Abstract
The pancreas is made from two distinct components: the exocrine pancreas, a reservoir of digestive enzymes, and the endocrine islets, the source of the vital metabolic hormone insulin. Human islets possess limited regenerative ability; loss of islet β-cells in diseases such as type 1 diabetes requires therapeutic intervention. The leading strategy for restoration of β-cell mass is through the generation and transplantation of new β-cells derived from human pluripotent stem cells. Other approaches include stimulating endogenous β-cell proliferation, reprogramming non-β-cells to β-like cells, and harvesting islets from genetically engineered animals. Together these approaches form a rich pipeline of therapeutic development for pancreatic regeneration.
The pancreas is central to the control of energy consumption and metabolism and is composed of two morphologically and functionally distinct components: the exocrine pancreas (acinar cells and ductal cells) and the endocrine pancreas (islets of Langerhans).Exocrine acinar cells produce an array of digestive enzymes, including lipases, proteinases, and amylases, which are secreted into pancreatic ducts and flow into the small intestine to break down fats, proteins, and carbohydrates for absorption. The endocrine islets represent less than 5% of total pancreatic mass but nevertheless number more than a billion cells in humans. Each of the five major types of islet cell synthesizes and secretes a principle hormone: insulin (β-cells), glucagon (α-cells), somatostatin (δ-cells), pancreatic polypeptide (PP cells), and ghrelin (ε-cells). Insulin and glucagon are released directly into the blood circulation through a dense intra-islet vascular network and have essential roles in the regulation of blood glucose levels
Distinct diseases afflict the exocrine and endocrine pancreas. Pancreatitis and pancreatic cancers, the majority of which are ductal carcinomas, originate from the exocrine pancreas whereas diabetes and rare pancreatic neuroendocrine tumours arise from the endocrine islets. Diabetes has been estimated to afflict well over 300 million people worldwide and is a major and growing health problem in the modern world. Complications resulting from long-term diabetes include kidney failure, peripheral vascular disease, stroke, and coronary artery disease; together, these complications create enormous medical and social burdens as well as causing premature deaths. The majority of diabetic patients suffer from type 2 diabetes (T2D), a disease attributed to insulin resistance by peripheral organs including liver, fat, and muscle. Recent genetic linkage studies and histological analyses have shown that patients with T2D also have significantly fewer islet β-cells than healthy individuals1–4. Type 1 diabetes (T1D), which makes up about 5–10% of all diabetes cases, is an autoimmune disease in which β-cells are selectively destroyed, leading to a severe insulin deficiency that must be treated with daily insulin injections for survival. Together, these diseases account for a large and growing patient population with pancreatic β-cell deficiency.
There is a long history of investigations into pancreatic regeneration, going back nearly a century5. The epidemic of diabetes in recent decades has spurred numerous studies on pancreas development, homeostasis, and regeneration. Animal studies have suggested that the exocrine pancreas possesses an intrinsic capacity for regeneration and thus can make a rapid and full recovery from exocrine diseases such as acute pancreatitis. By contrast, the endocrine islets have limited regenerative capacity in adults. Indeed, it remains unclear whether the adult human pancreas can spontaneously regenerate β-cells in any physiologically meaningful way. Substantial β-cell loss therefore results in permanent endocrine deficiency and irreversible diabetes. There is an increasing consensus that a regenerative medicine approach will be helpful, even essential, in treating certain forms of diabetes including T1D and possibly the subset of T2D in which there is substantial β-cell loss. Learning how to enhance or induce the intrinsic regenerative ability of endocrine islets and devising new strate-gies to produce insulin-secreting β-cells will have profound implications for developing therapeutic treatment for diabetes. Here we summarize our current understanding of pancreatic endocrine and exocrine regeneration and review the different strategies for therapeutic regeneration and repair.
Regeneration of the endocrine pancreas
The majority of studies on pancreas regeneration have focused on endocrine islets, owing to their central importance in diabetes. Historically, studies of islet regeneration relied on rodent injury models, including pancreatectomy, pancreatic duct ligation, and chemical ablation of islet cells. In pancreatectomy, removal of up to 90% of the rat pancreas does not affect glucose homeostasis, suggesting a large reserve capacity, as 10% of the islet mass is sufficient to maintain blood glucose control6–8. By contrast, resection of 50–60% of the pancreas in humans triggers insulin-dependent diabetes9,10. Young rodents show tissue growth and sprouting from the cut surface after pancreatectomy6,7. Observations of rare samples from children also suggest tissue growth after pancreatectomy11. The capacity for this type of regeneration, however, declines sharply in adult animals and is absent in adult humans8,10,12.
A second injury model used to study pancreas regeneration is duct ligation which mimics obstructive pancreatitis. Physical ligation of the pancreatic ducts causes widespread acinar cell death, but the endocrine islets are spared and no substantial endocrine regeneration is observed13,14. In a third injury model, pancreatic β-cells can be specifically ablated using streptozotocin (STZ) or alloxan, chemical toxins that structurally mimic glucose and are selectively imported into β-cells. Depending on drug dosage, the entire β-cell mass can be partially or nearly completely ablated in a few days. Extensive studies have found no convincing evidence for β-cell regeneration in adult animals following chemical ablation12,15.
Despite the lack of substantial islet regeneration in injury models, islet hyperplasia is observed during pregnancy, in obesity, or under insulin resistance conditions in animal models16–19. For instance, mouse pancreatic β-cell mass increases by 3–5-fold during pregnancy, stimulated at least partly by the pregnancy hormones placental lactogen and prolactin, and involving signalling through serotonin, Menin, and FoxM120–23. High-fat diet-induced obesity in mice is also accompanied by impressive increases in islet cell mass24 Experimentally induced insulin resistance, such as liver-specific knockout of insulin receptors, induces up to a tenfold increase in β-cell mass25. The molecular pathways that drive these increases in β-cell mass in obesity and insulin resistance have yet to be fully elucidated.
Self-replication maintains β-cell mass
The proliferative rate of β-cells is quite high in young rodents, but declines rapidly with age26,27. For example, one study estimated a proliferation rate of approximately 4% per day in one-month-old rats and 0.5% per day in seven-month-old rats28. In addition, marked islet hyperplasia can be induced in adult animals by pregnancy or obesity. What is the source of these additional islet cells? In a milestone study, genetic lineage tracing in mice using β-cell-specific drivers showed that the major mechanism for β-cell replenishment in homeostasis or after injury was replication of pre-existing β-cells29 (Fig. 1). The role of replication is much less clear in humans, as very few replicating human β-cells (assessed by histological staining of proliferative antigens such as Ki-67 and PCNA) can be found in pancreas samples taken during autopsy of healthy, injured, pregnant, or obese adult humans3,10,30,31.
Fig. 1 |. Natural regenerative responses of the endocrine pancreas.
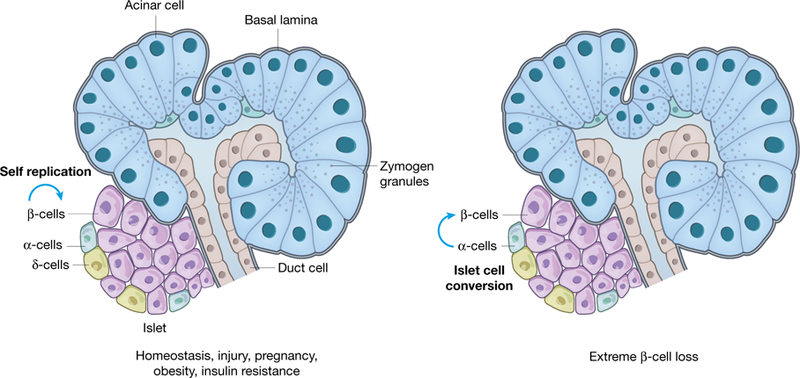
The adult endocrine pancreas (islets of Langerhans) is made up of four major endocrine cell types with each secreting a major hormone: insulin (β-cells), glucagon (α-cells), somatostatin (δ-cells), and pancreatic polypeptide (PP cells). Animal studies have shown that β-cell replication is a major mode of regeneration and repair in homeostasis, injury, pregnancy, obesity, and insulin resistance. Conversion of islet δ- and α-cells into β-cells has been reported after extreme β-cell loss using specific ablation methods in animal models. Significant regeneration of the endocrine pancreas is largely restricted to young children and young animals. Adult animals and adult humans have little, if any, ability to regenerate the endocrine pancreas.
α-cells and δ-cells may convert to β-cells
The five principle cell type of the islets, namely β-, α-, δ -, PP, and ε-cells, appear to be highly stable in normal homeostasis or in various injury models. For instance, selective ablation of β-cells with STZ or alloxan does not significantly affect the numbers or phenotypes of other islet cells. It came as a surprise that, when a diphtheria toxin-based β-cell ablation method was used to ablate more than 99% of β-cells in mice, slow but significant recovery of β-cell mass over several months was reported32. Lineage tracing studies suggested that the new insulin-producing cells arose from conversion of pancreatic α- or δ-cells, depending on the age of the mice32,33. The molecular mechanism of this conversion between islet cell types is unknown, as is whether such conversions also occur in humans. There is no clear evidence for this type of conversion in patients with T1D, but that could be either because it does not occur or because the converted cells are eliminated by the ongoing autoimmune process. Nevertheless, these studies suggest another mechanism that can potentially regenerate part of the endocrine compartment (Fig. 1).
The search for adult pancreatic stem cells
There is a long-standing hypothesis that pancreatic stem or progenitor cells might exist in the adult animal or even human pancreas34. This hypothesis was initially based on histological observations of single islet cells and small islets embedded in or closely associated with adult rodent and human pancreatic ducts, suggesting the emergence of new islet cells from ducts (referred to as neogenesis)34. However, genetic lineage-tracing studies using exocrine drivers (Muc1-CreER), acinar-specific drivers (Cela-CreER, Ptf1a-CreER), and duct-specific drivers (Sox9-CreER, Hnf1b-CreER)35–39 consistently indicated rare or no contribution from the exocrine to the endocrine compartment during normal homeostasis or in various injury models. The neogenesis hypothesis has been supported by a report that, after pancreatic duct ligation in mice, a rare population of NGN3+ endocrine precursor cells appeared in ductal structures40 and observations of NGN3+ cells around islets and ducts in experimental models of α-cell to β-cell transdifferentiation41,42. In summary, it remains unclear whether adult pancreatic stem cells exist.
Regeneration of the exocrine pancreas
The exocrine pancreas is composed of acinar cells and duct cells. The most common injury to the exocrine pancreas is pancreatitis, a painful inflammation triggered by various environmental (injury, alcohol, high fat diet and so on) or genetic (for example, cystic fibrosis) factors43. Understanding of exocrine damage and regeneration comes largely from rodent studies of experimental pancreatitis, the most common of which is supraphysiological stimulation of acinar secretion by caerulein, a mouse analogue of the hormone cholecystokinin44,45. Caerulein treatment leads to rapid apoptosis or necrosis of acinar cells and in addition, some acinar cells lose their abundant zymogen granules and shrink considerably to resemble duct cells in a process termed acinar-to-ductal metaplasia46. The animals recover from acute pancreatitis rapidly. Within a few weeks, the exocrine pancreas fully regains its normal cellular architecture and function. Examination of human exocrine tissues from patients with pancreatitis also shows ductal metaplasia and cell proliferation47,48. Although patients with acute pancreatitis can make a full recovery, it is unclear whether their exocrine pancreas undergoes similar spontaneous repair and regeneration to that seen in animal models.
Two distinct modes of regeneration have been proposed to occur in models of pancreatitis49 (Fig. 2). In the classical regeneration mode, new acinar cells are produced from proliferation of pre-existing acinar cells35,50,51. In the second regeneration mode, the degranulated and duct-like acinar cells are believed to ‘redifferentiate’ and revert back to a normal and functional acinar state. The dedifferentiated acinar cells have not been tracked with a lineage marker and their redifferentiation has been inferred by indirect means. Mechanistic studies in animal models have identified several genes and pathways required for the exocrine regenerative response in pancreatitis. Deletion of key components of the Hedgehog, Notch, and Wnt pathways from acinar cells severely disrupts exocrine regeneration52–54, as does deletion of the acinar-restricted transcription factors NR5A2 and PTF1A55,56. The dedifferentiated state of acinar cells appears to represent a vulnerability in which environmental and genetic factors could conspire to induce neoplastic transformation towards the deadly pancreatic cancers57,58. The mechanisms that control regeneration versus neoplastic transformation are not yet understood.
Fig. 2 |. Regeneration of the exocrine pancreas.
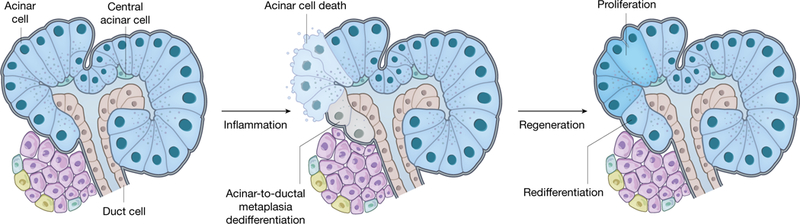
The exocrine pancreas is composed of acinar cells that synthesize and secrete digestive enzymes, ductal cells that funnel the enzymes into the small intestine, and central acinar cells. The exocrine pancreas can regenerate spontaneously and robustly in both animals and humans. Inflammatory injuries to the exocrine pancreas such as acute pancreatitis lead to acinar cell death and acinar dedifferentiation, which is characterized by degranulation and morphological transformation into duct-like cells in a process termed acinar-to-ductal metaplasia. Once inflammation subsides, acinar cells can rapidly regenerate by self-replication and possible redifferentiation of the metaplastic duct-like cells back into a normal and functional acinar state.
Strategies to produce new endocrine islet cells
Whereas adult mouse pancreatic islets show robust regeneration under physiological challenges such as obesity, insulin resistance, or pregnancy, it is uncertain whether the adult human pancreas can deploy an adaptive regenerative response and, even if it does, these responses are evidently not able to make a significant physiological impact. At the same time, the clinical need for β-cell regeneration therapy is enormous. Approximately 2.5 million people in the USA (and more than 20 million worldwide) suffer from T1D and many millions more patients with T2D have pancreatic β-cell deficiency. Both patient populations could benefit from therapies that restore functional β-cell mass, freeing them from daily insulin injections and avoiding the serious complications that develop from imprecise dosing. The need for β-cell regeneration in patients with T1D is particularly pressing as this disease preferentially affects children and the severe lack of β-cells in T1D can cause life-threatening fluctuations in blood glucose59,60.
Decades of clinical studies have established that cadaveric islet transplantation can be beneficial in patients with T1D, with some patients remaining free from insulin use for years61,62. Nevertheless, clinical cadaveric islet transplantation is used only on a small scale owing to the lack of suitable cadaveric islets and the requirement for long-term immune suppression to combat auto- and alloimmunity. To treat larger populations of patients, it would be beneficial to have a reliable and standardized source of human islets for transplantation, ideally without the need for immunosuppression. Alternatively, therapeutic interventions that stimulate endogenous islet regeneration could be used. In response to the enormous unmet medical need, several research efforts are now underway to evaluate strategies to produce new islets in vitro or stimulate islet regeneration in vivo (Fig. 3).
Fig. 3 |. Therapeutic strategies for regeneration and repair of the endocrine pancreas.
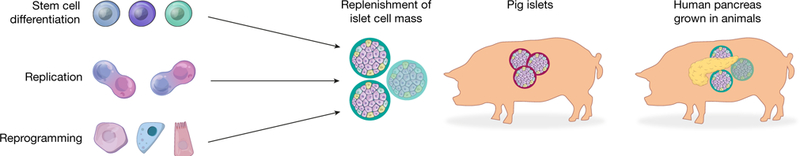
At present, the most advanced technology for making functional human insulin-secreting cells, and the only one to enter clinical trials, is derivation from human pluripotent stem cells. Other strategies include stimulating proliferation of residual β-cells in vivo, reprogramming of non-β-cells to β-like cells in vivo or in vitro, harvesting islets from genetically engineered pigs, and possibly growing entire human pancreata in animals followed by removal of human islets for transplantation.
Differentiation of pluripotent stem cells
Decades of developmental studies in frogs, fish, and mice have mapped out the key steps and critical signalling events that lead from a fertilized egg to the formation of mature islets in early childhood63–65. This deep understanding of pancreatic development was put to the service of regenerative medicine in 1998, when human embryonic stem cells (hES cells) were successfully cultured and opened the door to developing methods of deriving pancreatic islets from hES cells66. This advance was followed in 2006 by the groundbreaking discovery that induced pluripotent stem cells (iPS cells) can be derived from somatic cells such as skin fibroblasts, providing a pathway for generating patient-specific cell products67.
In the first major studies of deriving pancreatic endocrine cells from hES cells, a step-wise protocol was devised using combinations of signalling molecules to guide hES cell differentiation through four successive stages (definitive endoderm, pancreatic epithelium, endocrine progenitors, and β-like cells)68,69. The first differentiations of human stem cells into islet cells produced a population of cells with mixed hormone expression, but not mature or true human β-cells68. These studies, together with the decades of cellular and genetic studies of pancreatic development in animal models, created a blueprint for in vitro differentiation protocols that have been applied to pluripotent stem cells.
More recently, efforts have been directed towards the production of endocrine islets that can respond to glucose. More complicated differentiation protocols have been devised, with additional steps, optimized cocktails of inducing factors and chemicals, and the use of three-dimensional culture methods, which yield cellular clusters with remarkable morphological and functional resemblance to pancreatic islets70,71. Transplantation of these in vitro-derived cell clusters led to further functional maturation in vivo and robust rescue of experimental diabetes in mouse models70,71.
In addition to endocrine islets, pancreatic progenitor cells, some of which have the capacity to produce mature hormone-producing cells, have emerged as a candidate cell therapy product69. Preclinical studies showed that when hES cell-derived PDX1+ progenitors are transplanted into mice, some of these cells undergo growth and differentiation in vivo into functional β-cells that can reverse diabetes69,72. These advances have led to phase I and phase II clinical trials of pancreatic progenitors. More fully differentiated functional human islet clusters are set to enter trials in a few years. For both approaches, the in vitro-derived islet clusters contain both β-cells and other islet cell types (α-cells and δ-cells) that are known to fine-tune the function of β-cells. There are additional cell types present in native islets, including vascular cells and fibroblast-like cells, and incorporating these additional cells into clusters for transplantation may offer some benefit.
Despite marked advances in producing pancreatic endocrine cells from hES cells, important challenges remain. These include perfecting the differentiation protocols for manufacturing at a large scale, eliminating unwanted cells from the final product and, of course, providing protection against immune rejection. Both allo- and autoimmune rejection can in principle be avoided by physical protection in a small device: for example, encapsulation in alginate or a more durable biomaterial. Alternatively, it may be possible to reduce the immune attack by genetic modification of the transplanted cells and/or manipulating the immune system of the recipient.
Patient-specific cell products can be derived from iPS cells, which should avoid immune rejection and be immunologically compatible with the patient from whom the iPS cell was derived. This is, of course, primarily relevant for replacing β-cells in patients with T2D, as patients with T1D suffer from autoimmunity. Manufacturing patient-specific products, however, presents its own challenges, as it will require optimization of differentiation conditions for every batch of iPS cells, adding substantial costs and operational burden to the process.
β-cell replication
Stimulating β-cell proliferation is a simple and intuitive solution to replenishing β-cell mass. Indeed, a large number of growth factors and mitogenic agents have been shown to promote β-cell proliferation in animal models. These include parathyroid hormone-related protein, hepatocyte growth factor, glucagon-like peptide, insulin-like growth factors, gastrin, epidermal growth factors, platelet-derived growth factor, adenosine kinase inhibitors, and others16–18,73–75. However, these agents have generally failed to promote significant proliferation of human β-cells. Substantial proliferation of human β-cells appears to occur naturally only in early childhood (mostly the first year of life)76–79. In all, the weight of evidence indicates that human β-cells are resistant to proliferative stimuli.
There are structural and molecular differences between mouse and human islets. For instance, β-cells are concentrated in the core of mouse islets but are more evenly distributed in human islets. Human β-cells also express several factors, such as MAFB, that are absent from mouse β-cells80, and use GLUT1 rather than GLUT2 as the main glucose transporter81,82. In addition, although rodent β-cells are capable of substantial proliferation and growth in pregnancy, obesity, and insulin-resistant states, such proliferation is limited at best in adult humans. The failure of adult human β-cells to proliferate is puzzling, as they possess the necessary molecular elements that control cell cycle reentry (including cyclins, cyclin-dependent kinases (CDKs), E2F factors, and others). Direct manipulation of this molecular machinery can force human β-cells to proliferate; genetic mutations in cell cycle genes can also give rise to rare pancreatic endocrine hyperplasias such as insulinoma in humans83–85. Nevertheless, many of the cell cycle factors appear to be sequestered in the cytoplasm of mature β-cells86,87. It is unclear why this is the case or under what circumstances the cell cycle factors could be induced to traffic into the nucleus. Broad molecular and epigenetic changes occur as β-cells mature and age, with well-documented loss of EZH2 and BMI1, an increase in cell-cycle inhibitors such as P16INK4a and p18INK4c, and epigenetic changes15,88,89. Some of these changes seem to improve β-cell function90, but they may broadly suppress the ability of β-cells to respond to proliferative stimuli. There is longstanding evidence that insulin and glucose, both of which are elevated in obesity or insulin resistance, may directly stimulate β-cell proliferation91–93. But it remains unclear whether these are the key signals that drive islet hyperplasia.
A potentially important advance has come from high-throughput compound screens that identified inhibitors of dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) as reagents that can potently stimulate proliferation of cultured human β-cells in vitro and transplanted human β-cells in vivo. This provides the first concrete molecular target to manipulate human β-cell proliferation94–96. In addition, other pathways involved in human β-cell proliferation, such as calcineurin and SerpinB1, are being identifed97,98. To advance these reagents into clinics will require controlling the cell type specificity, targeting the intervention to islets, and ensuring that reagents that impinge on conserved cell cycle machineries do not raise the problem of tumour formation.
Reprogramming of non-β-cells to β-like cells
Observations of rare events in developmental cell fate changes go back many decades99. In the well-documented example of newt lens regeneration, removal of the lens leads to proliferation of pigmented epithelial cells surrounding the lens and regeneration of a new lens100. Molecular studies of master regulators of cell lineages such as MYOD further cemented the notion that powerful genetic factors could dictate cell fate choices101. And somatic cell nuclear transfer has demonstrated the potential of nearly every nucleus to be reprogrammed to another cell state102. Accordingly, there has been great interest in using master regulators of β-cell development to convert non-β-cells into insulin-producing cells. An early example is the induction of insulin expression from cultured mouse liver cells103. Other studies confirmed that insulin expression can be induced in non-β-cells, but these cells do not take on the morphological, molecular, and functional properties of pancreatic β-cells and are not reprogrammed to a β-like cell state104–108.
A combinatorial screening strategy showed that a combination of three developmental regulators of β-cells, NGN3, PDX1, and MAFA (referred to as NPM factors), could efficiently convert pancreatic acinar cells into β-like cells after delivery into the adult mouse pancreas using adenoviral vectors109. The induced β-like cells achieved long-term stability and acquired the ability to reverse diabetes110. Further screening identified gastrointestinal epithelial cells as another cell type that could be converted into β-like cells111. Cells from the antral stomach appear to be particularly amenable to such conversion112. In a separate study, conditional deletion of FOXO1 from NGN3+ intestinal endocrine progenitors also led to the formation of insulin-producing cells in the gut113. These studies together suggest that gastrointestinal epithelial cells are a potential source of functional insulin-expressing cells by reprogramming. Other examples of reprogramming mouse cells include cytokine-mediated conversion of acinar cells to insulin-expressing cells, conversion of duct cells to insulin-expressing cells byFBW7 deletion, and conversion of hepatocytes to insulin-producing cells by TGIF2114–116.
Extreme β-cell loss can trigger spontaneous conversion of pancreatic δ- and α-cells into β-cells32,33. Although the molecular mechanisms of these conversion events remain unknown, genetic deletion of ARX, a regulator of α-cell development, or forced expression of PAX4, a regulator of β-cell development, can convert α-cells into β-cells in mouse models117,118. A unique population of insulin-producing cells at the periphery of the islets has recently been proposed to be an inter-mediary in the transition from α-cells to β-cells119. Further studies have identified γ-aminobutyric acid (GABA) signalling as a potential facilitator of the reprogramming event. Long-term GABA treatment in mice led to impressive increase in β-cell mass42.
Despite proof-of-concept demonstrations of β-cell reprogramming in animal models, efforts to reprogram human cells have been less successful. Several studies have suggested that human α-cells in islets can be reprogrammed to become β-cells42,120. Changes in α-cell to β-cell ratios and the appearance of cells positive for both glucagon and insulin have provided some evidence for such conversions; however, in the absence of lineage tracing, direct evidence is still lacking. Other cell types such as pancreatic acinar cells, ductal cells, gall bladder cells, and intestinal cells have also been used to generate insulin-expressing cells, but these cells did not form long-term stable grafts after transplantation, suggesting incomplete cell fate conversion or an unstable epigenetic state121–124. At this point, the main challenge in translating approach into the clinic is to define reliable methods for efficient production of human β-like cells that can develop stable and functional transplants. Besides the in vitro reprogramming approach, in vivo reprogramming in human patients targeting pancreatic α-cells, acinar cells, or gastrointestinal epithelial cells may also be feasible. It will be challenging, however, to optimize in vivo reprogramming protocols for therapeutic use in humans.
Islets from genetically engineered animals
There has been long-standing interest in islet xenotransplantation, and several exploratory clinical xenotransplantation studies with pig islets were conducted decades ago125–127. However, severe immune rejection of xenograft materials by the human immune system and the presence of large numbers of pig retroviruses that may jump species pose substantial obstacles. Recent advances in genetic engineering have led to reconsideration of the possibility of using organs grown in pigs. Using CRISPR–Cas9 technology, a pool of 62 known pig retroviruses was deleted from pig skin cells128,129, which in principle could be used to make pig iPS cells, and subsequently, genetically ‘clean’ pigs as islet donors. Future clinical use of pig islets will depend on advances in encapsulation technology to protect the cells from human immune reactions while ensuring long-term survival and functionality.
Growing human pancreatic tissue in animals
The idea of growing human organs in animals for therapeutic use may seem futuristic. However, advances in stem cell technology and the identification of master regulators of organ formation have spurred efforts to explore this idea using animal models130–132. For example, genetic deletion of PDX1 in rats leads to specific loss of the entire pancreas due to a failure to create the embryonic pancreas. Injection of mouse embryonic stem cells into Pdx1− /− rat blastocysts created mouse–rat chimaeric animals in which all organs were made up of a mixture of mouse and rat cells except for the pancreas, which was derived from mouse cells. Thus, a mouse pancreas was grown in the body of a rat. The mouse islets from these rats can be harvested and transplanted back to diabetic mice to cure their diabetes132. This proof-of-concept experiment between two distinct rodent species provided a glimpse of what the future might hold for growing human organs in animal species. Nevertheless, this idea is still in its infancy. Preliminary studies have suggested that standard hES cells cannot make significant contributions to animal chimaeras133. Further mechanistic understanding may see the development of new methods to reduce species incompatibility and approaches that minimize or eliminate indiscriminate contribution of human cells to chimaeric animals. Aside from these technological challenges, societal consent is likely to be needed to move this technology towards clinical application.
Redifferentiating β-cells
Pancreatic β-cells become dysfunctional under a variety of stress conditions, such as prolonged hyperglycaemia and hyperlipidaemia (T2D), pancreatic inflammation due to chronic pancreatitis or pancreatic cancers (type 3c diabetes), or autoimmune-induced inflammation (T1D). Severe distress could lead to β-cell degranulation and down-regulation of β-cell genes. Recent studies have suggested that the loss of β-cell properties may represent dedifferentiation characterized by upregulation of genes that are typically expressed in embryonic islet progenitors (such as Neurog3)134. It is unclear whether dedifferentiation is a common characteristic of dysfunctional β-cells, and whether the dedifferentiation process, if it exists in humans, can be reversed. We do know that dysfunctional β-cells can recover in patients with T2D with proper management, such as diet, exercise, or intensive insulin therapy. If pharmacological means can be found to ‘redifferentiate’ the dedifferentiated β-cells, it could constitute a new therapeutic approach for diabetes and may be viewed as a distinct form of regenerative therapy, one that does not involve creation of new cells per se135. This therapy would be most relevant for T2D but could conceivably be helpful for early stage T1D as well.
Challenges of developing cell therapy for T1D
Developing cellular products to treat T1D faces the unique challenge of autoimmunity59,136. To protect the new β-cells, one can use immunosuppressants, the standard treatment for T1D patients that receive cadaveric islet transplants. However, many of these drugs are known to be toxic to β-cells, not to mention reducing the patient’s immune capacity. An alternative way to protect transplanted β-cells is encapsulation with engineered materials. Encapsulation physically separates β-cells from immune cells but it also separates β-cells from blood vessels, thus altering the kinetics of glucose sensing and oxygen and nutrient delivery, and potentially compromising the survival and function of the encapsulated cells. Innovative encapsulation materials are being developed to address these issues137–140. An alternative way to protect β-cells in patients with T1D is to modulate the immune system. Different immunotherapy regiments have been shown to attenuate or even completely arrest autoimmune attacks in the non-obese diabetic (NOD) mouse model141–143. Unfortunately, in clinical trials, these agents did not demonstrate a significant benefit for patients.
The current cell therapies for T1D require the use of encapsulation devices or immunosuppressive agents, with drawbacks and risks. Looking ahead, it would be ideal to find ways to produce islets that naturally resist autoimmunity. How might this be done? One important clue comes from studies of patients with longstanding T1D (such as the Joslin Medalist study), which made the surprising finding that a considerable number of these patients have detectable insulin production with preservation of glucose responsiveness, suggesting that some β-cells may evade autoimmunity and continue to function144,145. Another possibility is that new β-cells may be continuously produced in some patients. The autoimmune attack within the pancreas itself is also not uniform. Rather, some islets or even entire pancreatic lobes have been observed to escape immune destruction while surrounded by lobes depleted of β-cells60. These data suggest that human β-cells may be heterogeneous, and that a subpopulation of β-cells may resist the autoimmune attack. These clinical observations are consistent with accumulating evidence that mouse and human β-cells in normal islets are heterogeneous in their molecular signatures or proliferativepotential146–148. A subset of β-cells has also been proposed to serve as ‘hubs’ for initiating pulsatile insulin release149. At present, it is unclear whether the β-cells found in patients with T1D are newly created in response to autoimmunity or are for some reason resistant to immune elimination. Focused studies of these β-cells in human samples and deeper understanding of the heterogeneity of human β-cells may eventually yield molecular targets that allow the production of functional insulin-secreting cells that resist autoimmunity. β-Cells derived from reprogramming of α-cells have been shown to resist autoimmunity in mouse studies, providing another potential path to study and possibly produce autoimmune-resistant β-cells120. Finally, it may be possible to genetically modify β-cells so that they are able to avoid detection or elimination by the autoimmune cells.
Future perspectives
We have learned a great deal about how the pancreas develops during embryogenesis and the different regenerative responses the pancreas mounts to physiological challenges and injuries. These insights are now being used to formulate regenerative strategies by differentiating stem cells, reprogramming non-β-cells, and other approaches. Fundamental research into pancreas development and homeostasis will continue to provide new insights that inspire alternative therapeutic approaches. For instance, studies of islet formation during embryogenesis may help to refine protocols for differentiating hES cells into 3D islet clusters; deeper understanding of how islets transition from the immature to mature state in postnatal development should facilitate efforts to produce functionally mature β-cells in vitro; and investigation of signals that mediate physiological expansion of β-cell mass in obesity and insulin resistance could lead to novel β-cell proliferation reagents without significant tumorigenic risks.
In spite of major advances in our understanding of pancreas regeneration, key questions remain. To name just a few: is there convincing evidence for stem cells in the adult pancreas? How heterogeneous are pancreatic β-cells in terms of function and immunological properties? What mechanisms are used by the human pancreas for natural regeneration and repair? To answer these questions, a diverse array of model systems including rodents, zebrafish, large animals, primates, and others are likely to be informative. New technologies will play an important part in advancing these studies. Single-cell analysis will provide an unprecedented view of the heterogeneity of normal and diseased islet cells, capture rare cells relevant to endocrine regeneration or immune resistance, and define transitional states from hES cells to mature β-cells or from non-β-cells to β-like cells; live cell imaging at the single-cell level will enable direct visualization of calcium waves, insulin release, and immune interactions in intact islets in vivo; humanized mouse models and human organoids could serve as surrogates to study human pancreas biology; and human genetic studies and CRISPR–Cas technology may lead to the discovery of new factors in pancreas disease and regeneration.
Clinical trials of islet cell products derived from hES cells have begun. Other approaches, including β-cell proliferation and reprogramming, may also reach the point of therapeutic development. Each approach offers certain advantages (Table 1). Beyond the safety and efficacy of these cell products, how they will fare in the T1D autoimmune environment may be a crucial determinant for their success. We are again reminded here that the ultimate goal for T1D therapy is a cell product that will naturally resist or evade autoimmunity and requires no encapsulation or immunosuppression. Close collaboration between immunologists and β-cell biologists will be necessary to make timely progress on this goal and if we succeed, it will not only benefit patients with T1D, but also offer crucial lessons in finding cures for many other autoimmune diseases.
Table 1 |.
Comparison of current approaches to producing new β-cells
| Stem cell differentiation | Replication | In vivo reprogramming |
Ex vivo reprogramming |
Xenografts | Human pancreas | |
|---|---|---|---|---|---|---|
| Stage of development | Phase I/II clinical | Proof-of-concept with human islets |
Proof-of concept in animal models |
Proof-of-concept With human cells |
Proof-of-concept with pig cells |
Concept developmet in animal models |
| Advantages | Unlimited supply, Standardized productin |
No transplantation necessary |
No transplantation necessary |
Relatively simple Production process |
Unlimitedsupply, lower cost |
Unlimited supply |
| Tumour risk | Moderate (teratoma) | High | Unknown, potentially high |
Moderate | Low | Low |
| Patient specificity | Possible with iPS cells | Yes | Yes | Possible with patient cells |
No | Possible with iPScells |
| Issues | Complex production process |
Targeted delivery required |
Targeted delivery required |
Stability of cell product |
Targeted delivery required |
Feasibility unknown, ethical concerns |
Acknowledgements
We apologize that we were unable to cite many studies owing to space limitations. We thank past and present members of our laboratories and colleagues for their insights and contributions. Q.Z. and D.A.M. receive support from National Institute of Health (NIH) and Harvard Stem Cell Institute (HSCI), and D.A.M. from Howard Hughes Medical Institute (HHMI).
Footnotes
Competing interests D.A.M. is a founder of Semma Therapeutics Inc. Q.Z. declares no competing interests.
References
- 1.McCarthy MI Genomics, type 2 diabetes, and obesity. N. Engl. J. Med 363, 2339–2350 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Flannick J & Florez JC Type 2 diabetes: genetic data sharing to advance complex disease research. Nat. Rev. Genet 17, 535–549 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Butler AE et al. β-cell de cit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Rahier J, Guiot Y, Goebbels RM, Sempoux C & Henquin JC Pancreaticβ-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab 10 (Suppl. 4), 32–42 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Slack JM Developmental biology of the pancreas. Development 121, 1569–1580 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Lehv M & Fitzgerald PJ Pancreatic acinar cell regeneration. IV. Regeneration after resection. Am. J. Pathol 53, 513–535 (1968). [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner-Weir S, Trent DF & Weir GC Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J. Clin. Invest 71, 1544–1553 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, Saito H, Rychahou PG, Uchida T & Evers BM Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology 128, 1391–1404 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Kumar AF, Gruessner RW & Seaquist ER Risk of glucose intolerance and diabetes in hemipancreatectomized donors selected for normal preoperative glucose metabolism. Diabetes Care 31, 1639–1643 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menge BA et al. Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 57, 142–149 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Berrocal T, Luque AA, Pinilla I & Lassaletta L Pancreatic regeneration after near-total pancreatectomy in children with nesidioblastosis. Pediatr. Radiol 35,1066–1070 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Rankin MM & Kushner JA Adaptive β-cell proliferation is severely restricted with advanced age. Diabetes 58, 1365–1372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankin MM et al. β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes 62, 1634–1645 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao X et al. No evidence for β cell neogenesis in murine adult pancreas J. Clin. Invest 123, 2207–2217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tschen SI, Dhawan S, Gurlo T & Bhushan A Age-dependent decline in β-cell proliferation restricts the capacity of β-cell regeneration in mice. Diabetes 58, 1312–1320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezza T & Kulkarni RN The regulation of pre- and post-maturational plasticity of mammalian islet cell mass. Diabetologia 57, 1291–1303 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Saunders D & Powers AC Replicative capacity of β-cells and type 1 diabetes. J. Autoimmun 71, 59–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P et al. Diabetes mellitus—advances and challenges in human β-cell proliferation. Nat. Rev. Endocrino l 11, 201–212 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Rieck S & Kaestner KH Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab 21, 151–158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst S, Demirci C, Valle S, Velazquez-Garcia S & Garcia-Ocaña A Mechanisms in the adaptation of maternal β-cells during pregnancy. Diabetes Manag. (Lond.) 1, 239–248 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H et al. Serotonin regulates pancreatic β-cell mass during pregnancy. Nat. Med 16, 804–808 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H et al. Gestational diabetes mellitus resulting from impaired β-cell compensation in the absence of FoxM1, a novel downstream e ector of placental lactogen. Diabetes 59, 143–152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karnik SK et al. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318, 806–809 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Kahn SE, Hull RL & Utzschneider KM Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Michael MD et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000). [PubMed] [Google Scholar]
- 26.Finegood DT, Scaglia L & Bonner-Weir S Dynamics of β-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44, 249–256 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Teta M, Long SY, Wartschow LM, Rankin MM & Kushner JA Very slow turnover of β-cells in aged adult mice. Diabetes 54, 2557–2567 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Montanya E, Nacher V, Biarnés M & Soler J Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of β-cell hyperplasia and hypertrophy. Diabetes 49, 1341–1346 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Dor Y, Brown J, Martinez OI & Melton DA Adult pancreatic β-cells are formed by self-duplication rather than stem-cell di erentiation. Nature 429, 41–46 (2004).This paper used genetic lineage tracing in mouse models and convincingly demonstrated β-cell replication as a major mechanism for maintaining β-cell mass in homeostasis. [DOI] [PubMed] [Google Scholar]
- 30.Saisho Y et al. β-cell mass and turnover in humans: e ects of obesity and aging. Diabetes Care 36, 111–117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler AE et al. Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia 53, 2167–2176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorel F et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010).Data from this paper suggested that mouse pancreatic α-cells could naturally convert to β-cells after extreme β-cell loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chera S et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514, 503–507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguayo-Mazzucato C & Bonner-Weir S Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab 27, 57–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai BM et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet β cell, regeneration. J. Clin. Invest 117, 971–977 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopp JL et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138, 653–665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solar M et al. Pancreatic exocrine duct cells give rise to insulin-producing cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Pan FC et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140, 751–764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopinke D & Murtaugh LC Exocrine-to-endocrine di erentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev. Biol 10, 38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Al-Hasani K et al. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell 26, 86–100 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Ben-Othman N et al. Long-term GABA administration induces α-cell-mediated β-like cell neogenesis. Cell 168, 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Lowenfels AB, Sullivan T, Fiorianti J & Maisonneuve P The epidemiology and impact of pancreatic diseases in the United States. Curr. Gastroenterol. Rep 7, 90–95 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Willemer S, Elsässer HP & Adler G Hormone-induced pancreatitis. Eur. Surg. Res 24 (Suppl. 1), 29–39 (1992). [DOI] [PubMed] [Google Scholar]
- 45.Lerch MM & Gorelick FS Models of acute and chronic pancreatitis. Gastroenterology 144, 1180–1193 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Bockman DE Morphology of the exocrine pancreas related to pancreatitis. Microsc. Res. Tech 37, 509–519 (1997). [DOI] [PubMed] [Google Scholar]
- 47.Bockman DE, Boydston WR & Anderson MC Origin of tubular complexes in human chronic pancreatitis. Am. J. Surg 144, 243–249 (1982). [DOI] [PubMed] [Google Scholar]
- 48.Willemer S & Adler G Histochemical and ultrastructural characteristics of tubular complexes in human acute pancreatitis. Dig. Dis. Sci 34, 46–55 (1989). [DOI] [PubMed] [Google Scholar]
- 49.Murtaugh LC & Keefe MD Regeneration and repair of the exocrine pancreas. Annu. Rev. Physiol 77, 229–249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaine SA et al. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 137, 2289–2296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strobel O et al. In vivo lineage tracing de nes the role of acinar-to-ductal transdi erentiation in in ammatory ductal metaplasia. Gastroenterology 133, 1999–2009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris JP IV, Cano DA, Sekine S, Wang SC & Hebrok M β-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest 120, 508–520 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fendrich V et al. Hedgehog signaling is required for e ective regeneration of exocrine pancreas. Gastroenterology 135, 621–631 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siveke JT et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology 134, 544–555 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Hoang CQ et al. Transcriptional maintenance of pancreatic acinar identity differentiation, and homeostasis by PTF1A. Mol. Cell. Biol. 36, 3033–3047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Figura G, Morris JP IV, Wright CV & Hebrok M Nr5a2 maintains acinar cell di erentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut 63, 656–664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopp JL et al. Identi cation of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, 737–750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanger BZ & Hebrok M Control of cell identity in pancreas development and regeneration. Gastroenterology 144, 1170–1179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bluestone JA, Herold K & Eisenbarth G Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atkinson MA et al. How does type 1 diabetes develop? The notion of homicide or β-cell suicide revisited. Diabetes 60, 1370–1379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lakey JR, Mirbolooki M & Shapiro AM Current status of clinical islet cell transplantation. Methods Mol. Biol 333, 47–104 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Hering BJ et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 39, 1230–1240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arda HE, Benitez CM & Kim SK Gene regulatory networks governing pancreas development. Dev. Cell 25, 5–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCracken KW & Wells JM Molecular pathways controlling pancreas induction. Semin. Cell Dev. Biol 23, 656–662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murtaugh LC & Melton DA Genes, signals, and lineages in pancreas development. Annu. Rev. Cell Dev. Biol 19, 71–89 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Thomson JA et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Takahashi K et al. Induction of pluripotent stem cells from adult human broblasts by de ned factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 68.D’Amour KA et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 (2006).Refs 68 and 69 were among the rst to report diferentiation of hES cells toward pancreatic endocrine progenitors and islet cells. [DOI] [PubMed] [Google Scholar]
- 69.Kroon E et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol 26, 443–452 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Pagliuca FW et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).Refs 70 and 71 reported successful generation of glucose-sensitive islet clusters by in vitro diferentiation of hES and iPS cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rezania A et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol 32, 1121–1133 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Szot GL et al. Tolerance induction and reversal of diabetes in mice transplanted with human embryonic stem cell-derived pancreatic endoderm. Cell Stem Cell 16, 148–157 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Andersson O et al. Adenosine signaling promotes regeneration of pancreatic cells in vivo. Cell Metab 15, 885–894 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schulz N et al. Critical role for adenosine receptor A2a in β-cell proliferation. Mol. Metab 5, 1138–1146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Annes JP et al. Adenosine kinase inhibition selectively promotes rodent and porcine islet β-cell replication. Proc. Natl Acad. Sci. USA 109, 3915–3920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kassem SA, Ariel I, Thornton PS, Scheimberg I & Glaser B Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49, 1325–1333 (2000). [DOI] [PubMed] [Google Scholar]
- 77.Meier JJ et al. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57, 1584–1594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Köhler CU et al. Cell cycle control of β-cell replication in the prenatal and postnatal human pancreas. Am. J. Physiol. Endocrinol. Metab 300, E221–E230 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Gregg BE et al. Formation of a human β-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab 97, 3197–3206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai C et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55, 707–718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Vos A et al. Human and rat β cells di er in glucose transporter but not in glucokinase gene expression. J. Clin. Invest 96, 2489–2495 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrer J, Benito C & Gomis R Pancreatic islet GLUT2 glucose transporter mRNA and protein expression in humans with and without NIDDM. Diabetes 44, 1369–1374 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Kulkarni RN, Mizrachi EB, Ocana AG & Stewart AF Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61, 2205–2213 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernal-Mizrachi E et al. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes 63, 819–831 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stewart AF et al. Human β-cell proliferation and intracellular signaling: part 3. Diabetes 64, 1872–1885 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiaschi-Taesch NM et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes 62, 2450–2459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fiaschi-Taesch NM et al. Cytoplasmic-nuclear trafcking of G1/S cell cycle molecules and adult human β-cell replication: a revised model of human β-cell G1/S control. Diabetes 62, 2460–2470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnamurthy J et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Chen H et al. Polycomb protein Ezh2 regulates pancreatic β-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 23, 975–985 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Helman A et al. p16Ink4a-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med 22, 412–420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kulkarni RN New insights into the roles of insulin/IGF-I in the development and maintenance of β-cell mass. Rev. Endocr. Metab. Disord 6, 199–210 (2005). [DOI] [PubMed] [Google Scholar]
- 92.Dadon D et al. Glucose metabolism: key endogenous regulator of β-cell replication and survival. Diabetes Obes. Metab 14 (Suppl 3), 101–108 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Stamateris RE et al. Glucose induces mouse β-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes 65, 981–995 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang P et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic β cell replication. Nat. Med 21, 383–388 (2015).Refs 94, 95 and 96 identi ed DRYK1 inhibitors as reagents that stimulate human β-cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dirice E et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 65, 1660–1671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen W et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat. Commun 6, 8372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.ElOuaamari A et al. SerpinB1 promotes pancreatic β cell proliferation. Cell Metab 23, 194–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dai C et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J. Clin. Invest 127, 3835–3844 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slack JM Metaplasia and transdi erentiation: from pure biology to the clinic. Nat. Rev. Mol. Cell Biol 8, 369–378 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Eguchi G & Okada TS Di erentiation of lens tissue from the progeny of chick retinal pigment cells cultured in vitro: a demonstration of a switch of cell types in clonal cell culture. Proc. Natl Acad. Sci. USA 70, 1495–1499 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi J et al. MyoD converts primary dermal broblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc. Natl Acad. Sci. USA 87, 7988–7992 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gurdon JB From nuclear transfer to nuclear reprogramming: the reversal of cell di erentiation. Annu. Rev. Cell Dev. Biol 22, 1–22 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Ferber S et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med 6, 568–572 (2000). [DOI] [PubMed] [Google Scholar]
- 104.Heremans Y et al. Recapitulation of embryonic neuroendocrine di erentiation in adult human pancreatic duct cells expressing neurogenin 3. J. Cell Biol 159, 303–312 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gasa R et al. Proendocrine genes coordinate the pancreatic islet di erentiation program in vitro. Proc. Natl Acad. Sci. USA 101, 13245–13250 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaneto H et al. PDX-1/VP16 fusion protein, together with Neuro D or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 54, 1009–1022 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Minami K, Okano H, Okumachi A & Seino S Role of cadherin-mediated cell–cell adhesion in pancreatic exocrine-to-endocrine transdi erentiation. J. Biol. Chem 283, 13753–13761 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Baeyens L et al. In vitro generation of insulin-producing β cells from adult exocrine pancreatic cells. Diabetologia 48, 49–57 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Zhou Q, Brown J, Kanarek A, Rajagopal J & Melton DA In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 (2008).This paper showed that it is possible to directly convert pancreatic acinar cells to β-like cells in adult mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li W et al. Long-term persistence and development of induced pancreatic β cells generated by lineage conversion of acinar cells. Nat. Biotechnol 32, 1223–1230 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Chen YJ et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Reports 6, 1046–1058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ariyachet C et al. Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 18, 410–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Talchai C, Xuan S, Kitamura T, DePinho RA & Accili D Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet 44, 406–412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baeyens L et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat. Biotechno l 32, 76–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Sancho R, Gruber R, Gu G & Behrens A Loss of Fbw7 reprograms adult pancreatic ductal cells into α, δ, and β cells. Cell Stem Cell 15, 139–153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cerdá-Esteban N et al. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat. Commun 8, 14127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Courtney M et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet 9, e1003934 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Collombat P et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 138, 449–462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van der Meulen T et al. Virgin β cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab 25, 911–926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao X et al. Endogenous reprogramming of α cells into β cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell 22, 78–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee J et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 2, e00940 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bouchi R et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat. Commun 5, 4242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galivo F et al. Reprogramming human gallbladder cells into insulin-producing β-like cells. PLoS ONE 12, e0181812 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lemper M et al. Reprogramming of human pancreatic exocrine cells to β-like cells. Cell Death Difer 22, 1117–1130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun Y, Ma X, Zhou D, Vacek I & Sun AM Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J. Clin. Invest 98, 1417–1422 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dufrane D, Goebbels RM, Saliez A, Guiot Y & Gianello P Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 81, 1345–1353 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Elliott RB Towards xenotransplantation of pig islets in the clinic. Curr. Opin. Organ Transplant 16, 195–200 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Niu D et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR–Cas9. Science 357, 1303–1307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang L et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350, 1101–1104 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Kobayashi T et al. Generation of rat pancreas in mouse by interspeci c blastocyst injection of pluripotent stem cells. Cell 142, 787–799 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Rashid T, Kobayashi T & Nakauchi H Revisiting the ight of Icarus: making human organs from PSCs with large animal chimeras. Cell Stem Cell 15, 406–409 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Yamaguchi T et al. Interspecies organogenesis generates autologous functional islets. Nature 542, 191–196 (2017).This paper demonstrated the feasibility of harvesting interspecies-derived islets to control diabetes with rodent models. [DOI] [PubMed] [Google Scholar]
- 133.Wu J et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 168, 473–486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Talchai C, Xuan S, Lin HV, Sussel L & Accili D Pancreatic β cell dedi erentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 (2012).This paper suggested that dediferentiation is a potential major mechanism for β-cell failure in T2D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Accili D et al. When β-cells fail: lessons from dedi erentiation. Diabetes Obes Metab 18 (Suppl. 1), 117–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Orlando G et al. Cell replacement strategies aimed at reconstitution of the β-cell compartment in type 1 diabetes. Diabetes 63, 1433–1444 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Vegas AJ et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived β cells in immune-competent mice. Nat. Med 22, 306–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.An D et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl Acad. Sci. USA 115, E263–E272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Manzoli V et al. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am. J. Transplant 18, 590–603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen T et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am. J. Transplant 15, 618–627 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Shoda LK et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 23, 115–126 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Lernmark A & Larsson HE Immune therapy in type 1 diabetes mellitus. Nat. Rev. Endocrinol 9, 92–103 (2013). [DOI] [PubMed] [Google Scholar]
- 143.Reed JC & Herold KC Thinking bedside at the bench: the NOD mouse model of T1DM. Nat. Rev. Endocrinol 11, 308–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keenan HA et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 59, 2846–2853 (2010).This paper demonstrated the persistence of insulin-expressing cells in patients with long-term T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu EH et al. Pancreatic β cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a β cell allograft. Diabetologia 52, 1369–1380 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dorrell C et al. Human islets contain four distinct subtypes of β cells. Nat. Commun 7, 11756 (2016).Refs 146 and 147 suggested that islet β-cells are heterogeneous in their molecular and functional properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bader E et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535, 430–434 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Wang YJ et al. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab 24, 616–626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnston NR et al. β Cell hubs dictate pancreatic islet responses to glucose. Cell Metab 24, 389–401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


