Abstract
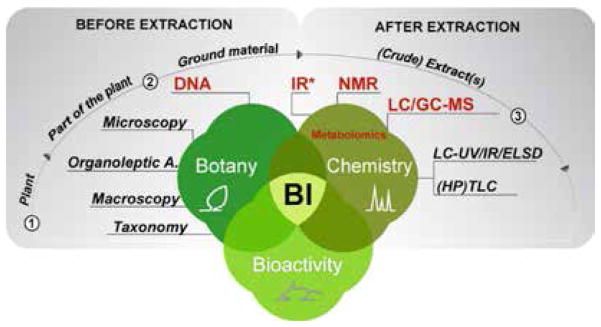
The concept of botanical integrity (BI), introduced previously in HerbalGram issue 106, involves the determination of identity, homogeneity, bioactivity, and safety of plant-derived materials designated for human consumption.1 It goes beyond previously established quality control principles. The inaugural article in this series described the three major domains of expertise that are required to assess BI (as noted in Figure 1): botanical examination (botany), phytochemical analysis (chemistry), and biological efficacy and safety assessments (bioactivity, which encompasses the fields of pharmacology and toxicology).
This article explores contemporary and comprehensive analytical techniques, focusing on the fields of botany and chemistry for the determination of BI. Recently, new approaches, such as authentication assays, have become available to characterize plant-derived material used in botanical dietary supplements (BDSs).
Botanical raw materials typically undergo post-harvest examination prior to extraction, and this process is fundamental to material authentication and the detection of adulteration. In addition, phytochemical analyses, such as chemical profiling and fingerprinting techniques, are required in order to study a plant’s metabolite composition. These analyses often are performed on extracts, but they also can be performed on botanical raw materials after sample preparation, which usually involves a small-scale extraction made in a laboratory. Phytochemical analyses are used to confirm the identity of the chosen plant and plant part. This can be accomplished by characterizing metabolites qualitatively and quantitatively and by targeting species-specific markers, bioactive constituents, and, sometimes, undesired compounds (negative markers).
Collectively, botanical identification and phytochemical characterization are techniques that are a traditional part of pharmacognosy — the study of medicines of natural origin. This scientific field has evolved considerably in terms of organisms covered and methodologies used (e.g., see www.pharmacognosy.us).
Analyses Performed on Plant Raw Materials
Traditional Methods: Organoleptic, Macroscopic, and Microscopic Examination
The first step in plant material identification requires an understanding of taxonomic principles. If possible, a reference herbarium voucher specimen or photograph of the source material should be obtained to facilitate a definitive taxonomic determination. The identification of whole or powdered botanical materials usually is achieved through organoleptic, macroscopic, and microscopic analyses performed by trained experts, such as botanists and pharmacognosists.
Organoleptic assessment of aroma, taste, and appearance characteristics can provide important clues about the identity, uniformity, and potential adulteration of the raw material. Macroscopic analysis involves the observation of morphological keys and the description of fruits, flowers, and vegetative parts (e.g., leaves and roots) obtained during cultivation or at the time of harvest (Step 1 in Figure 1). At this stage, the Latin binomial and plant part(s) used are documented.
Figure 1. Analytical Techniques that Support the Authentication and Phytochemical Characterization of Botanicals.
This article focuses on techniques related to botanical examination (botany) and phytochemical analysis (chemistry), which are used to determine the BI of a given plant material. The more recent approaches are highlighted in red, whereas more traditional techniques are in black. (Organoleptic A. = Organoleptic Analysis)
*IR (Infrared): This technique can be used on both ground plant material and corresponding extracts.
Botanical authentication is challenging when plant materials are powdered or extracted. Authentication of dried plant powders is usually performed with microscopic techniques such as normal light microscopy, scanning electron microscopy, or fluorescent microscopy. These methods are used to detect characteristic plant tissues or the presence of particular cell types such as hair, oil gland, secretory canal, vascular tissue, seed, starch grain, and pollen, or crystals in cells.
For most traditional herbal products (e.g., leaves of ginkgo [Ginkgo biloba, Ginkgoaceae] and aboveground parts of St. John’s wort [Hypericum perforatum, Hypericaceae]), these analyses are sufficient to identify the plant material. However, closely related plant species and hybrids usually share macroscopic and microscopic features. Thus, accurate botanical authentication requires the use of orthogonal or more specific analyses. These may include DNA methods (Step 2 in Figure 1), which test for underlying genetic differences between samples, and an array of phytochemical analyses that are used to determine characteristic metabolite profiles.2–5
Evolving Methodology: DNA Authentication
The use of DNA-based tools, including the Sanger method of DNA sequencing and high-resolution melting analysis, can be fundamental for the unambiguous identification of botanical materials.6,7 This is especially true for materials with a high risk of contamination or adulteration (e.g., due to misidentification), or if macroscopic and microscopic analyses are not sufficient to distinguish closely related species or hybrids.2
The roots of the three major species of licorice (Glycyrrhiza spp., Fabaceae), for example, have nearly identical macroscopic and microscopic features. In the absence of distinguishable taxonomic features, accurate botanical authentication of commercial licorice powder is possible through the use of DNA barcoding, complementing phytochemical analysis. In a 2015 study, DNA authentication was found to be important in the initial stages of building reliable BI assays for licorice.5 Subsequent phytochemical tests may be useful as well. However, since DNA degradation can occur during industrial processing, genetic analyses are generally more appropriate for the identification of unprocessed raw plant material, as detailed in the first article in this series.
Another potential limitation for the universal application of DNA-based botanical authentication is the reliability and overall comprehensiveness of available DNA barcode databases. Ideally, such databases should include reference sequences of plants that have been taxonomically identified and unambiguously vouchered. But, as stated by Coutinho Moraes et al., “at the present time, the number of DNA sequences for herbal and botanical products is insufficient.”7
Analyses Performed on Crude Extracts of Raw Materials
Traditional Methods: Targeted Chromatographic-based Analyses
Differences in cultivation methods, geographic origin, drying processes, and extraction methods yield botanical products with varying metabolic compositions. The documentation of these variables and development of appropriate and reproducible analytical methods for standardization are essential for the authentication and characterization of botanical extracts.4 The most widely used phytochemical methods for the characterization of extracts involve chromatographic separation of constituents by high-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC), gas chromatography (GC), and regular or high-performance thin-layer chromatography (TLC or HPTLC).
Chromatographic systems often are coupled (“hyphenated”) with suitable detection methods, such as ultraviolet (UV), fluorescence, refractive index (RI), evaporative light scattering detection (ELSD), charged aerosol detection (CAD), and mass spectrometry (MS). The resulting metabolite profiles depend on the type of separation and mode of detection. These conditions determine which kind of metabolic snapshot the chromatogram captures, and they should be chosen according to the physicochemical properties of the compounds expected to be present in the extract. Depending on the selectivity of the detection method, such choices can introduce a certain amount of “chemical bias” into the analysis.
Challenges in the phytochemical characterization of extracts are associated with various factors including the chemical diversity of plant metabolites, varying physicochemical properties, and their extended dynamic ranges, in terms of concentrations of the extracted compounds. Therefore, a combination of multiple analytical methods typically is required to detect different sets of compounds on the basis of their physicochemical properties (e.g., boiling point, volatility, presence of chromophores, and ionizability). (HP)TLC systems allow for the parallel comparison of multiple samples on a single plate, which then can undergo multiple detections with UV, MS, and/or various chemical or biological reagents. (HP)TLC systems generally are considered to be more practical, informative, economical, and easier to perform than other chromatographic systems. Thus, these systems are used frequently for routine quality control (QC) of plant material and as identification assays in pharmacopeias.
In general, traditional chromatographic analyses focus on the identification and quantification of select compounds for which the method is considered sufficiently specific. These designated marker compounds are then used to characterize the chemical composition of plant extracts. Such approaches are considered targeted phytochemical analyses and represent the gold standard in the QC of plant materials. When assessing herbs to be used in BDSs, the selected marker compounds should include specific bioactive phytochemicals. The frequent lack of commercially available reference standards required for this process is a significant practical challenge.3
Comprehensive Approaches: Techniques for Metabolomic Analyses
It is widely believed that BDSs and herbal medicines exert their health effects as a whole rather than by virtue of a few selected, potentially bioactive phytochemicals.8–13 Because of this, analytical approaches should have the capability to cover and characterize a much wider (ideally the entire) chemical composition of a given plant extract. This represents another major analytical challenge, which can be approached by the use of metabolomics.
Metabolomics is defined as the study of all chemicals (metabolites) produced by, and present in, a living organism.10,11 Hence, for the analysis of plant extracts, the primary goal of metabolomics is to gain insight into the chemical composition of the plant material at a specific time point. This leads to a better understanding of the molecular signature produced by diverse environmental influences, such as geographic origin, cultivation conditions, harvest time, storage methods, and industrial processing.12,14 As such, metabolomic analysis can aid in the development of standardization methods for cultivation, harvest, and extraction, among other processes.
Three main types of techniques are employed for metabolomic analysis of plant materials: LC- or GC-MS; nuclear magnetic resonance (NMR) spectroscopy; and vibrational spectroscopy, such as IR and Raman spectroscopy.
MS determines the mass-to-charge (m/z) ratio of the molecular ions, their fragmentation patterns, and the relative ion intensities of the compounds present in a plant extract (generally after chromatographic separation). With their high sensitivity (pM-nM) and inherent specificity, LC- and GC-based MS approaches are widely used for the identification and quantification of selected marker compounds, notably those found in trace amounts. With the improvement of MS instrumentation and data systems, LC/GC-MS techniques are also used for the profiling and metabolomic analysis of plant extracts, which requires only a small amount of the sample to be analyzed.
Historically, NMR and IR have been employed predominantly for the structural elucidation of individual isolated compounds. However, with the development of advanced software and the accessibility of statistical analyses of chemical information (chemometrics), the use of NMR and IR-based metabolomics has increased recently. Compared to chromatographic techniques, both NMR and IR are nondestructive, allowing a full recovery of the analyzed sample, and can readily accommodate crude plant extracts without physicochemical separation of their constituents.
NMR measures the resonance frequency of various nuclei, such as 1H, 13C, and 31P, under the influence of a magnetic field. NMR-based metabolomics of botanicals favor the measurement of protons (1H), which are characterized by a relatively high sensitivity, natural abundance, and nearly ubiquitous presence in phytochemicals. The 1H-NMR spectrum of a complex plant extract can be considered a metabolite fingerprint representing the superposition of spectra from all compounds present in the extract, at their relative abundance. Careful interpretation of the 1H spectrum may enable the simultaneous identification of the most abundant phytochemicals (low μM-mM) present, and, importantly, their quantification without the need for structurally identical reference standards. Currently, NMR and its quantitative form, qNMR, are considered robust and versatile tools for the direct unbiased, untargeted metabolomic analysis of botanical extracts.12,15,16
IR measures the stretching, wagging, and bending actions that occur within all molecules in an herbal material. There are three different types of IR analyses: near-IR (NIR; ~ 4,000–12,500 cm−1; vibrations and overtones), mid-IR (MIR; ~ 4,000–400 cm−1; bending and stretching vibrations), and far-IR (FIR; ~ 400–10 cm−1; lattice vibrations). Raman spectroscopy can operate between 4,000 and 400 cm−1 and below. MIR and Raman have been used for the QC of food products and, together with NIR, these techniques have become an alternative analytical tool for metabolomic analysis of raw botanicals (e.g., Panax ginseng, Araliaceae17; Digitalis purpurea, Plantaginaceae18), plant powders, and extracts. The IR spectrum offers an overall molecular fingerprint, representing characteristic chemical features of the most abundant botanical compounds. The implementation of chemometric analyses is required in order to compare spectra from different plant materials, calibrate classification models, and obtain meaningful qualitative/quantitative information.12,19 Following the validation of the classification models, IR methods allow a rapid identification of botanicals without extraction and sample destruction. Using these methods, the detection of adulterants in tested materials requires relatively large amounts of the contaminants (e.g., other plant species and undesired chemicals).
Metabolite Profiling versus Metabolite Fingerprinting
Since the introduction of metabolome research, there have been two main groups of metabolomic analyses: (1) metabolite profiling, which encompasses the identification and quantification of selected metabolites; and (2) metabolite/metabolic fingerprinting, which is dedicated to the comparison of chemical patterns in samples without the need for metabolite identification. Metabolite fingerprinting may be preferable when spectroscopic methods such as IR and NMR techniques have been used. Metabolite profiling, on the other hand, is associated more with LC-based methods. In the scientific literature, the terms metabolite/metabolic fingerprinting, metabolite profiling, and metabolomics are used interchangeably.10
Chemometrics
Metabolomics generally involves a large number of samples, a significant amount of numerical data from the chromatographic results, and spectra that have to be statistically processed and analyzed. Data mining resources, including statistics and multivariate analysis, are required to reduce both the dimension and complexity of datasets to determine the most relevant information. Chemometrics comprises multivariate data analyses of complex chemistry data sets and defines the use of these statistical methods to measure, simplify, and interpret a large amount of chemical information. Hence, the use of chemometrics is necessary to describe spectral differences and to evaluate global changes in large sets of MS profiles, or NMR spectra, so as to eventually achieve group classification.20 The most widely applied chemometric analyses that enable the distinction and classification of botanical extracts are principal component analysis (PCA); soft independent modeling of class analogy (SIMCA), a classification model based on PCA; hierarchical cluster analysis (HCA); and partial least squares discriminant analysis (PLS-DA). Such analyses can be performed with various software tools such as SIMCA-P (Umetrics; Umeå, Sweden), Pirouette (Infometrix; Bothell, Washington), AMIX-TOOLS (Bruker; Billerica, Massachusetts), and Matlab with Statistics Toolbox (MathWorks; Natick, Massachusetts), among others.9,14,21
Metabolomics and the Determination of Botanical Integrity
The phytochemical composition of a plant material is, in part, the result of its genomic expression and environmental factors, such as cultivation conditions, herbivory/microbial interactions, and the time of harvest. Hence, the metabolomic profiles of plant materials are inevitably subject to fluctuation even when created from what is considered the “exact same” authenticated plant specimen or species.
The production of representative metabolomic profiles and fingerprints requires the analysis of a significant number of botanically authenticated samples. In this regard, metabolomic and chemometric approaches enable a simultaneous comparison of multiple samples and integrate the information to produce representative classification (chemometric) models. Results obtained from such analytical platforms enable the assessment of the overall phytochemical composition of botanical material, the verification of botanical identity and distinction of plant species, the determination of parts used, the evaluation of geographic origin, and the objective comparison of botanical samples from various batches.
It is important to note that the building of robust chemometric models for metabolomic authentication requires an appropriate sample size. This is defined by the overall objective of the classification model, as determined by the investigators, and should ideally be representative of the geographic distribution of the botanicals. The more samples that are considered and included in the model, the more accurate (i.e., representative of the phytochemical variability) this model will be. Acquiring a representative amount of botanically verified reference materials to build accurate chemometric models remains a challenge. To the best of our knowledge, there is no consensus about the amount of samples per class or species required to build such classification models.
The chemometric models built through metabolomic analyses favor the selection of samples that display unusual chemical features and facilitate the detection of potential botanical or chemical adulteration. Using metabolomic approaches to assess the compositional quality of plant material, and comparing the results to representative classification models, can be implemented as QC measures and for the determination of BI.
Conclusion
Unambiguous identification of botanicals represents the first critical step for the determination of botanical integrity. DNA authentication is increasingly considered to be a helpful tool accompanying traditional macro- and microscopic examinations to identify botanicals accurately.2,6,7 Recently, work conducted at the authors’ Botanical Center at the College of Pharmacy at the University of Illinois at Chicago (UIC) has demonstrated that DNA authentication is a necessary tool for the identification of Glycyrrhiza hybrids (licorice), as well as for the detection of mixtures among Glycyrrhiza species.5 Furthermore, DNA identification has been shown to enable the selection of suitable botanical samples for the production of representative chemical fingerprints to build accurate chemometric classification models.
Chromatographic-based analyses, e.g., (HP)TLC and (U)HPLC-UV/ELSD/RI/MS, remain valuable techniques and industry gold standards for the phytochemical authentication and characterization of botanicals. These analyses focus on the generation of representative metabolite profiles with the identification and quantitation of selected marker compounds.
Metabolomic approaches can better address the phytochemical complexity and variability of plant materials, while satisfying the general need for a more holistic analysis. Currently, the most widely applied techniques for metabolomic analysis of botanicals are LC/GC-MS, (1H) NMR, and, to a lesser extent, IR. Notably, the excellent reproducibility and simultaneous quantification capability of NMR makes it of increasing interest as a QC tool. The building of chemometric models, based on any of these data, can foster the selection of plant material with desired chemical and biological features. Therefore, techniques for metabolomic analysis occupy a crucial place in the toolset for the holistic assessment of botanical integrity, aimed at ensuring quality and safety of botanical products.
Acknowledgments
The authors acknowledge support by grants P50 AT000155 and U41 AT 008706 from the National Center for Complementary and Integrative Health and the Office of Dietary Supplements of the US National Institutes of Health. The contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Footnotes
About the UIC/NIH Center for Botanical Dietary Supplements Research
Established in 1999, the UIC/NIH Center for Botanical Dietary Supplements Research addresses issues of standardization, quality, safety, and efficacy of botanical dietary supplements used for women’s health. Founded by the late Norman Farnsworth, PhD, and directed by Richard van Breemen, PhD, the Center’s leaders, including Judy Bolton, PhD, and Guido Pauli, PhD, take an integrated, collaborative approach to botanical research. Based on extensive preclinical studies involving botany, chemistry, and biology, the Center has completed clinical studies with black cohosh (Actaea racemosa, Ranunculaceae), red clover (Trifolium pratense, Fabaceae), and hops (Humulus lupulus, Cannabaceae), specifically addressing botanical safety and efficacy. Educating the next generation of pharmacognosists remains a primary goal of the Center, which has trained more than 60 pre- and post-doctoral researchers.
References
- 1.Simmler C, Chen S, Anderson J, et al. Botanical integrity: the importance of the integration of chemical, biological, and botanical analyses, and the role of DNA barcoding. HerbalGram. 2015;(106):56–58. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Pang X, Song J, et al. A renaissance in herbal medicine identification: from morphology to DNA. Biotechnol Adv. 2014;32(7):1237–1244. doi: 10.1016/j.biotechadv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Smillie TJ, Khan IA. A comprehensive approach to identifying and authenticating botanical products. Clin Pharmacol Ther. 2010;87(2):175–186. doi: 10.1038/clpt.2009.287. [DOI] [PubMed] [Google Scholar]
- 4.Applequist WL, Miller JS. Selection and authentication of botanical materials for the development of analytical methods. Anal Bioanal Chem. 2013;405:4419–4428. doi: 10.1007/s00216-012-6595-1. [DOI] [PubMed] [Google Scholar]
- 5.Simmler C, Anderson JR, Gauthier L, et al. Metabolite profiling and classification of DNA-authenticated licorice botanicals. J Nat Prod. 2015;78(8):2007–2022. doi: 10.1021/acs.jnatprod.5b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra P, Kumar A, Nagireddy A, et al. DNA barcoding: an efficient tool to overcome authentication challenges in the herbal market. Plant Biotechnol J. 2015 doi: 10.1111/pbi.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho Moraes DF, Still DW, Lum MR, Hirsch AM. DNA-based authentication of botanicals and plant-derived dietary supplements: where have we been and where are we going? Planta Med. 2015;81(9):687–695. doi: 10.1055/s-0035-1545843. doi:10.10551s-0035-1545843. [DOI] [PubMed] [Google Scholar]
- 8.Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem Anal. 2013;24(1):1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Choi YH, Verpoorte R. NMR-based plant metabolomics: where do we stand, where do we go? Trends Biotechnol. 2011;29(6):267–275. doi: 10.1016/j.tibtech.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 10.van der Kooy F, Maltese F, Choi YH, Kim HK, Verpoorte R. Quality control of herbal material and phytopharmaceuticals with MS and NMR based metabolic fingerprinting. Planta Med. 2009;75(7):763–775. doi: 10.1055/s-0029-1185450. doi:10.10551s-0029-1185450. [DOI] [PubMed] [Google Scholar]
- 11.Commisso M, Strazzer P, Toffali K, Stocchero M, Guzzo F. Untargeted metabolomics: an emerging approach to determine the composition of herbal products. Comput Struct Biotechnol J. 2013:4. doi: 10.5936/csbj.201301007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilia AR. Science meets regulation. J Ethnopharmacol. 2014;158:487–494. doi: 10.1016/j.jep.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Rather MA, Bhat BA, Qurishi MA. Multicomponent phytotherapeutic approach gaining momentum: Is the “one drug to fit all” model breaking down? Phytomedicine. 2013;21(1):1–14. doi: 10.1016/j.phymed.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Wolfender J-L, Rudaz S, Choi YH, Kim HK. Plant metabolomics: from holistic data to relevant biomarkers. Curr Med Chem. 2013;20(8):1056–1090. [PubMed] [Google Scholar]
- 15.Simmler C, Napolitano JG, McAlpine JB, Chen S-N, Pauli GF. Universal quantitative NMR analysis of complex natural samples. Curr Opin Biotechnol. 2014;25:51–59. doi: 10.1016/j.copbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes E, Tang H, Wang Y, Seger C. The assessment of plant metabolite profiles by NMR-based methodologies. Planta Med. 2006;72(9):771–785. doi: 10.1055/s-2006-946682. doi:10.10551s-2006-946682. [DOI] [PubMed] [Google Scholar]
- 17.Haibo B, Lixing N, Dan W, et al. Rapid determination of Panax ginseng by near-infrared spectroscopy. Anal Methods. 2013;5(23):6715. [Google Scholar]
- 18.Kudo M, Watt R, Moffat A. Rapid identification of Digitalis purpurea using near-infrared reflectance spectroscopy. J Pharm Pharmacol. 2000;52(10):1271–1277. doi: 10.1211/0022357001777252. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Yu Z. Species authentication and geographical origin discrimination of herbal medicines by near infrared spectroscopy: a review. J Pharm Anal. 2015:1–8. doi: 10.1016/j.jpha.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen JJ, Smit S, Hoefsloot HCJ, Smilde AK. The photographer and the greenhouse: how to analyse plant metabolomics data. Phytochem Anal. 2010;21(1):48–60. doi: 10.1002/pca.1181. [DOI] [PubMed] [Google Scholar]
- 21.Kim HK, Choi YH, Verpoorte R. NMR-based metabolomic analysis of plants. Nat Protoc. 2010;5(3):536–549. doi: 10.1038/nprot.2009.237. [DOI] [PubMed] [Google Scholar]