Abstract
The objective of this study was to examine the effects of immunosuppressive prednisolone therapy on pancreatic tissue and the concentration of serum canine pancreatic lipase immunoreactivity (cPLI) in healthy dogs. Six healthy beagle dogs were subcutaneously administered an immunosuppressive dose of prednisolone [4 mg/kg body weight (BW)] once daily for either 2 or 3 weeks. Serum cPLI concentration was measured before and after treatment. Ultrasonographic examination of the pancreas and laparoscopic biopsy and histopathological examination of the right pancreatic lobe and the liver were also conducted before and after treatment. The expression of pancreatic lipase messenger ribonucleic acid (mRNA) in the pancreas and liver was examined by polymerase chain reaction (PCR). Although the serum cPLI concentration was significantly higher on day 14 and on the day of the second laparoscopy than before treatment, it was classified as normal (≤ 200 μg/L) in 5 dogs and as abnormal (≥ 400 μg/L) in only 1 dog. None of the 6 dogs showed clinical signs of pancreatitis during the study period. After treatment, ultrasonographic examination of the pancreas showed no changes except for a hypoechoic pancreas in 1 dog. Histopathological examination of the right pancreatic lobe in all dogs showed no evidence of pancreatitis after treatment. Pancreatic lipase mRNA expression was detected in the pancreas, but not in the liver, before and after treatment. The administration of 4 mg/kg BW per day of prednisolone for 2 or 3 weeks increased the serum cPLI concentration without clinical signs of pancreatitis, although an abnormal cPLI concentration (≥ 400 μg/L) was observed in only 1 dog. No ultrasonographic or histological evidence of pancreatitis was observed in any of the dogs.
Résumé
L’objectif de la présente étude était d’examiner les effets d’une thérapie immunosuppressive par la prednisolone sur le tissu pancréatique et la concentration sérique canine de lipase pancréatique immunoréactive (cPLI) chez des chiens en santé. Six chiens beagle en santé ont reçu par voie sous-cutanée une dose immunosuppressive de prednisolone [4 mg/kg de poids corporel (PC)] une fois par jour pendant 2 ou 3 semaines. La concentration sérique de cPLI a été mesurée avant et après le traitement. Un examen échographique du pancréas et une biopsie suivie d’un examen histopathologique d’échantillons du lobe pancréatique droit ainsi que du foie obtenus par laparoscopie ont également été faits avant et après le traitement. L’expression de l’ARNm de la lipase pancréatique dans le pancréas et le foie a été examinée par réaction d’amplification en chaine par la polymérase. Bien que la concentration sérique de cPLI fût significativement plus élevée au jour 14 et le jour de la seconde laparoscopie qu’avant le traitement, elle était classée comme normale (≤ 200 μg/L) chez cinq chiens et comme anormale (≥ 400 μg/L) chez seulement un chien. Aucun des six chiens n’a présenté de signes cliniques de pancréatite durant la période d’étude. Après le traitement, l’examen échographique du pancréas ne démontrait aucun changement sauf pour un pancréas hypoéchogène chez un chien. L’examen histopathologique du lobe pancréatique droit chez tous les chiens n’a pas permis de mettre en évidence de pancréatite après le traitement. L’expression d’ARNm de lipase pancréatique fut détectée dans le pancréas, mais pas dans le foie, avant et après le traitement. L’administration de 4 mg/kg de PC par jour de prednisolone pendant 2 ou 3 semaines a fait augmenter la concentration sérique de cPLI sans signe clinique de pancréatite, bien qu’une concentration anormale de cPLI (≥ 400 μg/L) fût obtenue chez un chien. Aucune évidence échographique ou histologique de pancréatite ne fût observée chez les chiens de cette étude.
(Traduit par Docteur Serge Messier)
Introduction
Pancreatitis is the most common exocrine pancreatic disease in dogs. Accurate clinical diagnosis of pancreatitis remains challenging as there is no single noninvasive diagnostic method that is completely reliable (1). At present, canine pancreatic lipase immunoreactivity (cPLI) is widely used to diagnose canine pancreatitis and is thought to be a reliable serum marker for pancreatitis (2,3). Among various serum tests for diagnosing canine acute pancreatitis, determining the cPLI concentration is considered the most sensitive and specific assay, with a reported sensitivity of 71.7% to 77.8% and specificity of 80.5% to 88.0% (4).
The Spec cPL (IDEXX Laboratories, Westbrook, Maine, USA), a commercially available cPLI assay, is the most frequently used noninvasive biochemical test for diagnosing canine pancreatitis (4). Another rapid, in-clinic, semiquantitative, visually read test for estimating cPLI in serum has also been developed (SNAP cPL; IDEXX Laboratories). The SNAP cPL test has been shown to be highly sensitive (86% to 94%) in diagnosing acute pancreatitis (4), which means that a normal result makes a diagnosis of pancreatitis very unlikely (5).
The cPLI assay has inherent advantages over traditional serum lipase activity assays. It is reported that canine pancreatic lipase is exclusively expressed in pancreatic acinar cells and the cPLI assay is able to detect the unique 3-dimensional structure of pancreatic lipase without interference from other lipases (6). On the other hand, traditional lipase activity assays indiscriminately measure the activity of lipases originating from multiple organs, such as the stomach and liver. Additionally, 4 wk of peroral administration of prednisone [2.2 mg/kg body weight (BW) per day] to healthy dogs was not found to have any clinically significant effect on the serum cPLI concentration (7), although it has been reported that traditional lipase activity assays are influenced by such treatment (8,9).
In our recent study, serial cPLI monitoring in dogs with immunemediated disease treated with peroral prednisolone (2.0 to 2.2 mg/kg BW per day) showed various degrees of increased cPLI concentrations during the treatment period (10). One possible reason for the difference between the results of the previously mentioned study (7) and our recent study is differences in the formulae of prednisone and prednisolone. Prednisone is an inactive compound (or prodrug) until metabolized in the liver by the enzyme 11-β hydroxysteroid dehydrogenase type 1 (11). It had been thought that prednisolone and the prodrug prednisone are equivalent in terms of dosing when used as oral drugs. However, one study found that the relative bioavailability of prednisolone was only 65% when prednisone was administered compared with the administration of prednisolone (12), although it is not clear if the differences are due to decreased gastrointestinal absorption or to decreased hepatic conversion of prednisone to prednisolone. The oral bioavailability of prednisolone used in our study (2.0 to 2.2 mg/kg BW per day) could therefore be higher than that of prednisone used in the previous study (2.2 mg/kg BW per day) (7). These findings indicate that the dose of prednisolone might be an important factor in determining its effect on pancreatic tissue and serum cPLI concentration.
It is not known what causes the increased concentration of serum cPLI in dogs with immune-mediated disease that are treated with prednisolone. One possible reason is that pancreatic lipase is produced by extra-pancreatic tissue, such as in the liver. One study suggested that the increase in serum lipase activity could have resulted from dexamethasone-induced release from the liver, although this study referred to total lipase activity and not pancreatic lipase (8). One method for investigating the origin of pancreatic lipase is to examine the expression of pancreatic lipase messenger ribonucleic acid (mRNA) in extra-pancreatic tissues, such as the liver.
The purpose of this study was to examine the effects of prednisolone at the immunosuppressive dosage (4 mg/kg BW per day) on pancreatic tissue using histopathology and on the concentration of serum cPLI, as well as on ultrasonographic imaging of the pancreas and pancreatic lipase gene expression in the pancreas and liver of healthy dogs.
Materials and methods
Animals
Six healthy beagles that were part of a research colony owned by our laboratory were included in this study. All procedures were approved by the Laboratory Animal Experimentation Committee of the Graduate School of Veterinary Medicine at Hokkaido University (Accession number, 15-0033).
Study protocol
The first laparoscopy for biopsy of the right pancreatic lobe and liver was carried out before prednisolone was administered. Subcutaneous administration of 4 mg/kg BW per day of prednisolone (Prednisolone; Takeda Pharmaceutical, Osaka, Japan) was started 2 to 3 wk after the first laparoscopy and this dosage was maintained for either 2 or 3 wk. The prednisolone was discontinued at 2 wk if obvious side effects were observed, e.g., appearance of a pendulous abdomen. A second laparoscopic biopsy was conducted 2 wk (Dogs 1 to 3) or 3 wk (Dogs 4 to 6) after starting prednisolone. Serum cPLI was monitored and serum biochemistry examined at 1-week intervals until 2 wk (Dogs 1 to 3) or 3 wk (Dogs 4 to 6) after starting prednisolone.
After the second laparoscopy, the amount of prednisolone was tapered by 50% per week until the dosage reached 0.5 mg/kg BW per day. Cessation of prednisolone administration took a total of 3 wk.
Measurement of canine pancreatic lipase immunoreactivity
Serum cPLI was measured before prednisolone administration (day 0) and then at 1-week intervals for 2 wk in 3 dogs (Dogs 1 to 3) and for 3 wk in another 3 dogs (Dogs 4 to 6). Blood was collected by venipuncture, aliquots were centrifuged, and the serum was collected and stored at 4°C until analysis. The cPLI concentration was measured individually in all samples within 2 d of serum collection using a commercial enzyme-linked immunosorbent assay (ELISA) that has been analytically validated for use in dogs (13). The reference interval of the assay is 0 to 200 μg/L. A cPLI concentration of ≥ 400 μg/L is considered abnormal and indicates the possibility of pancreatitis, while a concentration of 201 to 399 μg/L is considered to be in the gray zone.
Serum biochemistry tests
Serum biochemistry tests were carried out before the first laparoscopy (baseline) and before prednisolone was administered (day 0) using a dry chemistry analyzer (FUJI DRI-CHEM 7000V; FujiFilm, Tokyo, Japan). These tests were repeated at 1-week intervals for 2 wk in Dogs 1 to 3 and for 3 wk in Dogs 4 to 6.
Ultrasonographic examination
Ultrasonographic examination of the pancreas was carried out before prednisolone administration and at the time of the second laparoscopy (day 14 for dogs 1 to 3 and day 21 for dogs 4 to 6). A complete abdominal ultrasound examination of each dog was done using a 12-MHz linear probe (PLT-1204BT; Toshiba Medical Systems, Tochigi, Japan). An image of the right pancreatic lobe was obtained in each dog using the right intercostal window or a right cranioventral approach (14). For consistency, transverse images of the right pancreatic limb were obtained in all dogs. The pancreatic lobe images of all 6 dogs were reviewed by one of the authors (H.O.) and the right pancreatic lobe echogenicity and height on the short axis were recorded. A representative image of the pancreas of each dog was subjectively graded as hypoechoic, isoechoic, or hyperechoic to the surrounding mesentery.
Laparoscopic procedure
After fasting for 24 h, the dogs were sedated by intravenous administration of midazolam (Midazolam Injection; SANDOZ, Yamagata, Japan), 0.01 mg/kg BW and butorphanol (Vetorphale; Meiji Seika Pharma, Tokyo, Japan), 0.02 mg/kg BW. General anesthesia was induced with propofol (PROPOFOL 1% Injection; Mylan, Osaka, Japan), 6 mg/kg BW and maintained with oxygen and isoflurane inhalation. Lactated Ringer’s solution (5 to 10 mL/kg BW per hour) was also administered intravenously with the dogs under anesthesia. The abdomen was aseptically prepared and a 1.5- to 2.0-cm-long skin and subcutaneous incision was made 2.0 cm caudal to the umbilicus. The midline was identified and a traction suture was placed on each side to lift the abdominal wall. A minilaparotomy incision was made with a surgical blade and the trocar (cannula) for a 5-mm laparoscope was passed through the incision. The abdomen was distended with CO2 through the cannula and the abdominal pressure was maintained at 8 mmHg using an automatic CO2 insufflator (UHI-3; Olympus, Tokyo, Japan). When adequate abdominal distention was achieved, a 5-mm telescope was placed inside the first cannula. A small skin incision about 1-cm long was made about 5 cm cranial and 5 cm lateral to the first trocar on both the right and left sides of the abdomen. Under visual control, a second and third cannula were placed through the right and left middle abdominal wall. Again under visual control, the abdomen was explored using a palpation probe.
Two biopsy samples were taken from the tail edge of the right pancreatic limb away from the pancreatic ducts using 5-mm punch biopsy forceps (A64620A; Olympus). Two additional biopsy samples were taken from the edge of the liver using 5-mm oval cup biopsy forceps (A64610A; Olympus). All instruments and the telescope were removed and the pneumoperitoneum was decompressed by opening one of the cannula valves and permitting the CO2 to escape. The cannulas were then removed and the puncture sites and skin were sutured using an absorbable suture (Coated VICRYL Suture; Ethicon, Cincinnati, Ohio, USA) and nonabsorbable monofilament suture (Suprylon; Vömel, Kronberg, Germany), respectively. All dogs received intravenous buprenorphine at 0.01 mg/kg BW (Lepetan; Otsuka Pharmaceutical, Tokyo, Japan) for analgesia and recovered with no complications. The respiratory rate, body temperature, arterial blood pressure, pulse oximetry, capnography, and electrocardiography of the dogs were monitored and recorded during the laparoscopic procedures.
Histological analysis of the pancreas and liver
Of the 2 biopsy specimens taken from the pancreas and the liver, 1 sample from each was immediately fixed with 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) for histopathological examination. The histological section of the pancreas and liver was examined by a Board-certified veterinary pathologist (Y.K.).
Gene expression analysis of pancreatic lipase in the pancreas and liver
Another biopsy specimen from the pancreas and liver was treated with RNAlater solution (Ambion, Foster City, California, USA) according to the manufacturer’s instructions and stored at −80°C until RNA isolation. Total RNA was extracted from the pancreas and liver using a commercially available RNA extraction kit (RNeasy Protect Mini Kit; Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. Genomic deoxyribonucleic acid (DNA) was removed from the samples with a commercially available kit (RNase-Free DNase Set; Qiagen). Complementary DNA (cDNA) was synthesized from 1.0 μg (pancreas) or 0.5 μg (liver) of total RNA using a commercially available cDNA synthesis kit (ReverTra Ace qPCR RT Master Mix; Toyobo, Osaka, Japan). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. The primer pair sequences used for polymerase chain reaction (PCR) amplification were: 5′-gttacactcaggcctcgcagaa-3′/5′-cgagtacccaaatgc tgactgaa-3′ for pancreatic lipase (Accession number, XM535023.4) and 5′-cattgccctcaatgaccact-3′/5′-tccttggaggccatgtagac-3′ for GAPDH (NM_001003142). Polymerase chain reaction was carried out with DNA polymerase (FastStart PCR Master; Roche, Indianapolis, Indiana, USA) according to the manufacturer’s instructions using the following steps: initial denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 10 s. Reactions were conducted in a 20-μL total volume containing 1 μL of cDNA, 500 nmol primers, and 10 μL of 2× Master Mix. Amplified fragments were visualized on a 3.0% agarose gel. Nucleotide sequences were confirmed by a DNA sequencing kit (BigDye Terminator v 3.1 Cycle Sequencing Kit; Applied Biosystems, Foster City, California, USA) with a genetic analyzer (ABI PRISM 3100 Genetic Analyzer; Applied Biosystems).
Statistical analysis
Statistical analyses were carried out using commercially available statistical software (JMP 13; SAS Institute, Cary, North Carolina, USA). The data set of the cPLI concentrations, serum biochemistry, thickness of the right pancreatic lobe measured by ultrasonography, and body weight was tested for normality using the Shapiro-Wilk W-test. A paired t-test was used to compare the serum biochemistry results at baseline and day 0. A paired t-test was used to compare the body weight on day 0 and on the day of the second laparoscopy. The Wilcoxon signed-rank test was used to compare the thickness of the right pancreatic lobe between day 0 and the day of the second laparoscopy. The Kruskal-Wallis test was used to check for overall differences in the cPLI concentration and serum biochemistry results on different days. The Dunn test was used to compare the cPLI concentration and serum biochemistry parameters between control (day 0) and day 7, day 14, or the day of the second laparoscopy. The Mann-Whitney U-test was used to compare the cPLI concentration of female and male dogs on the day of the second laparoscopy. Statistical significance was defined as P < 0.05.
Results
Animals
Three of the beagles were 4-year-old intact females (Dogs 1 to 3) and the other 3 were 2-year-old intact males (Dogs 4 to 6). The median body weight was 11.3 kg (range: 8.5 to 12.6 kg). None of the dogs had clinical signs of gastrointestinal disease or any apparent abnormalities as determined by blood testing, fecal examination, and abdominal ultrasound. The only exceptions were mildly elevated alkaline phosphatase (ALP) activity (385 U/L; reference interval: 47 to 254 U/L) in Dog 2 and alanine aminotransferase (ALT) activity (89 U/L; reference interval: 17 to 78 U/L) in Dog 3.
Three dogs (Dogs 1 to 3) were administered 4 mg/kg BW per day of prednisolone for 2 wk. Another 3 dogs (Dogs 4 to 6) were administered 4 mg/kg BW per day of prednisolone for 3 wk. Prednisolone was stopped if a dog developed a pendulous abdomen, which is an apparent side effect of prednisolone. None of the dogs showed clinical signs of gastrointestinal disease, such as vomiting, anorexia, or diarrhea, during the study period.
At the end of prednisolone treatment, 1 dog (Dog 1) had gained weight (7.3%) compared with day 0; the other 5 dogs had significantly lost weight compared with day 0 (P < 0.05). The amount of weight loss in each of the 5 dogs (Dogs 2 to 6) was 2.0%, 6.0%, 9.4%, 10.0%, and 10.0%, respectively. In addition, 1 dog (Dog 4) developed bacterial dermatitis on the left hind leg on day 21 of prednisolone treatment. This dog was treated with peroral administration of 5 mg/kg BW per day of enrofloxacin (Baytril; Bayer Yakuhin, Osaka, Japan) for 2 wk, after which the dermatitis was cured.
Measurement of canine pancreatic lipase immunoreactivity
The serum cPLI measurements in each dog before and after treatment are summarized in Table I. The second laparoscopy was carried out at the end of the prednisolone treatment, which was day 14 in Dogs 1 to 3 and day 21 in Dogs 4 to 6. Before administering prednisolone (day 0), the median cPLI concentration was 30 μg/L (range: 30 to 35 μg/L). The median cPLI concentration was significantly higher on day 14 (59 μg/L; range: 41 to 746 μg/L) and on the day of the second laparoscopy (68 μg/L; range: 30 to 746 μg/L) than on day 0 (P < 0.05). On day 14 and on the day of the second laparoscopy, the cPLI concentration was classified as normal (≤ 200 μg/L) in 5 dogs and abnormal (≥ 400 μg/L) in 1 dog. The serum cPLI concentrations on the day of the second laparoscopy were significantly higher in the 3 female dogs (Dogs 1 to 3) than in the 3 male dogs (Dogs 4 to 6) (P < 0.05).
Table I.
Median concentrations of canine pancreatic lipase immunoreactivitya (cPLI) in 6 healthy dogs before and after prednisolone treatmentb.
| Dog number | Day 0 | Day 7 | Day 14 | Day 21 | Day of second laparoscopyc |
|---|---|---|---|---|---|
| 1 | 35 | 137 | 746 | 746 | |
| 2 | 30 | 37 | 129 | 129 | |
| 3 | 30 | 30 | 71 | 71 | |
| 4 | 30 | 50 | 42 | 30 | 30 |
| 5 | 30 | 47 | 41 | 65 | 65 |
| 6 | 30 | 52 | 47 | 33 | 33 |
| Median (range) | 30 (30 to 35) | 48.5 (30 to 137) | 59 (41 to 746)d | 68 (30 to 746)d |
The reference interval of cPLI assay is 0 to 200 μg/L. A cPLI concentration of ≥ 400 μg/L is considered abnormal.
Prednisolone (4 mg/kg BW, once daily) was administered for 14 d in Dogs 1 to 3 and for 21 d in Dogs 4 to 6.
Day of second laparoscopy was day 14 in Dogs 1 to 3 and day 21 in Dogs 4 to 6.
Values differ significantly (P < 0.05) compared with the value for day 0.
Serum biochemistry tests
The results of the serum biochemistry examination in each dog are summarized in Table II. Only the alanine aminotransferase (ALT) concentration was higher on day 0 compared with baseline. The alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) concentrations were higher on day 14 and the on day of the second laparoscopy than on day 0. The lipase activity was abnormal on day 14 (476 U/L) in 1 dog (Dog 1) and normal on the day of the second laparoscopy in the other 5 dogs, although not statistically different from day 0. The creatinine concentration was lower on the day of the second laparoscopy than on day 0. The C-reactive protein concentration was increased (1.7 mg/dL) in 1 dog (Dog 4) on the day of the second laparoscopy (day 21).
Table II.
Median for results of serum biochemistry examination of 6 healthy dogs at baseline, day 0, and after prednisolone treatmenta.
| Variable | Reference interval | Baseline | Day 0 | Day 7 | Day 14 | Day of second laparoscopyb |
|---|---|---|---|---|---|---|
| ALT (U/L) | 17 to 78 | 39 (28 to 89) | 120 (47 to 183)d | 103 (59 to 333) | 117 (43 to 416) | 166 (34 to 416) |
| AST (U/L) | 17 to 44 | 31.5 (23 to 34) | 30.5 (22 to 39) | 33 (27 to 70) | 42.5 (24 to 65) | 40 (38 to 65) |
| ALP (U/L) | 47 to 254 | 114 (119 to 385) | 164 (139 to 433) | 1004 (319 to 1340) | 1615 (319 to 2560)e | 2268.5 (717 to 2560)e |
| GGT (U/L) | 5 to 14 | 7.5 (3 to 11) | 6.5 (5 to 9) | 14.5 (9 to 24) | 29.5 (6 to 68)e | 42.5 (21 to 68)e |
| T-Bil (mg/dL) | 0.1 to 0.5 | 0.2 (0.1 to 0.3) | 0.2 (0.1 to 0.4) | 0.3 (0.2 to 0.5) | 0.35 (0.2 to 0.4) | 0.4 (0.2 to 0.6) |
| T-Cho (mg/dL) | 111 to 312 | 174.5 (130 to 242) | 172 (107 to 221) | 175.5 (45 to 227) | 159 (50 to 234) | 166 (50 to 345) |
| TG (mg/dL) | 30 to 133 | 40 (21 to 89) | 42 (33 to 71) | 38.5 (27 to 132) | 60.5 (32 to 145) | 63.5 (32 to 145) |
| Lipase (U/L)c | 10 to 160 | 35 (23 to 45) | 36.5 (21 to 48) | 43.5 (23 to 83) | 56 (36 to 476) | 53 (27 to 476) |
| BUN (mg/dL) | 9.2 to 29.2 | 11.7 (9.4 to 18.3) | 12.5 (10.7 to 16.3) | 14.5 (11.8 to 17.1) | 13.9 (9.1 to 18.5) | 11.4 (8 to 20.4) |
| Crea (mg/dL) | 0.4 to 1.4 | 0.5 (0.3 to 0.8) | 0.5 (0.3 to 0.6) | 0.3 (0.2 to 0.4) | 0.3 (0.2 to 0.5) | 0.2 (0.2 to 0.5)e |
| CRP (mg/dL) | 0 to 1.0 | 0 (0 to 0.3) | 0.1 (0 to 0.5) | 0 (0) | 0.1 (0 to 0.8) | 0.3 (0 to 1.7) |
Prednisolone (4 mg/kg BW, subcutaneously, once daily) was administered for 14 d in Dogs 1 to 3 and for 21 d in Dogs 4 to 6.
Day of second laparoscopy was day 14 in Dogs 1 to 3 and day 21 in Dogs 4 to 6.
Lipase activity was measured by the v-LIP-P lipase dry-chemistry slide.
Values for day 0 differ significantly (P < 0.05) compared with the value for baseline.
Values differ significantly (P < 0.05) compared with the value for day 0.
ALP — alkaline phosphatase; ALT — alanine aminotransferase; AST — aspartate aminotransferase; BUN — blood urea nitrogen; Crea — creatinine; CRP — C-reactive protein; GGT — γ-glutamyltransferase; T-Bil — total bilirubin; T-Cho — total cholesterol; TG — triglyceride.
Ultrasonographic examination
The results of the ultrasonographic examination of the pancreas in all 6 dogs are summarized in Table III. Before the prednisolone was administered, the right pancreatic lobe was graded as isoechoic to the surrounding mesentery in all 6 dogs. After the prednisolone administration, the right pancreatic lobe was graded as isoechoic in 5 dogs and hypoechoic in 1 dog (Dog 5). There was no significant difference in the height of the right pancreatic lobe before (median, 11.3 mm; 8.1 to 13.5 mm) and after prednisolone treatment (median, 10.0 mm; 6.4 to 13.4 mm).
Table III.
Sonographic pancreatic appearance of 6 healthy dogs at day 0 and day of second laparoscopy.
| Day 0 | Day of second laparoscopya | |
|---|---|---|
| Thickness (mm) | ||
| median (range) | 11.3 (8.1 to 13.5) | 10.0 (6.4 to 13.4) |
| Ecogenicity | ||
| Isoechoic | 6 dogs | 5 dogs |
| Hyoechoic | 1 dog |
Day of second laparoscopy was day 14 in Dogs 1 to 3 and day 21 in Dogs 4 to 6.
Histological analysis
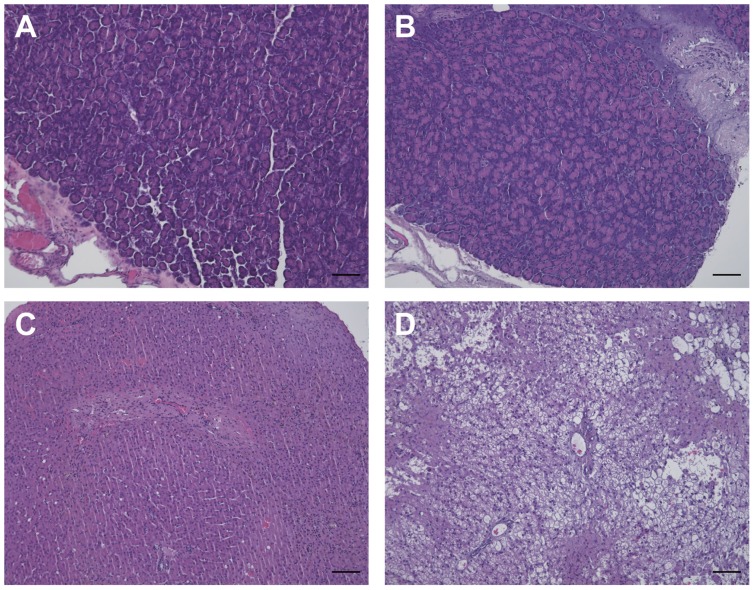
Before and after prednisolone was administered, histological analysis of the right pancreatic lobe revealed no histological abnormalities in any of the 6 dogs (Figures 1A and B). Before prednisolone was administered, no histological abnormalities were observed in the liver in all 6 dogs (Figure 1C). In contrast, histological analysis of the liver revealed vacuolar hepatopathy in all 6 dogs (Figure 1D).
Figure 1.
Histopathological examination of the pancreas and liver before and after subcutaneous administration of prednisolone. No histological abnormalities were observed in the pancreas before (A) and after (B) prednisolone treatment. No histological abnormalities were observed in the liver before prednisolone treatment (C). Marked vacuolar degeneration occurred in the liver after prednisolone treatment (D). Hematoxylin and eosin stain; bar = 100 μm.
Gene expression analysis of pancreatic lipase in the pancreas and liver
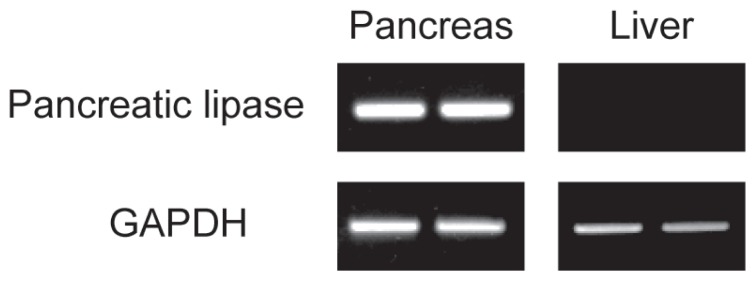
Before and after prednisolone was administered, pancreatic lipase gene expression was detected in the pancreas, but not in the liver in all 6 dogs (Figure 2).
Figure 2.
Upper panels show gene expression of pancreatic lipase in the pancreas and liver before (left) and after (right) prednisolone treatment. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene.
Discussion
This study demonstrated that subcutaneously administering prednisolone at the higher end of the immunosuppressive dose (4 mg/kg BW per day) for either 2 or 3 wk to 6 healthy beagle dogs caused a statistically significant increase in the serum cPLI concentration without clinical signs of pancreatitis, although the cPLI concentration increased into the abnormal range in only 1 dog.
The effects of corticosteroids on the pancreas are poorly understood and corticosteroid-induced pancreatitis is a difficult clinical condition to diagnose. The recurrence of pancreatitis after a rechallenge with a corticosteroid in humans has been described in only a few studies (15). Corticosteroids are not considered a risk factor for pancreatitis and are considered to be only a “possible/questionable” cause of drug-induced pancreatitis in humans (16).
On the other hand, the results of 2 studies examining the effect of corticosteroids on the serum cPLI concentration in dogs are inconsistent. One study showed that the serum cPLI concentration was not altered by 4 wk of peroral administration of 2.2 mg/kg BW per day of prednisone to 6 healthy young adult female heterozygous dogs with X-linked hereditary nephritis (7). In contrast, we recently reported that the serum cPLI concentration increased by various levels in 5 of 10 dogs with immune-mediated disease treated with an immunosuppressive dosage of prednisolone (2.0 to 2.2 mg/kg BW per day as the initial treatment) without clinical signs of pancreatitis (10). From the results of these 2 studies (7,10), we speculate that the differences between prednisone and prednisolone or the presence of underlying disorders might affect the results of administering corticosteroids on serum cPLI concentrations.
A possible explanation for the differences in the serum cPLI concentrations among 6 dogs treated with 2.2 mg/kg BW of prednisolone per day for 4 wk and 6 dogs treated with 4 mg/kg BW of prednisolone per day for 2 or 3 wk is that the glucocorticoid dosage used in the current study (4 mg/kg BW per day for 2 or 3 wk) was higher than that in the previous study using prednisone (2.2 mg/kg BW per day for 4 wk) (7). As mentioned previously, the relative bioavailability of prednisolone was reported to be only 65% when prednisone was administered compared with prednisolone in dogs, although the reason for this difference has not been fully elucidated (12). Additionally, we used the higher end of the immunosuppressive dose range of prednisolone (4 mg/kg per day), instead of 2.2 mg/kg BW per day of prednisone used in the previous study (7), although different routes of administration were used in the 2 studies. We used subcutaneous injection of prednisolone instead of oral administration to avoid incomplete absorption of the drug, which is one of the disadvantages of oral administration. Drug absorption is usually more predictable when using parenteral than oral administration. The bioavailability of prednisolone used in the current study (4 mg/kg BW per day, subcutaneously) could therefore be much higher than that of prednisone used in the previous study (2.2 mg/kg BW per day, orally) (7).
In another previous report, 3 of 19 dogs with neurological disease treated with dexamethasone had evidence of pancreatitis or peripancreatic fat necrosis at necropsy, although only 1 of the 3 dogs had clinical signs of pancreatitis (8). The results of this study indicate that pancreatitis may develop when dogs with neurological disease are treated with a corticosteroid. Possible explanations for the histopathological findings of pancreatitis in these 3 dogs are either that the stress associated with the underlying neurological condition leads to pancreatitis after dexamethasone treatment or that dogs with neurological disease are predisposed to developing pancreatitis.
In our previous study, 5 of 10 dogs with immune-mediated disease showed an increased cPLI concentration in the abnormal range without clinical signs of pancreatitis after prednisolone was administered, although histological examination of the pancreas was not carried out in any of the dogs (10). Two of these 5 dogs had idiopathic immune-mediated polyarthritis, 1 had meningoencephalomyelitis of unknown origin, 1 had immune-mediated polymyositis, and 1 had inflammatory colorectal polyps. It was difficult to make a definitive conclusion about the relationship between the disease category and abnormal cPLI concentrations after prednisolone was administered because of the small number of cases. Further studies using larger numbers of dogs with immune-mediated or neurological disease are needed to explore the relationship between the underlying disorders and the increase in the cPLI concentration to the abnormal range after prednisolone is administered.
It is not known what caused the serum cPLI concentration to increase in dogs treated with prednisolone. It may be a result of increased synthesis of this enzyme in the pancreas or increased cellular permeability to pancreatic lipase. It could also be caused by a decreased rate of removal of the enzyme from the serum by the kidney, although this is unlikely because the blood urea nitrogen (BUN) and creatinine concentrations were within the normal ranges in all dogs throughout the study period (17). The increase in the serum cPLI concentration could also have resulted from prednisolone-induced release from extra-pancreatic tissues, such as the liver, as lipase activity has been found in many organs, including the liver, stomach, and adipose tissue. Pancreatic lipase mRNA expression was not observed in the liver, however, even after prednisolone treatment. Pancreatic lipase synthesis and release from the liver is therefore not likely. The possibility of increased pancreatic lipase synthesis in other organs, such as the stomach and adipose tissue, however, cannot be ruled out based on the results of this study. In addition, the possibility of increased cellular permeability to pancreatic lipase cannot be ruled out based on the present results because we did not examine the cellular permeability of pancreatic acinar cells in dogs treated with prednisolone.
A previous study reported an association between the postprandial serum triglyceride concentration and the serum cPLI concentration in overweight and obese dogs (18). Another study reported a positive correlation between the serum triglyceride concentration and serum cPLI concentration in miniature schnauzers (19). While we initially considered that the increased cPLI concentration in the current study was caused by corticosteroid-induced hypertriglyceridemia, no association could be found between the serum cPLI concentration and serum triglyceride concentration because of the small number of dogs used in this study.
In this study, we used 6 healthy beagle dogs, consisting of 3 intact females and 3 intact males. On the day of the second laparoscopy, the serum cPLI concentrations in the 3 female dogs (2 wk after prednisolone was administered) were significantly higher than in the 3 male dogs (3 wk after prednisolone was administered). Hormonal differences between the sexes might have affected the serum cPLI concentration after prednisolone treatment, although the number of dogs was too small to achieve a definitive conclusion and the statistical power of the study was low. A further study is needed with a larger number of dogs to clarify the impact of sex hormones on the effect of administering 4 mg/kg BW per day of prednisolone on the serum cPLI concentration.
In addition to measuring serum cPLI concentration, we measured the serum lipase activity using slide-based catalytic lipase assay (v-LIP-P slide; FUJI DRI-CHEM). The results showed a tendency for the lipase activity to increase, similar to the results of the cPLI concentration, although the increase was not statistically significant. In addition, 1 dog (Dog 1) showed an abnormal range of lipase activity (476 IU/L) 2 wk after prednisolone was administered. In general, enzyme catalytic assays of lipase are thought to have low specificity for diagnosing canine pancreatitis. Lipase activity measured by v-LIP-P slide showed a relatively good correlation with the cPLI concentration (20), although several sample conditions could significantly affect the v-LIP-P slide results, e.g., hemolysis, icterus (21). Administering 4 mg/kg BW per day of prednisolone for 2 or 3 wk might have some effect on the lipase activity measured by v-LIP-P slide, although the power of this study was too low to determine this effect.
In 1 dog, the right pancreatic lobe was hypoechoic on ultrasonographic examination, but histologically normal after prednisolone was administered, which could be due to portal hypertension caused by portal vein thrombosis. Pancreatic edema is thought to be associated with pancreatitis, hypoalbuminemia, or portal hypertension (22). While administering a corticosteroid is a major predisposing factor for the formation of portal vein thrombosis (23), we detected no thrombi in the portal vein by abdominal ultrasound. The cause of the hypoechoic change in the right pancreatic lobe is therefore not known.
In the present study, liver enzymes increased slightly in 2 of 6 dogs at baseline (ALT in Dog 3 and ALP in Dog 2). While subclinical elevation of liver enzymes could have influenced the results of this study, no histopathological abnormalities were found in the livers of these 2 dogs at the time of the first laparoscopy. Moreover, as these 2 dogs did not include the dog with the highest cPLI concentration at the time of the second laparoscopy (Dog 1), it is unlikely that the mild increase in liver enzymes in these 2 dogs inappropriately influenced the effect of prednisolone on the pancreatic tissue and serum cPLI concentration. Another possible explanation for the increase in ALT on day 0 (2 to 3 wk after the first laparoscopy) compared with baseline was the effect of liver biopsy at the first laparoscopy.
In 1 dog (Dog 4), the C-reactive protein concentration was increased (1.7 mg/dL) on the day of the second laparoscopy. As this dog did not show clinical signs of gastrointestinal disease throughout the study period and the cPLI concentration on the day of the second laparoscopy was 30 μg/L, it is unlikely that acute pancreatitis caused this increase. The increase could have been caused by the bacterial dermatitis on this dog’s leg, although it is not known whether the dermatitis or the antibiotics given to treat it were the cause.
Five of the 6 dogs unexpectedly lost weight after prednisolone was administered, although their appetite and food consumption did not decrease during the study period. This dosage of prednisolone (4 mg/kg BW per day) might have been high enough to induce a breakdown of protein that resulted in muscle wasting. Because we did not expect muscle wasting, however, we did not monitor the body condition score or muscle condition score of the dogs. These scores may have been more suitable for accurately monitoring the adverse effects of this dose of prednisolone than the appearance of a pendulous abdomen.
The present study has some limitations. First, as we did not carry out a histological examination of the whole pancreas, the possibility of pancreatitis cannot be completely ruled out because pancreatic inflammation tends to occur in discrete areas within the pancreas rather than diffusely throughout the whole pancreas (24). Second, the effect of prolonged administration of an immunosuppressive dose of prednisolone on the pancreas and serum cPLI concentration remains unknown because we did not monitor the cPLI concentration for more than 3 wk. Third, a further study with more dogs is needed in order to increase the power of the study. Fourth, we did not conduct urinalysis in order to monitor the specific gravity of the urine in the 6 dogs, which would have been useful to assess side effects of prednisolone, such as polyuria/polydipsia, other than a pendulous abdomen. Finally, we cannot exclude the possibility of intra-individual variation in the serum cPLI concentration that is not associated with prednisolone treatment. A previous study demonstrated this possibility in 11 apparently healthy dogs, 2 of which had an abnormal cPLI concentration during the 12-week study period (25).
In conclusion, subcutaneous administration of 4 mg/kg BW per day of prednisolone to 6 healthy dogs increased the serum cPLI concentration without ultrasonographic or histological evidence of pancreatitis, although this increase was abnormal in only 1 dog. In addition, pancreatic lipase gene expression was detected in the pancreas, but not in the liver, of all dogs, both before and after prednisolone treatment.
References
- 1.Steiner JM. Is it pancreatitis? Vet Med. 2006;101:158–166. [Google Scholar]
- 2.Steiner JM, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9:263–273. [PubMed] [Google Scholar]
- 3.Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas-specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011;25:1241–1247. doi: 10.1111/j.1939-1676.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 4.McCord K, Morley PS, Armstrong J, et al. A multi-institutional study evaluating the diagnostic utility of the Spec cPL and SNAP cPL in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26:888–896. doi: 10.1111/j.1939-1676.2012.00951.x. [DOI] [PubMed] [Google Scholar]
- 5.Xenoulis PG, Steiner JM. SNAP tests for pancreatitis in dogs and cats: SNAP canine pancreatic lipase and SNAP feline pancreatic lipase. Top Companion Anim Med. 2016;31:134–139. doi: 10.1053/j.tcam.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Steiner JM, Berridge BR, Wojcieszyn J, Williams DA. Cellular immunolocalization of gastric and pancreatic lipase in various tissues obtained from dogs. Am J Vet Res. 2002;63:722–727. doi: 10.2460/ajvr.2002.63.722. [DOI] [PubMed] [Google Scholar]
- 7.Steiner JM, Teague SR, Lees GE, Willard MD, Williams DA, Ruaux CG. Stability of canine pancreatic lipase immunoreactivity concentration in serum samples and effects of long-term administration of prednisone to dogs on serum canine pancreatic lipase immunoreactivity concentrations. Am J Vet Res. 2009;70:1001–1005. doi: 10.2460/ajvr.70.8.1001. [DOI] [PubMed] [Google Scholar]
- 8.Parent J. Effects of dexamethasone on pancreatic tissue and on serum amylase and lipase activities in dogs. J Am Vet Med Assoc. 1982;180:743–746. [PubMed] [Google Scholar]
- 9.Fittschen C, Bellamy JE. Prednisone treatment alters the serum amylase and lipase activities in normal dogs without causing pancreatitis. Can J Comp Med. 1984;48:136–140. [PMC free article] [PubMed] [Google Scholar]
- 10.Ohta H, Morita T, Yokoyama N, et al. Serial measurement of pancreatic lipase immunoreactivity concentration in dogs with immune-mediated disease treated with prednisolone. J Small Anim Pract. 2017;58:342–347. doi: 10.1111/jsap.12652. [DOI] [PubMed] [Google Scholar]
- 11.Meikle AW, Weed JA, Tyler FH. Kinetics and interconversion of prednisolone and prednisone studied with new radioimmunoassays. J Clin Endocrinol Metab. 1975;41:717–721. doi: 10.1210/jcem-41-4-717. [DOI] [PubMed] [Google Scholar]
- 12.Colburn WA, Sibley CR, Buller RH. Comparative serum prednisone and prednisolone concentrations following prednisone or prednisolone administration to beagle dogs. J Pharm Sci. 1976;65:997–1001. doi: 10.1002/jps.2600650711. [DOI] [PubMed] [Google Scholar]
- 13.Huth SP, Relford R, Steiner JM, Strong-Townsend MI, Williams DA. Analytical validation of an ELISA for measurement of canine pancreatic-specific lipase. Vet Clin Pathol. 2010;39:346–353. doi: 10.1111/j.1939-165X.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 14.Granger LA, Hilferty M, Francis T, Steiner JM, Gaschen L. Variability in the ultrasonographic appearance of the pancreas in healthy dogs compared to dogs with hyperadrenocorticism. Vet Radiol Ultrasound. 2015;56:540–548. doi: 10.1111/vru.12261. [DOI] [PubMed] [Google Scholar]
- 15.Felig DM, Topazian M. Corticosteroid-induced pancreatitis. Ann Intern Med. 1996;124:1016. doi: 10.7326/0003-4819-124-11-199606010-00018. [DOI] [PubMed] [Google Scholar]
- 16.Topazian M, Pandol SJ. Acute pancreatitis. In: Yamada T, Alpers DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 5th ed. West Sussex, UK: Wiley-Blackwell; 2009. pp. 1761–1810. [Google Scholar]
- 17.Hulsebosch SE, Palm CA, Segev G, Cowgill LD, Kass PH, Marks SL. Evaluation of canine pancreas-specific lipase activity, lipase activity, and trypsin-like immunoreactivity in an experimental model of acute kidney injury in dogs. J Vet Intern Med. 2016;30:192–199. doi: 10.1111/jvim.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkest KR, Fleeman LM, Morton JM, et al. Association of postprandial serum triglyceride concentration and serum canine pancreatic lipase immunoreactivity in overweight and obese dogs. J Vet Intern Med. 2012;26:46–53. doi: 10.1111/j.1939-1676.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 19.Xenoulis PG, Suchodolski JS, Ruaux CG, Steiner JM. Association between serum triglyceride and canine pancreatic lipase immunoreactivity concentrations in miniature schnauzers. J Am Anim Hosp Assoc. 2010;46:229–234. doi: 10.5326/0460229. [DOI] [PubMed] [Google Scholar]
- 20.Ishioka K, Hayakawa N, Nakamura K, Terashima K. Patientside assay of lipase activity correlating with pancreatic lipase immunoreactivity in the dog. J Vet Med Sci. 2011;73:1481–1483. doi: 10.1292/jvms.11-0166. [DOI] [PubMed] [Google Scholar]
- 21.Steiner JM, Gomez R, Suchodolski JS, Lidbury JA. Specificity of, and influence of hemolysis, lipemia, and icterus on serum lipase activity as measured by the v-LIP-P slide. Vet Clin Pathol. 2017;46:508–515. doi: 10.1111/vcp.12517. [DOI] [PubMed] [Google Scholar]
- 22.d’Anjou MA, Penninck D. Pancreas. In: Penninck D, d’Anjou MA, editors. Atlas of Small Animal Ultrasonography. 2nd ed. Oxford, UK: Wiley-Blackwell; 2015. pp. 309–330. [Google Scholar]
- 23.Respess M, O’Toole TE, Taeymans O, Rogers CL, Johnston A, Webster CR. Portal vein thrombosis in 33 dogs: 1988–2011. J Vet Intern Med. 2012;26:230–237. doi: 10.1111/j.1939-1676.2012.00893.x. [DOI] [PubMed] [Google Scholar]
- 24.Newman S, Steiner J, Woosley K, Barton L, Ruaux C, Williams D. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med. 2004;18:488–493. doi: 10.1892/0891-6640(2004)18<488:lopian>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Carney PC, Ruaux CG, Suchodolski JS, Steiner JM. Biological variability of C-reactive protein and specific canine pancreatic lipase immunoreactivity in apparently healthy dogs. J Vet Intern Med. 2011;25:825–830. doi: 10.1111/j.1939-1676.2011.0729.x. [DOI] [PubMed] [Google Scholar]