Mitral valve prolapse (MVP) affects 1 in 40 people in the general population (1), and it is the primary indication for mitral valve (MV) surgery. Myxomatous degeneration, chordal rupture, and mitral regurgitation (MR) indicate the primary role of valvular disease. Traditionally, ventricular remodeling has been considered secondary to volume overload, by acting through b-adrenergic, cellular, and metabolic reductions in contractility leading to heart failure (2,3).
However, not all clinical observations are consistent with the concept that left ventricular (LV) involvement in MVP is purely secondary to volume load. Evidence of electrical instability (mainly ventricular ectopy and nonsustained ventricular tachycardia) supports the presence of an arrhythmogenic state with MVP (4). This fits with published reports that have recently expanded from case series and that link MVP and arrhythmic death (5). In cases of sudden death, MVP may be the only abnormality in an otherwise structurally normal heart (6). These observations may easily be coincidental in such a common condition as MVP, and a pathophysiological basis to explain the association has been lacking. However, over the last few years, cardiac magnetic resonance (CMR) has shown fibrotic changes in MV supporting structures, including papillary muscles (PMs) and basal myocardium (5,7,8), along with more diffuse fibrosis in patients with heart failure and arrhythmia (9,10). Such nonischemic fibrosis, studied in select patients, has been associated with sudden cardiac arrest (5,7,11,12) and ventricular remodeling (13–15).
In this issue of the Journal, Kitkungvan et al. (16) report their use of CMR in a larger-scale study of myocardial fibrosis in 365 referral patients with primary MR. Myocardial replacement fibrosis, detected by late gadolinium enhancement (LGE), was far more prevalent in patients with MVP, predominantly but not entirely in the inferobasal myocardium. Differences persisted over the range of LV volume and MR; fibrosis paralleled MR in MVP. In MVP, arrhythmic events and sudden cardiac arrest were more common in patients with fibrosis. These findings reinforce LGE as a powerful (17), technically robust arrhythmic prognosticator in nonischemic heart disease.
In this study, replacement fibrosis was also associated with LV dilation and reduced ejection fraction. These findings have potential impacts on the unresolved controversies about timing of interventions in MVP. Through prospective studies, nonischemic fibrosis may emerge as an important factor for risk stratification to improve decision making, preserve myocardial function, and prevent sudden cardiac death.
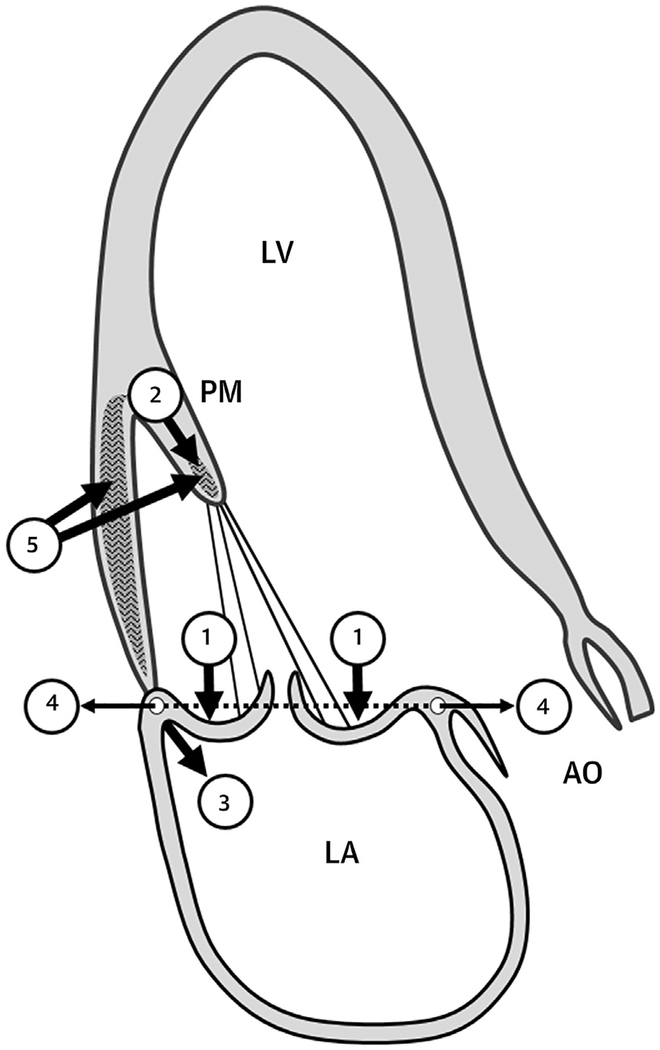
This study raises intriguing questions regarding the mechanism and possible prevention of MVP-associated fibrosis. Recently discovered causal mutations in DCHS1 (18) may induce fibrosis by altering fibroblast mechanosignaling through the Hippo pathway (19). Kitkungvan et al. (16) postulate mechanical induction of fibrosis localized to myocardium that is under increased stress and PM traction by the prolapsing leaflets (Figure 1) (20,21). Systolic annular expansion in MVP will increase PM and myocardial stress (8,22), as evidenced by reduced basal contraction that improves with repair (23). This postulate is supported by evidence for fibroblast mechanosignaling that induces myofibro-blast transformation and extracellular matrix production (24).
FIGURE 1.
Potential Stress-Induced Fibrotic Stimuli
Prolapse (1) induces papillary muscle (PM) traction (2) and basal LV wall tension (3), augmented by systolic annular expansion, (4) as potential fibrotic stimuli (5). (Courtesy of Mark Handschumacher.) AO = aorta; LA = left atrium; LV = left ventricle; PM = papillary muscle.
Future directions include exploring how these changes evolve: Does fibrosis precede LV remodeling, and is it associated with apoptosis? Does the “coarse” replacement fibrosis detected by LGE represent the “tip of the iceberg,” and can assessing diffuse interstitial fibrosis by CMR T1-mapping and quantification of extracellular volume expansion (25) detect fibrosis earlier with high sensitivity and better risk stratification (26)? Are CMR-assessed myocardial strain and fibrosis related (10)? Can MVP repair limit progressive fibrosis and electrical instability, or is there a point of no return? A prospective study is under way to ascertain how fibrosis affects post-repair outcomes (27). Basic studies have shown that within the myocardium, fibroblast differentiation to myofibroblasts triggers fibrogenesis, and fibrosis can be reversed by inhibiting this transition or by myofibroblast conversion into quiescent fibroblasts (28,29). These findings indicate the possibility of testing whether specifically reversing fibrosis can directly affect cardiac tissue in MVP and whether MV repair can be combined with antifibrotic therapy for better myocardial and electrical benefits. With the new molecular tools we have to modulate fibrosis, these important clinical questions can now be addressed.
In summary, this study reinforces a comprehensive view of MVP and its ventricular impact. This can provide additional therapeutic targets to prevent heart failure in this common condition.
Acknowledgments
This work was supported by National Institutes of Health grants R01HL128099 (R.A.L., M.J.H., and R.J.H.) and HL141917. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Editorials published in the Journal of the American College of Cardiology reflect the views of the author and do not necessarily represent the views of JACC or the American College of Cardiology.
R E F E R E N C E S
- 1.Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Pu M, Gao Z, Zhang X, et al. Impact of mitral regurgitation on left ventricular anatomic and molecular remodeling and systolic function: implication for outcome. Am J Physiol Heart Circ Physiol 2009;296:H1727–32. [DOI] [PubMed] [Google Scholar]
- 3.Beeri R, Yosefy C, Guerrero JL, et al. Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation. J Am Coll Cardiol 2008;51:476–86. [DOI] [PubMed] [Google Scholar]
- 4.Vohra J, Sathe S, Warren R, Tatoulis J, Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol 1993;16:387–93. [DOI] [PubMed] [Google Scholar]
- 5.Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–66. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009;119: 1085–92. [DOI] [PubMed] [Google Scholar]
- 7.Han Y, Peters DC, Kissinger KV, et al. Evaluation of papillary muscle function using cardiovascular magnetic resonance imaging in mitral valve pro-lapse. Am J Cardiol 2010;106:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuster V, Danielson MA, Robb RA, Broadbent JC, Brown AL Jr., Elveback LR. Quantitation of left ventricular myocardial fiber hyper-trophy and interstitial tissue in human hearts with chronically increased volume and pressure overload. Circulation 1977;55:504–8. [DOI] [PubMed] [Google Scholar]
- 10.Edwards NC, Moody WE, Yuan M, et al. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ Cardiovasc Imaging 2014;7: 946–53. [DOI] [PubMed] [Google Scholar]
- 11.Bui AH, Roujol S, Foppa M, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017;103:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulton BL, Liang JJ, Enriquez A, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol 2018; 29:146–53. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Kochav JD, Gurevich S, et al. Left ventricular geometric remodeling in relation to non-ischemic scar pattern on cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2014; 30:1559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schelbert EB, Piehler KM, Zareba KM, et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc 2015;4:e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Lueder TG, Wang BH, Kompa AR, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 2015;8:71–8. [DOI] [PubMed] [Google Scholar]
- 16.Kitkungvan D, Nabi F, Kim RJ, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol 2018;72:823–34. [DOI] [PubMed] [Google Scholar]
- 17.Dawson DK, Hawlisch K, Prescott G, et al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. J Am Coll Cardiol Img 2013;6:335–44. [DOI] [PubMed] [Google Scholar]
- 18.Clemenceau A, Bérubé JC, Bélanger P, et al. Deleterious variants in DCHS1 are prevalent in sporadic cases of mitral valve prolapse. Mol Genet Genomic Med 2018;6:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Lagares D, Choi KM, et al. Mechano-signaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 2015;308:L344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanfilippo AJ, Harrigan P, Popovic AD, Weyman AE, Levine RA. Papillary muscle traction in mitral valve prolapse: quantitation by two-dimensional echocardiography. J Am Coll Cardiol 1992;19:564–71. [DOI] [PubMed] [Google Scholar]
- 21.Morrel WG, Ge L, Zhang Z, Grossi EA, Guccione JM, Ratcliffe MB. Effect of mitral annuloplasty device shape and size on leaflet and myofiber stress following repair of posterior leaflet prolapse: a patient-specific finite element simulation. J Heart Valve Dis 2014;23:727–34. [PMC free article] [PubMed] [Google Scholar]
- 22.Clavel M-A, Mantovani F, Malouf J, et al. Dynamic phenotypes of degenerative myxomatous mitral valve disease: quantitative 3-dimensional echocardiographic study. Circ Cardiovasc Imaging 2015;8:e002989. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda S, Song JK, Mahara K, et al. Basal left ventricular dilatation and reduced contraction in patients with mitral valve prolapse can be secondary to annular dilatation: preoperative and postoperative speckle-tracking echocardiographic study on left ventricle and mitral valve annulus interaction. Circ Cardiovasc Imaging 2016;9:e005113. [DOI] [PubMed] [Google Scholar]
- 24.Molkentin JD, Bugg D, Ghearing N, et al. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation 2017;136:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avanesov M, Munch J, Weinrich J, et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur Radiol 2017;27:5136–45. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Edwards NC, Neal DAH, et al. A prospective study examining the role of myocardial Fibrosis in outcome following mitral valve repair IN DEgenerative mitral Regurgitation: rationale and design of the mitral FINDER study. BMC Cardiovasc Disord 2017;17:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez P, Sassi Y, Troncone L, et al. Deletion of delta-like 1 homologue accelerates fibroblast-myofibroblast differentiation and induces myocardial fibrosis. Eur Heart J 2018. April 13 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong D, Lee MA, Li Y, et al. Matricellular protein CCN5 reverses established cardiac fibrosis. J Am Coll Cardiol 2016;67:1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]