Abstract
Background
High altitude is a challenging condition caused by insufficient oxygen (O2) supply. Inability to adjust to hypoxia may lead to pulmonary edema, stroke, cardiovascular dysfunction and even death. Thus, understanding the molecular basis of adaptation to high altitude may reveal novel therapeutics to counteract the detrimental consequences of hypoxia.
Methods
Using high throughput unbiased metabolomic profiling, we report that the metabolic pathway responsible for production of erythrocyte 2,3-bisphosphoglycerate (2,3-BPG), a negative allosteric regulator of hemoglobin-O2 binding affinity, was significantly induced in 21 healthy humans within two hours of arrival at 5260m, and further increased following 16 days at 5260m.
Results
This finding led us to uncover discover that plasma adenosine concentrations and soluble CD73 (sCD73) activity rapidly increased at high altitude and were associated with elevated erythrocyte 2,3-BPG levels and O2 releasing capacity. Mouse genetic studies demonstrated that elevated CD73 contributed to hypoxia-induced adenosine accumulation and that elevated adenosine-mediated erythrocyte A2B adenosine receptor (ADORA2B) activation was beneficial by inducing 2,3-BPG production, triggering O2 release to prevent multiple tissue hypoxia, inflammation and pulmonary vascular leakage. Mechanistically, we demonstrated that erythrocyte AMP-activated protein kinase (AMPK) was activated in humans at high altitude and that AMPK is a key protein functioning downstream of ADORA2B, phosphorylating and activating BPG mutase and in this way inducing 2,3-BPG production and O2 release from erythrocytes. Significantly, preclinical studies demonstrated that activation of AMPK enhanced BPG mutase activation, 2,3-BPG production and O2 release capacity in CD73-deficient mice, in erythrocyte specific ADORA2B knockouts, and in wild type mice and in turn reduced tissue hypoxia, and inflammation.
Conclusions
Altogether, both human and mouse studies reveal novel mechanisms of hypoxia adaptation and potential therapeutic approaches for counteracting hypoxia-induced tissue damage.
Keywords: hypoxia, adenosine, AMP-activated protein kinase signal transduction, oxygen, adenosine receptor, erythrocyte, high-altitude acclimatization, altitudeOmics
Hypoxia is defined as inadequate oxygen (O2) supply to the whole body or a region of body. Hypoxia is commonly seen in patients with cardiovascular1–3, respiratory4, 5 and hemolytic6 diseases. Hypoxic conditions associated with these diseases are dangerous as decreased O2 availability promotes end organ damage and failure. Hypoxia also frequently occurs in healthy individuals when they travel to high altitude, or other low O2 content environments7, 8. It is widely observed that people differ in their ability to adapt to high altitude hypoxia8–11. Inability to adapt quickly to altitude hypoxia can result in pulmonary edema, stroke, cardiovascular dysfunction and even death3, 7, 12, 13. Thus, hypoxia is a highly dangerous condition for both normal individuals exposed to high altitude hypoxia and for patients with cardiovascular, respiratory and hemolytic diseases. However, current strategies to counteract hypoxia are limited due to a lack of fundamental understanding of molecular mechanisms underlying adaptation to hypoxia.
The erythrocyte is the most abundant circulating cell type and responsible for delivery of O2 to peripheral tissues. To function effectively in O2 uptake, transport and delivery, especially under hypoxia conditions, erythrocytes rely on sophisticated regulation of Hb-O2 affinity by endogenous allosteric modulators. One of the best known allosteric modulators is 2,3-BPG, a metabolic byproduct of glycolysis synthesized primarily in erythrocytes for the purpose of regulating Hb-O2 affinity14. It has been known for more than four decades that when humans ascend to high altitude the concentration of 2,3-BPG in erythrocytes increases significantly and its elevation is associated with an increased capacity of O2 release from hemoglobin15. Erythrocyte 2,3-BPG levels are also elevated in many cardiovascular diseases (CVD) associated with hypoxia16–18. However, the molecular basis underlying high altitude and disease-related hypoxia-induced erythrocyte 2,3-BPG remains unknown.
To address these questions, we recruited 21 young healthy human volunteers, and conducted high altitude studies. Our human studies showed that circulating adenosine levels and the activity of soluble ecto-5′-nucleotidase (CD73), a key enzyme to generate extracellular adenosine, were significantly induced at high altitude, and were associated with increased erythrocyte 2,3-BPG and O2 releasing capacity. In vivo genetic mouse studies coupled with in vitro human and mouse studies demonstrated that increased extracellular adenosine signaling via erythrocyte ADORA2B led to the sequential activation of AMPK (a well-known cellular energy sensor) and 2,3-BPG mutase, resulting in increased 2,3-BPG production and enhanced O2 release capacity to peripheral tissues and in turn reduced tissue hypoxia, inflammation and pulmonary injury. Thus, our findings highlight the importance of this newly identified erythrocyte adenosine signaling pathway via AMPK in human adaptation to high altitude and identify several attractive targets to counteract hypoxia in humans.
Methods
For expanded Methods sections, please refer to the Supplemental Materials.
Human Subjects
This study was conducted as part of the Altitudeomics project examining the integrative physiology of human responses to hypoxia19. In brief, all procedures conformed to the Declaration of Helsinki and were approved by the Universities of Colorado and Oregon Institutional Review Boards and the US Department of Defense Human Research Protection office. After informed written consent, recreationally active sea level (SL) habitants participated in the high altitude study. The participants were non-smokers, free from cardiorespiratory disease, born and raised at <1500 m, and had not travelled to elevations >1000 m in the 3 months prior to investigation.
Each subject was studied near SL (130 m, average PB=749 mmHg) and on the first and sixteenth days at Mt Chacaltaya, Bolivia (5260 m; average PB=406 mmHg; ALT1, ALT16). Baseline studies at SL were conducted over a two-week period in Eugene, OR, USA. Approximately one month after the SL studies, subjects traveled to Bolivia in pairs on successive days. Upon arrival at El Alto (4050m) after an overnight flight, subjects immediately descended to Coroico, Bolivia (1525 m; PB=639 mmHg). Subjects rested for 48 hours in Coroico to limit the effects of jet lag and were then driven over three hours to 5260 m. To provide an acute change in inspired PO2 from 1525 m to 5260 m, subjects breathed supplemental oxygen (2 L/min, nasal cannula or mask) during the drive. On arrival at 5260 m, the first subject immediately began the experimental protocol as previously described19.
Mouse Subjects
Eight to ten-week-old C57BL/6 wild-type (WT) mice were purchased from Harlan Laboratories (Indianapolis, IN). CD73-deficient (Cd73−/−) mice and A1, A2A, A2B and A3 adenosine receptor (ADORA1, ADORA2A, ADORA2B, and ADORA3)-deficient mice with C57BL/6 background were generated and genotyped as described before20, 21. A novel line of mice with erythrocyte-specific deletion of Adora2b was generated by crossing mice homozygous for a floxed Adora2b allele with mice expressing Cre recombinase under the control of erythropoietin receptor (EpoR) gene regulatory elements22. All protocols involving animal studies were reviewed and approved by the Institutional Animal Welfare Committee of the University of Texas Health Science Center at Houston.
Blood collection and preparation
Human and mouse blood were collected and stored as described before20, 23. Approximately four milliliter human blood were collected with heparin as an anti-coagulant for 2,3-bisphosphoglycerate (2,3-BPG) measurement using a commercial assay (Roche, USA) as previous described20. For plasma adenosine assay, one ml of EDTA collected blood was aliquoted to tubes containing 10 μM dipyridamole (an inhibitor of equilibrative nucleoside transporters) and 10 μM deoxycoformycin (an inhibitor of adenosine deaminase). Approximately one ml of mouse blood was collected and used for 2,3-BPG and plasma adenosine measurement similar to human blood as described above20, 23. High altitude human blood was collected and stored at −80°C after collection and during shipment from the field until assay.
Measurement of soluble CD73 activity in lowland volunteers
sCD73 enzyme activity in humans was measured by quantifying the rate of conversion of [3H]-AMP to [3H]-adenosine as described previously24, 25. Briefly, sCD73 activity was measured after incubation of plasma with 300 uM [3H]AMP (Quotient Bioresearch; GE Healthcare, Rushden, UK) for 60 min at 37°C. Premixture was then applied onto Alugram SIL G/UV254 sheets (Macherey-Nagel, Germany). Radiolabelled substrates and their products were separated by thin layer chromatography (TLC) and quantified by scintillation β-counting.
Plasma adenosine measurement
Plasma adenosine concentration was measured by reversed phase HPLC as previously described20. Briefly, 500 μl plasma was used to isolate adenosine by sequentially adding 0.6 M cold perchloric acid, 3 M KHCO3/3.6 N KOH, 1.8 M ammonium dihydrogen phosphate (pH 5.1) and phosphoric acid (30%). Next, the sample was centrifuged at 20,000 × g for 5 min and the supernatant was transferred to a new tube for reversed phase HPLC analysis. Adenosine content was normalized to volume and expressed as a concentration.
Measurement of P50 in lowland volunteers
Arterial bloods of high altitude human subjects were collected and used for P50 measurement. Blood PaO2 and pH were measured by blood gas analyzer, and SaO2 was measured by Co-Oximeter. The P50 was then calculated by using a Hill constant of 2.7 and the standard equation as described before26.
Statistics
All data are expressed as the mean ± SEM. Data were analyzed for statistical significance by using GraphPad Prism 5 software. Nonparametric equivalent Wilcoxon Rank-Sum tests were applied in two-group analysis. Differences between the means of multiple groups were compared by one-way ANOVA, followed by a Tukey’s multiple comparisons test. Comparison of the data obtained at different time points was analyzed by two-way repeated-measures, followed by the Tukey post hoc test. P value < 0.05 was considered significant.
Results
Metabolomic screening reveals that high altitude hypoxia activates the Rapoport-Luebering Shunt in erythrocytes resulting in increased production of 2,3-BPG and elevated oxygen release from hemoglobin
To identify metabolic alterations in erythrocytes in response to hypoxia, we conducted nonbiased high throughput metabolomic profiling of erythrocytes from humans at SL and at high altitude. Specifically, we recruited 21 young healthy lowland volunteers who were examined at SL and within two hours of arrival at an altitude of approximately 5260 meters (ALT1) and on day 16 at high altitude (ALT16) (Figure 1A). Overall, 233 metabolites were confidently identified and relatively quantified out of over 9000 detected features (mass to charge ratios) in erythrocytes under SL conditions and at high altitude (Supplementary Table 1).
Figure 1. Metabolomic profiling reveals high altitude hypoxia increases erythrocyte specific Rapoport-Luebering Shunt to induce 2,3-BPG production and P50 levels in humans.
(A) Table of human volunteer characteristics including age, height (HT), weight (WT), body mass index (BMI), and schema for high altitude human studies. (n=21) (B) Schematic drawing of erythrocyte-specific Rapoport-Luebering shunt occurring at a branch point in the pathway of anaerobic glycolysis for 2,3-bisphophoglycerate (2,3-BPG) production. (C–F) Metabolomic profiling reveals the significant changes in the relative erythrocyte concentration of glyceraldehyde-3-phosphate (G3P) (C), bisphosphoglycerate (BPG) (D), the monophosphoglycerates, 2-phosphoglycerate (2-PG) and 3-phosphoglycerate (3-PG) (E) and phophoenopyruvate (PEP) (F) at sea level (SL) and at high altitude on day 1 and day 16. (G–J) Erythrocyte 2,3-BPG concentration, P50 level, plasma adenosine concentration and soluble CD73 activity (sCD73) were elevated at high altitude hypoxia on ALT1 and ALT16 over SL. Erythrocyte 2,3-BPG concentration (G), P50 level (H), plasma adenosine concentration (I), and sCD73 (J). Data are expressed as mean ± SEM; *P<0.05 vs SL; **P<0.05 vs ALT1. AU (area under the peak)
Analysis of these metabolic changes revealed that the erythrocyte-specific Rapoport-Luebering shunt, occurring at a branch point in the pathway of glycolysis for production of 2,3-BPG from 1,3-bisphoglycerate (1,3-BPG) (Figure 1B), was rapidly activated by high-altitude hypoxia. Specifically, levels of BPG and its upstream glycolytic intermediate, glyceraldehyde-3-phosphate (G3P), were increased at ALT1 and were further elevated at ALT16 (Figure 1C and D). In contrast, the levels of the erythrocyte monophosphoglycerates (3-PG and 2-PG) and phospoenolpyruvate (PEP), the three immediate downstream products of 2,3-BPG, were significantly reduced at ALT1 and further reduced at ALT16 compared to SL (Figure 1E and F). To validate our metabolomic screening, we quantified erythrocyte 2,3-BPG concentrations in human samples. Consistently, we found that the erythrocyte 2,3-BPG concentration was significantly induced at ALT1 and further elevated at ALT16 (Figure 1G). Thus, our results indicate that in response to high altitude hypoxia the Rapoport-Luebering Shunt is activated resulting in a significant portion of G3P being utilized to produce 2,3-BPG rather than directly being metabolized to the glycolytic intermediate, 3-PG (Figure 1B).
Because 2,3-BPG is an allosteric modulator that decreases Hb-O2 binding affinity and thereby promotes O2 release14, it is possible that hypoxia-induced 2,3-BPG production in erythrocytes triggers O2 release. To test this possibility, we quantified the O2 release capacity of erythrocytes by measuring P50, the partial pressure of O2 required to achieve 50% Hb-O2 saturation, from the human subjects at high altitude. We found that erythrocyte P50 was significantly induced at ALT16 compared to ALT1, reflecting reduced Hb-O2 binding affinity and enhanced erythrocyte O2 release capacity at high altitude (Figure 1H). No significant differences were noted between male and female subjects (Supplementary Figure 1A and B). These findings provide evidence that high altitude hypoxia coordinately induces erythrocyte 2,3-BPG production and P50 levels in humans adapting to high altitude (Figure 1B).
Extracellular adenosine concentrations and soluble CD73 activity increase in humans at high altitude
Specific factors and signaling pathways involved in 2,3-BPG induction and subsequent O2 release from erythrocytes under high altitude have not been previously identified. Recent studies show that increased circulating adenosine is involved in 2,3-BPG induction in erythrocytes of individuals with sickle cell disease (SCD)20. However, whether adenosine signaling contributes to normal erythrocyte 2,3-BPG induction and subsequent O2 release underlying high altitude hypoxia adaptation remains unknown. To test this hypothesis, we used high performance liquid chromatography (HPLC) to measure plasma adenosine levels in the 21 lowland volunteers at SL and following ascent to high altitude. We found that the circulating adenosine levels were elevated at ALT1 compared to SL and were further elevated at ALT16 (Figure 1I). No significant differences in elevated plasma adenosine levels were observed between males and females (Supplementary Figure 1C).
CD73 is an ectoenzyme anchored to the cell surface that plays a key role in the synthesis of extracellular adenosine from AMP27. Under certain circumstances, the ectoenzyme can be cleaved from the cell surface and emerge in the circulation as a soluble nucleotidase28. Therefore, we measured soluble CD73 (sCD73) activity in normal individuals at SL and at high altitude. sCD73 activity was significantly increased in the human subjects at ALT1 compared to SL and further increased at ALT16 (Figure 1J). There was no significant differences in elevated sCD73 activity between males and females in response to high altitude hypoxia (Supplementary Figure 1D). Taken together, these results show that plasma adenosine concentration and sCD73 activity increase rapidly in response to high altitude hypoxia and that their induction is associated with increased erythrocyte 2,3-BPG concentrations and P50 levels.
Soluble CD73 activity is induced by hypoxia and elevated CD73 is essential for hypoxia-induced plasma adenosine, erythrocyte 2,3-BPG and oxygen release capacity
Although extracellular adenosine is well-known to orchestrate a physiological response to tissue injury29, nothing is known about the functional role of adenosine signaling in normal erythrocytes under hypoxia. Our human studies presented above raise an intriguing hypothesis that activated CD73-mediated elevated plasma adenosine has a previously unrecognized role in high altitude adaption by regulating 2,3-BPG production and Hb-O2 binding affinity in erythrocytes. To test this hypothesis, we exposed WT mice and Cd73−/− mice to normobaric hypoxia (10% oxygen, similar to that at 5260 m) for one week. Similar to the human high altitude studies, we found that CD73 activity, plasma adenosine, erythrocyte 2,3-BPG and O2 releasing capacity were significantly increased in WT mice following 1 week of hypoxia compared to normoxia (Figure 2A–D). In contrast, hypoxia-mediated increased plasma adenosine, erythrocyte 2,3-BPG and O2 releasing capacity were significantly impaired in Cd73−/− mice (Figure 2A–D). These results indicate that in mice CD73 activity is induced by hypoxia, as seen in human volunteers at high altitude, and that CD73 is required for hypoxia-induced plasma adenosine production and elevated erythrocyte 2,3-BPG concentration and O2 release capacity.
Figure 2. Elevated CD73 is essential for hypoxia-induced plasma adenosine, erythrocyte 2,3-BPG and oxygen release capacity to prevent tissue hypoxia, inflammation and lung damage in mice.
(A–D) CD73 is essential for hypoxia-induced plasma adenosine, erythrocyte 2,3-BPG concentration and P50 levels in mice. Plasma adenosine concentration (A), soluble CD73 activity (B), Erythrocyte 2,3-BPG (C), and P50 (D) in WT mice and Cd73−/− mice under normoxia or hypoxia (10% O2 1 week). Data are expressed as mean ± SEM; *P<0.05 vs WT under normoxia; **P<0.05 vs WT under hypoxia (n=10). (E–J) Deletion of Cd73−/− results in elevated tissue hypoxia and inflammation infiltration in multiple tissues, as well as pulmonary dysfunction under hypoxia (10% O2 1 week). Immunohistochemical analysis of tissue hypoxia by hypoxyprobe (E) and Myeloperoxidase activity (F) in kidney, lung and heart. Bronchoalveolar lavage fluid (BALF) total cell count (G), BALF albumin concentration (H), BALF interleukin 6 concentration (I) in WT mice and Cd73−/− mice. Data are expressed as mean ± SEM; *P<0.05 vs WT under normoxia; **P<0.05 vs WT under hypoxia (n=10; Scale bar=200μm).
CD73-mediated elevated plasma adenosine prevents tissue hypoxia, inflammation and lung injury in mice
To assess the functional importance of CD73-mediated increased plasma adenosine in tissue response to hypoxia, we measured tissue hypoxia levels using an immunohistochemical (IHC) analysis with hypoxyprobe in WT mice and Cd73−/− mice under normoxia and following one week of hypoxia as described above. No hypoxia signals were seen in tissue sections of Cd73−/− or WT mice under normoxia (Figure 2E). However, under hypoxia, hypoxyprobe revealed that multiple organs including kidneys, lungs and hearts displayed a mild hypoxia signal in WT mice (Figure 2E). In contrast, 1 week of 10% O2 led to severe hypoxia in various organs including kidneys, lungs and hearts of Cd73−/− mice (Figure 2E). Image quantification analysis demonstrated that the intensity of hypoxyprobe in the kidneys, lungs and hearts was significantly elevated in Cd73−/− mice compared to WT mice (Supplementary Figure 2B–D).
Besides analyzing tissue hypoxia, we further quantified neutrophils in multiple tissues since it was reported that hypoxia rapidly induces infiltration of neutrophils to tissue30, 31. Specifically, we measured the activity of myeloperoxidase (MPO), a specific enzyme expressed in neutrophils, in multiple tissues under normoxia and hypoxia in WT and Cd73−/− mice. Similar to tissue hypoxia staining (Figure 2E), we found that MPO activity was mildly induced by hypoxia in multiple tissues of WT mice, and was significantly induced further by hypoxia in multiple tissues of Cd73−/− mice compared to WT mice (Figure 2F). Thus, we conclude that CD73-mediated elevation of plasma adenosine is beneficial to prevent tissue hypoxia and inflammation.
Hypoxia is known to induce lung injury including pulmonary vascular leakage and inflammation19. Thus, we collected bronchoalveolar lavage fluid (BALF) of WT and Cd73−/− mice for evaluation of pulmonary damage and inflammation. Specifically, we quantified cell count, albumin concentration, and interleukin 6 (IL-6) concentration in BALF of WT and Cd73−/− mice under normoxia and hypoxia. Consistent with our tissue hypoxia staining and MPO activity measurements in the lung (Figure 2E–F), cell count, albumin and IL-6 concentrations in BALF were slightly induced by hypoxia in WT mice and their levels were significantly further increased in Cd73−/− mice (Figure 2G–I). Thus, these results show that CD73-mediated elevation of plasma adenosine plays a beneficial role in preventing hypoxia-induced lung damage and inflammation in mice.
ADORA2B underlies hypoxia-induced 2,3-BPG production and oxygen release capacity in in vitro cultured mouse erythrocytes
It is known that increased extracellular adenosine elicits physiological or pathological functions by engaging its four adenosine receptors including ADORA1, ADORA2A, ADORA2B, and ADORA332. Each adenosine receptor has a distinct cellular or tissue distribution and affinity for adenosine33. To identify which adenosine receptor is responsible for hypoxia-induced 2,3-BPG production and O2 release capacity in erythrocytes, we conducted genetic studies in four adenosine receptor-deficient mice. We found that hypoxia significantly induced 2,3-BPG production and O2 release capacity in cultured erythrocytes isolated from WT mice, Adora1-deficient mice, Adora2a-deficient mice and Adora3-deficient mice. However, hypoxia-mediated effects were absent in cultured erythrocytes isolated from Adora2b-deficient mice (Figure 3A and B), indicating that hypoxia-induced erythrocyte 2,3-BPG production and O2 release capacity is dependent on ADORA2B.
Figure 3. Erythrocyte ADORA2B activation induces erythrocyte 2,3-BPG production and oxygen release capacity to counteract tissue hypoxia, inflammation and lung damage in mice.
(A and B) Hypoxia induces 2,3-BPG production (A) and P50 (B) levels in cultured WT, Adora1−/−, Adora2a−/−, Adora3−/− but not Adora2b−/− mouse erythrocytes in a time-dependent manner. *P<0.05 for 3 hours hypoxia vs normoxia; **P<0.05 for 6 hours hypoxia vs 3 hours hypoxia (n=8). (C–E) Erythrocyte ADORA2B contributes to hypoxia-induced 2,3-BPG production and P50 levels. Plasma adenosine (C), erythrocyte 2,3-BPG (D) and P50 (E) of EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice under normoxia or hypoxia (10% O2 1 week). Data are expressed as mean ± SEM; *P<0.05 vs EpoR-Cre+ mice under normoxia; **P<0.05 vs EpoR-Cre+ mice under hypoxia (n=10). Targeted deletion of erythrocyte ADORA2B results in elevated tissue hypoxia and inflammation infiltration in multiple tissues, as well as pulmonary dysfunction under hypoxia (10% O2, 1 week). Immunohistochemical analysis of tissue hypoxia by hypoxyprobe (F) and Myeloperoxidase activity (G) in kidney, lung and heart. Bronchoalveolar lavage fluid (BALF) total cell count (H), BALF albumin concentration (I), BALF interleukin 6 concentration (J) in EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice. Data are expressed as mean ± SEM; *P<0.05 vs EpoR-Cre+ mice under normoxia; **P<0.05 vs EpoR-Cre+ mice under hypoxia (n=10; Scale bar=200μm).
Genetic deletion of erythrocyte ADORA2B attenuates hypoxia-induced 2,3-BPG production and oxygen release capacity in vivo
To precisely determine the importance of erythrocyte ADORA2B-mediated elevated 2,3-BPG production and O2 release in hypoxia, we generated mice with erythrocyte lineage-specific ablation of Adora2b genes by mating mice with floxed Adora2b alleles (Adora2bf/f) with mice containing a transgene expressing Cre recombinase only in the erythroid lineage (EpoR-Cre+) (Supplementary Figure 3A). First, we validated the specificity and efficiency of ablation of Adora2b in erythroid cells by EpoR-Cre using bone marrow (BM) cells isolated from Epo-Cre+, Adora2bf/f/EpoR-Cre+ and Adora2b−/− mice. The BM-derived hematopoietic progenitor cells (HPCs) were enriched by negative selection, followed by induction to erythroid progenitor cells in erythroid-differentiation medium containing erythropoietin (Supplementary Figure 3B). After two days, we found that over 96% of harvested HPCs isolated and enriched from Epo-Cre+, Adora2bf/f/EpoR-Cre+ and Adora2b−/− mice were differentiated into CD71+ erythroid progenitor cells, which is consistent with a previous publication (Supplementary Figure 3C)34. Moreover, qRT-PCR analysis showed that erythrocyte lineage Adora2b mRNA levels of Adora2bf/f/EpoR-Cre+ were reduced to levels similar to that of Adora2b−/− mice, whereas pulmonary Adora2b mRNA levels in Adora2bf/f/EpoR-Cre+ were still similar to control mice (EpoR-Cre+) (Supplementary Figure 3D and E). These results demonstrated that we had successfully generated mice with erythrocyte lineage specific deletion of Adora2b.
To test the functional role of erythrocyte ADORA2B signaling in response to hypoxia, we exposed EpoR-Cre mice and Adora2bf/f/EpoR-Cre+ mice to normoxia or hypoxia (10% O2) for one week. The levels of plasma adenosine, erythrocyte 2,3-BPG and P50 were similar in EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice under normoxia (Figure 3C–E). Following one week of hypoxia exposure, plasma adenosine concentrations increased to similar levels in EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice (Figure 3C). However, the hypoxia-induced erythrocyte 2,3-BPG production and P50 levels observed in the EpoR-Cre+ mice were significantly attenuated in Adora2bf/f/EpoR-Cre+ mice (Figure 3D and E). Thus, our studies provide strong genetic evidence that mouse erythrocyte ADORA2B is essential for elevated adenosine-mediated induction of erythrocyte 2,3-BPG and O2 releasing capacity under hypoxia.
Erythrocyte ADORA2B is essential to protect against tissue hypoxia, inflammation and lung damage
To further determine if erythrocyte ADORA2B signaling is tissue protective under hypoxia, we assessed tissue hypoxia by hypoxyprobe in EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice following one week of hypoxia (10% O2). We found that hypoxia treatment led to severe tissue hypoxia in various organs including kidneys, lungs and hearts in Adora2bf/f/EpoR-Cre+ mice, while only a mild hypoxia signal was present in those tissues of EpoR-Cre+ mice (Figure 3F). Image quantification analysis demonstrated that the intensity of hypoxyprobe signals in kidneys, lungs and hearts were significantly elevated in Adora2bf/f/EpoR-Cre+ mice compared to EpoR-Cre+ (Supplementary Figure 4B–D) following hypoxia challenge. We also observed greater elevation of MPO activity in kidney, lung and heart of Adora2bf/f/EpoR-Cre+ mice compared to EpoR-Cre+ mice after hypoxia exposure (Figure 3F). Furthermore, greater lung injury reflected by increased cell counts, albumin and IL-6 concentrations in BALF were detected in Adora2bf/f/EpoR-Cre+ mice after hypoxia challenge (Figure 3H–J). These findings demonstrate that erythrocyte ADORA2B is essential for tissue protection during hypoxia exposure.
AMPK functions downstream of erythrocyte ADORA2B in mice and underlies hypoxia-induced 2,3-BPG production by phosphorylation and activation of 2,3-BPG mutase
Erythrocyte 2,3-BPG is synthesized from the glycolytic intermediate 1,3-BPG by 2,3-BPG mutase (Figure 1B). Factors regulating 2,3-BPG production are poorly understood. A previous report identified that 2,3-BPG mutase is one of the substrates of AMPK in erythrocytes, and follow-up studies validated that purified AMPK directly phosphorylates purified 2,3-BPG mutase in a cell free system35. Additional studies showed that ADORA2B signaling activates AMPK in other cellular systems36. Like adenosine, AMPK plays an essential role in multiple cellular functions especially under conditions of energy depletion or limited O2 availability37. However, whether AMPK functions downstream of ADORA2B as a key enzyme responsible for hypoxia-induced 2,3-BPG production in erythrocytes has not been previously studied. To test this possibility, we exposed EpoR-Cre mice and Adora2bf/f/EpoR-Cre+ mice to hypoxia (10% oxygen) for different times. We found that erythrocyte p-AMPK, 2,3-BPG mutase activity, 2,3-BPG concentration and P50 level were significantly induced in EpoR-Cre mice under hypoxia in a time-dependent manner. However, hypoxia-induced time-dependent elevation of p-AMPK, 2,3-BPG mutase activity, 2,3-BPG and P50 were significantly attenuated in Adora2bf/f/EpoR-Cre+ mice (Figure 4A–D). Next, we conducted experiments to determine whether hypoxia activated AMPK interacts with and phosphorylates 2,3-BPG mutase in erythrocytes. Specifically, we assessed AMPK activation by monitoring its phosphorylation at T172 of the catalytic α subunit in erythrocytes. We found that basal erythrocyte p-AMPKα at T172 levels were similar in Adora2bf/f/EpoR-Cre+ mice and EpoR-Cre+ mice under normoxia (Figure 4E), while p-AMPKα at T172 was significantly induced in erythrocytes of EpoR-Cre+ mice following 1 week of hypoxia (10% O2). In contrast, hypoxia-induced p-AMPK α at T172 was significantly attenuated in the erythrocytes of Adora2bf/f/EpoR-Cre+ mice (Figure 4E). Then, using an antibody that recognizes p-AMPK substrates we showed that p-AMPK specifically phosphorylates 2,3-BPG mutase and that under normoxia the levels of AMPK-phosphorylated 2,3-BPG mutase were similar in Adora2bf/f/EpoR-Cre+ mice and EpoR-Cre+ mice (Figure 4F). Subsequently, we found that hypoxia significantly induced p-AMPK-phosphorylated 2,3-BPG mutase in erythrocytes of EpoR-Cre+ mice and that this phosphorylation was significantly attenuated in Adora2bf/f/EpoR-Cre+ mice (Figure 4F).
Figure 4. AMPK functions downstream of erythrocyte ADORA2B and underlies hypoxia-induced 2,3-BPG production by phosphorylation and activation of 2,3-BPG mutase in mice.
(A–D) Erythrocyte ADORA2B is essential for hypoxia-induced p-AMPK, 2,3-BPG mutase activity, 2,3-BPG concentration and P50 levels in vivo. Erythrocyte p-AMPKα (quantified by ELISA) (A), 2,3-BPG mutase activity (B), 2,3-BPG concentration (C) and P50 (D) of EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice under normoxia or hypoxia (10% O2, 90% N2) for 1 day, 3 days, and 7 days. Data are expressed as mean ± SEM; *P<0.05 vs EpoR-Cre+ mice under normoxia; **P<0.05 vs EpoR-Cre+ mice under hypoxia for 1 day; #P<0.05 Adora2bf/f/EpoR-Cre+ mice vs EpoR-Cre+ mice at the same time point (n=10). (E) Representative western blot and relative image quantification analysis of p-AMPKα in erythrocytes of EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice under normoxia or hypoxia (10% O2, 90% N2) for 1 week. (F) Representative western blot and relative image quantification analysis of p-AMPK phosphorylated 2,3-BPG mutase levels in the erythrocyte lysates in EpoR-Cre+ mice and Adora2bf/f/EpoR-Cre+ mice under normoxia or hypoxia (n=3). Data are expressed as mean ± SEM; *P<0.05 vs EpoR-Cre+ mice under normoxia; **P<0.05 vs EpoR-Cre+ mice under hypoxia. (G–J) AICAR treatment significantly stimulated hypoxia-induced erythrocyte AMPK phosphorylation (G), 2,3-BPG mutase activity (H), 2,3-BPG production (I) and P50 levels (J) compared to saline-treated group in Cd73−/− mice and Adora2bf/f/EpoR-Cre+ mice under hypoxia (10% O2, 90% N2) for 3 days. Data are expressed as mean ± SEM; *P<0.05 for AICAR-treated mice vs saline-treated mice (n=8).
Finally, to determine the essential role of AMPK activation in adenosine ADORA2B-mediated induction of 2,3-BPG, P50 and subsequent tissue protection, we treated Cd73−/− and Adora2bf/f/EpoR-Cre+ mice with AICAR (5-aminoimidazole-4-carboxamide ribonucleotide), a cell permeable AMPK activator38. Specifically, we treated these mice with AICAR under hypoxia (10% O2) for 3 days. Our results (Figure 4G–J) show that AICAR treatment restored erythrocyte AMPK phosphorylation, 2,3-BPG mutase activity, 2,3-BPG and P50 in both Cd73−/− and Adora2bf/f/EpoR-Cre+ mice under hypoxia. Thus, our results demonstrate that AICAR can override a genetic block to hypoxia-induced adenosine production (CD73 deficiency) or erythrocyte ADORA2B signaling (erythrocyte-specific ADORA2B deficiency) to stimulate erythrocyte 2,3-BPG production and O2 release capacity by the pharmacologic activation of AMPK. Taken together, we provide in vivo evidence that AMPK is a critical intermediate that functions downstream of erythrocyte ADORA2B underlying hypoxia-induced 2,3-BPG production by phosphorylation and activation of 2,3-BPG mutase.
AMPK activation induces erythrocyte 2,3-BPG mutase activity, 2,3-BPG production and oxygen release capacity in mice under normoxia
Our discovery that erythrocyte AMPK is activated and subsequently phosphorylates 2,3-BPG mutase in response to adenosine ADORA2B signaling suggests that activation of AMPK may be sufficient to induce 2,3-BPG production by activating 2,3-BPG mutase. To test this possibility, we conducted in vitro studies in cultured WT mouse erythrocytes with AICAR. Initially, we found that AICAR treatment significantly induced phosphorylation of AMPK and activity of 2,3-BPG mutase, 2,3-BPG levels and P50 in a time-dependent manner (Supplementary Figure 5A–D). To further test whether AICAR-mediated effects in cultured erythrocytes were dependent on AMPK, we utilized Compound C (dorsomorphin), a cell permeable inhibitor of AMPK38–40. Our results showed that Compound C co-treatment significantly attenuated AICAR-induced p-AMPK, 2,3-BPG mutase activity, 2,3-BPG and O2 releasing capacity in cultured mouse erythrocytes (Supplementary 5A-D). Thus, our in vitro studies demonstrated that AMPK activation is sufficient to induce 2,3-BPG production and enhanced O2 release capacity in mouse erythrocytes.
Next, we extended in vitro studies to in vivo to test if AICAR treatment in WT mice under normoxia is capable of inducing AMPK, 2,3-BPG mutase activity and 2,3-BPG production. As expected, we found that AICAR treatment directly induced phosphorylation of AMPK, 2,3-BPG mutase activity, 2,3-BPG production and P50 levels in WT mice under normoxia (Supplementary Figure 5 E–H).
In vivo beneficial effects of AMPK activation in hypoxia adaptation in mouse
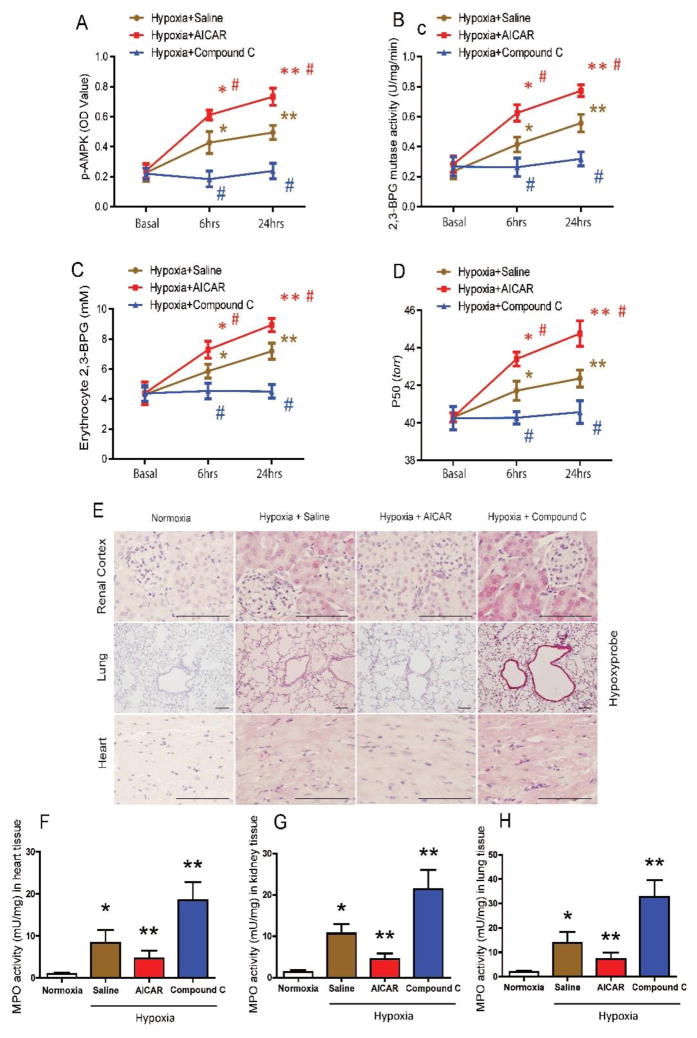
To determine the in vivo effect of AMPK activation in hypoxia adaptation in WT mice, we pre-treated WT mouse with AICAR prior to a hypoxic challenge. For these experiments, a lower O2 content (8% O2) was utilized for a shorter period of hypoxia exposure up to 24 hours for evaluating the therapeutic potential of AMPK activation in WT mice. We found that AICAR treatment induced greater elevation of erythrocyte 2,3-BPG and O2 releasing capacity by further stimulating erythrocyte p-AMPK and 2,3-BPG mutase activity in WT mice compared to the saline-treated group under hypoxia (Figure 5A–D). IHC with hypoxyprobe and MPO activity measurement showed that AICAR treatment significantly attenuated tissue hypoxia and inflammation in lung, kidney and heart in WT mice compared to the saline-treated group under hypoxia (Figure 5E–H and Supplementary Figure 6A–C).
Figure 5. In vivo effects of AICAR and Compound C treatment under hypoxia.
(A–D) AICAR treatment significantly stimulated erythrocyte p-AMPK (A), 2,3-BPG mutase activity (B), 2,3-BPG production (C) and P50 levels (D) in WT mice compared to saline-treated WT mice under hypoxia (8% O2) in a time-dependent manner, while Compound C treatment significantly attenuated erythrocyte p-AMPK (A), 2,3-BPG mutase activity (B), 2,3-BPG production (C) and P50 levels (D) in WT mice compared to saline-treated WT mice under hypoxia (8% O2). Data are expressed as mean ± SEM; *P<0.05 for 6-hour vs basal level, **P<0.05 for 24-hour vs 6-hour. #P<0.05 for AICAR-treated group vs saline group, and Compound C-treated group vs saline group at the same time point (n=8). (E–H) AICAR treatment prevented tissue hypoxia and inflammation infiltration, while Compound C treatment aggravated tissue hypoxia and MPO activity in WT mice under hypoxia for 24 hours. IHC analysis of tissue hypoxia by hypoxyprobe in kidney, lung and heart (E) (Scale bar=200μm). MPO activity in heart (F), kidney (G) and lung (H). AICAR or Compound C-treated WT mice after 24 hours hypoxia treatment (n=8). Data are expressed as mean ± SEM; *P<0.05 vs WT mice under normoxia; **P<0.05 vs WT mice with saline treatment under hypoxia.
Next, to validate AMPK activation is essential for hypoxia adaptation, we treated WT mice with Compound C under hypoxia. We found that Compound C treatment significantly suppressed hypoxia-induced p-AMPK, 2,3-BPG mutase activity, 2,3-BPG production and O2 releasing capacity in WT mouse erythrocytes, thereby resulting in enhanced tissue hypoxia and inflammation in lungs, kidneys and hearts compared to the saline-treated group under hypoxia (Figure 5E–H and Supplementary Figure 6A–C). These studies indicate that erythrocyte p-AMPK is critical for enhanced hypoxia adaptation in WT mice by rapidly inducing 2,3-BPG mutase activity, 2,3-BPG and subsequent O2 release to peripheral tissues.
Erythrocyte p-AMPK and 2,3-BPG mutase activity are induced in humans at high altitude, and AMPK activation induces erythrocyte 2,3-BPG mutase activity, 2,3-BPG production and oxygen release in cultured human erythrocytes
To translate our mouse findings to humans, we measured erythrocyte p-AMPK by western blot and ELISAs in human subjects at SL and at high altitude on ALT1 and ALT16. We found that erythrocyte p-AMPK levels were increased at ALT1 and were further elevated at ALT16 (Figure 6A and B). No significant differences in p-AMPK levels were observed between male and female volunteers in response to high altitude (Supplementary Figure 1E). Additionally, we found that erythrocyte 2,3-BPG mutase activity was induced at ALT1 and was further elevated at ALT16 (Figure 6C). Consistently, we observed that levels of p-AMPK-phosphorylated 2,3-BPG mutase in erythrocytes were significantly increased at ALT1, and were further enhanced at ALT16 (Figure 6D), implicating AMPK activation as associated with high altitude hypoxia-induced activation of erythrocyte 2,3-BPG in humans.
Figure 6. Erythrocyte p-AMPK and 2,3-BPG mutase activity are induced in humans at high altitude, and AMPK activation induces erythrocyte 2,3-BPG mutase activity, 2,3-BPG production and oxygen release in cultured human erythrocytes.
(A) Representative western blot and relative image quantification analysis (n=3 per group) of p-AMPKα subunit phosphorylated at threonine 172 (T172), total AMPKα subunit, and β-actin in the erythrocytes of humans at sea level (SL) and high altitude on day 1 and day 16. Erythrocyte p-AMPK levels quantified by ELISA (B) and 2,3-BPG mutase activity (C) at high altitude ALT1 and ALT16 over SL. (D) Representative western blot and relative image quantification analysis (n=3 per group) of p-AMPK phosphorylated 2,3-BPG mutase at SL and high altitude on day 1 and day 16 in erythrocyte lysates. Data are expressed as mean ± SEM; *P<0.05 for high altitude on day 1 vs SL; **P<0.05 for high altitude on day 16 vs day 1. (E–H) AMPK activator AICAR-induced phosphorylation of AMPK (E), 2,3-BPG mutase activity (F), 2,3-BPG concentration (G) and P50 levels (H) in cultured normal human erythrocytes under normoxia in a time-dependent manner. Compound C significantly attenuated the effect of AICAR treatment. Data are expressed as mean ± SEM; *P<0.05 for AICAR-treated 2-hour group vs 1-hour group, and for AICAR-treated 2-hour group vs DMSO-treated 2-hour group, **P<0.05 for AICAR-treated 4-hour group vs 2-hour group, and for AICAR-treated 4-hour group vs DMSO-treated 4-hour group. #P<0.05 for AICAR plus Compound C vs AICAR at the same time point (n=8). (I) Working model: CD73 is essential for high altitude hypoxia-induced plasma adenosine production. Elevated plasma adenosine prevents hypoxia-induced tissue inflammation and damage by activating ADORA2B on erythrocytes to induce 2,3-BPG mutase activity, 2,3-BPG production, and subsequently increased P50 and increased O2 release to peripheral hypoxic tissues. AMPK is a key enzyme that functions downstream of ADORA2B to activate 2,3-BPG mutase, promote 2,3-BPG production and O2 release from erythrocytes. Thus, enhancing the CD73-ADORA2B-AMPK signaling pathway is a promising therapeutic strategy to treat or prevent hypoxia-induced tissue damage.
To translate our mouse findings regarding the significance of AMPK activation in erythrocytes to humans, we conducted a series of in vitro studies by culturing normal human erythrocytes. First, we found that AICAR treatment induced p-AMPK, 2,3-BPG mutase activity, 2,3-BPG and subsequent O2 release in cultured normal human erythrocytes in a time-dependent manner (Figure 6E–H). To further test whether AICAR-mediated effects in cultured human erythrocytes are dependent on AMPK, we incubated AICAR-treated human normal erythrocytes with or without Compound C. We found that Compound C significantly attenuated AICAR-induced p-AMPK, 2,3-BPG mutase activity, 2,3-BPG levels and O2 release capacity in cultured human erythrocytes (Figure 6E–H). Taken together, these data show that high altitude hypoxia induces human erythrocyte p-AMPK and 2,3-BPG mutase activity in a time-dependent manner. In vitro experiments with cultured human erythrocytes showed that activation of AMPK induces 2,3-BPG mutase activity, 2,3-BPG production and subsequent O2 release in cultured human erythrocytes. Compound C inhibits AMPK activation and subsequently suppresses the induction of 2,3-BPG mutase activity, 2,3-BPG production and O2 release in cultured human erythrocytes. Thereby, we provide human evidence that AMPK activation triggers erythrocyte O2 release by promoting 2,3-BPG mutase activity and 2,3-BPG production.
Discussion
It has been known for more than four decades that when humans ascend to high altitudes the affinity of Hb for O2 decreases to make more O2 available to hypoxic peripheral tissues15, 41. It was also recognized early on that the reduced O2 affinity was due in part to the increase in erythrocyte 2,3-BPG which functioned as a negative allosteric regulator of Hb-O2 affinity14, 42, 43. The molecular mechanisms accounting for the high altitude-mediated regulation of erythrocyte 2,3-BPG concentration were not understood until we conducted non-biased metabolomic screening of erythrocytes in humans and mouse genetic studies. Both human and mouse studies reported here provide strong evidence that CD73-mediated adenosine signaling via ADORA2B in erythrocytes, plays a beneficial role in preventing hypoxia-induced tissue hypoxia, inflammation and lung damage by inducing 2,3-BPG production and triggering O2 release in an AMPK-dependent manner (Figure 6I). Erythrocyte 2,3-BPG levels are increased by high altitude hypoxia in normal individuals15 and in patients with CVD frequently facing hypoxia17–19. Here we found that adenosine is induced by high altitude and that our newly identified erythrocyte adenosine signaling cascade promotes O2 delivery to prevent tissue hypoxia. Thus, our findings are highly significant and highlight multiple new therapeutic avenues to prevent and treat hypoxia-induced tissue injury in both normal individuals at high altitude and CVD patients facing hypoxia.
Although extracellular adenosine is known to be induced under hypoxia, no prior study has reported that plasma adenosine levels are increased at high altitude, and a role for elevated plasma adenosine in high altitude adaptation has never been recognized prior to our study with young healthy human volunteers. Here we show for the first time that plasma adenosine levels were induced at ALT1 and increased further at ALT16. Consistent with the high altitude-dependent increase in plasma adenosine, we also found that the activity of sCD73, likely released from endothelial cells, was also significantly induced by high altitude hypoxia. Moreover, we confirmed early studies showing that erythrocyte 2,3-BPG and O2 releasing capacity were significantly elevated in humans at high altitude. The elevated plasma adenosine concentration is associated with increased erythrocyte 2,3-BPG concentration and O2 releasing capacity. Similar to humans at high altitude, we found that sCD73 activity, plasma adenosine levels, erythrocyte 2,3-BPG concentration and O2 release capacity were significantly elevated in WT mice under hypoxia (10% O2) for 1 week. However, genetic deletion of CD73 significantly reduced hypoxia-induced plasma adenosine, erythrocyte 2,3-BPG and O2 releasing capacity in mice. The decreased O2 availability to peripheral tissues resulted in multiple tissue hypoxia, enhanced inflammation and pulmonary damage, which is consistent with early studies showing that genetic deletion of CD73 leads to vascular leakage and increased immune cell infiltration in the lungs under hypoxia.27, 44 Erythrocyte specific ADORA2B knockouts allowed us to demonstrate for the first time that ADORA2B is essential for elevated plasma adenosine induced-2,3-BPG production and O2 releasing capacity from erythrocytes. The ablation of erythrocyte ADORA2B in mice results in severe tissue hypoxia, inflammation and lung injury, similar to CD73-deficient mice. Thus, we have determined that elevated plasma adenosine signaling via erythrocyte ADORA2B plays an important role in preventing multiple tissue hypoxia and injury by inducing erythrocyte 2,3-BPG concentration and triggering O2 release to local tissues. The discovery of this adenosine erythrocyte activated signaling pathway reveals new dimensions to the importance of adenosine signaling in adaptation to hypoxia and immediately suggest novel therapeutics to counteract tissue hypoxia in normal individuals at high altitude and CVD patients frequently suffering from hypoxia.
AMPK is a well-known metabolic stress-sensing kinase, which plays an essential role in regulating cellular energy metabolism.45, 46 In erythrocytes, AMPK plays critical roles in regulating oxidative stress and maintaining the integrity and life span of the cell47–50. A previous study used a proteomics approach to identify 2,3-BPG-mutase as a potential AMPK target in erythrocytes35. Our current work revealed that AMPK, functioning downstream of ADORA2B, phosphorylates and activates 2,3-BPG-mutase resulting in increased 2,3-BPG production, and elevated O2 release from erythrocytes. Moreover, we showed that AICAR induced phosphorylation of AMPK and the activation of 2,3-BPG mutase, 2,3-BPG production and O2 release capacity in cultured WT mouse erythrocytes. In contrast, the AMPK inhibitor, Compound C, significantly attenuated the effects of AICAR in cultured WT mouse erythrocytes. Because of the importance of AMPK in the induction of 2,3-BPG production in erythrocytes and broad usage of AICAR as an AMPK activator previously18, we conducted preclinical studies and found that pretreatment with AICAR induced erythrocyte 2,3-BPG concentration and O2 availability to local tissue both in Cd73−/− mice and Adora2bf/f/EpoR-Cre+ mice under hypoxia. Furthermore, we demonstrated that AICAR enhanced hypoxia adaptation by rapidly inducing erythrocyte AMPK phosphorylation, 2,3-BPG mutase activity, 2,3-BPG levels, and triggering O2 release to local tissue in WT mice. Similar to mouse findings, we showed that p-AMPK and 2,3-BPG mutase activity were significantly elevated in erythrocytes of humans facing high altitude hypoxia. The significance of our mouse finding is further supported by human translational studies showing that AICAR-induced p-AMPK, 2,3-BPG mutase activity, 2,3-BPG production and O2 releasing capacity in cultured normal human erythrocytes. In contrast, Compound C attenuates AICAR-induced p-AMPK, 2,3-BPG mutase activation, 2,3-BPG production and O2 releasing capacity in cultured normal human erythrocytes. Thus, preclinical studies in mice and translational studies with human volunteers provide a strong foundation for future clinical trials in humans to test the ability of AMPK activators to increase O2 availability in humans adapting to high altitude or facing other hypoxia challenges (Figure 6I).
In conclusion, we have identified a functional role of CD73-dependent elevated extracellular adenosine signaling in O2 release from Hb in erythrocytes in response to hypoxia. This discovery has revealed a previously unrecognized role of adenosine signaling in erythrocyte physiology and provides a novel molecular mechanism underlying adaptation to hypoxia. Moreover, our finding that AMPK, functioning downstream of ADORA2B in erythrocytes, activates 2,3-BPG mutase, subsequently induces 2,3-BPG production and O2 releasing capacity, has important clinical implications. Overall, our current studies have added significant insight to the molecular mechanisms underlying human adaptation to hypoxia to counteract tissue hypoxia, inflammation and injury and thereby have highlighted novel therapeutic possibilities for prevention and treatment of hypoxia-related conditions and diseases.
Supplementary Material
Clinical Perspective.
What is new?
Plasma adenosine concentrations and sCD73 activity are rapidly increased at high altitude and are associated with elevated erythrocyte 2,3-BPG levels and O2 releasing capacity.
Elevated CD73 contributes to hypoxia-induced adenosine and that elevated adenosine-mediated erythrocyte ADORA2B activation is beneficial by inducing 2,3-BPG production and triggering O2 release to prevent multiple tissue hypoxia, inflammation and lung vascular leakage.
Erythrocyte AMP-activated protein kinase (AMPK) is activated in humans at high altitude.
AMPK functions downstream of ADORA2B to phosphorylate and activate BPG mutase and in this way induces 2,3-BPG production and O2 release from erythrocytes.
What are the clinical implications?
Rapidly elevated adenosine signaling via ADORA2B coupled with AMPK activation-induced O2 deleivery is not limited to high altitude hypoxia but it is relevant to many pathological hypoxia conditions including cardiovascular, hemolytic and respiratory diseases.
Targeting ADOAR2B-AMPK may be beneficial to counteract tissue hypoxia by enhancing O2 availability in both normal individuals ascending to high altitude and patients with cardiovascular, hemolytic and respiratory diseases constantly facing hypoxia.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health Grants HL119549 (to MRB, HKE and YX), DK083559 (to YX), HL113574 (to YX), American Heart Association Grant 12IRG9150001 (to YX), and National Blood Foundation Early Career Grant 2016 cycle (to AD). Funding for the overall AltitudeOmics study was provided, in part, by grants from the U.S. Department of Defense [W81XWH-11-2-0040 Telemedicine & Advanced Technology Research Center (TATRC) to RCR and W81XWH-10-2-0114 to ATL]; Cardiopulmonary & Respiratory Physiology Laboratory, University of Oregon; and the Charles S. Houston Endowed Professorship at the Altitude Research Center, School of Medicine, University of Colorado.
Footnotes
Disclosures
None.
References
- 1.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JD, Honigman B. The effect of altitude-induced hypoxia on heart disease: Do acute, intermittent, and chronic exposures provide cardioprotection? High Alt Med Biol. 2011;12:45–55. doi: 10.1089/ham.2010.1021. [DOI] [PubMed] [Google Scholar]
- 4.Weissmann N, Sommer N, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Oxygen sensors in hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2006;71:620–629. doi: 10.1016/j.cardiores.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med (Berl) 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 6.Sun K, Xia Y. New insights into sickle cell disease: A disease of hypoxia. Curr Opin Hematol. 2013;20:215–221. doi: 10.1097/MOH.0b013e32835f55f9. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation. 2007;116:2191–2202. doi: 10.1161/CIRCULATIONAHA.106.650796. [DOI] [PubMed] [Google Scholar]
- 8.West JB. Paralysis and blindness during a balloon ascent to high altitude. High Alt Med Biol. 2004;5:453–456. doi: 10.1089/ham.2004.5.453. [DOI] [PubMed] [Google Scholar]
- 9.Petousi N, Robbins PA. Human adaptation to the hypoxia of high altitude: The tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol (1985) 2014;116:875–884. doi: 10.1152/japplphysiol.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storz JF. Evolution. Genes for high altitudes. Science. 2010;329:40–41. doi: 10.1126/science.1192481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisancho AR. Developmental functional adaptation to high altitude: Review. Am J Hum Biol. 2013;25:151–168. doi: 10.1002/ajhb.22367. [DOI] [PubMed] [Google Scholar]
- 12.Hackett PH, Roach RC. High-altitude illness. N Engl J Med. 2001;345:107–114. doi: 10.1056/NEJM200107123450206. [DOI] [PubMed] [Google Scholar]
- 13.Hackett PH, Creagh CE, Grover RF, Honigman B, Houston CS, Reeves JT, Sophocles AM, Van Hardenbroek M. High-altitude pulmonary edema in persons without the right pulmonary artery. N Engl J Med. 1980;302:1070–1073. doi: 10.1056/NEJM198005083021907. [DOI] [PubMed] [Google Scholar]
- 14.Brewer GJ. 2,3-dpg and erythrocyte oxygen affinity. Annu Rev Med. 1974;25:29–38. doi: 10.1146/annurev.me.25.020174.000333. [DOI] [PubMed] [Google Scholar]
- 15.Lenfant C, Torrance J, English E, Finch CA, Reynafarje C, Ramos J, Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968;47:2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shappell SD, Murray JA, Nasser MG, Wills RE, Torrance JD, Lenfant CJ. Acute change in hemoglobin affinity for oxygen during angina pectoris. N Engl J Med. 1970;282:1219–1224. doi: 10.1056/NEJM197005282822201. [DOI] [PubMed] [Google Scholar]
- 17.Woodson RD, Torrance JD, Shappell SD, Lenfant C. The effect of cardiac disease on hemoglobin-oxygen binding. J Clin Invest. 1970;49:1349–1356. doi: 10.1172/JCI106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bersin RM, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, DeMarco T, Wolfe C, Chatterjee K. Importance of oxygen-haemoglobin binding to oxygen transport in congestive heart failure. Br Heart J. 1993;70:443–447. doi: 10.1136/hrt.70.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subudhi AW, Bourdillon N, Bucher J, Davis C, Elliott JE, Eutermoster M, Evero O, Fan JL, Jameson-Van Houten S, Julian CG, Kark J, Kark S, Kayser B, Kern JP, Kim SE, Lathan C, Laurie SS, Lovering AT, Paterson R, Polaner DM, Ryan BJ, Spira JL, Tsao JW, Wachsmuth NB, Roach RC. Altitudeomics: The integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PLoS One. 2014;9:e92191. doi: 10.1371/journal.pone.0092191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, Carter-Dawson L, Lewis DE, Eltzschig HK, Kellems RE, Blackburn MR, Juneja HS, Xia Y. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via a2b adenosine receptor contributes to chronic hypertension. Circ Res. 2013;112:1466–1478. doi: 10.1161/CIRCRESAHA.111.300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrich AC, Pelanda R, Klingmuller U. A mouse model for visualization and conditional mutations in the erythroid lineage. Blood. 2004;104:659–666. doi: 10.1182/blood-2003-05-1442. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Berka V, Song A, Sun K, Wang W, Zhang W, Ning C, Li C, Zhang Q, Bogdanov M, Alexander DC, Milburn MV, Ahmed MH, Lin H, Idowu M, Zhang J, Kato GJ, Abdulmalik OY, Dowhan W, Kellems RE, Zhang P, Jin J, Safo M, Tsai AL, Juneja HS, Xia Y. Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J Clin Invest. 2014;124:2750–2761. doi: 10.1172/JCI74604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzalez-Alonso J. Intravascular adp and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. The Journal of physiology. 2007;579:553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yegutkin GG, Wieringa B, Robson SC, Jalkanen S. Metabolism of circulating adp in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and ntpdase1/cd39 activities. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:3875–3883. doi: 10.1096/fj.12-205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtman MA, Murphy MS, Adamson JW. Detection of mutant hemoglobins with altered affinity for oxygen. A simplified technique. Ann Intern Med. 1976;84:517–520. doi: 10.7326/0003-4819-84-5-517. [DOI] [PubMed] [Google Scholar]
- 27.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (cd73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine a2b receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linden J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 30.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM. Hypoxia-induced exocytosis of endothelial cell weibel-palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest. 1996;97:493–500. doi: 10.1172/JCI118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2b adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Xia Y. Beneficial and detrimental role of adenosine signaling in diseases and therapy. J Appl Physiol (1985) 2015;119:1173–1182. doi: 10.1152/japplphysiol.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J. International union of pharmacology. Xxv. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 34.Shuga J, Zhang J, Samson LD, Lodish HF, Griffith LG. In vitro erythropoiesis from bone marrow-derived progenitors provides a physiological assay for toxic and mutagenic compounds. Proc Natl Acad Sci U S A. 2007;104:8737–8742. doi: 10.1073/pnas.0701829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thali RF, Tuerk RD, Scholz R, Yoho-Auchli Y, Brunisholz RA, Neumann D. Novel candidate substrates of amp-activated protein kinase identified in red blood cell lysates. Biochem Biophys Res Commun. 2010;398:296–301. doi: 10.1016/j.bbrc.2010.06.084. [DOI] [PubMed] [Google Scholar]
- 36.Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of amp-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdulrahman RM, Boon MR, Sips HC, Guigas B, Rensen PC, Smit JW, Hovens GC. Impact of metformin and compound c on nis expression and iodine uptake in vitro and in vivo: A role for cre in ampk modulation of thyroid function. Thyroid. 2014;24:78–87. doi: 10.1089/thy.2013.0041. [DOI] [PubMed] [Google Scholar]
- 40.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenfant C, Sullivan K. Adaptation to high altitude. N Engl J Med. 1971;284:1298–1309. doi: 10.1056/NEJM197106102842305. [DOI] [PubMed] [Google Scholar]
- 42.Valeri CR, Yarnoz M, Vecchione JJ, Dennis RC, Anastasi J, Valeri DA, Pivacek LE, Hechtman HB, Emerson CP, Berger RL. Improved oxygen delivery to the myocardium during hypothermia by perfusion with 2,3 dpg-enriched red blood cells. Ann Thorac Surg. 1980;30:527–535. doi: 10.1016/s0003-4975(10)61725-0. [DOI] [PubMed] [Google Scholar]
- 43.Finch CA, Lenfant C. Oxygen transport in man. The New England journal of medicine. 1972;286:407–415. doi: 10.1056/NEJM197202242860806. [DOI] [PubMed] [Google Scholar]
- 44.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases cd39 and cd73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 45.Long YC, Zierath JR. Amp-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardie DG, Ross FA, Hawley SA. Ampk: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Dale GL, Song P, Viollet B, Zou MH. Ampkalpha1 deletion shortens erythrocyte life span in mice: Role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foller M, Sopjani M, Koka S, Gu S, Mahmud H, Wang K, Floride E, Schleicher E, Schulz E, Munzel T, Lang F. Regulation of erythrocyte survival by amp-activated protein kinase. FASEB J. 2009;23:1072–1080. doi: 10.1096/fj.08-121772. [DOI] [PubMed] [Google Scholar]
- 49.Foretz M, Hebrard S, Guihard S, Leclerc J, Do Cruzeiro M, Hamard G, Niedergang F, Gaudry M, Viollet B. The ampkgamma1 subunit plays an essential role in erythrocyte membrane elasticity, and its genetic inactivation induces splenomegaly and anemia. FASEB J. 2011;25:337–347. doi: 10.1096/fj.10-169383. [DOI] [PubMed] [Google Scholar]
- 50.Foretz M, Guihard S, Leclerc J, Fauveau V, Couty JP, Andris F, Gaudry M, Andreelli F, Vaulont S, Viollet B. Maintenance of red blood cell integrity by amp-activated protein kinase alpha1 catalytic subunit. FEBS Lett. 2010;584:3667–3671. doi: 10.1016/j.febslet.2010.07.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.