Abstract
Background and Purpose—
Intracerebral hemorrhage (ICH) is a devastating disease without a proven therapy to improve long-term outcome. Considerable controversy about the role of surgery remains. Minimally invasive endoscopic surgery for ICH offers the potential of improved neurological outcome.
Methods—
We tested the hypothesis that intraoperative computerized tomographic image–guided endoscopic surgery is safe and effectively removes the majority of the hematoma rapidly. A prospective randomized controlled study was performed on 20 subjects (14 surgical and 4 medical) with primary ICH of >20 mL volume within 48 hours of ICH onset. We prospectively used a contemporaneous medical control cohort (n=36) from the MISTIE trial (Minimally Invasive Surgery and r-tPA for ICH Evacuation). We evaluated surgical safety and neurological outcomes at 6 months and 1 year.
Results—
The intraoperative computerized tomographic image–guided endoscopic surgery procedure resulted in immediate reduction of hemorrhagic volume by 68±21.6% (interquartile range 59–84.5) within 29 hours of hemorrhage onset. Surgery was successfully completed in all cases, with a mean operative time of 1.9 hours (interquartile range 1.5–2.2 hours). One surgically related bleed occurred peri-operatively, but no patient met surgical safety stopping threshold end points for intraoperative hemorrhage, infection, or death. The surgical intervention group had a greater percentage of patients with good neurological outcome (modified Rankin scale score 0–3) at 180 and 365 days as compared with medical control subjects (42.9% versus 23.7%; P=0.19).
Conclusions—
Early computerized tomographic image–guided endoscopic surgery is a safe and effective method to remove acute intracerebral hematomas, with a potential to enhance neurological recovery.
Keywords: coma, endoscopic surgery, hemorrhagic, intracerebral hemorrhage, stroke
Intracerebral hemorrhage (ICH) is an important neurological emergency with high societal impact.1 Hemorrhage volume and, to an extent, total edema volume independently influence outcome after ICH.2,3 The effects of the primary bleed, progressive hemorrhagic expansion, and necrosis of brain tissue have long been thought to irreversibly influence outcome.4 However, secondary injury of retained blood and the blood breakdown products are thought to lead to slowly ensuing damage to perihematomal tissue because of mass effect, excitotoxic edema, and progressive neurotoxicity.5–7 Preliminary studies suggest the possibility that stereotactic minimally invasive hematoma removal may reduce secondary neurotoxicity,8–10 but the effects of hematoma reduction on clinical outcome remain unclear.
To date, routine craniotomy surgery to remove hematomas has not resulted in proven benefit.11,12 A series of nonrandomized studies suggest that endoscopic surgery can be safely performed after ICH,13 but the procedure remains unproven. Many questions exist regarding the surgical optimization of endoscopic surgical technique. Given these concerns, we hypothesized that an endoscopic removal of ICH could be safely performed in a multicenter study with adherence to the structured surgical protocol in the acute period after ICH. The main outcome of the study is surgical safety (ie, mortality and recurrent bleeding). Secondary outcomes included surgical variability measures: total percentage hematoma evacuated by the surgeon, timing of surgery, and global compliance with the a priori surgical protocol. Exploratory outcomes for purposes of future planning included neurological outcome as measured by the modified Rankin outcome score at 180 and 365 days.
Methods
This was a multicenter, randomized controlled trial that was approved by the University of California, Los Angeles (UCLA), Institutional Review Board and the local Institutional Review Boards at each participating institution. The eligibility criteria are shown in Table I in the online-only Data Supplement. The detailed methods are in the online-only Data Supplement. After obtaining informed consent form signatures, eligible patients with a stable ICH volume of >20 mL were randomized 3:1 to endoscopic surgery or standard medical management and were managed per the protocol and within local institutional guidelines. Subjects randomized to endoscopic surgery underwent the procedure within 48 hours of the time of the computed tomography (CT) scan diagnosing the hemorrhage. Both medical and surgical patients received similar standard of care as outlined in a structured guideline for treatment identical in both the MISTIE (Minimally Invasive Surgery and r-tPA for ICH Evacuation) and ICES studies (Intraoperative Stereotactic CT-Guided Endoscopic Surgery). A standardized imaging assessment was used in each subject to determine the presence/absence of a vascular malformation, stability of the hemorrhage volume, and stereotactic volumetric assessment of the hemorrhage. CT imaging was performed on admission, 6 hours after the initial CT, preoperatively, postoperatively, and then daily ≤7 days. Neurological assessments were performed using the modified Rankin Scale (mRS) at 30, 90, 180, 270, and 365 days after hemorrhage onset.
Imaging, Screening, and Stability Protocol
Patients were identified on admission to the emergency room at participating centers using structured inclusion/exclusion criteria and underwent a diagnostic brain CT scan on admission. Six or more hours later, a stability CT was done 6 hours later to ensure that the ICH clot was not expanding, as defined by no increase in clot size >5 mL, using the ABC/2 method between 2 sequential scans. A volumetric stereotactic CT was obtained just before surgery and used for intraoperative stereotactic guidance.
Endoscopic Surgical Protocol
Operative Procedure
All surgeons performed a stereotactic endoscopic surgery after receiving detailed training by the surgical principal investigator (NAM). Briefly, the ideal trajectory, which bisected is parallel to the long axis of the hematoma, was selected using the image guidance probe positioned over the candidate entry point. One of three preapproved approaches were selected: (1) anterior frontal lobe approach, (2) posterior parietal lobe approach, (3) surface cortical approach, each of which were designed to be parallel and in the middle of the long axis of the hematoma while avoiding the internal capsule, sylvian fissure, eloquent white matter tracts, and ventricles (Figure I in the online-only Data Supplement). The approach was preplanned and agreed to by the surgical center (SC) principal investigators before surgery. After a standard burr hole opening, the endoscope sheath (Storz, El Segundo, CA) with obturator in place was connected to the Mitaka/Storz hydraulic fixation arm (Storz, El Segundo, CA; see Table II in the online-only Data Supplement), and the endoscope was introduced into the cortex manually after releasing the hydraulic brake. The endoscope sheath was introduced to a point approximately two thirds of the way to the distal margin of the hematoma parallel to the long axis of the hematoma. For the typical ovoid hemorrhage, the tip of the sheath is placed two thirds of the way along the long axis of the hematoma (point no 1). The hematoma was removed by a technique using irrigation and aspiration. Once hemostasis was obtained, the Mitaka arm was released and the endoscope sheath backed out to a point approximately one third of the way into the hematoma cavity (point no 2), and the aspiration and irrigation technique was repeated until 75% to 80% of the hematoma volume was removed. The endoscope was introduced to observe the volume of irrigation to be sure that there was no sign of any ongoing streams of blood coming from any vessels, which might require coagulation. The dura and skin were closed in a routine manner. An immediate postoperative CT scan was obtained.
Scoring Surgical Protocol Compliance
Each surgery was documented using a structured report form and a standard-of-care operative note. Adjudication of the surgery by the SC principal investigators included review of the structure report form, the operative note, and postoperative CT image to determine if the planned trajectory was used. Compliance with the surgical protocol was evaluated and scored using an ordinal scale. The variability of surgical techniques (such as suction pressure, duration of surgery, etc) was judged based on this review.
Medical Treatment Protocol
All subjects were managed using the American Heart Association recommendations for the treatment of spontaneous ICH.14,15 Patients randomized to receive standard medical care received CT scans and other monitoring assessments on the same schedule as those randomized to receive the ICES procedure. Mean hourly intracranial pressure (ICP) values, when monitored for clinical care, were compared between surgical and medical patients.
Follow-Up
Subjects were followed with a magnetic resonance imaging scan at day 7. Subjects were followed at regular intervals up to day 365 and assessed by a certified examiner who assessed mRS, Barthel Index, Stroke Impact Scale, Glasgow Outcome Scale, extended Glasgow Outcome Scale, National Institutes of Health Stroke Scale (clinic visit only), and repeat CT (days 30 and 180 only).
Image Analysis
To optimize accuracy and minimize investigator bias, ICH and intraventricular hemorrhage volumes were analyzed by a core laboratory using semiautomated segmentation at the threshold of 40 Hounsfield units.16 This approach has been validated for accuracy and interrater reliability.17 Core laboratory values were used in all analyses. Core laboratory defined location as either lobar or deep (putamen or thalamus).
Statistical Analysis
Primary and Secondary Outcomes
The aim of the endoscopic treatment was to achieve near total clot removal without procedure-related safety events that would endanger the lives of the patients beyond the risks associated with intensive medical treatment. The primary safety outcomes are the following: the 30-day rate of mortality, the 7-day rate of procedure-related mortality, the 30-day rate of bacterial brain infection, and the rate of symptomatic bleeding within the 72 hours post last dose timeframe. The primary efficacy outcome is the 180-day dichotomized mRS score 0 to 3 versus 4 to 6. Secondary efficacy outcomes are the 180day ordinal mRS, the 365-day ordinal mRS, and the difference in clot-size reduction at the end of the treatment period for each group. All adverse and serious adverse events were reported during the acute treatment period and all serious adverse events through the end of the follow-up period. Adverse events remained below the prespecified thresholds for the 30-day mortality (70%), symptomatic brain bleeding (35%), and bacterial brain infection (15%), as shown in Table 1. We tested rates of safety events across groups by Fisher exact test and calculated exact binomial confidence intervals for the rate of events. The method of Kaplan and Meier was used to estimate the survival functions (±95% confidence interval) for patients in the ICES treatment and ICES and MISTIE medical management groups.
Table 1.
Mortality and Adverse Events Among Surgically and Medically Managed Patients
| Event | Study-Stop Threshold | MISTIE II Medical (N=36) | ICES Medical (N=6) | ITT Surgical (N=14) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |||
| Died within 0–7 days | 10% | 0 (0%) | (0%, 9.7%) | 0 (0%) | (0%, 45.9%) | 0 (0%) | (0%, 23.2%) | … |
| Died within 0–30 days | 70% | 4 (9.5%) | (1.8%, 22.5%) | 0 (0%) | (0%, 45.9%) | 1 (7.1%) | (1.8%, 33.9%) | 0.685 |
| Bacterial brain infection 0–30 days* | 15% | 1 (2.8%) | (0.07%, 14.5%) | 0 (0%) | (0%, 45.9%) | 0 (0%) | (0%, 23.2%) | 0.759 |
| Symptomatic bleed 72 h post last dose† | 35% | 1 (2.8%) | (0.07%, 14.5%) | 0 (0%) | (0%, 45.9%) | 0 (0%) | (0%, 23.2%) | 0.759 |
| Asymptomatic bleed 72 h post last dose‡ | n/a | 3 (8.3%) | (1.8%, 22.5%) | 1 (16.7%) | (0.4%, 64.1%) | 3 (21.4%) | (4.7%, 50.8%) | 0.530 |
| Symptomatic or asymptomatic bleed 72 h post last dose§ | n/a | 4 (11.1%) | (3.1%, 26.1%) | 1 (16.7%) | (0.4%, 64.1%) | 3 (21.4%) | (4.7%, 50.8%) | 0.719 |
Primary safety events, with thresholds for randomized medical (MISTIE II vs ICES) and ITT surgical with 95% CI. CI indicates confidence interval; CT, computed tomography; GCS, Glasgow coma score; ICES, Intraoperative Stereotactic CT-Guided Endoscopic Surgery; ICH, intracerebral hemorrhage; ITT, intention-to-treat; MISTIE II, Minimally Invasive Surgery and r-tPA for ICH Evacuation Phase II; and r-tPA, recombinant tissue-type plasminogen activator.
Bacterial brain infection criteria included cultured organism identification in the presence of fever, relevant laboratory values, and associated clinical symptoms.
Symptomatic bleeding criteria included radiographic evidence of an increase in clot volume (>5 mL increase) associated with a decrease in the GCS motor scale score of >2 points sustained for a minimum of 8 hours or associated clinical symptoms in the opinion of the site investigator.
Asymptomatic brain bleeding was reported and adjudicated to include those events where clot size increase (>5 mL increase) was confirmed by the core laboratory on volumetric measurement of the CT scan by comparison to the most previous CT scan, but where no alteration of GCS was noted.
Symptomatic or asymptomatic bleed is the total of all adjudicated bleeding events.
Power Analysis
We powered the study based on the probability of recurrent bleeding because of surgery and based our estimates on the prior MISTIE pilot studies, which demonstrated a 14.6% bleeding rate with minimally invasive surgery for ICH. To achieve a 90% power to observe at least 1 symptomatic bleed, we estimated a sample size of 15 subjects exposed to active endoscopic for safety reasons.
Intention-to-Treat Efficacy Analysis
The secondary aim (efficacy aim) of the trial was to assess the preliminary efficacy of the ICES procedure at day 180 after hemorrhage onset. The intention-to-treat (ITT) study size was 20 subjects (14 randomized to surgical and 6 to medical management). The 4 run-in, nonrandomized, surgical subjects were training cases and not included in the ITT analyses. We a priori planned to compare the medical control subjects from the main MISTIE study (n=36) with the ICES cohort (n=6).
We estimated the average benefit comparing ICES treatment versus medical control using the ITT principle, that is, using all randomized participants. Specifically, we estimated the difference between the probability of having 180-day mRS ≤3, referred to as a good outcome, under treatment versus control. This average treatment effect was estimated using a simple difference in proportions (the unadjusted estimator). We also created multivariable logistic regression models to adjust for imbalance in the covariates between groups that are predictive of outcome. The variables considered in the multivariate regression analyses, in addition to treatment, were those deemed to be predictive of 180-day mRS in the univariate analyses (P<0.10) and based on clinical considerations.
Results
The trial opened enrollment in August 2009 and closed to enrollment in April 2012 after enrolling the planned 24 subjects (4 pilot run-in subjects and 20 ITT subjects) (Figure II in the online-only Data Supplement). The demographic and baseline characteristics of the randomized subjects are shown in Table III in the onlineonly Data Supplement. There were 17 males and 7 females enrolled, with 18 surgical and 6 medical subjects. An additional 36 medical control subjects from the MISTIE-medical were included in the ITT analyses. The ICES and MISTIE-medical subjects were similar with regards to age, National Institutes of Health Stroke Scale, Glasgow coma score, sex, hemorrhage location, hemorrhage hemisphere, initial hemorrhage volume, and admission systolic blood pressure. The ICES surgical patients had greater prevalence of deep hematomas (85.7%) with marginally more left hemisphere hematomas (57%).
Surgical Findings and Variability
A prospective assessment of surgical details was completed for each surgery using a structured case report form. These results are highlighted in Table IV in the online-only Data Supplement. There was a strong negative correlation between starting ICH volume size and suction pressure necessary to remove the hematoma (r=−0.8) with median suction pressure 150 mm Hg (Figure III in the online-only Data Supplement). The mean duration of surgery was 1.9 hours (interquartile range 1.4–2.6). The duration of irrigation ranged from 30 to 65 minutes. Active bleeding was visible in 35.7% of cases. Control of bleeding required irrigation only in 64.3% of cases, electrocautery in 21.4% of cases, and use of intravenous desmopressin in 42.9% of cases. Surgical trajectory and depth was acceptable in 90% of cases. Overall, surgical compliance with the protocol occurred in 19/20 cases, with a single case of noncompliance keeping the endoscope stationary during the evacuation.
Imaging Analysis
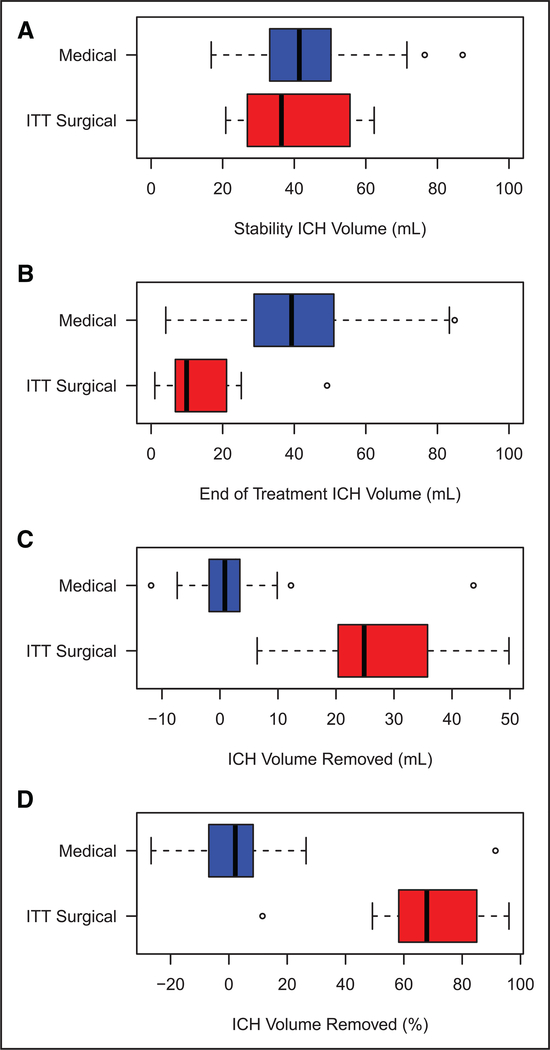
The mean initial hematoma volume and stability hematoma volumes were similar in the ICES surgical and medical groups (Table 2). Surgery resulted in a 71.2% (interquartile range 61–84.7) reduction in ICH (Figure 1). Recurrent ICH occurred in a single case in which a surgical protocol deviation occurred. By 72 hours, the surgical group (16.6±16) had smaller ICH volumes (mean±SD) as compared with the medical group (37.7±17) with a difference (ICES-medical) of −21.1 (95% confidence interval −31.5, −10.73; P=0.0002). A comparison of persistence of ICH volume in the medical group as compared with a reduction of ICH volume in the surgery group at the end of treatment is shown in Figure IV in the online-only Data Supplement. (Post-operative CT and magnetic resonance imaging scans [at 7 days] demonstrated clinically silent tract hematomas in 2 patients, tract edema >5 mL in 2 patients, and no new strokes in the surgical patients.) A single medical-arm patient had a new left middle cerebral artery ischemic stroke on the side opposite from the ICH hemisphere.
Table 2.
Multivariate Analysis
| Variable | Univariate (Unadjusted) | Multivariate Model 1 | Multivariate Model 2 | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| ICES | 2.417 | 0.661, 8.832 | 4.974 | 0.832,29.744 | 1.676 | 0.109, 25.786 |
| Age | 0.942 | 0.890, 0.998 | 0.841 | 0.751, 0.940 | 0.844 | 0.752, 0.949 |
| GCS | 1.560 | 1.165, 2.089 | 2.236 | 1.335, 3.745 | 2.034 | 1.205, 3.435 |
| EOT Vol | 0.942 | 0.903, 0.984 | 0.963 | 0.884, 1.050 | ||
CI indicates confidence interval; CT, computed tomography; EOT, end-of-treatment; GCS, Glasgow coma score; ICES, Intraoperative Stereotactic CT-Guided Endoscopic Surgery; and OR, odds ratio.
Figure 1.
Distribution of hematoma reduction by patient group. The stability volume (A), end of treatment volume (B), volume removed (C), and percent volume removed (D) for the medical and surgical groups are shown. ICH indicates intracerebral hemorrhage; and ITT, intention-to-treat.
ITT Efficacy Analysis
Mortality remained below the prespecified safety thresholds of 10% (procedural) and 30% (30-day), respectively, and there were no surgery-related mortalities. Table 1 demonstrates the mortality rates at each of the prespecified time points. The secondary safety end points of brain infection and surgically related bleeding were all below the prespecified safety thresholds. The serious adverse events were similar between the surgical and medical groups (Table V in the online-only Data Supplement). We performed random effects regression analyses of ICP values over time for the first 6 days post ictus among those patients with ICP monitoring data. A total of 8 medical and 7 ICES surgical patients had ICP monitoring data for these first 6 days. An eighth surgical patient had ICP monitoring data for days 7 to 10 post ictus only. These ICP data were not considered in these analyses because this was the only patient with ICP data beyond 6 days post ictus. In the random effects models, an indicator for group assigned, both linear and nonlinear, quadratic terms in time, and time-by-treatment group interaction terms were considered. Based on the regression analysis, we found no statistical difference in mean ICP values by group. Similarly, no significant time-by-treatment effects were seen in the regression models considered (Figure V in the onlineonly Data Supplement).
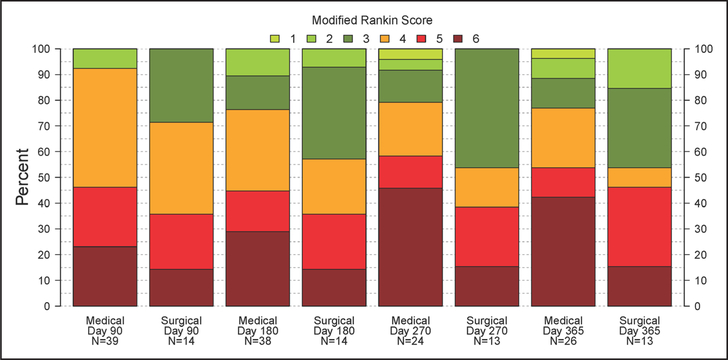
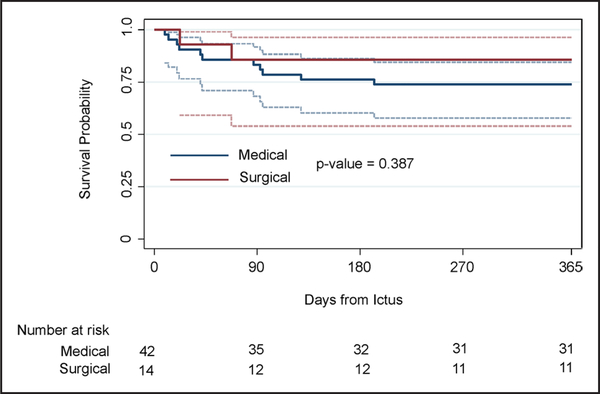
The long-term outcome was assessed by the mRS score at 30, 90, 180, 270, and 365 days. Figure 2 and Table VI in the online-only Data Supplement demonstrate the mRS at the prespecified time points. The patients who underwent endoscopic evacuation had a higher proportion of good outcomes, as defined by mRS score 0 to 3 at 365 days as compared with medical controls (P=0.23). The unadjusted estimate of the absolute benefit (ie, the difference between the probability of having 180-day mRS ≤3 under treatment versus control) is 19.2% (95% confidence interval, −10.1% to 48.4%; Table VI in the online-only Data Supplement). Figure 3 summarizes mortality in the surgical and medical groups.
Figure 2.
Distribution of modified Rankin Scale (mRS) over the 365-day follow-up period. mRS scores determined at 90, 180, 270, and 365 days after symptom onset are shown by treatment group. The mRS score of 0 is not displayed because none of the subjects achieved this score during the follow-up period.
Figure 3.
Kaplan–Meier. Summary of mortality in the surgical and medical groups.
Discussion
The main results of this study were the following: (1) endoscopic surgery can be safely and successfully performed at multiple sites using the SC-guided approach; (2) endoscopic surgery removes more than two thirds of the ICH volume immediately; and (3) patients treated with endoscopic surgery have a 12% greater likelihood of a good functional outcome (mRS 0–3) at 1 year as compared with medically treated patients. These results suggest that endoscopic surgery is safe to perform using the stability protocol and may be therapeutic for patients with ICH.
Overall, the surgical protocol was performed well across all surgeons and at all sites using the SC-guided approach. However, there was an imbalance of experience between centers, with one center performing the majority of the operations. Despite this imbalance, performance metrics were similar between the senior surgeon and other surgeons (Table VII in the online-only Data Supplement). The majority of operations used an optimal trajectory, and in those case, more complete hematoma removal occurred, but in 6/14 cases, the trajectory was not optimal single surgery deviated with respect to lateral rotation of the endoscope to laterally explore the edges of the hematoma. Unfortunately, in that single case, recurrent bleeding into the hematoma cavity occurred presumably because of incomplete hemostasis.
We did observe variation among surgeons with respect to several techniques, including the duration of irrigation and overall duration of the surgery. It is not clear if the variations observed were associated with surgeon preference, associated with intraoperative difficulty with hemostasis, or because of other factors.
Image analysis revealed that over two thirds of the hematoma can be immediately removed with endoscopic surgery. There appeared to be minimal surgically induced damage with only clinically silent tract hematomas or brain edema in the majority of patients. This is an important finding because this suggests that minimally invasive surgery is not causing additional traumatic injury to the brain.
The clinical outcome results suggest that surgery may play a therapeutic role after ICH. Although the present study was not sufficiently powered to be definitive, the results indicate a 23.1% advantage of surgery for a good clinical outcome at 365 days.
Hemorrhage location and size, variability in surgical technique, the amount of hematoma evacuation, and timing of surgery all may influence outcome.17 Hyperacute surgery has been associated with recurrent bleeding.14 In the current study, we took a stability-first approach, which provided time for blood pressure control, surgical planning, supervision of the approach by the surgical center and time to complete the surgery under optimal condition. In aggregate, the data suggest that the cautious, slower approach may be adequate. However, these data beg the question of whether the stability approach can be accelerated with earlier surgery, thereby, limiting the time for secondary injury from the hematoma. The optimum extent of hematoma evacuation is presently unknown. We designed the surgical evacuation to end once 80% of the clot was evacuated. This resulted in 68% of our subjects with an end-of-treatment volume <15 mL. We hypothesize that the extent of evacuation will correlate with clinical outcome and that 15 mL may be an important target. Finally, our data suggest that this type of trajectory-controlled, minimally invasive procedure is generalizable to multiple sites and surgeons. In the absence of randomized, monitored data, we are unsure about the comparability of ICES and MISTIE procedures to other types of procedures using US Food and Drug Administration-approved devices but performed without a standard protocol or the oversight of a surgical center.
New unexpected questions have been generated by our study: (1) What is the optimal duration of surgery, including the duration of irrigation to ensure hemostasis? (2) What is the optimal trajectory to avoid traversing important white matter tracts? (3) What is the true effect size of ICES type surgery, given the range of outcomes noted in this safety-oriented pilot study? (4) What is the effect of hemorrhage location and interruption of white matter tracts on outcome?17–20 These questions may be testable in a phase II B study that tests an ideal endoscopic technique consisting of these elements: a trajectory designed to avoid major white matter connections accessing the middle of the long axis of the hematoma, minimal pressure suction with irrigation of the hematoma bed to clear the hematoma, and minimal exploration of the hematoma bed with the endoscope without lateral rotation of the endoscope sheath. The phase II study should be powered to address location heterogeneity because the effect size is likely dependent on the initial hematoma location and size. When such phase II results are combined with the expected results from MISTIE III, such a study will provide evidence to assess the effectiveness of a mechanically aggressive procedure that removes the hematoma right away (ICES) versus the gentle irrigation approach of MISITE III, which removes the hematoma by gradual thrombolysis. If effects are comparable or better, then the possible benefits of acutely performing the ICES procedure can be tested in the appropriate phase III study.
Caveats and Limitations of This Study
Although MISTIE and ICES sites were required to meet the same eligibility criteria and to follow American Heart Association treatment guidelines, it is possible that there may be differences in patient populations or clinical practice between MISTIE and ICES sites that could potentially introduce bias. Because of the sample sizes involved, statistical comparisons may not be adequately powered to detect some differences in observed patient characteristics that may relate to outcomes, and patients could also differ with respect to unmeasured factors.
Conclusions
Image-guided endoscopic evacuation of ICH seems to be safe and effectively reduces ICH volume. The long-term outcome data suggest the potential of ICES to enhance clinical outcome and possibly our clinical armamentarium for ICH, but our sample size is too small to draw meaningful conclusions.
Supplementary Material
Acknowledgments
We thank the patients and families who volunteered for this study,
Sources of Funding
ICES was part of the overall MISTIE II study, which was supported by grant R01NS046309 awarded to Dr Daniel Hanley from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS). ICES was a component of the UCLA Spotrias Grant awarded to Drs Neil Martin and Paul (NINDS NS 044378).
Footnotes
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00224770.
Disclosures
Dr Martin receives paid consultant to Storz; Patent pending Endoscopic Surgery. The other authors report no conflicts.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA. 116.013837/-/DC1.
References
- 1.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 3.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 4.Gebel JM Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631–2635. [DOI] [PubMed] [Google Scholar]
- 5.Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–882, discussion 882. [DOI] [PubMed] [Google Scholar]
- 6.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 7.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. [DOI] [PubMed] [Google Scholar]
- 8.Vespa P, McArthur D, Miller C, O’Phelan K, Frazee J, Kidwell C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2:274–281. doi: 10.1385/NCC:2:3:274. [DOI] [PubMed] [Google Scholar]
- 9.Miller CM, Vespa PM, McArthur DL, Hirt D, Etchepare M. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduced levels of extracellular cerebral glutamate and unchanged lactate pyruvate ratios. Neurocrit Care. 2007;6:22–29. doi: 10.1385/NCC:6:1:22. [DOI] [PubMed] [Google Scholar]
- 10.Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al. ; MISTIE Investigators. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013;44:627–634. doi: 10.1161/STROKEAHA.111.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. ; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 12.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM; STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vespa PM, Martin N, Zuccarello M, Awad I, Hanley DF. Surgical trials in intracerebral hemorrhage. Stroke. 2013;44(6 suppl 1):S79–S82. doi: 10.1161/STROKEAHA.113.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359–1363. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. ; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK, et al. ; CLEAR and VISTA Investigators. The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 2013;44:635–641. doi: 10.1161/STROKEAHA.112.670653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC; VISTAICH Collaborators. Perihematomal edema and functional outcomes in intracerebral hemorrhage: influence of hematoma volume and location. Stroke. 2015;46:3088–3092. doi: 10.1161/STROKEAHA.115.010054. [DOI] [PubMed] [Google Scholar]
- 18.Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the Minimally-Invasive Surgery Plus rtPA for Intracerebral Hemorrhage Evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147–151. [DOI] [PubMed] [Google Scholar]
- 19.Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. J Neurosurg. 2012;117:767–773. doi: 10.3171/2012.7.JNS111567. [DOI] [PubMed] [Google Scholar]
- 20.Goh SY, Irimia A, Torgerson CM, Tubi MA, Real CR, Hanley DF, et al. Longitudinal quantification and visualization of intracerebral haemorrhage using multimodal magnetic resonance and diffusion tensor imaging. Brain Inj. 2015;29:438–445. doi: 10.3109/02699052.2014.989907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.