Abstract
Background:
Within the dual systems perspective, high reward sensitivity and low punishment sensitivity in conjunction with deficits in cognitive control may contribute to high levels of risk taking, such as substance use.
Objective:
The current study examined whether the individual components of effortful control (inhibitory control, attentional control, and activation control) serve as regulators and moderate the association between reward or punishment sensitivity and substance use behaviors.
Method:
A total of 1,808 emerging adults from a university setting (Mean age = 19.48; 72% female) completed self-report measures of reward and punishment sensitivity, effortful control, and substance use.
Results:
Findings indicated significant two-way interactions for punishment sensitivity and inhibitory control for alcohol and marijuana use. The form of these interactions revealed a significant negative association between punishment sensitivity and alcohol and marijuana use at low levels of inhibitory control. No significant interactions emerged for reward sensitivity or other components of effortful control.
Conclusions:
The current findings provide preliminary evidence suggesting the dual systems theorized to influence risk taking behavior interact to make joint contributions to health risk behaviors such as substance use in emerging adults.
Keywords: dual system, punishment sensitivity, reward sensitivity, effortful control, risk taking, substance use, emerging adulthood
Emerging adulthood (Arnett, 2007) marks a time period from approximately 18 to 25 years in which substance use tends to be problematic. For instance, the prevalence of substance use is higher during emerging adulthood than other developmental periods, with rates of use and misuse peaking at 49% among 19 to 20 year olds (Arnett, 2007; SAMHSA, 2014; Johnston et al., 2009). The consequences of substance use behaviors among emerging adults are also disconcerting; heavy use during this time leads to alcohol use disorders (O’Neill, Parra, & Sher, 2001) and is linked to increased injury and death (Fingerhut & Anderson, 2008). Thus, successful navigation through this developmental period of vulnerability is important for positive long-term adult outcomes (Schulenberg, O’Malley, Bachman, & Johnston, 2004) and prompts the need for more research that will enhance our ability to identify markers of such risky behaviors.
To better understand the emergence and maintenance of risk-taking behavior such as substance use, researchers have developed various forms of a dual systems model to explain the differing developmental trajectories that influence risky decision making (e.g., Carver, Johnson, & Joorman, 2009; Casey, Getz, & Galvan, 2008; Steinberg, 2010). Carver and colleagues’ (2009) dual systems model of self-regulation posits that the nervous system is structured to allow humans to simultaneously process information from experiences via two distinct methods; a reflexive lower order system and a reflective higher order system. The reflexive system is reactive or heuristic in nature and governs approach and avoidance, including reward and punishment sensitivity. In contrast, the reflective system is more strategic or deliberate and enacts cognitive control. From this perspective, increases in reward sensitivity and decreases in punishment sensitivity in conjunction with deficits in cognitive control may contribute to higher levels of risk taking, such as substance use (Carver, Johnson, & Joorman, 2008; Steinberg, 2010).
While previous studies have examined the direct roles of the reflexive and reflective systems in risky behaviors, research has often fallen short in examining how these dual systems may interact to predict risky decision making. The current study sought to examine whether the components of effortful control, a temperamental dimension of self-regulation or cognitive control, serve as regulators, rather than direct predictors, and moderate the association between reward and punishment sensitivity and substance use behaviors in emerging adulthood.
Reward and Punishment Sensitivity
One way the reflexive system or network associated with reward sensitivity and punishment sensitivity has been operationalized relies on Gray’s (1987) reinforcement sensitivity theory, which asserts that there are two statistically independent motivational systems involved in the inhibition or activation of behavior. The first is the behavioral approach system (BAS) that is sensitive to conditioned reward stimuli and responds with appetitive motivation (or approach), and the second is the behavioral inhibition system (BIS) that is sensitive to risk stimuli and responds with aversive motivation (or avoidance) to conditioned cues signaling punishment. From this theory, risk-taking can be viewed as resulting not only from sensitivity to reward but also from an inability to inhibit behaviors that will likely result in punishment (also see Ernst & Fudge, 2009).
Reward sensitivity increases dramatically from early adolescence into emerging adulthood (Urošević et al., 2012) and the rewarding effects of substance use during this developmental period likely plays a role in substance misuse among individuals higher in reward sensitivity (Bijttebier, Beck, Claes, & Vandereycken, 2009). Indeed, self-report measures of BAS are often associated with substance use and misuse in emerging adult populations (e.g., Braddock et al., 2011; Franken & Muris, 2006; O’Connor & Colder, 2009; Wardell, Read, Colder, & Merrill, 2012). Other work in emerging adults has found that BAS is a predictor of lifetime drug and alcohol use disorder diagnoses (Johnson, Turner, & Iwata, 2003).
Punishment sensitivity shows some continuity across childhood into adulthood (Fox, Henderson, Marshall, Nichols, & Ghera, 2005) and in contrast to reward sensitivity, it is believed that punishment sensitivity should be related to less substance use (Bijttebier et al., 2009). Indeed some research has shown that high BIS is related to low alcohol and drug use among emerging adults (Franken & Muris, 2006; Kimbrel, Nelson-Gray, & Mitchell, 2007; Pardo, Aguilar, Molineuvo, & Torrubia, 2007; Wardell et al., 2012), However, other research has failed to find associations between BIS and alcohol use in emerging adults (e.g., Braddock et al., 2011; Johnson et al., 2003; Jorm et al., 1998). Overall, evidence supporting direct associations between reward sensitivity and substance use is consistent, while associations between punishment sensitivity and substance use outcomes are less clear. However, evaluating the interaction between these two networks (reward and punishment) and a reflective higher order system (e.g., effortful control) may provide crucial insight into understanding individual differences in substance use behaviors.
Effortful Control
The reflective system, or network associated with the cognitive control system, is a regulatory system that includes subcomponent processes of self-regulation and self-control (Baumeister & Vohs, 2003) and is characterized by deliberate top down processes (Carver et al., 2009). In the developmental subdiscipline, self-regulation is studied within a temperament framework that includes effortful control (Rothbart, Derryberry, & Posner, 1994). A multidimensional construct, effortful control refers to an individual’s ability to inhibit dominant responses in order to perform a less pronounced response that may provide a better outcome in the long-term as well as detect errors and engage in planning behavior (Rothbart et al., 1994). Effortful control is comprised of three subcomponents: inhibitory control, suppression of undesirable behavior; attentional control, the ability to shift and focus attention; and activation control, the ability to perform an action despite having a strong urge to avoid it (Rothbart et al., 2000). Thus, effortful control is dependent on executive functions (Carver et al., 2008) and the conceptualization of the dual systems model centers around effortful control as a unique temperament system that works alongside and interacts with the basic temperament systems of approach (BAS) and avoidance (BIS) during reward or punishment cues (e.g., Rothbart, Ahadi, & Evans, 2000; Nigg, 2006). Thus, processing occurs from both a reflexive lower order system that is reactive in nature (e.g., reward and punishment sensitivity) and a reflective or regulatory higher order system that is more strategic or deliberate (e.g., effortful control). Within these dynamic systems, effortful control regulates the operation of the reflexive system in the service of goal-directed behavior. Therefore, risk-taking behaviors such as substance use can be viewed as resulting not only from an inability to inhibit behaviors that pursue reward seeking but also from an inability to inhibit behaviors that will likely result in punishment.
Most of the existing research examining the association between effortful control and substance use has examined only direct associations primarily based on self-report measures, and findings are mixed in emerging adult populations with some studies reporting modest associations (r = .12 to .25; Ellingson, Richmond-Rakerd, & Slutske, 2015; Wong & Rowland, 2013) and others reporting no association (r = −.04; Meehan et al., 2013). Despite the equivocal evidence supporting a direct association between effortful control components and substance use outcomes in emerging adult populations, the moderating effects of effortful control have not been extensively studied. To the authors’ knowledge, there are only a few studies that examined the moderating role of effortful control in the link between reward and punishment sensitivity and substance use, and they are limited in that most only focused on the inhibitory control component (based on behavioral task performance) using small samples (i.e., N ≤ 72). For example, in a sample of female college students, Patrick and colleagues (2008) reported a non-significant trend (p < .10) for an interaction effect revealing higher levels of alcohol use may be associated with high approach sensitivity and poor inhibitory control (Patrick, Blair, & Maggs, 2008). In another study of college students, Jonker and colleagues found a significant interaction between executive control and punishment sensitivity, suggesting that low punishment sensitivity was significantly related to high alcohol use among those with weak attentional control but not among those with strong attentional control (Jonker, Ostafin, Glashouwer, van Hemel-Ruiter, & de Jong, 2014). The interaction between these effortful control components and reward and punishment sensitivity explained about 5 to 8% of the variance in substance use outcomes.
Finally, a recent neuroimaging study (Kim-Spoon et al., in press) indicated that neural activation during cognitive control moderated the link between neural activation during risk processing (conceptually related to punishment sensitivity) and late adolescents’ health risk behaviors. Specifically, the significant interaction between cognitive control and risk processing suggested that hemodynamic activity in the anterior insula related to risk avoidance predicted health risk behaviors (i.e., higher severity and earlier onset of substance use and risky sexual behaviors) among late adolescents exhibiting greater dorsal anterior cingulate cortex activity during a cognitive interference task (explaining 41% variance), but not among late adolescents exhibiting less dorsal anterior cingulate cortex activity (explaining 1% variance). Taken together, research thus far indicates equivocal evidence for the direct association between effortful control and substance use in emerging adult populations. In contrast, there is reason to believe the moderating role of effortful control on the link between reward and punishment sensitivity and substance use is a promising line of research.
Most research examining the role of effortful control in emerging adult populations (e.g., Meehan, De Panfilis, Cain, & Clarkin, 2013) used a composite measure of effortful control. However, it may be useful to examine the independent elements of effortful control in order to determine if they function differentially as moderators of reward and punishment sensitivity. For instance, research on the unity/diversity framework of executive functioning (a construct closely related to effortful control) demonstrates that inhibition correlates virtually perfectly with ‘unity’ across the three domains (i.e., inhibition, shifting, and updating) of executive functioning (Miyake & Friedman, 2012) and is the primary source of predictive power in explaining individual differences in behavioral disinhibition (Young et al., 2009). Thus, one of the main goals of this study was to test for specificity and functionality of the three components of effortful control (inhibitory, attentional, and activation control) in the prediction of substance use outcomes.
Current Study
In the current study, we sought to expand existing research by examining whether effortful control moderates the association between reward and punishment sensitivity and substance use behaviors in a large sample of emerging adults, focusing on two of the most commonly used substances among college students: alcohol and marijuana (O’Malley & Johnston, 2002). Based on past research, we hypothesized that low levels of BIS and high levels of BAS would be associated with higher levels of alcohol and marijuana use. Our main hypothesis was that the association between reward (BAS) and punishment (BIS) sensitivity and substance use behaviors would be moderated by level of effortful control, such that the associations between BIS/BAS and substance use behaviors would be significantly stronger for individuals with lower effortful control compared to those with higher effortful control. We examined the moderating roles of the three distinct components of effortful control (i.e., inhibitory, attentional, and activation control) to test the differential functionality of these components for predicting substance use in an emerging adult population.
Method
Participants
Participants (n = 1,903) were recruited from a university in Southwestern Virginia to participate in an online survey in exchange for extra course credit. As recommended by Meade and Craig (2012), participants who responded incorrectly on two attention check items were eliminated from further analyses (n = 44, 2.4%). An additional n = 40 individuals only partially completed the survey and thus were eliminated from further analyses. Finally, we restricted our sample to include only emerging adults, thus eliminating an additional n = 11 (0.6%) participants. Thus, the final sample included 1,808 emerging adults (72% female, coded 0 = male and 1 = female) ranging in age from 18 to 25 years (M = 19.48, SD = 1.30). The primary race category was Caucasian (78.3%) with the remaining sample identifying as Asian or Asian-American (10.1%), African-American (3.3%), Hispanic or Latino (3.3%), Biracial or Multiracial (2.4%), and Other (2.3%). For the purposes of analyses, race was dichotomized into two groups: Caucasian = 1 vs. All other = 0. Participants provided information on their perception of family economic status by indicating how well-off they believed their family to be on a five-point Likert-type scale ranging from 1 (very poor) to 5 (upper middle class) with M = 4.20 (SD = 0.71).
Measures
Behavioral Inhibition/Behavioral Activation System Scale (BIS/BAS; Carver & White, 1994).
The BIS/BAS scale consists of 20 items rated on a 4-point Likert-type scale ranging from 1 (Very true for me) to 4 (Very false for me). The BIS scale consists of seven items and includes statements such as “I worry about making mistakes”. The BAS portion contains three subscales (drive, fun-seeking, and reward responsiveness), is composed of 13 items, and consists of statements such as “I often act on the spur of the moment” (fun seeking), “I go out of my way to get things I want” (drive), and “When I see an opportunity for something I like I get excited right away” (reward responsiveness). In the current sample the internal consistency was α = .76 and α = .81 for the BIS and BAS composite respectively.
Adult Temperament Questionnaire (ATQ; Evans & Rothbart, 2007).
A shortened 62-item version of the ATQ was used and items are rated on a 7-point Likert-type scale ranging from 1 (Extremely untrue of you) to 7 (Extremely true of you). The current analyses utilized the effortful control factor of the ATQ that contains three subscales (inhibitory control, attentional control, and activation control), is composed of 19 items, and consists of statements such as “I can easily resist talking out of turn, even when I’m excited and want to express an idea” (inhibitory control), “It’s often hard for me to alternate between two different tasks” (attentional control), and “I can keep performing a task even when I would rather not do it” (activation control). For the current sample, the internal consistency was α = .551, α = .72, α = .72, for inhibitory, attentional, and activation control subscales, respectively.
Substance Use Behaviors.
To assess substance use behaviors, participants completed two items assessing typical frequency ranging from 1 (Never) to 6 (Usually use every day), use during the last 30 days ranging from 1 (0 days) to 7 (all 30 days), and age of initiation ranging from 1 (never) to 7 (8 years or younger) of alcohol and marijuana use (CDC, 2012; Wills, Yaeger, & Sandy, 2003). Scores were standardized and averaged within type of substance to produce an alcohol use composite (r = .44 to .77; z-score range of −2.10 – 3.07; α = .81) and a marijuana use composite (r = .51 to .81; z-score range of −.77 to 3.85; α = .87).
Data Analytic Plan
Data analysis was conducted in SPSS Version 22. For all study variables, descriptive analyses were conducted to determine normality of distributions and univariate and multivariate outliers. Skewness and kurtosis were examined for all variable distributions with acceptable levels of skewness to be less than 3 and acceptable levels of kurtosis less than 10 (Kline, 2011). In addition, general linear modeling (GLM) was used to identify any multivariate predictors of the endogenous variables among the demographic variables and any variables with significant Wilks’ Lambda coefficients were used as covariates. Multiple regression analyses were conducted to test for interaction effects between BIS, BAS, and effortful control as well as main effects of individual variables on the outcome measures of substance use, including alcohol and marijuana use. All predictor variables were mean-centered prior to analyses and product terms were computed using these centered scores. All variables were entered into a single multiple regression model. Significant interactions were explored using computational tools recommended by Preacher and colleagues (2006) to test the significance of the simple slopes of the dependent variable at both high (1 SD above the mean) and low (1 SD below the mean) values of the predictors (Preacher, Curran, & Bauer, 2006).
To control for the false positive rate in multiple comparisons we used the Benjamini-Hochberg method (Benjamini & Hochberg, 1995) for all multiple regression models, which is a modification of the Bonferroni procedure. The Benjamini-Hochberg method is a sequentially rejective approach based on false discovery rate (the proportion of erroneous rejections to the total number of rejections) and does not compromise statistical power as severely as the Bonferonni method. To aid in the interpretation of a moderating effect when an interaction was significant, the Johnson-Neyman technique (Johnson & Fay, 1950) was employed in order to identify higher and lower regions of significance (ROS) through use of computational tools recommended by Preacher and colleagues (Preacher et al., 2006). These regions of significance identified the upper and lower values of the moderating variable (effortful control) where BIS and BAS were significantly associated with the outcome variable of interest and thus identified the exact range(s) of the moderator variable where simple slopes differ significantly from zero.
Results
Descriptive statistics are presented in Table 1 and correlations between all main study variables are presented in Table 2. As predicted, BAS was positively associated with use of both alcohol and marijuana, and BIS was negatively correlated with use of alcohol and marijuana. Regarding the use of substances in this population, severity of alcohol use was higher than that for marijuana. For example, the average for alcohol use in the last 30 days was consistent with using 3 to 5 days per month. For marijuana the average for the last 30 days corresponded to using 1 to 2 days per month. Similarly, the mean age of initiation for alcohol was approximately 15–16 years old, while the mean age of initiation for marijuana was 17 years or older. Two cases were identified as multivariate outliers due to significant Mahalanobis Distances (p < .001); however, the pattern of results did not change when running the analyses with and without the identified cases. Therefore, these outliers were not excluded. Multivariate general linear modeling analysis with potential demographic covariates predicting substance use behaviors revealed significant effects for age, sex, race, and family economic status (all p < .001), indicating that older, male, White, and higher family economic status were related to higher levels of substance use. Thus, these variables were included as covariates in all further analyses. Multiple regression analyses included age, gender, race, and family economic status as covariates, main effects of BIS, BAS, and individual measures of effortful control (inhibitory, attentional, and activation control), and two-way interaction terms between BIS/BAS and ATQ dimensions and between BIS and BAS.
Table 1.
Descriptive statistics for effortful control, substance use, and demographic variables
| M / % | SD / N | Range | |
|---|---|---|---|
| Age | 19.48 | 1.30 | 18 – 25 |
| Sex | 71.6% | 1295 | 0 – 1 |
| Race | 78.3% | 1416 | 0 – 1 |
| FES | 4.20 | .71 | 1.00 – 5.00 |
| BIS | 3.12 | .48 | 1.29 – 4.00 |
| BAS | 3.11 | .39 | 1.73 – 4.00 |
| ATQ-IC | 4.21 | .86 | 1.00 – 6.57 |
| ATQ-AT | 3.83 | 1.03 | 1.00 – 7.00 |
| ATQ-AC | 4.72 | .95 | 1.29 – 7.00 |
| Alcohol frequency | 3.77 | 1.19 | 1.00 – 6.00 |
| Alcohol initiation | 2.55 | .97 | 1.00 – 7.00 |
| Alcohol 30 day use | 2.95 | 1.38 | 1.00 – 7.00 |
| Marijuana frequency | 2.09 | 1.36 | 1.00 – 6.00 |
| Marijuana initiation | 1.75 | .92 | 1.00 – 7.00 |
| Marijuana 30 day use | 1.49 | 1.17 | 1.00 – 7.00 |
Note. FES = family economic status; BIS = Behavioral inhibition scale; BAS = Behavioral activation scale composite; ATQ = adult temperament questionnaire; ATQ-IC = inhibitory control; ATQ-AT = attentional control; ATQ-AC = activation control. Sex is coded male = 0 and female = 1. Race is coded Caucasian = 1 and other = 0.
Table 2.
Correlations between effortful control, substance use, and demographic variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||
| 2. Sex | .02 | |||||||||
| 3. Race | −.02 | .04 | ||||||||
| 4. FES | −.08 | −.04 | .12 | |||||||
| 5. BIS | −.04 | .36 | .00 | −.02 | ||||||
| 6. BAS | −.04 | .00 | .00 | .09 | .00 | |||||
| 7. ATQ-IC | .08 | −.16 | .00 | −.04 | −.18 | −.28 | ||||
| 8. ATQ-AT | .02 | −.08 | .06 | .02 | −.34 | −.08 | .46 | |||
| 9. ATQ-AC | −.04 | .01 | .06 | .03 | −.12 | .01 | .36 | .52 | ||
| 10. Alcohol Use | .16 | −.02 | .18 | .15 | −.07 | .19 | −.13 | −.06 | −.12 | |
| 11. Marijuana Use | .06 | −.15 | .06 | .05 | −.07 | .14 | −.08 | −.07 | −.16 | .51 |
Note. Correlations in bold significant at p < .05. FES = family economic status; BIS = Behavioral inhibition scale; BAS = Behavioral activation scale; ATQ = adult temperament questionnaire; ATQ-IC = inhibitory control; ATQ-AT = attentional control; ATQ-AC = activation control. Sex is coded male = 0 and female = 1. Race is coded Caucasian = 1 and other = 0.
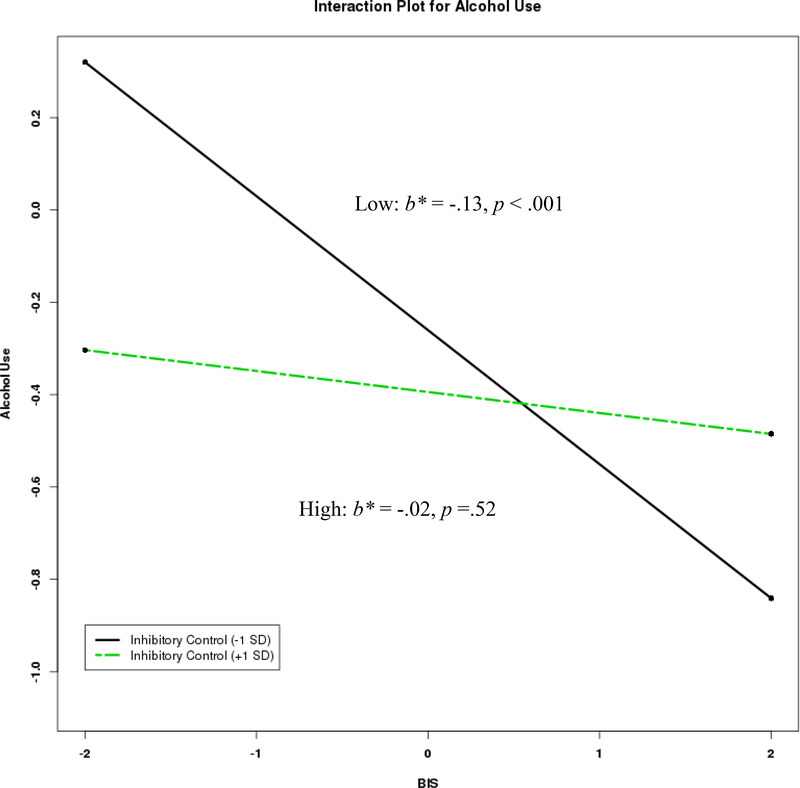
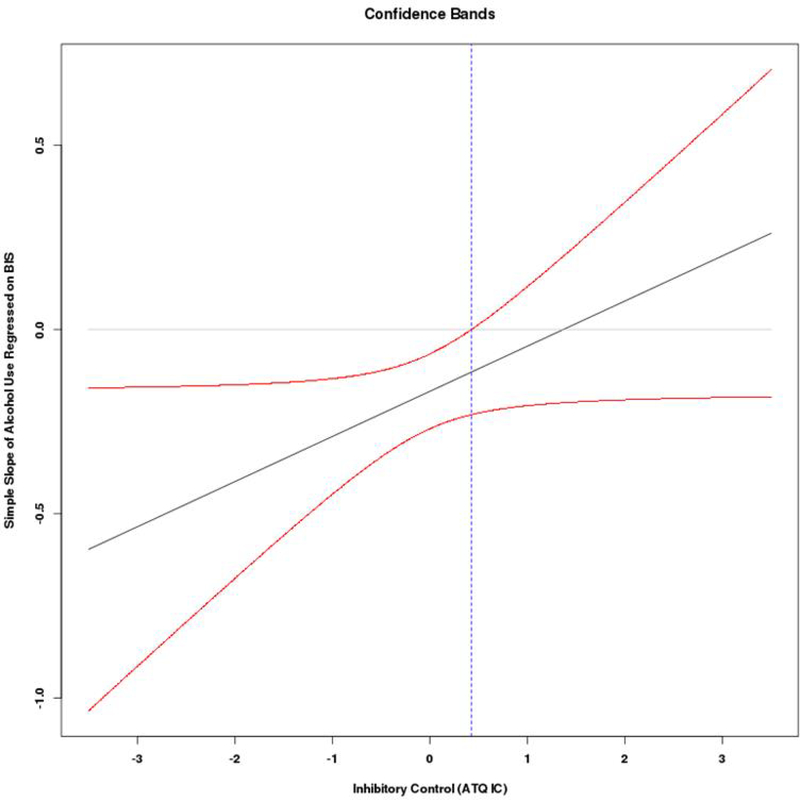
Coefficients from the final models are presented in Table 3. For alcohol use, there were significant main effects for BIS, BAS, inhibitory control, and activation control as well as a significant interaction that emerged between BIS and inhibitory control. As expected, higher BAS and lower activation control were related to higher alcohol use. The form of the significant interaction between BIS and inhibitory control (Figure 1a) revealed that lower BIS scores were associated with greater alcohol use among those with lower levels of inhibitory control, b = −.26, SE = .07, p < .001, 95% CI [−.406, −.117]. There was no significant association between BIS and alcohol use at high levels of inhibitory control, b = −.05, SE = .08, p = .52, 95% CI [−.198, .100]. Regions of significance calculations (Figure 1b) indicated the region of significance extended from 0.43 and below, indicating any simple slope at or below this value is statistically significant, and this included 63% of the current sample. However, the significant interaction effect for alcohol did not hold after controlling for multiple comparisons, thus these interaction results should be interpreted with caution. The other interactions involving attentional or activation control and the interaction between BIS and BAS were not significant.
Table 3.
Multiple regression analyses of BIS, BAS, and effortful control predicting alcohol and marijuana use
| Variables | Alcohol Use | Marijuana Use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b [95%CI] | SE | b* | p | R2 | b [95% CI] | SE | b* | p | R2 | |||
| Age | .21 [.159, .257] | .03 | .18* | .000 | .08 [.025, .127] | .03 | .07* | .004 | ||||
| Sex | −.03 [−.129, .079] | .05 | −.01 | .740 | −.31 [−.421, −.206] | .06 | −.14* | .000 | ||||
| Race | .45 [.340, .551] | .05 | .18* | .000 | .18 [.072, .291] | .06 | .07* | .001 | ||||
| FES | .13 [.085, .172] | .02 | .13* | .000 | .08 | .03 [−.013, .078] | .02 | .03 | .158 | .03 | ||
| BIS | −.16 [−.257, −.054] | .05 | −.08* | .000 | −.07 [−.178, .032] | .05 | −.04 | .175 | ||||
| BAS | .15 [.111, .189] | .02 | .17* | .000 | .12 [.077, .158] | .02 | .14* | .000 | ||||
| ATQ-IC | −.07 [−.131, −.009] | .03 | −.06 | .026 | −.03 [−.091, .036] | .03 | −.02 | .397 | ||||
| ATQ-AT | .00 [−.055, .054] | .03 | .00 | .995 | .01 [−.042, .071] | .03 | .02 | .619 | ||||
| ATQ-AC | −.13 [−.180, −.070] | .03 | −.12* | .000 | .14 | −.18 [−.234, −.121] | .03 | −.17* | .000 | .07 | ||
| BIS X BAS | .04 [−.039, .123] | .04 | .03 | .308 | .00 [−.085, .082] | .04 | .00 | .971 | ||||
| BIS X ATQ-IC | .12 [.001, .247] | .06 | .05 | .048 | .17 [.043, .298] | .07 | .07* | .009 | ||||
| BIS X ATQ-AT | −.06 [−.156, .047] | .05 | −.03 | .291 | −.05 [−.155, .055] | .05 | −.03 | .350 | ||||
| BIS X ATQ-AC | .03 [−.080, .135] | .06 | .01 | .617 | .05 [−.063, .055] | .06 | .02 | .396 | ||||
| BAS X ATQ-IC | .01 [−.041, .053] | .02 | .01 | .809 | −.01 [−.063, .035] | .03 | −.02 | .568 | ||||
| BAS X ATQ-AT | −.01 [−.055, .034] | .02 | −.01 | .640 | −.03 [−.072, .020] | .02 | −.03 | .272 | ||||
| BAS X ATQ-AC | .01 [−.036, .056] | .02 | .01 | .671 | .15 | .03 [−.014, .081] | .02 | .04 | .166 | .08 | ||
Note. Coefficients in bold significant at p < .05; * p < .05 after controlling for false discovery rate; b* = standardized regression coefficient; b = unstandardized coefficient; CI = confidence interval; FES = family economic status; BIS = Behavioral inhibition scale; BAS = Behavioral activation scale; ATQ = adult temperament questionnaire; ATQ-IC = inhibitory control; ATQ-AT = attentional control; ATQ-AC = activation control. Sex is coded male = 0 and female = 1. Race is coded Caucasian = 1 and other = 0.
Figure 1a.
Interaction between behavioral inhibition and inhibitory control in the prediction of alcohol use with standardized estimates.
Figure 1b.
Regions of significance and confidence bands for interaction between behavioral inhibition and inhibitory control predicting alcohol use. The region of significance extends from 0.43 and below, indicating any simple slope at or below this value is statistically significant.
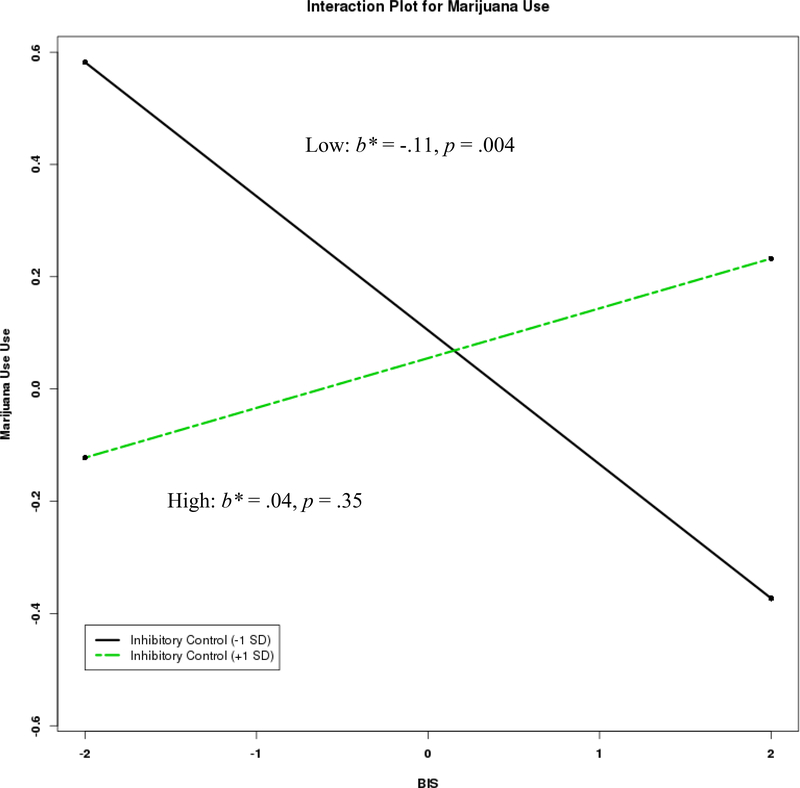
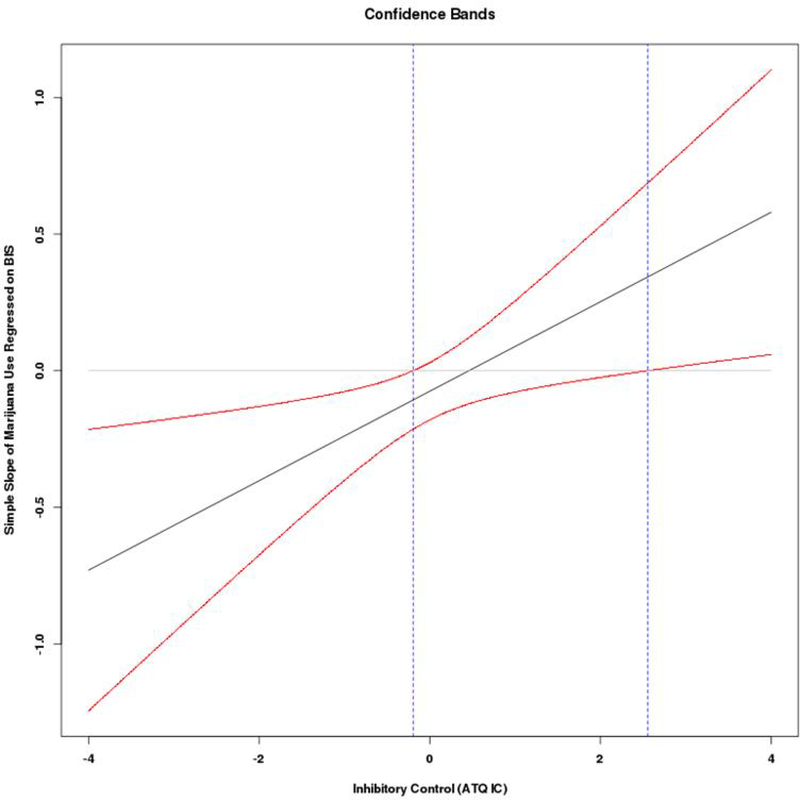
For marijuana use, results were similar; with significant main effects of BAS and activation control and a significant interaction that emerged between BIS and inhibitory control. Higher BAS and lower activation control were related to higher marijuana use. The form of the significant interaction between BIS and inhibitory control (Figure 2a) revealed lower BIS scores were associated with marijuana use among those with lower inhibitory control, b = −.22, SE = .08, p = .004, 95% CI [−.369, −.069]. There was no significant association between BIS and marijuana use at high levels of inhibitory control, b = .07, SE = .08, p = .35, 95% CI [−.081, .227]. Regions of significance calculations (Figure 2b) on the moderator (inhibitory control) produced a lower and upper region of significance of −0.20 and 2.56 respectively, indicating that any simple slope outside this range was statistically significant, which included 52% of the current sample. Considering the range of inhibitory control for the current sample was −3.23 to 2.35, the effect of BIS on marijuana use is significant for only the lower bound values of inhibitory control (−0.20 and below). No other interactions were significant.
Figure 2a.
Interaction between behavioral inhibition and inhibitory control in the prediction of marijuana use with standardized estimates.
Figure 2b.
Regions of significance and confidence bands for interaction between behavioral inhibition and inhibitory control predicting marijuana use. The lower and upper regions of significance are −0.20 and 2.56 respectively, indicating that any simple slope outside this range was statistically significant. Considering the range of inhibitory control for the current sample was −3.23 to 2.35, the effect of BIS on marijuana use is significant for only the lower bound values of inhibitory control (−0.20 and below).
Discussion
The current study investigated whether components of effortful control serve unique roles in moderating the link between reward and punishment sensitivity and substance use behaviors. In this sample of emerging adults, inhibitory control was the only component of effortful control that emerged as a significant moderator. Specifically, we observed significant, yet modest, interactions that revealed in emerging adults with low levels of inhibitory control, low levels of punishment sensitivity (as measured by BIS) are associated with higher alcohol and marijuana use. We note that after controlling for multiple comparisons, the interaction for alcohol use was no longer significant; thus inferences made from alcohol results need to be interpreted with caution and replication of the finding is warranted. In contrast, punishment sensitivity is not associated with alcohol or marijuana use for emerging adults with higher levels of inhibitory control. These findings suggest that inhibitory control acts as a regulator such that when inhibitory control is high, emerging adults are able to regulate punishment sensitivity and avoid substance use. In contrast, when inhibitory control is low, emerging adults have difficulty regulating punishment sensitivity, which increases the likelihood that they will engage in substance use.
Results from the current study support the role of BIS interacting with inhibitory control to predict emerging adults’ substance use outcomes (particularly alcohol and marijuana). Reinforcement sensitivity theory (Gray, 1987) as well as the triadic model of the neurobiology of motivated behavior (Ernst & Fudge, 2009) would suggest that both BIS and BAS are important in the initiation and maintenance of risk-taking behaviors. Although previous research on the direct association between BAS and risk-taking behavior has been relatively consistent (e.g., Braddock et al., 2011; Wardell et al., 2012), the connection between BIS and risk-taking behavior has been less clear (e.g., Braddock et al., 2011; Pardo et al., 2007). Our results help to elucidate the interactive role of BIS with inhibitory control and the resulting contribution to substance use behaviors.
While our findings confirm the direct association between BAS and substance use behavior, the lack of interactive effects for BAS in the current and recent research (e.g., Jonker et al., 2014) warrants closer consideration of the competing nature of the BIS and BAS systems and how they interact with the regulatory system (i.e., effortful control; Carver et al., 2008), at least with regard to substance use outcomes. The BIS and BAS motivational systems compete and one system can override or dominate the other and potentially guide behavior, especially when the regulatory system or effortful control processes are not adequate (Carver et al., 2008). Coupled with this competition, positive affective states like happiness or hopefulness are tied to BAS (approach system), whereas negative emotions like fear, anxiety, or sadness are tied to the BIS (avoidance system; Gray, 1987). It may be that the BIS system, responsible for negative affect, draws upon the regulatory system or effortful control processes more often than the BAS. For example, it has been theorized that negative affective states may increase regulatory processes in an effort to repair or improve upon these negative feelings (Diamond & Aspinwall, 2003). At the same time, positive affective states may lead an individual to underestimate or ignore consequences or negative outcomes, thus leading to less effective self-regulation (Aspinwall, 1998). Essentially, negative mood or emotions may result in more extensive information processing, cueing properties of self-regulation, compared to positive moods or emotions (e.g., Schwarz & Clore, 1996). Our findings suggest that operation of the regulatory system across different affective states may be an important area to explore in future research.
One of the unique contributions of our results is to demonstrate specificity for the moderating effects by inhibitory control, rather than attentional or activation control. The findings support the theoretical perspective suggesting inhibitory control as the core aspect of executive control (Zhou, Chen, & Main, 2012) and parallel the prior research indicating inhibitory control as the strongest predictor of disinhibitory psychopathology, compared to other dimensions of executive functions (Young et al., 2009). Our findings are also in line with work by Jonker and colleagues (2014) who found executive control network scores moderated the negative association between self-reported punishment sensitivity and alcohol use. Therefore, despite differences in methodology between the study by Jonker and colleagues (2014) and the current study, results collectively highlight the importance of exploring the unique effects of inhibitory control as regulators between punishment sensitivity and substance use, particularly within an emerging adult population. We also note that, consistent with mixed findings from past research (e.g., Ellingson et al., 2015; Meehan et al., 2013; Wong & Rowland, 2013), the main effects of inhibitory control were rather small and inconsistent in predicting alcohol and marijuana use. Instead, we found that activation control showed consistent main effects on alcohol and marijuana use.
Our results fill the gap in the literature by providing evidence for the main effects of activation control, the ability to persist and complete tasks even if undesired, as a negative predictor of alcohol and marijuana use. Little research has explored the relationship between activation control, specifically, and maladaptive outcomes or health risk behaviors. However, activation control may be adaptive particularly when an individual experiences stress or negative emotions, as it would require one to force themselves into action in order to resolve situations (Clements & Bailey, 2010), reducing the likelihood to use drugs to cope. Indeed, small-to-moderate sized correlations (r = −.20 to −.36) have been reported for psychopathological outcomes including eating disorder symptoms (Claes, Bijttebier, Mitchell, de Zwaan, & Mueller, 2011) and anxiety levels (Clements & Bailey, 2010) among college students. In the current study, activation control was consistently and negatively related to alcohol and marijuana use as shown in Table 3 even after taking into account the main effects of the other two effortful control components. Thus, our findings suggest activation control may play an important role in predicting substance use in emerging adult populations. Future research would benefit from continuing to explore the dynamics behind the association between activation control and substance use within this population.
Certain limitations qualify the findings in the present study. First, the cross-sectional design and analyses used in the current study do not allow us to infer causality. For example, although the hypotheses we present frame components of effortful control as preceding the use of substances, it is also possible that regular substance use can influence an individual’s effortful control. Thus, future work using longitudinal designs is warranted. Second, we relied on self-report measures in this study, which increased the possibility of shared method variance inflating associations between the study variables. Related to the use of self-report, the frequency of alcohol use in the last thirty days (the mean/median falling in the category of 3–5 days per month) was slightly below compared to the average that reported in a national survey by Substance Abuse and Mental Health Services Administration (SAMHSA; Lipardi & Jean-Francois, 2016), possibly indicating underreporting. However, there were similar numbers reported 3–5 days/month (24%) versus 6–9 days/month (23%) in our sample. In addition, according to national survey results from the Monitoring the Future (MTF) project (Johnston, O’Malley, Bachman, Schulenberg, & Miech, 2015), 35% of college students in the MTF project engaged in heavy drinking (defined by five or more drinks in a row) in the past two weeks versus 58% in our sample reported heavy drinking in the past month. Therefore, although the number of days of alcohol use seemed to be slightly lower in our sample compared to the SAMHSA survey, college students in our sample may have been engaging more in heavy drinking compared to those in the MTF survey. Third, the effect sizes for the significant interactions between inhibitory control and substance use were relatively small in size. However, our results were consistent with recent work examining interaction effects that relied on neuroimaging or laboratory measures (Jonker et al., 2014; Kim-Spoon et al., in press), confirming the robustness of our findings. Fourth, reliability estimates were relatively low for the inhibitory control subscale. However, low reliability has a downward bias on estimating effects (Furr & Bacharach, 2008). Thus, effects found in the current study could have been stronger, not weaker, if the inhibitory control score had demonstrated higher reliability. Finally, we relied upon a convenience sample of individuals drawn from a university setting of emerging adults where the majority was female and Caucasian. Although we controlled for potential gender and racial effects in our analyses, we acknowledge limitations in the generalizability of our findings and need for replications using different age groups and more ethnically diverse samples.
Despite these limitations, findings from this study enhance our understanding of the dual systems model of risk-taking among emerging adults. Although much of the existing research on the dual systems model has focused on adolescents, existing literature suggests that brain regions that underlie these systems are still undergoing transformation during emerging adulthood (Steinberg, 2010). For instance, the cognitive control network includes regions of the prefrontal cortex that are undergoing maturation, including increased myelination and experience-dependent synaptogenesis and pruning, throughout adolescence and into emerging adulthood (Paus, 2005). Within this network, inhibitory control may be the most prominent component of effortful control that modulates the motivation system (avoidance, in particular) in predicting substance use outcomes during emerging adulthood. Given that this age group has the highest rates of substance use compared to other age groups (SAMHSA, 2014), it is important to explore how the dual systems models apply to emerging adults. Longitudinal research spanning adolescence through emerging adulthood would help provide critical information about how the networks involving reward and punishment sensitivity and cognitive control change over time and potentially isolate crucial periods of development to inform targets for intervention or prevention programs.
Acknowledgment:
We thank Julee Farley, Andre Plate, and Jeanette Walters for their help with data collection. We are grateful to those who participated in our study.
Preparation of this manuscript was supported by grants from the National Institutes of Health (DA036017 to JK and BK; MH091872 to PC) and from Virginia Tech Institute for Society, Culture, and Environment (ISCE).
Footnotes
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
A confirmatory factor analysis was conducted on the three subscale latent factors of the ATQ [χ2 (143) = 795.86, p < .001, CFI = .90, RMSEA = .05] and found that all of the factor loadings were significant at p < .001. Seven correlations between residual variances were freed to be estimated using modification indices in order to achieve acceptable CFI. Based on these results and for the purpose of keeping the current result comparable to previous research, we used the subscale as derived in the original validation of the ATQ (Evans & Rothbart, 2007). The alpha coefficient for the ATQ IC in the current sample is in line with results from the original validation study (α = .60) and numerous other studies reporting alpha levels ranging from .33 to .56 (e.g., Gomez, Kyriakides, & Devlin 2014; Willem, Vasey, Beckers, Claes, & Bijttebier 2013).
References
- Arnett JJ (2007). Emerging adulthood: What is it, and what is it good for? Child Development Perspectives, 1, 68–73. doi: 10.1111/j.1750-8606.2007.00016.x [DOI] [Google Scholar]
- Aspinwall LG (1998). Rethinking the role of positive affect in self-regulation. Motivation and Emotion, 22, 1–32. doi: 10.1023/A:1023080224401 [DOI] [Google Scholar]
- Baumeister RF, & Vohs KD (2003). Self-regulation and the executive function of the self In Leary MR & Tangney JP (Eds.), Handbook of self and identity (pp. 197–217). New York, NY: Guilford Press. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society - Series B (Methodological), 57, 289–300. Retrieved from: http://www.jstor.org/stable/2346101. [Google Scholar]
- Bijttebier P, Beck I, Claes L, & Vandereycken W (2009). Gray’s Reinforcement Sensitivity Theory as a framework for research on personality–psychopathology associations. Clinical Psychology Review, 29, 421–430. doi: 10.1016/j.cpr.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Braddock KH, Dillard JP, Voigt DC, Stephenson MT, Sopory P, & Anderson JW (2011). Impulsivity partially mediates the relationship between BIS/BAS and risky health behaviors. Journal of Personality, 79, 793–810. doi: 10.1111/j.1467-6494.2011.00699.x [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, & Joormann J (2008). Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin, 134, 912–943. doi: 10.1037/a0013740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, & Joormann J (2009). Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Current Directions in Psychological Science, 18, 195–199. doi: 10.1111/j.1467-8721.2009.01635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. doi: 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review, 28, 62–77. doi: 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2012). Youth Risk Behavior Survey. Retrieved from: http://www.cdc.gov/Features/YRBS/
- Claes L, Bijttebier P, Mitchell JE, de Zwaan M, & Müller A (2011). The relationship between compulsive buying, eating disorder symptoms, and temperament in a sample of female students. Comprehensive Psychiatry, 52, 50–55. doi: 10.1016/j.comppsych.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Clements AD, & Bailey BA (2010). The relationship between temperament and anxiety phase I in the development of a risk screening model to predict stress-related health problems. Journal of Health Psychology, 15, 515–525. doi: 10.1177/1359105309355340 [DOI] [PubMed] [Google Scholar]
- Diamond LM, & Aspinwall LG (2003). Emotion regulation across the life span: An integrative perspective emphasizing self-regulation, positive affect, and dyadic processes. Motivation and Emotion, 27, 125–156. doi: 10.1023/A:1024521920068 [DOI] [Google Scholar]
- Ellingson JM, Richmond-Rakerd LS, & Slutske WS (2015). Brief report: Cognitive control helps explain comorbidity between alcohol use disorder and internalizing disorders. Journal of Studies on Alcohol and Drugs, 76, 89–94. [PMC free article] [PubMed] [Google Scholar]
- Ernst M & Fudge JL (2009). A developmental neurobiological model of motivated behavior: Anatomy, connectivity, and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews, 33, 367–382. doi: 10.1016/j.neubiorev.2008.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, & Rothbart MK (2007). Developing a model for adult temperament. Journal of Research in Personality, 41, 868–888. doi: 10.1016/j.jrp.2006.11.002 [DOI] [Google Scholar]
- Fingerhut LA, & Anderson RN (2008). The three leading causes of injury mortality in the United States, 1999–2005. National Center for Health Statistics: Health E Stats; [Online]. Retrieved December 4, 2008, from www.cdc.gov/nchs/products/pubs/pubd/hestats/injury99-05/injury99-05.htm [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, & Ghera MM (2005). Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology, 56, 235–262. doi: 10.1146/annurev.psych.55.090902.141532 [DOI] [PubMed] [Google Scholar]
- Franken IH, & Muris P (2006). BIS/BAS personality characteristics and college students’ substance use. Personality and Individual Differences, 40, 1497–1503. doi: 10.1016/j.paid.2005.12.005 [DOI] [Google Scholar]
- Furr RM & Bacharach VR (2008). Psychometrics: An introduction. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Gomez R, Kyriakides C, & Devlin E (2014). Attention-Deficit/Hyperactivity Disorder symptoms in an adult sample: Associations with Rothbart’s temperament dimensions. Personality and Individual Differences, 60, 73–78. doi: 10.1016/j.paid.2013.12.023 [DOI] [Google Scholar]
- Gray JA (1987). The psychology of fear and stress. London, Cambridge University Press. [Google Scholar]
- Johnson PO, & Fay LC (1950). The Johnson-Neyman technique, its theory and application. Psychometrika, 15, 349–367. doi: 10.1007/BF02288864 [DOI] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, & Iwata N (2003). BIS/BAS levels and psychiatric disorder: An epidemiological study. Journal of Psychopathology and Behavioral Assessment, 25, 25–36. doi: 10.1023/A:1022247919288 [DOI] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, & Schulenberg JE (2009). Monitoring the Future National Survey Results on Drug Use, 1975–2008 Volume II: College students & adults ages 19–50. (NIH Publication No. 09–7403). Bethesda, MD: National Institute on Drug Abuse. [Google Scholar]
- Johnston LD, O’Malley PM, Bachamn JG, Schulenberg JE, & Miech RA (2015). Monitoring the Future National Survey Results on Drug Use, 1975–2014 Volume II: College students & adults ages 19–55. Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- Jonker NC, Ostafin BD, Glashouwer KA, van Hemel-Ruiter ME, & de Jong PJ (2014). Reward and punishment sensitivity and alcohol use: The moderating role of executive control. Addictive Behaviors, 39, 945–948. doi: 10.1016/j.addbeh.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, & Rodgers B (1998). Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity and norms in a large community sample. Personality and Individual Differences, 26, 49–58. doi: 10.1016/S0191-8869(98)00143-3 [DOI] [Google Scholar]
- Kimbrel NA, Nelson-Gray RO, & Mitchell JT (2007). Reinforcement sensitivity and maternal style as predictors of psychopathology. Personality and Individual Differences, 42, 1139–1149. doi: 10.1016/j.paid.2006.06.028 [DOI] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Lauharatanahirun N, Farley JP, Chiu P, Bickel WK, & King-Casas B (in press). Neural interaction between risk sensitivity and cognitive control predicting health risk behaviors among late adolescents. Journal of Research on Adolescence. doi: 10.1111/jora.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB, (2011). Principles and practice of structural equation modeling (3rd ed.). New York: Guilford Press. [Google Scholar]
- Lipari RN, & Jean-Francois B (2016). A day in the life of college students aged 18 to 22: Substance use facts, The CBHSQ Report: May 26, 2016 Center for Behavioral Health Statistics and Quality. Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Meade AW, & Craig SB (2012). Identifying careless responses in survey data. Psychological Methods, 17, 437–455. doi: 10.1037/a0028085 [DOI] [PubMed] [Google Scholar]
- Meehan KB, De Panfilis C, Cain NM, & Clarkin JF (2013). Effortful control and externalizing problems in young adults. Personality and Individual Differences, 55, 553–558. doi: 10.1016/j.paid.2013.04.019 [DOI] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science, 21, 8–14. doi: 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47, 395–422. doi: 10.1111/j.1469-7610.2006.01612.x [DOI] [PubMed] [Google Scholar]
- O’Connor RM, & Colder CR (2009). Influence of Alcohol Use Experience and Motivational Drive on College Students’ Alcohol‐Related Cognition. Alcoholism: Clinical and Experimental Research, 33, 1430–1439. doi: 10.1111/j.1530-0277.2009.00973.x [DOI] [PubMed] [Google Scholar]
- O’Neill SE, Parra GR, & Sher KJ (2001). Clinical relevance of heavy drinking during the college years: Cross-sectional and prospective perspectives. Psychology of Addictive Behaviors, 15, 350–359. doi: 10.1037//0893-164X.15.4.350 [DOI] [PubMed] [Google Scholar]
- O’Malley PM, & Johnston LD (2002). Epidemiology of alcohol and other drug use among American college students. Journal of Studies on Alcohol Supplement, 14, 23–39. doi: 10.15288/jsas.2002.s14.23 [DOI] [PubMed] [Google Scholar]
- Pardo Y, Aguilar R, Molinuevo B, & Torrubia R (2007). Alcohol use as a behavioral sign of disinhibition: Evidence from J. A. Gray’s model of personality. Addictive Behaviors, 32, 2398–2403. doi: 10.1016/j.addbeh.2007.02.010 [DOI] [PubMed] [Google Scholar]
- Patrick ME, Blair C, & Maggs JL (2008). Executive function, approach sensitivity, and emotional decision making as influences on risk behaviors in young adults. Journal of Clinical and Experimental Neuropsychology, 30, 449–462. doi: 10.1080/13803390701523109 [DOI] [PubMed] [Google Scholar]
- Paus T (2005). Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences, 9, 60–68. doi: 10.1016/j.tics.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. doi: 10.3102/10769986031004437 [DOI] [Google Scholar]
- Rothbart MK, Ahadi SA, & Evans DE (2000). Temperament and personality: origins and outcomes. Journal of Personality and Social Psychology, 78, 122–135. doi: 10.1037/0022-3514.78.1.122 [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D, & Posner MI (1994). A psychobiological approach to the development of temperament. Temperament: Individual differences at the interface of biology and behavior, 83–116. doi: 10.1037/10149-003 [DOI] [Google Scholar]
- SAMHSA (2014). Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD.
- Schulenberg JE, O’Malley PM, Bachman JG, & Johnston LD (2004). Early adult transitions and their relation to well-being and substance use In Settersten RA, Furstenberg FF, & Rumbaut RG (Eds.), On the frontier of adulthood: Theory, research, and public policy (pp. 417–453). Chicago, IL: University of Chicago Press. doi: 10.7208/chicago/9780226748924.003.0013 [DOI] [Google Scholar]
- Schwarz N, & Clore GL (1996). Feelings and phenomenal experiences In Higgins ET & Kruglanski AW (Eds.), Social psychology: Handbook of basic principles (pp. 433–465). New York: Guilford. [Google Scholar]
- Steinberg L (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52, 216–224. doi: 10.1002/dev.20445 [DOI] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim K, & Luciana M (2012). Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology, 48, 1488–1500. doi: 10.1037/a0027502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, Read JP, Colder CR, & Merrill JE (2012). Positive alcohol expectancies mediate the influence of the behavioral activation system on alcohol use: A prospective path analysis. Addictive Behaviors, 37, 435–443. doi: 10.1016/j.addbeh.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem L, Vasey MW, Beckers T, Claes L, & Bijttebier P (2013). Cognitive biases and alcohol use in adolescence and young adulthood: The moderating role of gender, attentional control and inhibitory control. Personality and Individual Differences, 54, 925–930. doi: 10.1016/j.paid.2013.01.015 [DOI] [Google Scholar]
- Wills TA, Yaeger AM, & Sandy JM (2003). Buffering effect of religiosity for adolescent substance use. Psychology of Addictive Behaviors, 17, 24–31. doi: 10.1037/0893-164X.17.1.24 [DOI] [PubMed] [Google Scholar]
- Wong MM, & Rowland SE (2013). Self‐Determination and Substance Use: Is Effortful Control a Mediator? Alcoholism: Clinical and Experimental Research, 37, 1040–1047. doi: 10.1111/acer.12062 [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118, 117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chen SH, & Main A (2012). Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives, 6, 112–121. doi: 10.1111/j.1750-8606.2011.00176.x [DOI] [Google Scholar]