Abstract
Rationale:
Episodic bouts of social stress can precede the initiation, escalation or relapse to disordered alcohol intake. Social stress may engender neuroadaptations in the hypothalamic-pituitary-adrenal (HPA) axis and in extrahypothalamic stress circuitry to promote the escalation of alcohol intake.
Objectives:
We aimed to (1) confirm a pattern of escalated drinking in socially defeated mice, and to (2) test drugs that target distinct aspects of the hypothalamic-pituitary adrenal (HPA) axis and extrahypothalamic neural substrates for their effectiveness in reducing murine, stress-escalated drinking.
Methods:
Male C57BL/6J (B6) mice were socially defeated by resident Swiss-derived males for ten consecutive days receiving 30 bites/day. Ten days after the final defeat, cohorts of B6 mice received continuous or intermittent access to 20% EtOH (w/v) and water. After four weeks of drinking, mice were injected with weekly, systemic doses of the CRF-R1 antagonist, CP376395; the glucocorticoid receptor antagonist, mifepristone; the 11-beta-hydroxylase inhibitor, metyrapone; or the 5-alpha-reductase inhibitor, finasteride.
Results:
Prior to drug treatments, defeated mice reliably consumed more EtOH than non-defeated controls, and mice given alcohol intermittently consumed more EtOH than those with continuous access. CP376395 (17-30 mg/kg) reduced continuous, but not intermittent EtOH intake (g/kg) in socially defeated mice. Mifepristone (100 mg/kg), however, increased drinking by defeated mice with intermittent access to alcohol while reducing drinking during continuous access. When administered finasteride (100 mg/kg) or metyrapone (50 mg/kg), all mice reduced their EtOH intake while increasing their water consumption.
Conclusions:
Mice with a history of episodic social defeat stress were selectively sensitive to the effects of CRF-R1 antagonism, suggesting that CRF-R1 may be a potential target for treating alcohol use disorders in individuals who escalate their drinking after exposure to repeated bouts of psychosocial stress. Future studies will clarify how social defeat stress may alter the expression of extrahypothalamic CRF-R1 and glucocorticoid receptors.
Keywords: social defeat stress, alcohol, intermittent access to alcohol, CRF-R1, CP376395, glucocorticoids, metyrapone, mifepristone, finasteride, C57BL/6J mice
The onset of escalated alcohol consumption and relapse to disordered drinking is often preceded by episodes of social stress (Boden et al. 2014; Brown et al. 1990; Field and Powell 2007; Laws et al. 2017; Miller et al. 1974; Sinha 2001; Uhart and Wand 2009). Similarly, in some preclinical rodent models, animals subjected to daily social defeats subsequently escalate their alcohol consumption compared to non-defeated or dominant individuals (Albrechet-Souza et al. 2017; Caldwell and Riccio 2010; Croft et al. 2005; Hwa et al. 2016a; Karlsson et al. 2017; Kudryavtseva et al. 2006; Kudryavtseva et al. 1991; Nelson et al. 2017; Norman et al. 2015; Ribeiro Do et al. 2009). Converging evidence suggests that this pattern of drinking may arise from stress-induced neuroadaptations, culminating in the sensitization of hypothalamic, extrahypothalamic and mesocorticolimbic stress- and reward-related circuitries (Covington et al. 2005; Han et al. 2017; Holly et al. 2016; Hwa et al. 2016a; Laine et al. 2017; Martinez et al. 2002; Matsuda et al. 1996; Miczek et al. 2008; Nikulina et al. 2004; Nikulina et al. 2008). Here, we use a mouse model of episodic social defeat stress-escalated drinking to examine pharmacological interventions that target distinct aspects of the hypothalamic-pituitary-adrenal (HPA) axis and extrahypothalamic stress circuitry. In addition, we assessed whether these effects are specific to the schedule of chronic alcohol access by administering drugs to socially defeated and non-defeated mice with either continuous or intermittent access to alcohol.
Activation of the HPA axis prompts corticotropin-releasing factor (CRF, i.e. CRH) release from median eminence terminals originating in the paraventricular nucleus of the hypothalamus. CRF subsequently stimulates adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary and glucocorticoid secretion from the adrenal cortex. In addition to other physiological effects, glucocorticoids regulate HPA axis activity and stress responding through negative feedback. In contrast, distinct CRF signaling in extrahypothalamic brain regions is associated with the regulation of affective behavior (Heinrichs and Koob 2004). Within the extended amygdala, CRF is upregulated upon exposure to stress or alcohol, and sensitization of the extrahypothalamic CRF system contributes to the anxiogenic effects of alcohol withdrawal (Gilpin and Roberto 2012). Peripherally, CRF-R1 antagonists block ACTH secretion (Rivier et al. 1984; Rivier et al. 1999; Webster et al. 1996), while central CRF-R1 antagonism has been proposed to act via the extended amygdala to normalize stress-escalated drug-taking (Gilpin and Roberto 2012).
Though consistent evidence demonstrates that CRF-R1 blockade reduces alcohol intake in animal models of chronic or binge-like drinking (Cippitelli et al. 2012; Correia et al. 2015; Gehlert et al. 2007; Gilpin et al. 2008; Hwa et al. 2013; Hwa et al. 2016a; Hwa et al. 2016b; Koob 2010; Lodge and Lawrence 2003; Lowery et al. 2010; Rinker et al. 2017; Sparta et al. 2008; Sparta et al. 2009), so far, similar compounds have been ineffective in suppressing stress-induced alcohol craving in samples of anxious, alcohol-dependent patients (Kwako et al. 2015; Schwandt et al. 2016). We propose that stress-induced dysregulation of glucocorticoid secretion, and of CRF and dopamine release in response to ethanol may escalate intake by individuals with a stress history, and that targeting CRF signaling may underlie the potentially therapeutic effects of CRF-R1 antagonists (Molander et al. 2012; Sinha 2008). To clarify the potential role of CRF-R1 in stress-escalated continuous or intermittent alcohol intake, we treated socially defeated and non-defeated mice with doses of the CRF-R1 antagonist, CP376395.
Previously, we and others have reported escalated levels of circulating corticosterone in mice subjected to moderate, episodic social defeat stress (Croft et al. 2008; Norman et al. 2015). In animal models, self-administered corticosterone escalates local dopamine release in the ventral striatum, and promotes alcohol consumption (Fahlke and Hansen 1999; Piazza et al. 1993; Piazza et al. 1996) while acutely administered alcohol can increase cortical and hippocampal glucocorticoid concentrations (Porcu et al. 2014). In rats, blocking glucocorticoid receptors (GR) with mifepristone suppresses alcohol drinking and stress-induced alcohol seeking (Koenig and Olive 2004; Simms et al. 2012; Vendruscolo et al. 2015). Similarly, in alcohol-dependent patients, mifepristone significantly diminishes alcohol craving (Vendruscolo et al. 2015). We hypothesized that elevated levels of circulating glucocorticoids may increase the rewarding value of alcohol to promote alcohol consumption by mice with a history of episodic social defeat stress. To assess glucocorticoid functioning in stress-escalated drinking, defeated and non-defeated male mice received either systemic doses of the 11β-hydroxylase inhibitor, metyrapone, to block synthesis of corticosterone, or doses of the GR antagonist, mifepristone, to prevent receptor activation by corticosterone.
Finally, we tested the 5α-reductase inhibitor, finasteride, in our murine model of stress-escalated chronic alcohol drinking. Inhibiting allopregnanolone and tetrahydrodeoxycorticosterone (THDOC) synthesis with acute doses of finasteride can transiently suppress alcohol intake (Ford et al. 2005a; Ford et al. 2008; Ramaker et al. 2011); conversely, low doses of the neurosteroid allopregnanolone, a potent positive allosteric modulator of GABAA receptors, can increase voluntary alcohol consumption in C57BL/6J mice (Ford et al. 2005b; Ford et al. 2007; Sinnott et al. 2002). Stress exposure, whether in the form of episodic social defeat or intermittent periods of forced abstinence from chronic alcohol may alter how neurosteroids interact with the GABAA receptors (Sarkar et al. 2011). To address the potential role of neurosteroid synthesis in mice with a history of repeated social stress, animals maintained on continuous or intermittent schedules of alcohol access were treated systemically with acute doses of finasteride.
In the present experimental work, we assessed the role of (1) signaling via CRF-R1 receptors, (2) corticosterone synthesis and its actions on glucocorticoid receptors, and (3) endogenous neurosteroid synthesis in a murine model of social defeat stress-escalated alcohol intake. The 11β-hydroxylase inhibitor, metyrapone, and the 5α-reductase inhibitor, finasteride, reduced alcohol intake in mice, regardless of their stress history. In contrast, the glucocorticoid receptor antagonist, mifepristone, increased alcohol intake in mice with a stress history and intermittent access to alcohol while the same dose of mifepristone decreased drinking in mice with continuous alcohol access. Upon receiving the CRF-R1 antagonist, CP376395, socially defeated mice with continuous access reduced their alcohol drinking, adding to the accumulating evidence that CP376395 may diminish chronically escalated alcohol consumption. In light of these findings, we propose that CP376395 may be most effective in individuals who initiate or intensify their drinking after experiencing episodes of psychosocial stress.
Materials and Methods
Animals
Eight-week-old male C57BL/6J mice (B6 mice; Jackson Laboratories, Bar Harbor, ME, USA) were housed individually. Aggressive residents were adult Swiss Webster (CFW) males (Charles River Laboratories, Wilmington, MA, USA) maintained in breeding pairs with CFW females for at least three weeks before aggressive encounters to facilitate territorial agonistic behavior toward male conspecifics (Miczek and O’Donnell 1978). All mice were housed in clear polycarbonate cages (28×17×14 cm) lined with pine shavings, and had unlimited access to tap water and rodent chow (Purina LabDiet 5001) through stainless-steel wire mesh cage lids. Food, water and bedding were changed weekly at least 24 hours prior to systemic drug injections and behavioral testing. The vivarium was temperature-regulated and maintained on a 12-hour reverse light/dark photocycle (lights off from 0700 to 1900 hours). Mice were cared for according to the NIH Guide for the Care and Use of Laboratory Animals (National Research Council 2011) and procedures were approved by the Institutional Animal Care and Use Committee of Tufts University.
Drugs
Ninety-five percent ethyl alcohol (EtOH; Pharmco-AAPER Products) was diluted with tap water to achieve a concentration of 20% EtOH (w/v). EtOH solutions were made weekly and any remaining solution was disposed of at the end of each week.
The CRF-R1 antagonist, CP376395 (Tocris, Minneapolis, MN, USA) was dissolved in 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) in dH2O, and administered in doses of 10, 17 and 30 mg/kg. Metyrapone (Sigma-Aldrich, St. Louis, MO, USA), an 11β-hydroxylase inhibitor, was dissolved in 0.9% saline and injected in doses of 10, 30 and 50 mg/kg. Twenty percent (2-hydroxypropyl)-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA) in dH2O served as the vehicle for suspensions of the glucocorticoid receptor antagonist, mifepristone (i.e. RU38486; Sigma-Aldrich, St. Louis, MO, USA; 17, 30, 56, and 100 mg/kg). Twenty percent β-cyclodextrin vehicle was also used to prepare suspensions of the 5α-reductase inhibitor, finasteride (Sigma-Aldrich, St. Louis, MO, USA; 10, 30, and 100 mg/kg). Drug doses, selected based on previous research and on pilot studies (Hwa et al. 2016b; Lowery et al. 2010; Ramaker et al. 2011), were prepared immediately prior to their administration and given systemically in an injection volume of 10 mL per kg of body weight.
Moderate Episodic Social Defeat Stress
Male B6 mice were randomly assigned to the non-defeated, control group or to the moderate episodic social defeat stress group (Table 1). Control mice were weighed daily and returned to their home cage. Moderate episodic social defeat stress occurred as previously described (Norman et al. 2015; Yap et al. 2005). Briefly, social defeat episodes occurred every 24 hours for ten consecutive days; each episode consisted of a 5-minute pre-defeat threat period, followed by the social defeat, and concluding with a 5-minute post-defeat threat period. First, the female and any pups were temporarily removed from a resident CFW’s home cage and replaced by a B6 male in a perforated, protective cage (15×7×7 cm). Immediately after this pre-defeat threat, the B6 male was removed from the protective cage and returned to the resident’s home cage. This social defeat was terminated either after the experimental mouse received 30 bites, after five minutes had elapsed, or in the rare case that the B6 mouse attacked the resident male. Following the defeat, the B6 mouse was placed back in the protective cage for a post-defeat threat period in the resident’s home cage. After the post-defeat threat period, B6 mice were returned to their home cages until the next social defeat episode on the following day. B6 mice that were aggressive toward a resident CFW (n=6 of 100) could not be defeated, and were therefore excluded from further experimental work.
Table 1.
Sample sizes (n /group)
| CAA | IAA | |
|---|---|---|
| CP376395 | ||
| Non-defeated | 12 | 10 |
| Socially defeated | 12 | 11 |
| Metyrapone | ||
| Non-defeated | 8 | 8 |
| Socially defeated | 15 | 10 |
| Mifepristone | ||
| Non-defeated | 12 | 12 |
| Socially defeated | 11 | 11 |
| Finasteride | ||
| Non-defeated | 7 | 10 |
| Socially defeated | 14 | 10 |
| BEC analysis | ||
| Non-defeated | 23 | 16 |
| Socially defeated | 26 | 20 |
Continuous access to alcohol (CAA); Intermittent access to alcohol (IAA); Blood ethanol concentration (BEC)
Voluntary EtOH Intake: Intermittent or Continuous Access to 20% EtOH (w/v)
Ten days after the final social defeat episode, defeated and non-defeated B6 males received either intermittent or continuous access 20% EtOH (w/v) and water (i.e. two-bottle choice), as described previously (Hwa et al. 2011). Defeated and non-defeated mice with intermittent access to alcohol (IAA) were presented with 20% EtOH and water, three hours into the dark photocycle on Mondays, Wednesdays and Fridays. On all other days, both bottles dispensed water. Separate groups of defeated and non-defeated mice were assigned to receive daily, continuous access to 20% EtOH and water (CAA). For both continuous and intermittent access protocols, EtOH presentation alternated between the left and right side of the cage lid to prevent the development of a side preference. Body weights were recorded every 48 hours, and bottles were weighed prior to and after each 24-hour EtOH access period. To control for fluid loss not due to drinking (i.e. drip values), bottle measurements were recorded from an empty cage; these values were subtracted from the recorded intake values of each mouse to account for leakage.
Stress-Escalated Voluntary EtOH Intake and Systemic Pharmacology
During the third week of continuous or intermittent access to EtOH, mice were habituated every 48 hours to handling and intraperitoneal (IP) vehicle injections. Weekly IP injections of CP376395, metyrapone, mifepristone or finasteride began during the fourth week of intermittent or continuous EtOH access (Table 1). Mice continued to consume alcohol, with weekly drug treatments, for up to six additional weeks (i.e. 10 weeks total; Fig. 1). Drugs were administered two hours into the dark phase of the photocycle. An hour later, bottles were emptied, refilled with 20% EtOH (w/v) and water, weighed and returned, signifying the onset of the EtOH access period (i.e. 0 hours, Fig. S1). EtOH and water intake and drip values were recorded after 2, 4, and 24 hours of EtOH access to evaluate brief and long-lasting drug effects on fluid consumption. Animals received weekly, IP drug doses in a non-systematic order with the highest doses administered last to minimize the potential for carry-over effects. Mice were maintained on two-bottle choice protocols, allowing us to determine if effective drug doses selectively reduced alcohol consumption or if they were non-selective and reduced both alcohol and water intake, possibly due to sedative effects or effects on thirst or fluid regulation.
Figure 1.
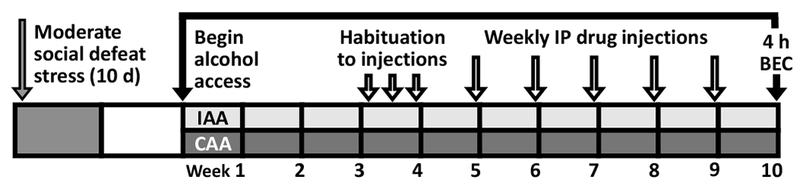
Male C57BL/6J (B6) mice underwent ten consecutive days of moderate, episodic social defeat stress, receiving 30 bites/day from an aggressive resident CFW male; alternatively, non-defeated B6 control mice were weighed daily. Ten days after the final social defeat session, defeated and non-defeated B6 males received either intermittent or continuous access to 20% EtOH (w/v) and water. Mice with intermittent access to alcohol (IAA) received 20% EtOH and water on Mondays, Wednesdays and Fridays, and two bottles of water on the remaining days. Mice with continuous access to alcohol (CAA) received 20% EtOH and water daily. During the third week of IAA or CAA, mice were habituated to intraperitoneal (IP) injections on Mondays, Wednesdays and Fridays one hour prior to the onset of the alcohol access period. Beginning in the fourth week of IAA or CAA, mice received weekly IP injections of CP376395 (0, 10, 17, 30 mg/kg), metyrapone (0, 10, 30, 50), mifepristone (0, 17, 30, 56, 100), or finasteride (0, 10, 30, 100). At least one week after the final IP drug treatment, blood samples were collected from the submandibular vein 4-hours after the onset of the alcohol access period and analyzed for blood plasma ethanol concentration (BEC).
After receiving their final drug treatment, a subset of mice (Table 1) continued to drink with either continuous or intermittent access to alcohol for at least one more week. Blood was then collected from the submandibular vein after 4-hour access to EtOH and water to determine blood ethanol concentration (BEC; mg/dL). Samples were centrifuged for 10 minutes (3000 rpm, 4°C) and plasma (5µL) was extracted and analyzed with the Analox AM1 Alcohol Analyzer (Analox Instruments Inc., Lunenberg, MA, USA).
Statistics
Twenty-four-hour alcohol consumption is presented as grams of EtOH consumed per kilogram of body weight (g/kg; (mL EtOH intake*0.2)/body weight in kg)) and as percent preference (100*(mL EtOH intake/ mL EtOH intake+mL water intake)). For the first four weeks of intermittent or continuous access to EtOH, average individual daily intake values were analyzed by 2-way analyses of variance (2-way ANOVA) to detect interactions between the EtOH access protocol and history of social defeat stress. To detect changes in drinking over time, average individual EtOH intake (g/kg) and percent preference values were calculated for the first and fourth weeks of intermittent or continuous access; these data were analyzed by 2-way repeated measures ANOVA (2-way RM ANOVA). EtOH intake (g/kg), water intake (mL) and BEC (mg/dl) data collected after IP drug administration were analyzed within drug group and drinking protocol. Main effects and interactions between drug and social defeat history were detected by 2-way RM ANOVA. Statistically significant main effects were followed by Dunnett’s post-hoc comparisons, conducted to identify differences between vehicle and specific drug doses and between socially defeated and non-defeated groups. A square root transformation was used when assumptions of normality or equal variance were not met; figures and tables portray the back-transformed data. All statistical analyses were conducted with α set at 0.05 using SigmaPlot v13.0 (Systat Software Inc., San Jose, CA, USA).
Results
Stress-escalated voluntary EtOH intake
A history of moderate, episodic social defeat stress and intermittent alcohol access both, independently escalated 20% EtOH intake by male C57BL/6J mice. Two-way ANOVA revealed significant main effects of stress history (F[1,169]=71.98, p<0.001) and the schedule of EtOH access (F[1,169]= 215.23, p<0.001) on EtOH consumption (g/kg; Fig. 2A, 2B). Specifically, mice that underwent moderate episodic social defeat stress significantly increased their EtOH consumption compared to non-defeated control mice, and intermittent access to EtOH yielded greater levels of drinking compared to continuous access. Likewise, social defeat stress (F[1,169]=31.94, p<0.001) and intermittent EtOH access (F[1,169]=205.6, p<0.001) significantly increased preference for 20% EtOH over water (Fig. 2C, 2D). Only mice with intermittent access to alcohol showed an escalation in their EtOH intake (F[1,80]=95.46, p<0.001) and EtOH preference (F[1,80]=119.70, p<0.001) from the first to the fourth week of drinking. In accordance with these findings, blood ethanol concentrations (BECs) taken from a representative subset of mice revealed higher BECs in animals with intermittent access to alcohol (F[1,81]=53.82, p<0.001) and in those with a history of moderate, episodic social defeat stress (F[1,81]=4.52, p=0.037; Table 2).
Figure 2.
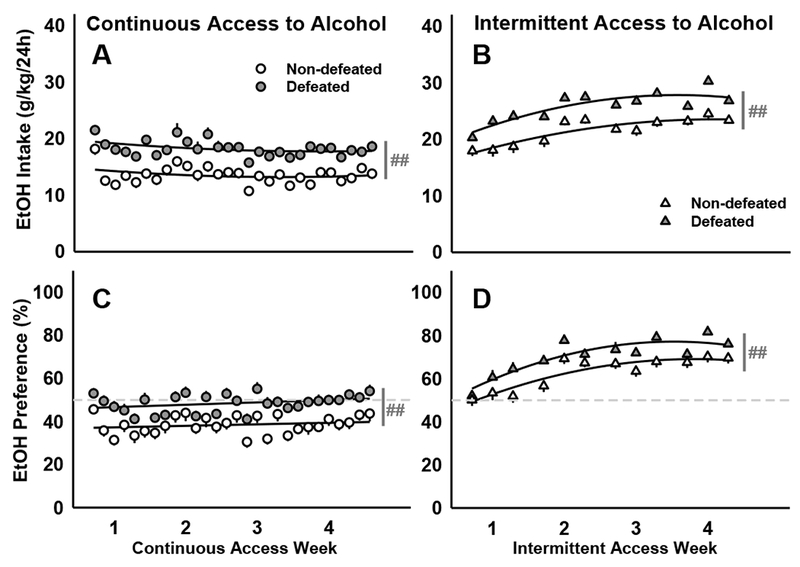
Socially defeated and non-defeated C57BL/6J males received 20% EtOH and water for four weeks prior to receiving weekly, systemic drug injections. Daily mean ± SEM intake (g/kg/24h) for mice with (A) continuous access to alcohol or (B) intermittent access to alcohol. The amount of alcohol consumed as a percentage of the total daily intake (EtOH Preference) is depicted as daily mean ±SEM for mice with (C) continuous or (D) intermittent access to alcohol. Best-fit curves are in black. Dark gray lines represent significant main effects: ##p<0.01 non-defeated vs. defeated. Dashed lines mark the preference cutoff: values >50% indicate a preference for alcohol over water while values <50% indicate a preference for water over alcohol.
Table 2.
BECs (mg/dl) after 4-hour access to 20% EtOH and water
Mean ± SEM; blood ethanol concentration (BEC); continuous access alcohol (CAA); intermittent access alcohol (IAA);
p <0.05, CAA vs. IAA;
p <0.05, socially defeated
Selective suppression of stress-escalated, continuous access EtOH drinking by CRF-R1 antagonism
In socially defeated mice with continuous access to alcohol, injections of the CRF-R1 antagonist, CP376395, reduced daily alcohol intake. Two-way RM ANOVA revealed a significant interaction between stress history and CP376395 (F[3,66]= 3.74, p=0.015) and a main effect of drug treatment (F[3,66]=4.08, p=0.01), with 17 and 30 mg/kg doses selectively reducing daily alcohol intake in mice with a history of moderate, episodic social defeat stress (Fig. 3A). This interaction developed over the course of the 24-hour access period following drug administration, but was not detectable early in the drinking episode. Rather, CRF-R1 antagonism reduced 2-and 4-hour alcohol intake by all mice, regardless of stress history (2hr: F[3,66]= 9.37, p<0.001; 4hr: F[3,66]= 5.93, p=0.001; Table 3). Importantly, water drinking was not suppressed by drug administration, suggesting that CP376395 specifically reduces alcohol consumption (Table S1). By four hours into the access period, defeated mice consumed significantly more alcohol than their non-defeated counterparts (F[1,22]= 7.67, p=0.011); this effect persisted for the entire, 24-hour access period in defeated mice injected with vehicle or the lowest dose of CP376395 (10 mg/kg; Fig. 3A). In our analysis of water consumption (mL), we found that social defeat stress significantly interacted with CP376395 (F[3,66]= 3.01, p=0.036). In general, stress-escalated alcohol intake was associated with lower levels of water drinking compared to the control condition. However, upon receiving doses of CP376395 that effectively reduced their alcohol consumption, defeated mice compensated by drinking slightly more water while controls consumed slightly less water than in the vehicle condition (Table S1, Figure S2A).
Figure 3.
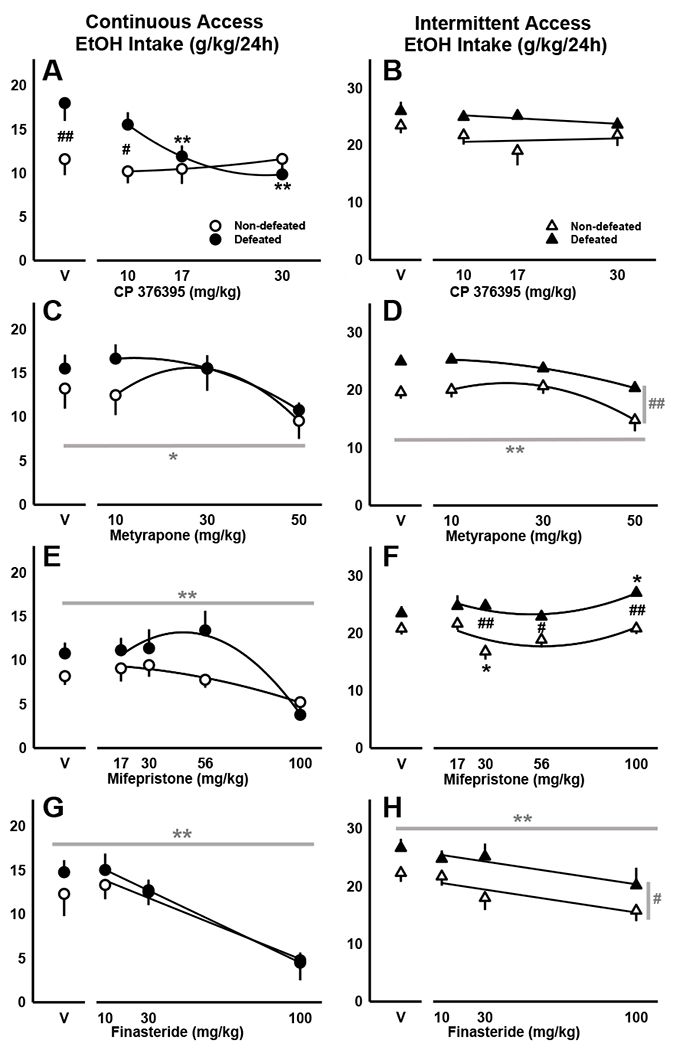
Daily alcohol intake (g/kg/24h) shown as mean ±SEM for socially defeated and non-defeated C57BL/6J males with either (A, C, E, G) continuous or (B, D, F, H) intermittent access to alcohol. Mice received systemic doses of (A, B) CP376395, (C, D) metyrapone, (E, F) mifepristone, or (G, H) finasteride prior to the onset of the alcohol access period. For interactions between social defeat stress and drug treatment, significant post-hoc comparisons are depicted as: *p<0.05, **p<0.01 vehicle vs. drug; #p<0.05, ##p<0.01 non-defeated vs. defeated. Best-fit curves are in black. Dark gray lines represent significant post-hoc comparisons in the presence of main effects: *p<0.05, **p<0.01 vehicle vs. drug; #p<0.05, ##p<0.01 non-defeated vs. defeated.
Table 3.
Two- and four-hour continuous access alcohol intake (g/kg) by socially defeated or non-defeated mice after systemic injections of CP376395, metyrapone, or mifepristone
| Non-defeated | Socially defeated | ||||
|---|---|---|---|---|---|
| Drug | mg/kg | 2hr | 4hr | 2hr | 4hr |
| CP 376395d | veh | 2.60 ± 0.43 | 3.93 ± 0.77 | 3.46 ± 0.38 | 6.75 ± 0.95 |
| 10a | 1.56 ± 0.32 | 2.90 ± 0.50 | 2.19 ± 0.25 | 4.29 ± 0.42 | |
| 17ab | 1.48 ± 0.27 | 2.98 ± 0.45 | 1.60 ± 0.25 | 3.55 ± 0.46 | |
| 30ab | 1.81 ± 0.35 | 2.72 ± 0.56 | 1.25 ± 0.28 | 2.59 ± 0.51 | |
| Metyrapone | veh | 1.43 ± 0.34 | 4.69 ± 1.13 | 2.04 ± 0.26 | 4.03 ± 0.41 |
| 10 | 2.12 ± 0.32 | 3.74 ± 0.61 | 2.61 ± 0.47 | 5.26 ± 0.74 | |
| 30 | 1.99 ± 0.51 | 4.31 ± 0.93 | 1.80 ± 0.43 | 4.16 ± 0.61 | |
| 50ab | 1.25 ± 0.35 | 2.65 ± 0.55 | 0.99 ± 0.12 | 2.64 ± 0.29 | |
| Mifepristone | veh | 1.32 ± 0.21 | 3.50 ± 0.55 | 1.31 ± 0.34 | 3.20 ± 0.92 |
| 17 | 1.23 ± 0.32 | 3.40 ± 0.92 | 1.16 ± 0.31 | 3.12 ± 0.63 | |
| 30 | 1.50 ± 0.39 | 3.28 ± 0.51 | 1.11 ± 0.41 | 2.57 ± 0.63 | |
| 56 | 0.62 ± 0.16 | 2.08 ± 0.56 | 1.01 ± 0.30 | 3.05 ± 0.62 | |
| 100ab | 0.16 ± 0.06 | 0.35 ± 0.13 | 0.40 ± 0.19 | 0.86 ± 0.52 | |
Mean ± SEM; main effects of drug followed by post-hoc comparisons of vehicle vs. drug dose:
p <0.05 at 2hr,
p <0.05 at 4hr; main effects of social stress followed by post-hoc comparisons of socially defeated vs. non-defeated:
p <0.05 at 2hr,
p <0.05 at 4hr
CRF-R1 antagonism decreased 2- and 4-hour alcohol intake in defeated and non-defeated mice with intermittent access to alcohol
Antagonism of CRF-R1 with CP376395 reduced 2- and 4-hour alcohol intake by mice maintained on the intermittent access schedule (2hr: F[3,48]= 21.63, p<0.001; 4hr: F[3,48]= 15.83, p<0.001). This drug effect was no longer evident by 24 hours of two-bottle choice (Fig. 3B, Table 4). In general, defeated animals consuming less water than controls, especially during the first two hours of access (2hr: F[1,16]=8.42, p=0.01). Though the CRF-R1 antagonist selectively reduced alcohol intake without suppressing water consumption, 2-way RM ANOVA detected a significant interaction between defeat stress and CP376395 (24hr: F[3,48]= 2.94, p=0.042). Given the 17.0 mg/kg dose, non-defeated mice drank more water compared to defeated animals (Table S2, Figure S2A), though intake did not significantly differ from the vehicle condition for either group.
Table 4.
Two- and four-hour intermittent access alcohol intake (g/kg) by socially defeated or non-defeated mice after systemic injections of CP376395, metyrapone, mifepristone or finasteride
| Non-defeated | Socially Defeated | ||||
|---|---|---|---|---|---|
| Drug | mg/kg | 2hr | 4hr | 2hr | 4hr |
| CP 376395 | veh | 3.67 ± 0.26 | 6.20 ± 0.28 | 4.72 ± 0.42 | 7.09 ± 0.60 |
| 10a | 3.65 ± 0.72 | 6.44 ± 0.75 | 3.03 ± 0.55 | 5.19 ± 0.58 | |
| 17ab | 1.65 ± 0.38 | 4.42 ± 1.02 | 2.37 ± 0.33 | 4.21 ± 0.50 | |
| 30ab | 1.38 ± 0.31 | 2.27 ± 0.55 | 1.19 ± 0.26 | 3.22 ± 0.59 | |
| Metyraponecd | veh | 2.41 ± 0.35 | 3.95 ± 0.47 | 3.65 ± 0.23 | 5.24 ± 0.45 |
| 10 | 2.27 ± 0.32 | 3.44 ± 0.53 | 3.73 ± 0.27 | 5.36 ± 0.44 | |
| 30 | 2.42 ± 0.36 | 3.63 ± 0.70 | 3.10 ± 0.43 | 4.58 ± 0.47 | |
| 50ab | 1.58 ± 0.37 | 1.80 ± 0.42 | 2.21 ± 0.28 | 3.66 ± 0.27 | |
| Mifepristonecd | veh | 1.78 ± 0.29 | 4.17 ± 0.68 | 2.61 ± 0.28 | 5.20 ± 0.71 |
| 17 | 1.71 ± 0.34 | 5.19 ± 0.81 | 2.43 ± 0.36 | 4.73 ± 0.93 | |
| 30 | 1.55 ± 0.28 | 2.92 ± 0.53 | 2.18 ± 0.29 | 4.99 ± 0.59 | |
| 56 | 1.40 ± 0.33 | 3.36 ± 0.66 | 1.68 ± 0.42 | 4.10 ± 0.77 | |
| 100 | 1.40 ± 0.20 | 3.02 ± 0.37 | 2.46 ± 0.23 | 4.56 ± 0.49 | |
| Finasteridecd | veh | 1.66 ± 0.38 | 3.46 ± 0.85 | 2.75 ± 0.39 | 6.06 ± 0.68 |
| 10 | 1.88 ± 0.41 | 4.11 ± 0.66 | 2.19 ± 0.45 | 5.17 ± 0.84 | |
| 30ab | 0.50 ± 0.16 | 1.94 ± 0.46 | 1.84 ± 0.48 | 4.58 ± 0.99 | |
| 100ab | 0.29 ± 0.20 | 0.97 ± 0.32 | 0.58 ± 0.38 | 2.21 ± 0.58 | |
Mean ± SEM; main effects of drug followed by post-hoc comparisons of vehicle vs. drug dose:
p <0.05 at 2hr,
p <0.05 at 4hr; main effects of social stress followed by post-hoc comparisons of socially defeated vs. non-defeated:
p <0.05 at 2hr,
p <0.05 at 4hr
Inhibition of 11β-hydroxylase reduced alcohol intake in defeated and non-defeated mice
Treatment with the highest dose of the 11β-hydroxylase inhibitor, metyrapone (50 mg/kg), diminished alcohol drinking during 2, 4 and 24 hours of continuous access (2hr: F[3,60]= 6.23, p<0.001; 4hr: F[3,60]= 5.55, p=0.002; 24hr: F[3,60]= 5.00, p=0.004; Fig. 3C, Table 3). Mice consumed less alcohol after receiving metyrapone, and compensated by increasing their water intake, but only for the initial 2 and 4-hour access periods (2hr: F[3,60]= 8.86, p<0.001; 4hr: F[3,60]= 3.94, p=0.012; Table S1, Fig. S2B).
Animals with intermittent access also reduced their alcohol intake upon receiving the highest dose of metyrapone (50 mg/kg; Fig. 3D). Both stressed and non-stressed mice were sensitive to this effect during 2, 4 and 24 hours of alcohol access (2hr: F[3,48]= 7.31, p<0.001; 4hr: F[3,48]= 9.16, p<0.001; 24hr: F[3,48]= 16.95, p<0.001; Table 4). Stress-escalated alcohol intake was observed at every time point in socially defeated mice compared to their non-defeated counterparts (2hr: F[1,16]= 9.80, p=0.006; 4hr: F[1,16]= 10.33, p=0.005; 24hr: F[1,16]= 29.11, p<0.001). An analysis of water intake data revealed a significant interaction between stress history and metyrapone (2hr: F[3,48]= 2.93, p=0.043; 4hr: F[3,48]= 3.09, p=0.036; Table S2). While metyrapone (50 mg/kg) reduced alcohol intake by all mice, only non-defeated animals compensated by drinking more water during the initial 2- and 4-hour access periods. Though alcohol intake remained suppressed, water intake was no longer elevated by the 24-hour time point (Fig. S2B).
Glucocorticoid receptor antagonism increased EtOH intake by defeated mice and decreased EtOH intake by non-defeated mice with intermittent access to alcohol
For animals with intermittent access to alcohol, stress history interacted with mifepristone to increase alcohol consumption (24hr: F[4,84]=2.65, p=0.039). Specifically, mice subjected to episodic social defeat stress consumed significantly more alcohol than controls when given the highest drug dose (100 mg/kg). In contrast, given a moderate dose of mifepristone (30 mg/kg), non-defeated animals reduced their alcohol intake (Fig. 3F, Table 4). Stress-escalated drinking was evident after 2, 4, and 24 hours of alcohol access (2hr: F[1,21]=8.81, p=0.007; 4hr: F[1,21]=4.63, p=0.043; 24hr: F[1,21]=17.18, p<0.001; Fig. 3F), while a main effect of mifepristone was only detected for the full, 24-hour access period (F[4,84]=4.03, p=0.005).
In analyzing water intake during the initial two hours of intermittent alcohol access, 2-way RM ANOVA identified a significant interaction between defeat stress history and mifepristone (F[4,84]=2.52, p=0.047), a main effect of reduced water intake by defeated mice (F[1,21]=8.85, p=0.007), and a main effect of drug treatment (F[4,84]=5.73, p<0.001). Compared to vehicle, non-defeated mice diminished their water intake after receiving moderate doses of mifepristone (17, 56 mg/kg), whereas defeated animals were only sensitive to this effect at higher doses (56, 100 mg/kg; Table S2). By four hours of alcohol access, all doses of mifepristone significantly reduced water intake regardless of stress history (F[4,84]=5.89, p<0.001). This effect was no longer detectable by the 24-hour time point, though the main effect of defeat stress persisted (F[1,21]=4.34, p=0.049; Fig. S2C).
Mifepristone diminished alcohol consumption in mice with continuous access to alcohol
Regardless of their stress history, mice with continuous access to alcohol reduced their 2-, 4- and 24-hour EtOH intake after receiving the highest dose of mifepristone (100 mg/kg; 2hr: F[4,80]= 8.42, p<0.001; 4hr: F[4,80]= 13.86, p<0.001; 24hr: F[4,80]= 9.34, p<0.001; Fig. 3E, Table 3). For water intake, 2-way RM ANOVA identified a significant interaction between social stress and mifepristone treatment (2hr: F[4,80]= 2.88, p=0.028; 4hr: F[4,80]= 3.46, p=0.012; 24hr: F[4,80]= 3.86, p=0.006; Table S1). While non-defeated mice generally consumed more water than their socially defeated counterparts (2hr: F[1,20]= 7.19, p=0.014; 4hr: F[1,20]= 4.38, p=0.049; Table S1), mifepristone (100 mg/kg) selectively increased water drinking by defeated animals, yielding values comparable to controls (Fig. S2C). Mifepristone (56, 100 mg/kg) also briefly diminished water drinking by controls, though this effect was no longer evident by the 24-hour time point (Fig. S2C, Table S1).
Inhibition of 5α-reductase suppressed daily alcohol consumption by defeated and non-defeated mice
Given intermittent access to alcohol, defeated and non-defeated mice treated with finasteride (100 mg/kg) reduced their 2-, 4- and 24-hour alcohol intake (2hr: F[3,54]=12.59, p<0.001; 4hr: F[3,54]=14.54, p<0.001; 24hr: F[3,54]=7.42, p<0.001; Fig. 3H, Table 4). While the moderate dose (30 mg/kg) also suppressed 2- and 4-hour alcohol drinking, only the highest dose (100 mg/kg) suppressed 24-hour intake (Table 4). During the first two hours of access, finasteride diminished both alcohol (g/kg) and water (mL) consumption (EtOH: F[3,54]=12.59, p<0.001; H2O: F[3,54]=8.36, p<0.001; Table 4, Table S2); however, by 24 hours, finasteride-treated animals selectively increased their water drinking (F[3,54]=5.56, p=0.002; Fig. S2D), while maintaining low alcohol intake (F[3,54]=7.42, p<0.001; Fig. 3H; Table 4). Two-way RM ANOVA also revealed significant main effects of escalated 2-, 4- and 24-hour alcohol intake by mice with a history of defeat stress (2hr: F[1,18]=4.92, p=0.04; 4hr: F[1,18]=6.62, p=0.019; 24hr: F[1,18]=4.82, p=0.042). During the first two hours of intermittent access, stress-escalated alcohol intake was concurrent with reduced water consumption (F[1,18]=7.5, p=0.013).
As in the intermittent access group, mice with continuous access reduced their daily alcohol consumption upon receiving finasteride (100 mg/kg; F[3,63]=40.78, p<0.001; Fig. 3G, Table 3). This effect was specific for alcohol, as finasteride did not suppress 24-hour water intake (Fig. S2D, Table S1). For this experiment, only 24-hour drinking data were collected, and thus we cannot exclude the possibility of a non-specific effect of finasteride early in the access period, as observed in the intermittent access experiment.
Discussion
In some individuals, social stress experiences precede an escalation in alcohol drinking (Sinha 2001; Sinha 2008). Here, we first confirm our earlier findings, showing that episodic social defeat stress can promote persistently escalated alcohol consumption in male C57BL/6J mice (Hwa et al. 2016a); likewise, we demonstrate that intermittent access to alcohol can produce significantly higher levels of voluntary alcohol intake and BECs compared to continuous access, as illustrated in our previous work (Hwa et al. 2011; Hwa et al. 2013; Hwa et al. 2016a). After establishing a pattern of stress-escalated drinking, mice were administered drugs targeting specific aspects of the HPA axis, including CRF-R1 receptors, glucocorticoid synthesis and receptor activation, and neurosteroid synthesis. We found that: (1) the CRF-R1 antagonist, CP376395, selectively reduced voluntary alcohol intake by mice with continuous alcohol access and a history of episodic social defeat stress, (2) blocking glucocorticoid receptors increased alcohol intake by socially defeated mice with intermittent access to alcohol, and (3) inhibiting corticosterone or neurosteroid synthesis diminished alcohol intake, regardless of stress history or the schedule of alcohol access.
Interactions between stress neuropeptides and mesocorticolimbic dopamine signaling may mediate stress-escalated alcohol consumption (Melis et al. 2009; Spanagel et al. 2014). Acute alcohol intake promotes HPA axis activation and CRF and corticosterone contribute to dopamine release in the nucleus accumbens (Fahlke et al. 1996; Lemos et al. 2012; Piazza et al. 1996; Richardson et al. 2008). Importantly, a history of repeated social defeat stress also increases mesolimbic CRF and plasma corticosterone, and results in cross-sensitization to alcohol, a phenomenon characterized by increased locomotor behavior and amplified accumbal dopamine release in response to acute drug challenge (Han et al. 2017; Holly et al. 2016; Norman et al. 2015; Phillips et al. 1997; Roberts et al. 1995; Yavich and Tiihonen 2000). An increase in alcohol-induced dopamine release after exposure to episodic social defeat stress may promote the rapid escalation of drinking by stressed mice (Phillips et al. 1997; Quadir et al. 2016). By targeting BNST-VTA-NAcc-mPFC circuitry, CRF-R1 antagonism may selectively reduce the escalation of alcohol consumption in stress-experienced animals (Albrechet-Souza et al. 2017; Hwa et al. 2016a; Silberman et al. 2013).
In previous work, we and others have shown that chronic intermittent access to alcohol can promote significantly escalated voluntary alcohol drinking by C57BL/6J mice relative to consumption during continuous access (Hwa et al. 2011; Hwa et al. 2013; Hwa et al. 2016a; Melendez 2011; Newman et al. 2016; Osterndorff-Kahanek et al. 2013; Rosenwasser et al. 2013; but see Crabbe et al. 2012). In dependence-inducing protocols, alternating bouts of exposure to ethanol vapor and withdrawal can upregulate extrahypothalamic Crh and Crhr1 gene expression and suppress hypothalamic Crh mRNA while increasing subsequent voluntary intake. These intermittent exposure effects contrast with those seen after continuous ethanol vapor exposure (in rats: O’Dell et al. 2004; Richardson et al. 2008; Sommer et al. 2008; in mice: Eisenhardt et al. 2015). Intermittency may sustain escalated, persistent alcohol consumption by reducing CRF-mediated glucocorticoid secretion and negative feedback in the HPA axis (Breese et al. 2011; Edwards et al. 2015; Rivier et al. 1984) and by increasing CRF signaling and sensitivity in extrahypothalamic brain areas associated with negative affect in withdrawal (Koob and Kreek 2007).
Blocking CRF-R1 receptors with CP376395 reduced alcohol intake in stressed mice with continuous access without suppressing their water consumption. Yet, CP376395 only transiently diminished alcohol drinking by animals given alcohol intermittently, a schedule of access shown to escalate alcohol consumption compared to continuous access. Previous reports suggest that CRF-R1 antagonists may selectively diminish intake by animals classified as high drinkers; we hypothesize that the alcohol- and stress-induced neuroplastic changes in extrahypothalamic versus hypothalamic CRF systems may be more indicative of later responding to CRF-R1 antagonists than alcohol intake alone (Correia et al. 2015; Heilig and Koob 2007; Hwa et al. 2013; Sparta et al. 2008). Because hypothalamic and extrahypothalamic CRF sites undergo divergent modifications during chronic alcohol drinking, the effectiveness of systemically administered CRF-R1 antagonists may depend on the extent to which receptors have been upregulated in the extended amygdala and downregulated along the HPA axis (Edwards et al. 2015; Eisenhardt et al. 2015; O’Dell et al. 2004; Richardson et al. 2008; Sommer et al. 2008). This possibility is supported through studies in Crhr1 knockout mice; while a global gene knockout promotes social stress-escalated alcohol consumption (Molander et al. 2012; Sillaber et al. 2002), a conditional, forebrain-specific Crhr1 knockout blocks stress-escalated drinking. These observations suggest that CRF-R1 in the HPA axis may counteract stress effects on drinking whereas extrahypothalamic CRF-R1 activation may stimulate drinking by mice subjected to episodic social defeat stress.
Considerable preclinical work shows that CRF-R1 antagonists can effectively suppress measures of alcohol intake (Koob 2010). However, two recent clinical trials conducted in patients with alcohol use disorders (AUD) report no effect of CRF-R1 antagonism on alcohol cue-induced craving after inpatient detoxification (Kwako et al. 2015; Schwandt et al. 2016; but see Shaham and de Wit 2016, Pomrenze et al. 2017). CRF-R1 antagonists may be specifically effective in reducing alcohol intake by individuals who are currently drinking, and possibly, in those who escalate their drinking after experiencing episodic stress. Elevated circulating cortisol is characteristic of individuals who consume considerable amounts of alcohol, whereas patients in early abstinence tend to have low levels of cortisol; therefore, different drugs may be necessary to normalize glucocorticoid production depending on current drinking status. Reducing glucocorticoid levels with a CRF-R1 antagonist may suppress drinking and the likelihood of relapse during acute withdrawal; conversely, compounds that can increase cortisol levels may diminish cravings during early abstinence (Stephens and Wand 2012). As such, antagonists targeting CRF-R1 should be tested in future clinical trials conducted in non-treatment seeking individuals diagnosed with AUDs (Pomrenze et al. 2017), and in those who escalate their drinking after exposure to repeated bouts of psychosocial stress.
Studies using the glucocorticoid receptor antagonist, mifepristone, demonstrate suppressed alcohol intake and alcohol-cued craving after drug administration in patients suffering from AUDs and reduced alcohol consumption by dependent rats (Vendruscolo et al. 2012; Vendruscolo et al. 2015) whereas mifepristone appears to have no effect on drinking in nondependent rats (Fahlke et al. 1995; Fahlke et al. 1996; Vendruscolo et al. 2012; Vendruscolo et al. 2015). In the present work, we show that a high dose of mifepristone (100 mg/kg) can actually increase intermittent alcohol consumption by defeated mice while a modest dose (30 mg/kg), administered to non-defeated animals, can reduce intermittent alcohol intake. Interestingly, we found that the same dose of mifepristone that escalated drinking in stressed mice with intermittent access effectively reduced alcohol intake in defeated and non-defeated mice with continuous access to alcohol. These findings suggest that moderate antagonism of glucocorticoid receptors may be effective in reducing drinking while higher doses may interfere with HPA axis negative feedback mechanisms; however, the effectiveness of mifepristone appears to depend on individual differences in stress and the pattern of alcohol intake.
Mifepristone may escalate drinking in stressed mice by blunting corticosterone-mediated negative feedback of the HPA axis. Animals with a history of social defeat stress exhibit a potentiated dopamine response to acute alcohol (Yavich and Tiihonen 2000), and may experience greater subjective reinforcing effects of the drug. In repeated bouts of alcohol intake, the eventual downregulation of HPA axis glucocorticoid signaling may accelerate stress-induced drinking, which could be sustained through sensitization of extrahypothalamic brain areas associated with negative affect in withdrawal (Breese et al. 2011; Edwards et al. 2015; Koob and Kreek 2007; Rivier et al. 1984). This profile of neuroadaptations may cause mifepristone to further impair negative feedback, particularly in animals that escalate their drinking on an intermittent schedule of access. As such, increased levels of circulating corticosterone would subsequently stimulate CRF transcription in the extended amygdala, thus worsening negative affect and increasing alcohol consumption (Shepard et al. 2006; Tran and Greenwood-Van Meerveld 2012). While antagonism of glucocorticoid receptors with mifepristone may have therapeutic effects in some alcohol-dependent patients, its use may be limited by the patient’s history of psychosocial stress exposure and their pattern of ongoing alcohol consumption.
Consistent with previous findings (Fahlke et al. 1994; Koenig and Olive 2004; Simms et al. 2012; Vendruscolo et al. 2015), we found a suppression of alcohol intake when glucocorticoid synthesis was inhibited with systemically administered doses of metyrapone. Likewise, inhibiting peripheral glucocorticoid secretion through adrenalectomy suppresses alcohol consumption by rats, and drinking is recovered through systemic or intracerebral injections of corticosterone but not aldosterone (Fahlke et al. 1994; Fahlke et al. 1995; Lamblin and De Witte 1996), indicating that metyrapone likely diminishes intake by blocking corticosterone synthesis, and not through its inhibition of mineralocorticoid synthesis. Importantly, when alcohol drinking was suppressed in our experiments, animals compensated by consuming greater volumes of water, suggesting that metyrapone selectively diminishes voluntary alcohol intake by nondependent animals.
Similarly, finasteride treatment suppressed alcohol intake by defeated and non-defeated mice that received continuous or intermittent access to alcohol. This effect may result from the inhibition of allopregnanolone and THDOC synthesis; however, finasteride treatment may also cause an accumulation of precursor and recruitment of an alternative neurosteroid synthetic pathway. Thus, a reduction in allopregnanolone and THDOC concentrations may precede an increase in concentrations of neurosteroids that act as negative allosteric modulators of the GABAA receptor (Morrow et al. 1990). While the inhibition of 5α-reductase effectively suppressed chronic, voluntary drinking in C57BL/6J mice, the mechanism by which finasteride affects alcohol drinking requires further investigation.
In mice with continuous alcohol access, CP376395 selectively suppressed social stress-escalated drinking. Neuroadaptations in glucocorticoid receptor expression during intermittent access may preclude CRF-R1 antagonists from reducing stress-escalated drinking. Because activation of glucocorticoid receptors can modulate Crh transcription in the extended amygdala (Shepard et al. 2006; Tran and Greenwood-Van Meerveld 2012), intermittency-induced neuroadaptations in GR expression may also promote mifepristone-escalated drinking in defeated mice. Interestingly, intra-VTA infusions of CP376395 can selectively reduce alcohol intake in mice with a history of episodic stress that receive alcohol intermittently (Hwa et al. 2016a). These findings along with our present results suggest that local inhibition of CRF-R1 receptors within the VTA may target circuitry that is distinct from regions that undergo neuroadaptive changes in response to intermittency-escalated drinking, possibly within the extended amygdala. Using a combination of pharmacological tools that target CRF/urocortin 1 and optogenetics in CRF-Cre mice, we plan to identify stress-associated neuroadaptations occurring in CRF-expressing neurons projecting from the CeA and BNST to the VTA (Silberman et al. 2013).
The current studies explore the potential roles of several molecular targets, highlighting CRF-R1 antagonism as a promising candidate for the treatment of stress-escalated alcohol drinking. We continue to characterize the effectiveness of CRF-R1 antagonists by using an ethologically relevant defeat protocol to investigate CRF mechanisms of stress-escalated alcohol drinking in females that have been repeatedly defeated by aggressive, female conspecifics. Using this protocol, our ongoing work explores the role of sexually dimorphic brain regions such as the BNST that may be involved in stress-escalated drinking (Allen and Gorski 1990). Here, we show that CP376395 can reduce stress-escalated alcohol consumption in a dose range that does not influence concurrent water intake. Further studies are necessary to examine the effects of social defeat stress and CRF-R1 antagonism in mice with a prior history of alcohol consumption (e.g. Karlsson et al. 2017), and to determine if CRF-R1 antagonism generally suppresses the motivation to obtain and consume natural rewards or if such compounds can selectively reduce chronic, stress-escalated alcohol drinking.
Supplementary Material
Acknowledgements
The authors would like to thank J. Thomas Sopko for his assistance during manuscript preparation, and Tufts University research assistants, Madeline Kuppe, Ariana Nestler, Kyle Aronson, Janine Elya, Tom Fatkin, Amanda Ho, Diana Sapashnik, Madison Steele, Victoria Stoj, Marissa Heyer, Lena Walton, and Meghan Lauzé for their excellent contributions.
Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism under award numbers R01AA013983 (K.A.M.), F31AA025827 (E.L.N.), and F31AA021622 (L.S.H.), and by CAPES-Brazil under award number PAJT 88887.096822/2015–00 (L. A-S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures
The authors declare no biomedical financial interests or potential conflicts of interest.
References
- Albrechet-Souza L, Viola TW, Grassi-Oliveira R, Miczek KA, de Almeida RMM (2017) Corticotropin releasing factor in the bed nucleus of the stria terminalis in socially defeated and non-stressed mice with a history of chronic alcohol intake. Front Pharmacol 8:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Gorski RA (1990) Sex Difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 302:697–706 [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ (2014) Associations between exposure to stressful life events and alcohol use disorder in a longitudinal birth cohort studied to age 30. Drug Alcohol Depend 142:154–160 [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M (2011) Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther 129:149–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I (1990) Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol 99:344–348 [DOI] [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC (2010) Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44:265–274 [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M (2012) Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav 100:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia D, Martynhak BJ, Pereira M, Siba IP, Ribeiro AF, Camarini R, Boerngen-Lacerda R (2015) Reduction of ethanol intake by corticotropin-releasing factor receptor-1 antagonist in “heavy-drinking” mice in a free-choice paradigm. Psychopharmacology 232:2731–2739 [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Miczek KA (2005) Brief social defeat stress: long lasting effects on cocaine taking during a binge and Zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology 30:310–321 [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P (2012) Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol Alcohol 47:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ (2005) Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology 183:163–170 [DOI] [PubMed] [Google Scholar]
- Croft AP, O’Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ (2008) Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res 1238:12–22 [DOI] [PubMed] [Google Scholar]
- Edwards S, Little HJ, Richardson HN, Vendruscolo LF (2015) Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol 49:811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhardt M, Leixner S, Lujan R, Spanagel R, Bilbao A (2015) Glutamate receptors within the mesolimbic dopamine system mediate alcohol relapse behavior. J Neurosci 35:15523–15538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, Hard E, Soderpalm B (1994) Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol 11:195–202 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hansen S (1999) Effect of local intracerebral corticosterone implants on alcohol intake in the rat. Alcohol Alcohol 34:851–861 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ, Engel JA, Hansen S (1995) Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology 117:216–224 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Hansen S (1996) Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology 127:133–139 [DOI] [PubMed] [Google Scholar]
- Field M, Powell H (2007) Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol Alcohol 42:560–566 [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA (2005a) Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol 37:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA (2005b) Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res 29: 1630–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA (2007) Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res 179: 265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, Finn DA (2008) Inhibition of 5α-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res 32:1408–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M (2007) 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci 27:2718–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF (2008) Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res 32:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M (2012) Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev 36:873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, DeBold JF, Miczek KA (2017) Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology 234:2813–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311:427–440 [DOI] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA (2016) Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci 36:4093–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35:1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, DeBold JF, Miczek KA (2013) Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN. Psychopharmacology 225:313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA (2016a) Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology 233:681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Shimamoto A, Kayyali T, Norman KJ, Valentino RJ, DeBold JF, Miczek KA (2016b) Dissociation of μ-opioid receptor and CRF-R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addict Biol 21:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Schank JR, Rehman F, Stojakovic A, Bjork K, Barbier E, Solomon M, Tapocik J, Engblom D, Thorsell A, Heilig M (2017) Proinflammatory signaling regulates voluntary alcohol intake and stress-induced consumption after exposure to social defeat stress in mice. Addict Biol 22:1279–1288 [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF (2004) The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology 29:999–1003 [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2010) The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva N, Gerrits MA, Avgustinovich DF, Tenditnik MV, van Ree JM (2006) Anxiety and ethanol consumption in victorious and defeated mice; effect of κ-opioid receptor activation. Eur Neuropsychopharmacol 16:504–511 [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Madorskaya IA, Bakshtanovskaya IV (1991) Social success and voluntary ethanol consumption in mice of C57BL/6J and CBA/Lac strains. Physiol Behav 50:143–146 [DOI] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M (2015) The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 40:1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine MA, Sokolowska E, Dudek M, Callan SA, Hyytia P, Hovatta I (2017) Brain activation induced by chronic psychosocial stress in mice. Sci Rep 7:15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamblin F, De Witte P (1996) Adrenalectomy prevents the development of alcohol preference in male rats. Alcohol 13:233–238 [DOI] [PubMed] [Google Scholar]
- Laws HB, Ellerbeck NE, Rodrigues AS, Simmons JA, Ansell EB (2017) Social rejection and alcohol use in daily life. Alcohol Clin Exp Res 41:820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ (2003) The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience 117:243–247 [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE (2010) CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology 35:1241–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J (2002) Mapping brain response to social stress in rodents with c-fos expression: a review. Stress 5:3–13 [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M (1996) Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res 26:157–170 [PubMed] [Google Scholar]
- Melendez RI (2011) Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Diana M, Enrico P, Marinelli M, Brodie MS (2009) Ethanol and acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol 43:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM (1978) Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and L-dopa. Psychopharmacology 57:47–55 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Hersen M, Eisler RM, Hilsman G (1974) Effects of social stress on operant drinking of alcoholics and social drinkers. Behav Res Ther 12:67–72 [DOI] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R (2012) Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology 37:1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM (1990) Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol 37: 263–270 [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the care and use of laboratory animals: eighth edition. National Academy Press, Washington DC [Google Scholar]
- Nelson BS, Sequeira MK, Schank JR (2017) Bidirectional relationship between alcohol intake and sensitivity to social defeat: association with Tacr1 and Avp expression. Addict Biol 10.1111/adb.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Gunner G, Huynh P, Gachette D, Moss SJ, Smart TG, Rudolph U, DeBold JF, Miczek KA (2016) Effects of Gabra2 point mutations on alcohol intake: increased binge-like and blunted chronic drinking by mice. Alcohol Clin Exp Res 40:2445–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP (2008) Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of μ-opioid receptor mRNA and FosB/ΔFosB immunoreactivity. Eur J Neurosci 27:2272–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, Ganschow L, Hammer RP, Miczek KA (2004) Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience 123:857–865 [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA (2015) Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology 232:991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28:1676–1682 [DOI] [PubMed] [Google Scholar]
- Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA (2013) Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One 8:e59870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN (1997) Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav 57:487–493 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H (1993) Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A 90:11738–11742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M (1996) Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A 93:8716–8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Fetterly TL, Winder DG, Messing RO (2017) The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcohol Clin Exp Res 41:1986–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Locci A, Santoru F, Berretti R, Morrow AL, Concas A (2014) Failure of acute ethanol administration to alter cerebrocortical and hippocampal allopregnanolone levels in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res 38:948–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, Szumlinski KK (2016) Homer2 regulates alcohol and stress cross-sensitization. Addict Biol 21:613–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA (2011) Alteration of ethanol drinking in mice via modulation of the GABAA receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res 35:1994–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do CB, Aguilar MA, Lluch J, Rodriguez-Arias M, Minarro J (2009) Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology 207:485–498 [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL (2008) Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci 28:1641–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE (2017) Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake. Biol Psychiatry 81:930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W (1984) Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther 229:127–131 [PubMed] [Google Scholar]
- Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL (1999) Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem 42:3175–3182 [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Lessov CN, Phillips TJ (1995) Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther 275:790–797 [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S (2013) Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol 18:496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J (2011) Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci 31:18198–18210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, Grigoriadis DE, Pich EM, Leggio L, Heilig M (2016) The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology 41:2818–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, de Wit H (2016) Lost in translation: CRF1 receptor antagonists and addiction treatment. Neuropsychopharmacology 41:2795–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA (2006) Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res 174:193–196 [DOI] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG (2013) A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci 33:950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R (2002) Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science 296:931–933 [DOI] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE (2012) Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology 37:906–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–359 [DOI] [PubMed] [Google Scholar]
- Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA (2002) Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology 162:438–447 [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA (2008) Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry 63:139–145 [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M (2014) Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci 37:219–227 [DOI] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM III, Fee JR, Knapp DJ, Breese GR, Thiele TE (2009) The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res 33:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE (2008) Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res 32:259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MC, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34:468–483 [PMC free article] [PubMed] [Google Scholar]
- Tran L, Greenwood-Van Meerveld B (2012) Altered expression of glucocorticoid receptor and corticotropin-releasing factor in the central amygdala in response to elevated corticosterone. Behav Brain Res 234:380–385 [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS (2009) Stress, alcohol and drug interaction: an update of human research. Addict Biol 14:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF (2012) Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32:7563–7571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ (2015) Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 125:3193–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP (1996) In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137:5747–5750 [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, Gale MC, Datta R, Miczek KA (2005) Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology 179:230–239 [DOI] [PubMed] [Google Scholar]
- Yavich L, Tiihonen J (2000) Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol 401:365–373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


