Abstract
Plants defend themselves against pathogens by activating an array of immune responses. Unfortunately, immunity programs may also cause unintended collateral damage to the plant itself. The quantitative disease resistance gene ACCELERATED CELL DEATH 6 (ACD6) serves to balance growth and pathogen resistance in natural populations of Arabidopsis thaliana. An autoimmune allele, ACD6-Est, which strongly reduces growth under specific laboratory conditions, is found in over 10% of wild strains. There is, however, extensive variation in the strength of the autoimmune phenotype expressed by strains with an ACD6-Est allele, indicative of genetic modifiers. Quantitative genetic analysis suggests that ACD6 activity can be modulated in diverse ways, with different strains often carrying different large-effect modifiers. One modifier is SUPPRESSOR OF NPR1-1, CONSTITUTIVE 1 (SNC1), located in a highly polymorphic cluster of nucleotide-binding domain and leucine-rich repeat (NLR) immune receptor genes, which are prototypes for qualitative disease resistance genes. Allelic variation at SNC1 correlates with ACD6-Est activity in multiple accessions, and a common structural variant affecting the NL linker sequence can explain differences in SNC1 activity. Taken together, we find that an NLR gene can mask the activity of an ACD6 autoimmune allele in natural A. thaliana populations, thereby linking different arms of the plant immune system.
Author summary
Plants defend themselves against pathogens by activating immune responses. Unfortunately, these can cause unintended collateral damage to the plant itself. Nevertheless, some wild plants have genetic variants that confer a low threshold for the activation of immunity. While these enable a plant to respond particularly quickly to pathogen attack, such variants might be potentially dangerous. We are investigating one such variant of the immune gene ACCELERATED CELL DEATH 6 (ACD6) in the plant Arabidopsis thaliana. We discovered that there are variants at other genetic loci that can mask the effects of an overly active ACD6 gene. One of these genes, SUPPRESSOR OF NPR1-1, CONSTITUTIVE 1 (SNC1), codes for a known immune receptor. The SNC1 variant that attenuates ACD6 activity is rather common in A. thaliana populations, suggesting that new combinations of the hyperactive ACD6 variant and this antagonistic SNC1 variant will often arise by natural crosses. Similarly, because the two genes are unlinked, outcrossing will often lead to the hyperactive ACD6 variants being unmasked again. We propose that allelic diversity at SNC1 contributes to the maintenance of the hyperactive ACD6 variant in natural A. thaliana populations.
Introduction
Plants rely on a sophisticated immune system to defend themselves against pathogens. A central challenge for plants is how to achieve a fast, effective response upon pathogen attack, while at the same time preventing spontaneous firing of the signaling machinery in the absence of danger [1]. Inappropriate activation of immune signaling can reduce growth or even damage the plant’s own cells, while an inefficient immune response makes plants more likely to succumb to pathogen attack [2–6]. Although highly effective immune alleles may have background activity, they will nevertheless be favored when pathogen pressure is high. In contrast, in locations and years with low pathogen pressure, such alleles tend to be selected against [7]. If differently active alleles exist at the same locus, such temporal or spatial variation in pathogen pressure will maintain both types of alleles at ratios that reflect the prevalence of the different environments; this is one example of the phenomenon of balancing selection [8–10]. Very different alleles have been described for several disease resistance (R) genes of the nucleotide binding site leucine-rich repeat (NLR) class, although formal evidence for balancing selection is still rare [11–15]. NLR immune receptors detect the presence of so-called pathogen effector molecules, leading to effector-triggered immunity (ETI). NLRs do so in several different ways, and the biochemical and structural basis for effector detection, including direct interaction with effectors or with effector-modified host proteins, is increasingly well understood [16,17]. Variation in NLR immune receptors is linked to the fact that effectors are rarely essential for pathogen survival, and that even closely related pathogens can greatly vary in effector content [16,18].
In mutant screens, gain-of-function alleles have been identified for several NLR genes, based on their autoimmune phenotypes. The mutant proteins are active regardless of effector presence, and thus can increase resistance to a range of unrelated pathogens [19–26]. Whether highly active autoimmune alleles of NLR genes are being deployed to enhance broad-spectrum pathogen resistance in nature is unknown, but the presence of NLR alleles with modest autoimmune activity in breeding programs is well documented, and phenotypic signs of autoimmunity have been exploited to follow resistance genes in segregating crop populations (e.g., [27]).
In addition to specialized NLRs, which often engender very strong, qualitative disease resistance, plants employ various genes for quantitative resistance [28–31]. An example in the wild plant A. thaliana is ACCELERATED CELL DEATH 6 (ACD6) [32–35]. Although ACD6 does not encode an NLR immune receptor, the locus features extensive copy number and sequence variation in wild populations, reminiscent of what is found at many NLR loci. Importantly, natural populations segregate for ACD6 allele classes with clear functional differences [36–38]. One class, first identified by genetic analysis of the natural Est-1 accession, protects in the greenhouse against a wide range of unrelated pathogens, from microbes to insects [36]. This unusually large benefit of the hyperactive ACD6 allele appears to be due to enhanced and partially constitutive defense responses. On the other hand, the autoimmunity seen in plants with the ACD6-Est allele substantially compromises growth under specific laboratory conditions, reducing both plant size and the tempo with which new leaves are being produced [36,39,40]. That the allele is found at a frequency of over 10% in natural populations is compatible with the idea that selection maintains this allele despite a fitness trade-off [36–38]. The frequency of functionally distinct ACD6 alleles differs between local populations, suggestive of a role of ACD6 in local adaptation [37,38]. Alternatively, since the expression of immune and growth traits often differ between laboratory and field conditions [41–43], the effects the ACD6-Est allele in nature might be modified by the environment, and there is evidence from other laboratories that the expression of ACD6-Est associated lesions is environment-dependent [39,40,44,45].
ACD6 encodes a transmembrane protein with intracellular ankyrin repeats, which positively regulates cell death and defense, acting in part via the immune hormone salicylic acid (SA) and the SA transducer NONEXPRESSER OF PR GENES 1 (NPR1) [32,33]. The precise mechanism of ACD6 biochemical action remains enigmatic, but ACD6 is found in a complex with several immune-related proteins, including pathogen-associated-molecular-pattern (PAMP) receptors, indicating a role for ACD6 in PAMP triggered immunity (PTI) [34,35]. While PTI and ETI have often been considered as different arms of the plant immune system, the distinction between ETI and PTI is becoming more and more blurred, not only because of substantial overlap in downstream responses, but also because not all effectors are highly variable, and not all PAMPs are highly conserved [46].
Here, we report that natural alleles at several independent loci can modulate the activity of the ACD6-Est autoimmune allele. We have characterized one locus, encoding the NLR protein SUPPRESSOR OF NPR1-1, CONSTITUTIVE 1 (SNC1) in detail and show that a common structural variant affecting the NL linker explains differences in ACD6-Est dependent SNC1 activity. We propose that allelic diversity at SNC1 and other loci contributes to the maintenance of the ACD6-Est autoimmune allele in natural A. thaliana populations.
Results
Incidence of the ACD6-Est autoimmune allele in natural A. thaliana populations
Sanger sequencing analysis of a limited collection of 96 natural accessions had shown that 19, or 20%, had the ACD6-Est allele, which triggers autoimmunity and reduced growth under specific laboratory conditions [36]. To extend our knowledge of the global distribution of the ACD6-Est allele, we attempted to use Illumina short read data of 1,135 accessions [47,48], with the goal of ascertaining the presence of two codons that are causal for ACD6-Est autoimmune activity [36]. Likely because of linked sequence diversity and copy number variation, the first codon, encoding amino acid 566 in the reference sequence, GCA (Ala) in Col-0 and AAC (Asn) in Est-1, could not be confidently typed with short reads. At the second codon, encoding amino acid 634 in the reference, 823 accessions could be assigned to have either Col-0- or Est-1-like codons. Of these, 721, or 88%, had CTT (Leu), diagnostic for the Col-0 reference, and 102, or 12%, had TTT (Phe), diagnostic for Est-1, confirming that ACD6-Est alleles are not uncommon in the global A. thaliana population.
Of the 19 accessions with an ACD6-Est allele examined by Todesco and colleagues [36], two did not show any necrotic lesions, an obvious sign of autoimmunity. The remaining 17 accessions differed in their phenotypic severity as well, with three classified as expressing mild, five intermediate, and nine strong necrosis. We extended this analysis to a total of 54 accessions for which we had confirmed the presence of an ACD6-Est allele by Sanger sequencing (S1 Table). Seven had no or only very mild lesions, 22 had intermediate lesions, and 25 were similarly affected as the Est-1 strain, in which this allele had been originally identified (Fig 1A).
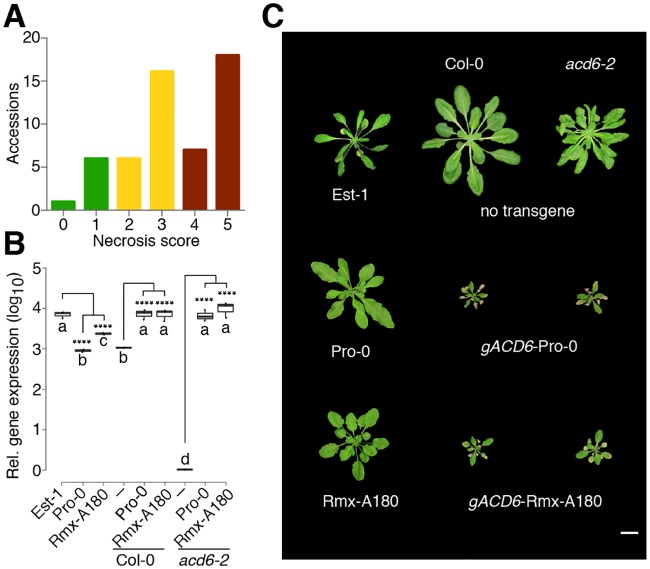
Fig 1. Genetic background dependence of ACD6-Est effect.
(A) Variation of necrosis in 54 natural accessions (n = 6 each) with ACD6-Est alleles. Broad-sense heritability H2 is 0.89. (B) Accumulation of ACD6 mRNA in various genetic backgrounds (n = 3), as measured by qRT-PCR. Asterisks indicate significant differences in pairwise Student’s t-tests (p<0.0001; Holm adjusted). Letters indicate significantly different groups (p<0.0001; post hoc Tukey’s HSD). (C) Representative non-transgenic and ACD6 transgenic lines at 5 weeks of age in Col-0 and acd6-2 backgrounds. Est-1, Pro-0 and Rmx-A180 are shown for comparison on the left. Scale bar, 10 mm.
Evidence for extragenic suppression of ACD6-Est alleles
While we could confirm that there is substantial variation in the extent of autoimmune phenotypes exhibited by ACD6-Est carriers, this observation did not inform about the genetic cause of this variation. To determine whether this was due to sequence differences at the ACD6 locus itself, we directly tested ACD6 activity in the first two ACD6-Est accessions identified as lacking necrotic lesions, Pro-0 and Rmx-A180 [36]. ACD6 was expressed well in both accessions, indicating that suppression of the ACD6-Est phenotype was not due to reduced RNA accumulation (Fig 1B). We introduced full-length genomic ACD6 fragments from both accessions into the Col-0 reference strain, which carries a standard ACD6 allele, and into acd6-2, a Col-0 derivative with a T-DNA insertion in ACD6. All four classes of transformants from 10 T1 lines had small rosettes and necrotic lesions, similar to what has been reported for Est-1 [36] (Fig 1C). Together, these results demonstrated that the ACD6 alleles from Pro-0 and Rmx-A180 have similar activity as the original ACD6-Est allele outside their native genetic backgrounds, suggesting that accessions such as Pro-0 and Rmx-A-180 carry extragenic suppressors of ACD6-Est activity.
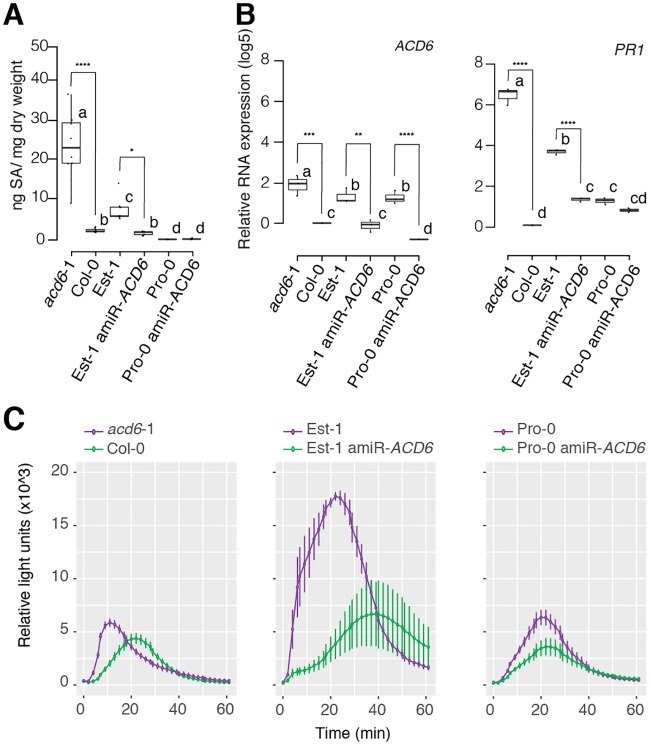
ACD6 acts in a feed-forward loop that regulates the accumulation of SA, a key hormone in both disease signaling and autoimmunity [49]. We therefore asked whether the suppressed autoimmunity of the ACD6-Est allele in Pro-0 was accompanied by reduced SA levels. Indeed, Pro-0 had much less SA than the ACD6-Est reference strain Est-1 or acd6-1, which carries an EMS-induced hyperactive ACD6 allele in the Col-0 background [33]. SA levels in Pro-0 were even lower than in Col-0, which carries the standard ACD6 allele (Fig 2A). Similarly, the expression of SA responsive marker gene PR1 was reduced in Pro-0 compared to Est-1 (Fig 2B). As expected, knocking down ACD6 with an artificial miRNA [36,50] resulted in greatly reduced SA levels in both acd6-1 and Est-1, while the effect in Pro-0 was much smaller (Fig 2A). In agreement, knocking-down ACD6 in Pro-0 did not substantially affect PR1 expression either (Fig 2B). Finally, to assess not only autoimmunity but also true immune responses, we measured H2O2 accumulation after stimulation with the PAMP flg22 [51]. Est-1 responded strongly to flg22, with the H2O2 production being greatly reduced by knocking down ACD6. Pro-0 responded much more weakly, and knocking down ACD6 was of little consequence (Fig 2C). In conclusion, these results confirmed attenuation of the effects of the hyperactive ACD6-Est allele in Pro-0.
Fig 2. ACD6-dependent suppression of salicylic acid accumulation and flg22 responses in Pro-0.
(A) SA levels in lines with different ACD6 alleles, n = 10 per line. Asterisks indicate pairwise Student’s t-test results (p<0.0001; Holm adjusted). Letters indicate significantly different groups (p<0.0001; post hoc Tukey’s HSD). (B) ACD6 and PR1 expression in different T1 individuals (n = 4), normalized to Col-0. Asterisks indicate significance in pairwise Student’s t-tests (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; Holm adjusted). Letters indicate significantly different groups (p<0.0001; post hoc Tukey’s HSD). (C) H2O2 production in response to flg22 (n = 4). Error bars represent standard errors. This experiment was repeated four times with similar results.
Modulation of ACD6 hyperactivity by RPP4/5 alleles
To understand the population genetic architecture underlying extragenic suppression of the activity of ACD6-Est-type alleles, we crossed three accessions that carried such alleles, but did not show necrosis, to the necrotic Est-1 accession. The ratios of necrotic and non-necrotic F2 individuals suggested more than one locus in all mapping populations (S2 Table). We used RAD-seq [52] followed by QTL mapping to identify causal regions of the genome in these accessions. As expected, we identified multiple significant QTL in all mapping populations (Fig 3A). The QTLs explained 4% to 23% of the observed phenotypic variation, further supporting the quantitative nature of phenotypic suppression of ACD6-Est alleles (S3 Table). In most, but not all, cases did the Est-1 allele increase necrosis (S1 Fig).
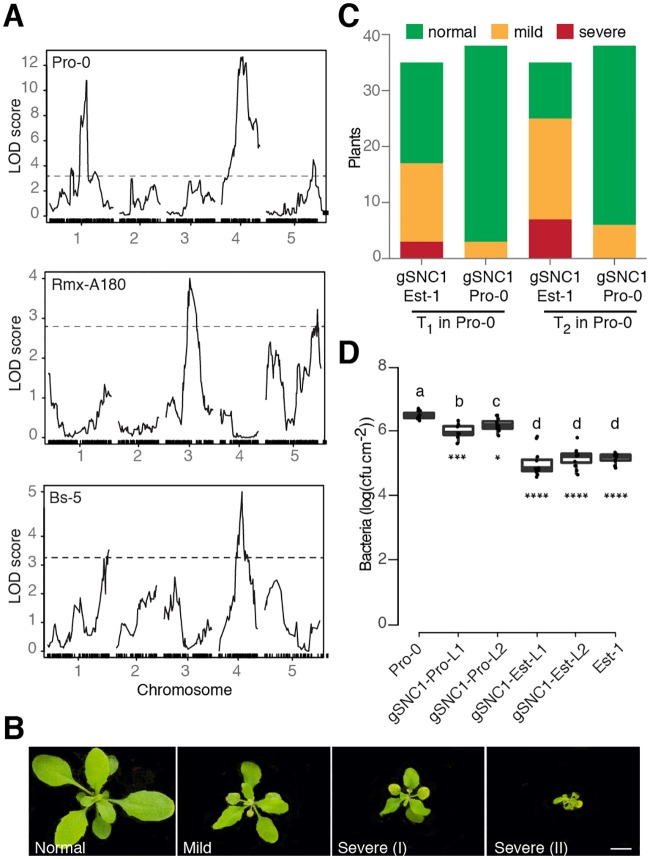
Fig 3. Genetic analysis of ACD6 modifiers in natural accessions.
(A) Necrosis QTL mapping in three F2 populations. Dashed lines indicate LOD score significance thresholds of p<0.05 (from 1,000 permutations). (B) Representative phenotypes of transformants expressing genomic SNC1 fragments. Scale bar, 10 mm. (C) Necrosis in transgenic Pro-0 plants. The effects of SNC1-Est-1 and SNC1-Pro-0 transgenes were significantly different (chi-square test, p<0.001). (D) Growth of Pst DC3000 after infection at OD600 = 0.0001. Bacterial growth in Pro-0 [gSNC1-Pro-0] was significantly different from either Pro-0 [gSNC1-Est-1] or nontransgenic Pro-0 on day 3. Asterisks indicate significance in pairwise Student’s t-tests against Pro-0 (*p<0.05, ***p<0.001, **** p<0.0001; Holm adjusted). Letters indicate significantly different groups (p<0.0001; post hoc Tukey’s HSD). L1/L2 designates two independent transgenic lines for both constructs.
We decided to focus on the QTL in the center of chromosome 4, near ACD6, which explained 18% of phenotypic variance in the Pro-0 x Est-1 cross, because the confidence interval (Chr 4: 7.5–9.7 Mb, LOD = 13.1, p<0.0001) contained a well-studied locus that had been linked to suppression of autoimmunity. Laboratory-induced loss-of-function alleles of this locus, SNC1, can fully or partially suppress the phenotypic defects of different autoimmune mutants [53,54]. In addition, SNC1 gain-of-function alleles reduce plant size, similar to ACD6 hyperactivity, while down-regulation of SNC1 homologs increases plant size [19,55]. SNC1 is located in a complex NLR gene cluster, RECOGNITION OF PERONOSPORA PARASITICA 4/5 (RPP4/5), which has alleles that mediate resistance to different strains of the oomycete pathogen Hyaloperonospora arabidopsidis [56,57]. SNC1 and different RPP4 and RPP5 alleles are closely related in sequence [19,55,56,58].
To test whether SNC1 contributes to the suppressed leaf necrosis in Pro-0, and whether SNC1 can modulate ACD6 activity, we took a transgene approach. We first introduced a SNC1-Pro-0 genomic fragment into Pro-0. This transgene caused autoimmunity, consistent with additional NLR copies often leading to spontaneous activation of immunity, but the degree of autoimmunity was very modest [59–61]. In contrast, when we introduced a chimeric transgene containing the SNC1 promoter from Pro-0, but coding sequences from Est-1, into Pro-0, we observed strong necrosis and dwarfism, both in primary transformants and in the T2 generation (Fig 3B and 3C, S2 Fig). In agreement, Pro-0 plants transformed with the SNC1-Est allele were more resistant to infection by P. syringae pv. tomato strain DC3000 (Pto DC3000) than untransformed Pro-0 plants or Pro-0 plants carrying an extra SNC1-Pro copy (p<0.0001) (Fig 3D). This suggests that differences in the transcribed portion of SNC1, most likely differences between the SNC1-Est and SNC1-Pro proteins, are causal for attenuated effects of ACD6-Est alleles. However, since we did not have Near Isogenic Lines (NILs) at our disposal, with which we could directly compare the phenotypic effects of the QTL region with the SNC1 transgenes, we cannot exclude that the QTL on chromosome 4 includes further genes that contribute to attenuated ACD6-Est activity in Pro-0.
Correlation of a common SNC1 structural variant with attenuation of ACD6 activity
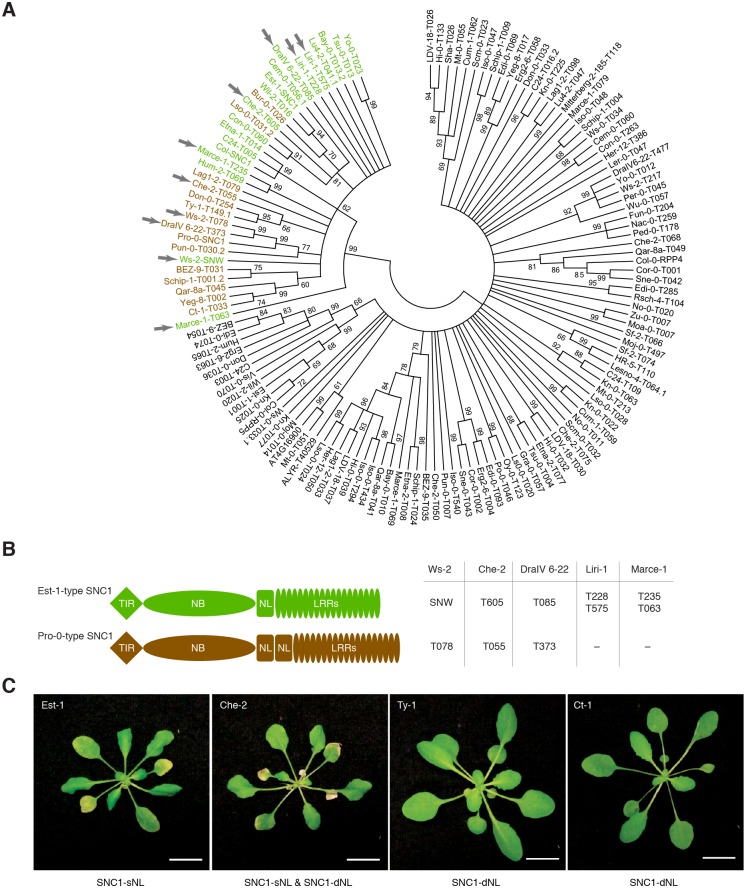
To gauge whether attenuation of ACD6-Est activity by variant SNC1 alleles is likely to be common, we wanted to assess the population frequency of different SNC1 alleles. We compared the SNC1 alleles from Est-1 and Pro-0 with RPP4/RPP5/SNC1-like sequences from a REN-seq dataset of 65 A. thaliana accessions [62]. A protein-phylogeny of 142 RPP4/RPP5/SNC1 homologs indicated that 34 genes, from 29 accessions, form a single clade of SNC1 orthologs, which are predicted to encode highly similar Toll-Interleukin-1 receptor (TIR) domains. Five accessions, Ws-2, Liri-1, Marce-1, DraIV 6–22 and MNF-Che-2, have two SNC1 homologs each (Fig 4A, S3 Fig, S5 Table). The most noticeable sequence differences among SNC1 proteins affect the NL linker between the nucleotide binding (NB) and leucine-rich repeat (LRR) domains [19]. The NL linker (amino acids 544–671 in SNC1-Col-0) is duplicated in 15 of the 34 SNC1 proteins (Fig 4A and 4B, S3 and S4 Figs). A SNC1 allele that differs functionally from the Col-0 reference allele has been identified in Ws-2, SNW [59]. The SNC1 homolog that we found in addition to SNW in Ws-2 (Ws-2-T078) has a duplicated linker and is highly similar to the Pro-0 variant (Fig 4B, S5 Fig). Similarly, the MNF-Che-2 and DraIV 6–22 accessions have two SNC1 genes, one each encoding a single and a duplicated linker (Fig 4B). Overall, our analysis revealed a complex history of SNC1 diversification within and between accessions.
Fig 4. Correlation between SNC1 alleles and ACD6-Est-1 effects in natural accessions.
(A) Phylogeny of 136 RPP4/5 and SNC1 proteins from 65 accessions. Bootstrap values over 60% are indicated. The SNC1 clade is highlighted in color, with single NL linker (SNC1-sNL) genes green and duplicated NL linker (SNC1-dNL) genes brown. Arrows highlight five accessions with two SNC1 homologs. (B) Diagrams of SNC1 proteins with single and duplicated NL linkers. Gene IDs for five accessions with two SNC1 homologs on the right. (C) Accessions with ACD6-Est-like alleles; SNC1 types indicated below. Scale bar, 10 mm.
To address whether SNC1 linker variation is associated with suppression of ACD6 activity across accessions, we determined the ACD6 allele type in all 15 accessions with the duplicated SNC1 linker. Three accessions, Ty-1, Ct-1 and MNF-Che-2, had ACD6-Est alleles (S6 Fig), but only MNF-Che-2 had symptoms of necrosis. This is in agreement with our hypothesis that SNC1-Pro allele can function as genetic suppressors of ACD6 hyperactivity (Fig 4C). That ACD6-Est induced necrosis was not suppressed in MNF-Che-2 may be due to the additional SNC1 homolog without a duplicated linker in this accession (Fig 4C).
Experimental evidence for SNC1 NL linker affecting ACD6 activity
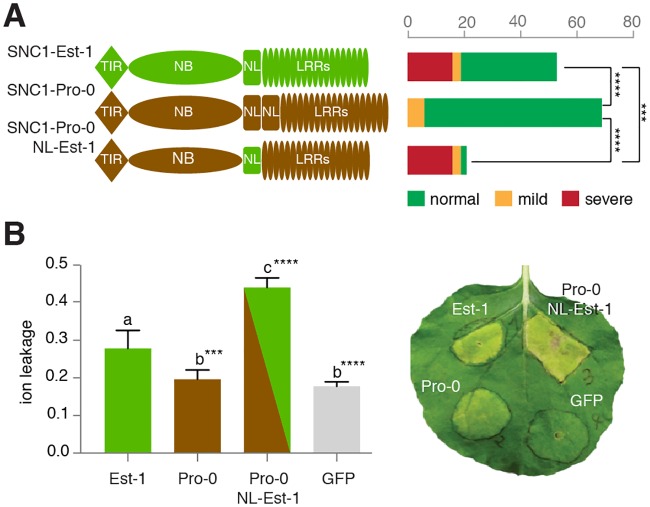
A gain-of-function allele of SNC1 induced by EMS mutagenesis, snc1-1, has a single amino acid substitution in the NL linker domain, pointing to the functional importance of the NL linker [19]. Since the NL linkers of SNC1 from Pro-0 and Est-1 differ notably, we suspected that they might be responsible for differential ACD6-Est effects in different backgrounds. To test this hypothesis, we introduced three different SNC1 constructs into Col-0: a genomic SNC1-Pro fragment, a genomic SNC1-Est fragment, and a chimeric SNC1-Pro fragment with the dNL linker of Pro-0 replaced with the sNL linker from Est-1. Sixteen of 53 SNC1-Est T1 transgenic plants had a severe autoimmune phenotype (Fig 5A). However, none of 70 SNC1-Pro T1 transgenic plants showed severe, and only 10% showed mild phenotypes. The ability of SNC1-Pro in triggering leaf necrosis was fully restored when introducing the SNC1-Est linker in an otherwise Pro-0 protein (SNC1-Pro-NL-Est) (Fig 5A).
Fig 5. Contribution of NL linker variation to SNC1 activity.
(A) Distribution of necrosis in Col-0 T1 transformants expressing gSNC1-Pro-0 with different NL linkers. Asterisks indicate pairwise chi-square test results (***p<0.001, ****p<0.0001; Holm adjusted). (B) Left, ion leakage, an indicator of cell death, in N. benthamiana leaves transiently transformed with different gSNC1 transgenes 4 d after Agrobacterium infiltration. Asterisks indicate significance in pairwise Student’s t-tests against Est-1 (***p<0.001, ****p<0.0001; Holm adjusted). Letters indicate significantly different groups (p<0.0001; post hoc Tukey’s HSD). The leaf samples were collected from six independent plants. Right, image of a representative 7-day old leaf after infiltration.
To further assess the contribution of the NL linker on SNC1-induced cell-death signaling, we transiently expressed both SNC1-Est and SNC1-Pro in N. benthamiana and monitored chlorosis indicative of cell death 7 days after infiltration. Expression of SNC1-Pro resulted in a much weaker cell death and significantly less ion leakage than either SNC1-Est or SNC1-Pro-NL-Est (Fig 5B, S7 Fig). Taken together, we conclude that polymorphisms in the NL linker region explain functional differences between SNC1-Est and SNC1-Pro.
Discussion
The naturally hyperactive ACD6-Est allele in A. thaliana can induce an autoimmune syndrome associated with necrosis and reduced growth in many, but not all, accessions with this allele [36]. We have shown that variation in the effects of ACD6-Est alleles does not map to the ACD6 locus itself, but rather is caused by extragenic modifiers of the ACD6-Est phenotype. Notably, alleles at several different loci can have such an effect, including a common allele at the NLR locus SNC1.
Genetic mapping experiments with three different accessions carrying an ACD6-Est allele identified a minimum of six genomic regions that can modulate ACD6-Est effects. The identified QTL only explain a minority of observed phenotypic variation, suggesting that several additional minor-effect loci contribute to modulation of ACD6 activity. An attractive hypothesis is that suppressors of ACD6-Est-like activity have arisen as a consequence of excessive deleterious effects of ACD6-Est and perhaps hyperimmunity alleles at other loci. Alternatively, the ACD6-Est allele itself may first have evolved on a background that masked its effects. We found that about a quarter of A. thaliana accessions share the SNC1 allele with a duplicated NL linker, which can attenuate ACD6-Est effects. The fraction of accessions with an ACD6-Est allele among those with this SNC1 allele is similar to the frequency of ACD6-Est alleles in the global A. thaliana population. While there is no obvious indication for linkage between specific SNC1 and ACD6 alleles, the numbers examined so far are very small, and the situation could be different in local A. thaliana populations. Another explanation for why we did not see an enrichment of specific SNC1 alleles among ACD6-Est carriers might be that other modifiers are more important in natural populations.
It has been argued that tandem duplication of NLR genes within clusters can provide an effective mechanism to acquire novel NLR specificities without sacrificing old ones [63]. SNC1 features both relevant structural and copy number variation, and functionally distinct variants can occur in the same genome, offering an opportunity to test such hypotheses about NLR evolution. Since SNC1 has a dosage effect on plant innate immunity [59], it will be interesting to ask whether there are situations in which it is advantageous to have two functionally different SNC1 copies, as we have seen in MNF-Che-2, Ws-2 and DraIV 6–22.
Mutations in other NLRs can either trigger autoimmunity [19,21–26,64–69] or suppress autoimmunity in various mutants with a range of biochemical defects [67,70–77]. As for SNC1 itself, the lethality of bon1 (bonzai1) bon3 double mutants is caused by inappropriate activation of at least five NLRs, including SNC1 [77]. Some of the other QTL regions that modify ACD6-Est effects include NLR genes as well (S8 Fig), and it will be interesting to learn whether the situation for ACD6-Est is similar as for bon1 bon3 mutants, and whether ACD6-Est activity can be attenuated by alleles at NLR loci other than SNC1.
In summary, our genetic analyses of accessions with a hyperactive ACD6 allele have shown that natural A. thaliana populations frequently harbor extragenic modulators of ACD6 activity, underscoring the importance of epistatic interactions for natural phenotypes [29,78–83]. In addition, our work has revealed a new connection between ACD6, which has before been primarily linked to PAMP recognition and PTI [34,35,84], and NLR proteins, which are central to ETI. There is already genetic evidence for cell death triggered by PAMP receptor complexes being suppressed by the NLR SNC1 [59,85]. An arbitrary selection of evidence for the distinction between PTI and ETI being fluid [46] includes the overlap in the signaling network downstream of AvrRpt2-triggered ETI and the network activated by the well-known PAMP flg22 [86], or MAPK3 and MAPK6 as widely shared downstream signaling components for both PTI and ETI [86–88]. Together, our findings illustrate the potential of studies of naturally occurring autoimmunity in either A. thaliana inbred strains or hybrids [89] to contribute to our understanding of the immune system in healthy and diseased plants.
Methods
Plant material and growth conditions
Seeds of Arabidopsis thaliana accessions (S1 and S5 Tables) were from stocks available in the lab. Seeds were germinated and cultivated in growth rooms at a constant temperature of 23°C (temperature variability about ± 0.1°C), air humidity of 65% and long days (LD, 16 hr day length) or short days (SD, 8 hr day length), with light (125 to 175 μmol m-2 s-1) provided by a 1:1 mixture of Cool White and Gro-Lux Wide Spectrum fluorescent lights (Luxline plus F36W/840, Sylvania, Erlangen, Germany). Leaf necrosis was scored in SD at 23°C.
qRT-PCR
RNA was extracted from three biological replicates (4- to 5-week-old entire rosettes) using TRIzol Reagent (Thermo Scientific, Waltham, MA) and treated with DNase I (Thermo Scientific, Waltham, MA, USA). 2 μg total RNA was used as a template for reverse transcription (M-MLV reverse transcriptase kit; Thermo Scientific, Waltham, MA, USA). Quantitative real-time PCR reactions were performed using Maxima SYBR Green Master Mix (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s directions on a CFX384 real time instrument (Bio-Rad, Hercules, CA). Transcript abundance was normalized to the TUBULIN BETA CHAIN 2 (At5g62690) transcript. Relative expression compared to the control (usually Col-0) was quantified based on [90]. Primers used for qRT-PCR are listed in S7 Table.
H2O2 assay
Leaf discs (5 mm diameter) were punched from 5-week-old 23°C SD-grown plants and immediately floated with the adaxial side up in individual wells of a 96-well plate (Greiner Bio-One, Frickenhausen, Germany) containing 200 μL of water. They were incubated overnight (12–16 hr) covered with a transparent lid, to recover. The water was replaced with the elicitation solution containing 10 μM Luminol (Sigma-Aldrich, MO), 10 μg mL-1 horseradish peroxidase and 100 nM flg22 (QRLSTGSRINSAKDDAAGLQIA, >85% purity; EZBiolab, Westfield, IN [91]). H2O2 production was monitored by luminescence immediately after the elicitation solution was added, for at least 90 minutes (Tecan Infinite PRO multimode reader, Tecan, Männedorf, Switzerland). Controls were mock treated with the same solution without flg22. Each genotype was assayed at least three independent times, with leaves coming from 4 biological replicates each time.
Salicylic acid (SA) quantification
Each sample consisted of 10 (±1) mg freeze-dried, ground plant material of 4-week-old rosettes, with 8 biological replicates per genotype, of plants that had been grown in randomized complete block design at 23°C SD. SA was extracted twice with 400 μl 20% methanol (LCMS-grade) / 0.1% hydrofluoroalkane by 5 min ultrasonic extraction, followed by 20 min incubation on ice, and removal of solids by centrifugation for 10 min at 13,500 g. 320 μl supernatant were removed after each extraction step and combined in a new vial. A third extraction step with 400 μl of 100% methanol (conditions see above) was performed and the supernatant was combined with the previous ones. The total volume was split in half before drying in a speed vac. For analysis of the conjugated and free salicylic acid the pellets were redissolved in 30 μl 50% methanol / 0,1% formic acid. Ultra Performance Liquid Chromatography Mass Spectrometry (UPLC-MS) analysis was performed on a Waters Acquity UPLC system coupled to a SYNAPT G2 QTOF mass spectrometer equipped with an ESI-Source (Waters Corporation, Milford, MA) at the University of Tübingen—ZMBP Analytics Laboratory. MassLynx v4.1 was used to control the LCMS system and TargetLynx (Waters Corporation) to perform data integration.
RAD-seq and whole genome genotyping
RAD-seq library preparation using a set of 192 adaptor sequences containing PstI and Mse1 recognition sequences were prepared according to [52,92]. Ninety six or 192 individual samples were pooled per library and sequenced on one Illumina HiSeq 2000 lane (100 bp single-end reads). Raw reads were pre-processed with the SHORE (https://sourceforge.net/projects/shore) pipeline. Default parameters were used for de-multiplexing, read trimming and mapping. The BWA [93] option, also with default parameters, was used to map the reads to the TAIR9 Col-0 reference genome. The consensus sequences were computed, which were used to create a matrix containing the genotypes at specific marker positions for all F2 individuals (S4 Table). We only considered bi-allelic SNPs that agreed with SNPs from both parents and the F1 hybrid of a given cross. Scripts are available at https://github.com/mzaidem/ACD6_suppressors_mapping_RADseq_scripts/tree/master.
QTL mapping and analysis
Pro-0 x Est-1, Rmx-A180 x Est-1 and Bs-5 x Est-1 F2 individuals were phenotyped for late-onset necrosis in a binary manner. QTL mapping and testing for QTL effects and interactions were performed using the R/qtl package [94]. Lod scores were calculated under a single-QTL model using the function ‘‘scanone”. Lod score significance thresholds were established using 1,000 permutations. Bayesian credible intervals were estimated for individual QTL. The standard expectation-maximization algorithm was always used as ‘‘method.” Using the markers closest to the two highest LOD scores, designated as Q1 and Q2, we conducted direct linear modeling. The “Full” model has the equation: y ~ Q1 + Q2 + Q1:Q2, where the additive effect as well as the interaction effect of the selected QTL’s are used to calculate the amount of necrotic phenotype variation explained by the model. The “Additive” model (y ~ Q1 + Q2), calculates how much of the necrotic phenotype variation observed is only due to the additive effect of Q1 and Q2. While the “Interaction” model (y ~ Q1:Q2), accounts for how much of the necrotic phenotype variation observed is due to the interaction of Q1 and Q2.
Transgenic lines
ACD6 fragments were amplified from genomic DNA with PCR primers designed based on ACD6 from Est-1 ([36], S7 Table). The Pro-0 (Rmx-A160) genomic DNA fragment was 9.1 (10.3) kb long, including 2.5 (1.8) kb sequences upstream of the initiation codon and 2.8 (3.2) kb downstream of the stop codon. Restriction enzyme sites of Gibson Assembly (NEB, Ipswich, the USA) were used to generate chimeric constructs [95]. An amiRNA against ACD6 (TTAATGGTGACTAAAGGCCGT) [36] was used to knock down ACD6. All constructs were individually cloned into the Gateway entry vector pCR8/GW/TOPO (Invitrogen) and moved into binary vector pFK206 by LR reaction (Thermo Scientific, Waltham, MA, USA). Constructs in pFK206 were transferred to Agrobacterium tumefaciens strain ASE and plants were transformed by floral dipping [96] (S6 Table).
Phylogenetic analysis of RPP4/RPP5/SNC1 protein sequences
SNC1 (AT4G16890), RPP4 (AT4G16860), and RPP5 (AT4G16950) like protein sequences from 63 accessions were extracted from an in-house NLR database of Araport II. These sequences and the single RPP4/5 homolog of Arabidopsis lyrata were used as template to query the database with exonerate v2.2.0 allowing a minimal mapping score of 95% [97]. We used MEGA6 [98] to realign these sequences with SNC1-Est-1, SNC1-Pro-0, SNC1-Ws-2 (SNW, GenBank accession AY510018), RPP5-Ler-0 (AF180942) and the RPP4/RPP5/SNC1 homolog At4g16900 from Col-0 (Araport II). One-hundred thirty-six RPP4/5 protein sequences were used to infer phylogenetic relationships with both Neighbor-Joining (NJ) and Maximum likelihood (ML) approaches in MEGA6, either over their entire lengths or only the TIR domain (positions 1–226 in SNC1-Col-0). Node confidence was estimated with 1,000 bootstrap replications. All sequences that we could confidently assign to the SNC1 clade were aligned separately to identify functional polymorphisms. A similar approach was used to generate a phylogenetic tree of ACD6 protein sequences extracted from the in-house NLR database, using ACD6 (AT4G14400) and the adjacent ACD6 homolog AT4G14390 from Araport II as references.
Bacterial infection
Pseudomonas syringae pv tomato strain DC3000 was grown to OD600 around 3.0, collected and resuspended in 10 mM MgCl2 at 5 × 105 colony-forming units (cfu)/ml. The bacterial suspension was infiltrated into 4-week-old leaves with a needle-less syringe. Bacterial growth was determined 3 days post inoculation (dpi).
Transient expression in N. benthamiana and ion-leakage measurements
Agrobacterium tumefaciens with binary plasmids was grown to OD600 of 1.6, and incubated in induction medium (10 mM MES pH 5.6, 10 mM MgCl2 and 150 μM acetosyringone) for three hours. Cell suspensions were normalized to OD600 of 0.8 for co-infiltration into the abaxial side of leaves of N. benthamiana leaves that had been grown under SD at 23°C. Images were taken 7 days after infiltration. Conductivity assays to measure ion leakage were performed according to [58]. Six independent leaves were infiltrated with A. tumefaciens with binary constructs encompassing different SNC1 alleles and two discs (0.6 mm diameter) were harvested per leaf 5 days after infiltration. The fresh leaves were briefly rinsed with ddH2O and placed in a tube with 8 ml ddH2O. Samples were gently shaken for 24 hr before measuring ion content (C1) using an Orion Conductivity (Thermo Scientific, Waltham, MA, USA) instrument. Total ion content (C2) was determined from leaf samples that had been boiled for 15 min. C1/C2 ratios were calculated as indicators of ion leakage. Nicotiana benthamiana leaves expressing GFP only were used as control. Statistic differences were calculated by one-way-ANOVA.
Supporting information
(TIF)
(TIF)
Gray indicates structural variants including small and large deletions.
(TIF)
(TIF)
(TIF)
Bootstrap values over 60% are indicated. Est-1-like clade highlighted in red.
(TIF)
Samples were harvested 2 d after infiltration.
(TIF)
QTL intervals are indicated by unfilled triangles.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank the members of the GBMF-funded Ren-seq Team, Freddy Monteiro, Jeff Dangl, Oliver Furzer, Kamil Witek and Jonathan Jones, for their help in generating the Ren-seq NLR database. We thank the ZMBP Analytics Laboratory from the University of Tübingen for help in salicylic acid quantification. We thank Chang Liu, Oliver Furzer, Jeffery Dangl, Mark Stahl and the Weigel lab for comments.
Data Availability
The sequences of the ACD6 from various strains are available through Genbank accession numbers MH042080-MH042081. The sequences of the SNC1 and other members from RPP4/5 cluster from various strains are available through Genbank accession numbers MH042082-MH042105 and MH042112-MH042229.
Funding Statement
This work was supported by European Research Council, Advanced Grants, IMMUNEMESIS, and the Max Planck Society. The NLR RenSeq database was generated with generous support from the Gordon and Betty Moore Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Maekawa T, Kufer TA, Schulze-Lefert P. NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol. 2011;12: 817–826. 10.1038/ni.2083 [DOI] [PubMed] [Google Scholar]
- 2.Huot B, Yao J, Montgomery BL, He SY. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol Plant. 2014;7: 1267–1287. 10.1093/mp/ssu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ning Y, Liu W, Wang G-L. Balancing Immunity and Yield in Crop Plants. Trends Plant Sci. 2017; 10.1016/j.tplants.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 4.Brown JKM. Yield penalties of disease resistance in crops. Curr Opin Plant Biol. 2002;5: 339–344. [DOI] [PubMed] [Google Scholar]
- 5.Heil M, Baldwin IT. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 2002;7: 61–67. [DOI] [PubMed] [Google Scholar]
- 6.Burdon JJ, Thrall PH. The fitness costs to plants of resistance to pathogens. Genome Biol. 2003;4: 227 10.1186/gb-2003-4-9-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis JG, Lagudah ES, Spielmeyer W, Dodds PN. The past, present and future of breeding rust resistant wheat. Front Plant Sci. 2014;5: 641 10.3389/fpls.2014.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tellier A, Brown JKM. Spatial heterogeneity, frequency-dependent selection and polymorphism in host-parasite interactions. BMC Evol Biol. 2011;11: 319 10.1186/1471-2148-11-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroymann J, Mitchell-Olds T. Epistasis and balanced polymorphism influencing complex trait variation. Nature. 2005;435: 95–98. 10.1038/nature03480 [DOI] [PubMed] [Google Scholar]
- 10.Tian D, Araki H, Stahl E, Bergelson J, Kreitman M. Signature of balancing selection in Arabidopsis. Proc Natl Acad Sci U S A. 2002;99: 11525–11530. 10.1073/pnas.172203599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature. 2003;423: 74–77. 10.1038/nature01588 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto E, Takashi T, Morinaka Y, Lin S, Wu J, Matsumoto T, et al. Gain of deleterious function causes an autoimmune response and Bateson-Dobzhansky-Muller incompatibility in rice. Mol Genet Genomics. 2010;283: 305–315. 10.1007/s00438-010-0514-y [DOI] [PubMed] [Google Scholar]
- 13.Karasov TL, Kniskern JM, Gao L, DeYoung BJ, Ding J, Dubiella U, et al. The long-term maintenance of a resistance polymorphism through diffuse interactions. Nature. 2014;512: 436–440. 10.1038/nature13439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicard A, Kappel C, Josephs EB, Lee YW, Marona C, Stinchcombe JR, et al. Divergent sorting of a balanced ancestral polymorphism underlies the establishment of gene-flow barriers in Capsella. Nat Commun. Nature Publishing Group; 2015;6: ncomms8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Periyannan S, Moore J, Ayliffe M, Bansal U, Wang X, Huang L, et al. The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science. 2013;341: 786–788. 10.1126/science.1239028 [DOI] [PubMed] [Google Scholar]
- 16.Jones JDG, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354 10.1126/science.aaf6395 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Dodds PN, Bernoux M. What Do We Know About NOD-Like Receptors In Plant Immunity? Annu Rev Phytopathol. 2017; 10.1146/annurev-phyto-080516-035250 [DOI] [PubMed] [Google Scholar]
- 18.Baggs E, Dagdas G, Krasileva KV. NLR diversity, helpers and integrated domains: making sense of the NLR IDentity. Curr Opin Plant Biol. 2017/August;38: 59–67. 10.1016/j.pbi.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Goritschnig S, Dong X, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003;15: 2636–2646. 10.1105/tpc.015842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Z, Gao Z, Chung E-H, Eitas TK, Dangl JL. Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc Natl Acad Sci U S A. 2011;108: 7619–7624. 10.1073/pnas.1104410108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirano Y, Kachroo P, Shah J, Klessig DF. A gain-of-function mutation in an Arabidopsis Toll Interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell. 2002;14: 3149–3162. 10.1105/tpc.005348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noutoshi Y, Ito T, Seki M, Nakashita H, Yoshida S, Marco Y, et al. A single amino acid insertion in the WRKY domain of the Arabidopsis TIR-NBS-LRR-WRKY-type disease resistance protein SLH1 (sensitive to low humidity 1) causes activation of defense responses and hypersensitive cell death. Plant J. 2005;43: 873–888. 10.1111/j.1365-313X.2005.02500.x [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. Plant J. 2010;63: 283–296. 10.1111/j.1365-313X.2010.04241.x [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Li J, Bao F, Zhang X, Yang S. A gain-of-function mutation in the Arabidopsis disease resistance gene RPP4 confers sensitivity to low temperature. Plant Physiol. 2010;154: 796–809. 10.1104/pp.110.157610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igari K, Endo S, Hibara K-I, Aida M, Sakakibara H, Kawasaki T, et al. Constitutive activation of a CC-NB-LRR protein alters morphogenesis through the cytokinin pathway in Arabidopsis. Plant J. 2008;55: 14–27. 10.1111/j.1365-313X.2008.03466.x [DOI] [PubMed] [Google Scholar]
- 26.Bi D, Johnson KCM, Zhu Z, Huang Y, Chen F, Zhang Y, et al. Mutations in an Atypical TIR-NB-LRR-LIM Resistance Protein Confer Autoimmunity. Front Plant Sci. 2011;2: 71 10.3389/fpls.2011.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah SJA, Hussain S, Ahmad M, Farhatullah, Ali I, Ibrahim M. Using leaf tip necrosis as a phenotypic marker to predict the presence of durable rust resistance gene pair Lr34/Yr18 in wheat. J Gen Plant Pathol. 2011;77: 174–177. 10.1007/s10327-011-0301-6 [DOI] [Google Scholar]
- 28.Corwin JA, Kliebenstein DJ. Quantitative Resistance: More Than Just Perception of a Pathogen. Plant Cell. 2017;29: 655–665. 10.1105/tpc.16.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iakovidis M, Teixeira PJPL, Exposito-Alonso M, Cowper MG, Law TF, Liu Q, et al. Effector-Triggered Immune Response in Arabidopsis thaliana Is a Quantitative Trait. Genetics. 2016;204: 337–353. 10.1534/genetics.116.190678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Balint-Kurti P, Xu M. Quantitative Disease Resistance: Dissection and Adoption in Maize. Mol Plant. 2017;10: 402–413. 10.1016/j.molp.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Kou Y, Wang S. Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol. 2010;13: 181–185. 10.1016/j.pbi.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 32.Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT. The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell. 1999;11: 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H, Rate DN, Song JT, Greenberg JT. ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell. 2003;15: 2408–2420. 10.1105/tpc.015412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tateda C, Zhang Z, Shrestha J, Jelenska J, Chinchilla D, Greenberg JT. Salicylic acid regulates Arabidopsis microbial pattern receptor kinase levels and signaling. Plant Cell. 2014;26: 4171–4187. 10.1105/tpc.114.131938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Tateda C, Jiang S-C, Shrestha J, Jelenska J, Speed DJ, et al. A suite of receptor-like kinases and a putative mechano-sensitive channel are involved in autoimmunity and plasma membrane-based defenses in Arabidopsis. Mol Plant Microbe Interact. 2017; 10.1094/MPMI-09-16-0184-R [DOI] [PubMed] [Google Scholar]
- 36.Todesco M, Balasubramanian S, Hu TT, Traw MB, Horton M, Epple P, et al. Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature. 2010;465: 632–636. 10.1038/nature09083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Świadek M, Proost S, Sieh D, Yu J, Todesco M, Jorzig C, et al. Novel allelic variants in ACD6 cause hybrid necrosis in local collection of Arabidopsis thaliana. New Phytol. 2016; 10.1111/nph.14155 [DOI] [PubMed] [Google Scholar]
- 38.Todesco M, Kim S-T, Chae E, Bomblies K, Zaidem M, Smith LM, et al. Activation of the Arabidopsis thaliana immune system by combinations of common ACD6 alleles. PLoS Genet. 2014;10: e1004459 10.1371/journal.pgen.1004459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fusari CM, Kooke R, Lauxmann MA, Annunziata MG, Encke B, Hoehne M, et al. Genome-wide Association Mapping Reveals that Specific and Pleiotropic Regulatory Mechanisms Fine-tune Central Metabolism and Growth in Arabidopsis. Plant Cell. American Society of Plant Biologists; 2017; 10.1105/tpc.17.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flood PJ. Natural genetic variation in Arabidopsis thaliana photosynthesis. http://edepot.wur.nl/361162
- 41.Kerwin R, Feusier J, Corwin J, Rubin M, Lin C, Muok A, et al. Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. Elife. 2015;4 10.7554/eLife.05604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinhold A, Karimi Dorcheh E, Li R, Rameshkumar N, Baldwin IT. Antimicrobial peptide expression in a wild tobacco plant reveals the limits of host-microbe-manipulations in the field. Elife. 2018;7 10.7554/eLife.28715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malmberg RL, Held S, Waits A, Mauricio R. Epistasis for fitness-related quantitative traits in Arabidopsis thaliana grown in the field and in the greenhouse. Genetics. 2005;171: 2013–2027. 10.1534/genetics.105.046078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corwin JA, Copeland D, Feusier J, Subedy A, Eshbaugh R, Palmer C, et al. The Quantitative Basis of the Arabidopsis Innate Immune System to Endemic Pathogens Depends on Pathogen Genetics. PLoS Genet. 2016;12: e1005789 10.1371/journal.pgen.1005789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang N, Lariviere A, Tonsor SJ, Traw MB. Constitutive camalexin production and environmental stress response variation in Arabidopsis populations from the Iberian Peninsula. Plant Sci. 2014;225: 77–85. 10.1016/j.plantsci.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 46.Thomma BP, Nürnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell. 2011;23: 4–15. 10.1105/tpc.110.082602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S, et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17: 132 10.1186/s13059-016-0997-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.1001 Genomes Consortium. 1,135 Genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell. 2016;166: 481–491. 10.1016/j.cell.2016.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol. 2012;12: 89–100. 10.1038/nri3141 [DOI] [PubMed] [Google Scholar]
- 50.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18: 1121–1133. 10.1105/tpc.105.039834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60: 379–406. 10.1146/annurev.arplant.57.032905.105346 [DOI] [PubMed] [Google Scholar]
- 52.Rowan BA, Seymour DK, Chae E, Lundberg DS, Weigel D. Methods for Genotyping-by-Sequencing. Methods Mol Biol. 2017;1492: 221–242. 10.1007/978-1-4939-6442-0_16 [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Li S, Bi D, Cheng YT, Li X, Zhang Y. SRFR1 negatively regulates plant NB-LRR resistance protein accumulation to prevent autoimmunity. PLoS Pathog. 2010;6: e1001111 10.1371/journal.ppat.1001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gou M, Hua J. Complex regulation of an R gene SNC1 revealed by auto-immune mutants. Plant Signal Behav. 2012;7: 213–216. 10.4161/psb.18884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi H, Richards EJ. A cluster of disease resistance genes in Arabidopsis is coordinately regulated by transcriptional activation and RNA silencing. Plant Cell. 2007;19: 2929–2939. 10.1105/tpc.107.051821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Biezen EA, Freddie CT, Kahn K, Parker JE, Jones JDG. Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confers downy mildew resistance through multiple signalling components. Plant J. 2002;29: 439–451. [DOI] [PubMed] [Google Scholar]
- 57.Parker JE, Coleman MJ, Szabò V, Frost LN, Schmidt R, van der Biezen EA, et al. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell. 1997;9: 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Bernoux M, Bentham AR, Newman TE, Ve T, Casey LW, et al. Multiple functional self-association interfaces in plant TIR domains. Proc Natl Acad Sci U S A. 2017;114: E2046–E2052. 10.1073/pnas.1621248114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S, Hua J. A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell. 2004;16: 1060–1071. 10.1105/tpc.020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stokes TL, Kunkel BN, Richards EJ. Epigenetic variation in Arabidopsis disease resistance. Genes Dev. 2002;16: 171–182. 10.1101/gad.952102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Kapos P, Zhang Y. NLRs in plants. Curr Opin Immunol. 2015;32: 114–121. 10.1016/j.coi.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 62.Jupe F, Witek K, Verweij W, Sliwka J, Pritchard L, Etherington GJ, et al. Resistance gene enrichment sequencing (RenSeq) enables reannotation of the NB-LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J. 2013;76: 530–544. 10.1111/tpj.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urbach JM, Ausubel FM. The NBS-LRR architectures of plant R-proteins and metazoan NLRs evolved in independent events. Proc Natl Acad Sci U S A. 2017; 10.1073/pnas.1619730114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K, Dangl JL. Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc Natl Acad Sci U S A. 2011;108: 16463–16468. 10.1073/pnas.1113726108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts M, Tang S, Stallmann A, Dangl JL, Bonardi V. Genetic requirements for signaling from an autoactive plant NB-LRR intracellular innate immune receptor. PLoS Genet. 2013;9: e1003465 10.1371/journal.pgen.1003465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zbierzak AM, Porfirova S, Griebel T, Melzer M, Parker JE, Dörmann P. A TIR-NBS protein encoded by Arabidopsis Chilling Sensitive 1 (CHS1) limits chloroplast damage and cell death at low temperature. Plant J. 2013;75: 539–552. 10.1111/tpj.12219 [DOI] [PubMed] [Google Scholar]
- 67.Atanasov KE, Liu C, Erban A, Kopka J, Parker JE, Alcázar R. NLR mutations suppressing immune hybrid incompatibility and their effects on disease resistance. Plant Physiol. 2018; 10.1104/pp.18.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berkey R, Zhang Y, Ma X, King H, Zhang Q, Wang W, et al. Homologues of the RPW8 Resistance Protein Are Localized to the Extrahaustorial Membrane that Is Likely Synthesized De Novo. Plant Physiol. 2017;173: 600–613. 10.1104/pp.16.01539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang J, Zhu X, Wang Y, Liu L, Xu B, Li F, et al. Semi-dominant mutations in the CC-NB-LRR-type R gene, NLS1, lead to constitutive activation of defense responses in rice. Plant J. 2011;66: 996–1007. 10.1111/j.1365-313X.2011.04557.x [DOI] [PubMed] [Google Scholar]
- 70.Palma K, Thorgrimsen S, Malinovsky FG, Fiil BK, Nielsen HB, Brodersen P, et al. Autoimmunity in Arabidopsis acd11 is mediated by epigenetic regulation of an immune receptor. PLoS Pathog. 2010;6: e1001137 10.1371/journal.ppat.1001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lolle S, Greeff C, Petersen K, Roux M, Jensen MK, Bressendorff S, et al. Matching NLR Immune Receptors to Autoimmunity in camta3 Mutants Using Antimorphic NLR Alleles. Cell Host Microbe. 2017;21: 518–529.e4. 10.1016/j.chom.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 72.Zhao T, Rui L, Li J, Nishimura MT, Vogel JP, Liu N, et al. A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet. 2015;11: e1004945 10.1371/journal.pgen.1004945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bao Z, Yang H, Hua J. Perturbation of cell cycle regulation triggers plant immune response via activation of disease resistance genes. Proc Natl Acad Sci U S A. 2013;110: 2407–2412. 10.1073/pnas.1217024110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Wang Y, Liu J, Ding Y, Wang S, Zhang X, et al. Temperature-dependent autoimmunity mediated by chs1 requires its neighboring TNL gene SOC3. New Phytol. 2017;213: 1330–1345. 10.1111/nph.14216 [DOI] [PubMed] [Google Scholar]
- 75.Stuttmann J, Peine N, Garcia AV, Wagner C, Choudhury SR, Wang Y, et al. Arabidopsis thaliana DM2h (R8) within the Landsberg RPP1-like Resistance Locus Underlies Three Different Cases of EDS1-Conditioned Autoimmunity. PLoS Genet. 2016;12: e1005990 10.1371/journal.pgen.1005990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, et al. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe. 2012;11: 253–263. 10.1016/j.chom.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Pennington BO, Hua J. Multiple R-like genes are negatively regulated by BON1 and BON3 in arabidopsis. Mol Plant Microbe Interact. 2009;22: 840–848. 10.1094/MPMI-22-7-0840 [DOI] [PubMed] [Google Scholar]
- 78.Xing Z, Tan F, Hua P, Sun L, Xu G, Zhang Q. Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor Appl Genet. 2002;105: 248–257. 10.1007/s00122-002-0952-y [DOI] [PubMed] [Google Scholar]
- 79.Paaby AB, Rockman MV. Cryptic genetic variation: evolution’s hidden substrate. Nat Rev Genet. 2014;15: 247–258. 10.1038/nrg3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chow CY, Kelsey KJP, Wolfner MF, Clark AG. Candidate genetic modifiers of retinitis pigmentosa identified by exploiting natural variation in Drosophila. Hum Mol Genet. 2016;25: 651–659. 10.1093/hmg/ddv502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang W, Richards S, Carbone MA, Zhu D, Anholt RRH, Ayroles JF, et al. Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc Natl Acad Sci U S A. 2012;109: 15553–15559. 10.1073/pnas.1213423109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan AI, Dinh DM, Schneider D, Lenski RE, Cooper TF. Negative epistasis between beneficial mutations in an evolving bacterial population. Science. 2011;332: 1193–1196. 10.1126/science.1203801 [DOI] [PubMed] [Google Scholar]
- 83.Chae E, Bomblies K, Kim S-T, Karelina D, Zaidem M, Ossowski S, et al. Species-wide genetic incompatibility analysis identifies immune genes as hot spots of deleterious epistasis. Cell. 2014;159: 1341–1351. 10.1016/j.cell.2014.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Z, Shrestha J, Tateda C, Greenberg JT. Salicylic acid signaling controls the maturation and localization of the arabidopsis defense protein ACCELERATED CELL DEATH6. Mol Plant. 2014;7: 1365–1383. 10.1093/mp/ssu072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartels S, Anderson JC, González Besteiro MA, Carreri A, Hirt H, Buchala A, et al. MAP kinase phosphatase1 and protein tyrosine phosphatase1 are repressors of salicylic acid synthesis and SNC1-mediated responses in Arabidopsis. Plant Cell. 2009;21: 2884–2897. 10.1105/tpc.109.067678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. Network properties of robust immunity in plants. PLoS Genet. 2009;5: e1000772 10.1371/journal.pgen.1000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Underwood W, Zhang S, He SY. The Pseudomonas syringae type III effector tyrosine phosphatase HopAO1 suppresses innate immunity in Arabidopsis thaliana. Plant J. 2007;52: 658–672. 10.1111/j.1365-313X.2007.03262.x [DOI] [PubMed] [Google Scholar]
- 88.Sato M, Tsuda K, Wang L, Coller J, Watanabe Y, Glazebrook J, et al. Network modeling reveals prevalent negative regulatory relationships between signaling sectors in Arabidopsis immune signaling. PLoS Pathog. 2010;6: e1001011 10.1371/journal.ppat.1001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chae E, Tran DTN, Weigel D. Cooperation and Conflict in the Plant Immune System. PLoS Pathog. 2016;12: e1005452 10.1371/journal.ppat.1005452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18: 265–276. 10.1046/j.1365-313X.1999.00265.x [DOI] [PubMed] [Google Scholar]
- 92.Poland JA, Brown PJ, Sorrells ME, Jannink J-L. Development of High-Density Genetic Maps for Barley and Wheat Using a Novel Two-Enzyme Genotyping-by-Sequencing Approach. PLoS One. 2012;7: e32253 10.1371/journal.pone.0032253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19: 889–890. [DOI] [PubMed] [Google Scholar]
- 95.Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6: 343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 96.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16: 735–743. [DOI] [PubMed] [Google Scholar]
- 97.Slater GSC, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;6: 31 10.1186/1471-2105-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Gray indicates structural variants including small and large deletions.
(TIF)
(TIF)
(TIF)
Bootstrap values over 60% are indicated. Est-1-like clade highlighted in red.
(TIF)
Samples were harvested 2 d after infiltration.
(TIF)
QTL intervals are indicated by unfilled triangles.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The sequences of the ACD6 from various strains are available through Genbank accession numbers MH042080-MH042081. The sequences of the SNC1 and other members from RPP4/5 cluster from various strains are available through Genbank accession numbers MH042082-MH042105 and MH042112-MH042229.