those who did not; however, we recognize that this begs the question of whether a specific survival benefit was conferred by gefitinib, which would require a randomized trial.
Neoadjuvant therapy with gefitinib induced a profound response in a subset of patients with aggressive CSCC, indicating that the identification of biomarkers correlating with response to gefitinib may lead to personalized treatment options. Unfortunately, none of the EGFR markers we evaluated statistically correlated with response to gefitinib; the identification of biomarkers predictive of response to EGFR TKIs is a focus of our current and planned research in this patient population. Our correlative analyses were limited by our small sample size, in that more than half of the patients in the trial did not have tissue for correlative analysis, and by not having a baseline and a post-treatment biopsy from each patient.
The lack of correlation between EGFR FISH-positivity and EGFR expression that we observed has also been reported in other malignancies (43, 44). Interestingly, in the 1 patient for whom both baseline and postinduction specimens were available, the pEGFR expression decreased from a score of 180 at baseline to 0 after induction therapy. Although this patient had SD, gefitinib exposure may have contributed to this drop in pEGFR levels and will be studied in future trials. Previous studies have suggested that erlotinib decreases pEGFR expression in tumor and skin biopsies from patients with HNSCC, and this decrease is associated with a clinical benefit (45).
Although 4 tumors in our study were EGFR FISH-positive, FISH-positivity did not correlate with either response to gefitinib induction or survival. This finding is consistent with recent literature regarding EGFR molecular markers and response to gefitinib in non-small cell lung cancer; studies have identified EGFR mutation status, but not EGFR-FISH status, as a predictor of survival (46, 47). In studies demonstrating a higher objective response rate to gefitinib in non-small cell lung cancer patients with a high EGFR copy number than in those without, the presence of a mutation in the EGFR catalytic domain was correlated with both the objective response rate and PFS, indicating that the latter has more clinical utility (48). However, to our knowledge, ours is the first to evaluate EGFR-FISH status in CSCC; its potential for prognostic importance in CSCC, as in mucosal HNSCC, is suggested by the observation that 2 of 4 (50%) patients in our study with FISH-positive disease died of recurrent or persistent disease. In contrast, among the patients with FISH-negative tumors, one third died of their disease. The significance of this observation is limited by the small sample size. Thus, we intend to further investigate the EGFR-FISH status, as well as other biomarkers, in future trials.
Gefitinib in the neoadjuvant setting for advanced CSCC is a well-tolerated treatment that achieved a 45.5% response rate in patients with aggressive CSCC, a rate not dissimilar to that achieved with more toxic combination chemotherapy. Gefitinib therapy did not hinder subsequent definitive resection and/or radiation. Further, it is remarkable that a subset of patients experienced pathologic CR at a dose not generally recognized to produce serum levels that effectively inhibit wild-type EGFR. Given that gefitinib is not currently marketed in the U.S. and our desire to build on this experience, we have an ongoing clinical trial of erlotinib in neoadjuvant setting for patients with aggressive CSCC. As a priority in this ongoing trial, we hope to improve the collection of tumor specimens to build on our experience with molecular profiling of aggressive CSCC. The clinical and translational data from this trial may shed light on predictive markers and facilitate the evolution of personalized therapy for patients with CSCC.
Supplementary Material
Supplemental figure 1. A. Microphotographs of representative examples of EGFR gene copy number gene analysis by FISH of tumor tissue specimens showing high-polysomy (FISH+) in case 1, and normal gene copies (FISH-) in cases 8 and 15 (red signal, EGFR gene probe; green signal, centromeric probe, 200x). B. Representative microphotographs of immunohistochemical analysis of tumor tissues specimens of EGFR and p-EGFR showing membrane and cytoplasmic immunostainign of malignant cells in 3 tumor cases, and postive and negative controls (400x).
STATEMENT OF TRANSLATIONAL RELEVANCE.
Cutaneous squamous cell carcinoma (CSCC) is highly prevalent, with specific risk factors adversely affecting disease-specific survival. Preclinical studies have indicated a potential benefit of epidermal growth factor receptor (EGFR) inhibitors in CSCC. We have shown that neoadjuvant administration of an EGFR tyrosine kinase inhibitor (TKI), gefitinib, resulted in a clinical response rate similar to that achieved with combination chemotherapy. In addition, gefitinib therapy did not hinder subsequent definitive treatment and was well-tolerated. Although the biomarkers we examined were not correlated with a clinical benefit, the fact that neoadjuvant therapy with gefitinib induced a profound response in a subset of patients with aggressive CSCC indicates that the identification of biomarkers that correlate with response to EGFR TKIs may allow us to personalize treatment options for CSCC patients.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contribution of Shirley Taylor, BSN, who coordinated study patients and collected and organized data.
GRANT SUPPORT
Gefitinib was kindly provided by AstraZeneca Pharmaceuticals and the National Cancer Institute, NIH22.
Footnotes
Disclosure:
This study was sponsored by the University of Texas MD Anderson Cancer Center (MDA-2004–0204) and the National Cancer Institute (NCI protocol 6012). Gefitinib was provided by AstraZeneca Pharmaceuticals. None of the authors have a conflict of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010. September-October;60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 2.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001. March 29;344(13):975–83. [DOI] [PubMed] [Google Scholar]
- 3.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994. May;30(5 Pt 1):774–8. [DOI] [PubMed] [Google Scholar]
- 4.Karagas MR, Greenberg ER, Spencer SK, Stukel TA, Mott LA. Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. New Hampshire Skin Cancer Study Group. Int J Cancer. 1999. May 17;81(4):555–9. [DOI] [PubMed] [Google Scholar]
- 5.Clayman GL, Lee JJ, Holsinger FC, Zhou X, Duvic M, El-Naggar AK, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005. February 1;23(4):759–65. [DOI] [PubMed] [Google Scholar]
- 6.Moore BA, Weber RS, Prieto V, El-Naggar A, Holsinger FC, Zhou X, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005. September;115(9):1561–7. [DOI] [PubMed] [Google Scholar]
- 7.Veness MJ, Porceddu S, Palme CE, Morgan GJ. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head Neck. 2007. July;29(7):621–31. [DOI] [PubMed] [Google Scholar]
- 8.Baltaci M, Fritsch P, Weber F, Tzankov A, Sogner P, Derler AM, et al. Treatment with gefitinib (ZD 1839) in a patient with advanced cutaneous squamous cell carcinoma. Br J Dermatol. 2005. July;153(1):234–6. [DOI] [PubMed] [Google Scholar]
- 9.Jalili A, Pinc A, Pieczkowski F, Karlhofer FM, Stingl G, Wagner SN. Combination of an EGFR blocker and a COX-2 inhibitor for the treatment of advanced cutaneous squamous cell carcinoma. J Dtsch Dermatol Ges. 2008. December;6(12):1066–9. [DOI] [PubMed] [Google Scholar]
- 10.Suen JK, Bressler L, Shord SS, Warso M, Villano JL. Cutaneous squamous cell carcinoma responding serially to single-agent cetuximab. Anticancer Drugs. 2007. August;18(7):827–9. [DOI] [PubMed] [Google Scholar]
- 11.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003. July 15;21(14):2787–99. [DOI] [PubMed] [Google Scholar]
- 12.Harari PM, Huang S. Radiation combined with EGFR signal inhibitors: head and neck cancer focus. Semin Radiat Oncol. 2006. January;16(1):38–44. [DOI] [PubMed] [Google Scholar]
- 13.El-Abaseri TB, Fuhrman J, Trempus C, Shendrik I, Tennant RW, Hansen LA. Chemoprevention of UV light-induced skin tumorigenesis by inhibition of the epidermal growth factor receptor. Cancer Res. 2005. May 1;65(9):3958–65. [DOI] [PubMed] [Google Scholar]
- 14.Barnes CJ, Bagheri-Yarmand R, Mandal M, Yang Z, Clayman GL, Hong WK, et al. Suppression of epidermal growth factor receptor, mitogen-activated protein kinase, and Pak1 pathways and invasiveness of human cutaneous squamous cancer cells by the tyrosine kinase inhibitor ZD1839 (Iressa). Mol Cancer Ther. 2003. April;2(4):345–51. [PubMed] [Google Scholar]
- 15.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. September 3;361(10):947–57. [DOI] [PubMed] [Google Scholar]
- 16.Cohen EE, Haraf DJ, Kunnavakkam R, Stenson KM, Blair EA, Brockstein B, et al. Epidermal Growth Factor Receptor Inhibitor Gefitinib Added to Chemoradiotherapy in Locally Advanced Head and Neck Cancer. J Clin Oncol. 2010. May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saarilahti K, Bono P, Kajanti M, Back L, Leivo I, Joensuu T, et al. Phase II prospective trial of gefitinib given concurrently with cisplatin and radiotherapy in patients with locally advanced head and neck cancer. J Otolaryngol Head Neck Surg. 2010. June;39(3):269–76. [PubMed] [Google Scholar]
- 18.Cohen EE, Halpern AB, Kasza K, Kocherginsky M, Williams R, Vokes EE. Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2009. October;45(10):e155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petak I, Schwab R, Orfi L, Kopper L, Keri G. Integrating molecular diagnostics into anticancer drug discovery. Nat Rev Drug Discov. 2010. June 7;9(7):523–35. [DOI] [PubMed] [Google Scholar]
- 20.Cappuzzo F, Ligorio C, Janne PA, Toschi L, Rossi E, Trisolini R, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol. 2007. June 1;25(16):2248–55. [DOI] [PubMed] [Google Scholar]
- 21.Carlson JJ, Garrison LP, Ramsey SD, Veenstra DL. Epidermal growth factor receptor genomic variation in NSCLC patients receiving tyrosine kinase inhibitor therapy: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2009. November;135(11):1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makinson A, Pujol JL, Le Moing V, Peyriere H, Reynes J. Interactions between cytotoxic chemotherapy and antiretroviral treatment in human immunodeficiency virus-infected patients with lung cancer. J Thorac Oncol. 2010. April;5(4):562–71. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. February 2;92(3):205–16. [DOI] [PubMed] [Google Scholar]
- 24.Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff IH. Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest. 1990;8(2):147–59. [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Varella-Garcia M, Xavier AC, Massarelli E, Ozburn N, Moran C, et al. Epidermal growth factor receptor abnormalities in the pathogenesis and progression of lung adenocarcinomas. Cancer Prev Res. 2008. August;1(3):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varella-Garcia M, Diebold J, Eberhard DA, Geenen K, Hirschmann A, Kockx M, et al. EGFR fluorescence in situ hybridisation assay: guidelines for application to non-small-cell lung cancer. J Clin Pathol. 2009. November;62(11):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005. May 4;97(9):643–55. [DOI] [PubMed] [Google Scholar]
- 28.Khansur T, Kennedy A. Cisplatin and 5-fluorouracil for advanced locoregional and metastatic squamous cell carcinoma of the skin. Cancer. 1991. April 15;67(8):2030–2. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie TH Jr., McElveen LJ, Porubsky ES, Harmon JD. Cisplatin and doxorubicin. An effective chemotherapy combination in the treatment of advanced basal cell and squamous carcinoma of the skin. Cancer. 1985. April 15;55(8):1629–32. [DOI] [PubMed] [Google Scholar]
- 30.Ikegawa S, Saida T, Obayashi H, Sasaki A, Esumi H, Ikeda S, et al. Cisplatin combination chemotherapy in squamous cell carcinoma and adenoid cystic carcinoma of the skin. J Dermatol. 1989. June;16(3):227–30. [DOI] [PubMed] [Google Scholar]
- 31.Lippman SM, Meyskens FL Jr. Treatment of advanced squamous cell carcinoma of the skin with isotretinoin. Ann Intern Med. 1987. October;107(4):499–502. [DOI] [PubMed] [Google Scholar]
- 32.Guthrie TH Jr., Porubsky ES, Luxenberg MN, Shah KJ, Wurtz KL, Watson PR. Cisplatin-based chemotherapy in advanced basal and squamous cell carcinomas of the skin: results in 28 patients including 13 patients receiving multimodality therapy. J Clin Oncol 1990. February;8(2):342–6. [DOI] [PubMed] [Google Scholar]
- 33.Denic S Preoperative treatment of advanced skin carcinoma with cisplatin and bleomycin. Am J Clin Oncol 1999. February;22(1):32–4. [DOI] [PubMed] [Google Scholar]
- 34.Sadek H, Azli N, Wendling JL, Cvitkovic E, Rahal M, Mamelle G, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer. 1990. October 15;66(8):1692–6. [DOI] [PubMed] [Google Scholar]
- 35.Lippman SM, Parkinson DR, Itri LM, Weber RS, Schantz SP, Ota DM, et al. 13-cis-retinoic acid and interferon alpha-2a: effective combination therapy for advanced squamous cell carcinoma of the skin. J Natl Cancer Inst. 1992. February 19;84(4):235–41. [DOI] [PubMed] [Google Scholar]
- 36.Brewster AM, Lee JJ, Clayman GL, Clifford JL, Reyes MJ, Zhou X, et al. Randomized trial of adjuvant 13-cis-retinoic acid and interferon alfa for patients with aggressive skin squamous cell carcinoma. J Clin Oncol. 2007. May 20;25(15):1974–8. [DOI] [PubMed] [Google Scholar]
- 37.Shin DM, Glisson BS, Khuri FR, Clifford JL, Clayman G, Benner SE, et al. Phase II and biologic study of interferon alfa, retinoic acid, and cisplatin in advanced squamous skin cancer. J Clin Oncol. 2002. January 15;20(2):364–70. [DOI] [PubMed] [Google Scholar]
- 38.Cohen EE, Haraf DJ, Kunnavakkam R, Stenson KM, Blair EA, Brockstein B, et al. Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2010. July 10;28(20):3336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hainsworth JD, Spigel DR, Burris HA 3rd, Markus TM, Shipley D, Kuzur M, et al. Neoadjuvant chemotherapy/gefitinib followed by concurrent chemotherapy/radiation therapy/gefitinib for patients with locally advanced squamous carcinoma of the head and neck. Cancer. 2009. May 15;115(10):2138–46. [DOI] [PubMed] [Google Scholar]
- 40.Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 2009. April 10;27(11):1864–71. [DOI] [PubMed] [Google Scholar]
- 41.Glisson BS, Kim ES, Kies MS, Francisco M, Blumenschein GR, Tsao AS, et al. Phase II study of gefitinib in patients with metastatic/recurrent squamous cell carcinoma of the skin. Proc Am Soc Clin Oncol. 2006;24(18S):287s (#5531). [Google Scholar]
- 42.Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 2002. January 1;20(1):110–24. [DOI] [PubMed] [Google Scholar]
- 43.Helfrich BA, Raben D, Varella-Garcia M, Gustafson D, Chan DC, Bemis L, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006. December 1;12(23):7117–25. [DOI] [PubMed] [Google Scholar]
- 44.Pinter F, Papay J, Almasi A, Sapi Z, Szabo E, Kanya M, et al. Epidermal growth factor receptor (EGFR) high gene copy number and activating mutations in lung adenocarcinomas are not consistently accompanied by positivity for EGFR protein by standard immunohistochemistry. J Mol Diagn. 2008. March;10(2):160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calvo E, Malik SN, Siu LL, Baillargeon GM, Irish J, Chin SF, et al. Assessment of erlotinib pharmacodynamics in tumors and skin of patients with head and neck cancer. Ann Oncol. 2007. April;18(4):761–7. [DOI] [PubMed] [Google Scholar]
- 46.Tiseo M, Rossi G, Capelletti M, Sartori G, Spiritelli E, Marchioni A, et al. Predictors of gefitinib outcomes in advanced non-small cell lung cancer (NSCLC): study of a comprehensive panel of molecular markers. Lung Cancer. 2010. March;67(3):355–60. [DOI] [PubMed] [Google Scholar]
- 47.Bonanno L, Schiavon M, Nardo G, Bertorelle R, Bonaldi L, Galligioni A, et al. Prognostic and predictive implications of EGFR mutations, EGFR copy number and KRAS mutations in advanced stage lung adenocarcinoma. Anticancer Res. 2010. December;30(12):5121–8. [PubMed] [Google Scholar]
- 48.Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010. February 10;28(5):744–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. A. Microphotographs of representative examples of EGFR gene copy number gene analysis by FISH of tumor tissue specimens showing high-polysomy (FISH+) in case 1, and normal gene copies (FISH-) in cases 8 and 15 (red signal, EGFR gene probe; green signal, centromeric probe, 200x). B. Representative microphotographs of immunohistochemical analysis of tumor tissues specimens of EGFR and p-EGFR showing membrane and cytoplasmic immunostainign of malignant cells in 3 tumor cases, and postive and negative controls (400x).