Abstract
Background:
There are a number of different implant choices for surgical treatment of distal radius fractures, often determined by surgeon preference or availability. Although no one volar plate demonstrates superior outcomes, there are significant cost differences absorbed by hospitals and surgical centers. This purpose of this study is to characterize the economic implications of implant selection in the surgical management of distal radius fractures.
Methods:
A retrospective review of billing records at a mid-size community surgicenter was conducted for CPT codes 25607, 25608, and 25609 between 1/1/2014 and 6/1/2014, and associated implant costs and facility reimbursements were collected. A unique stochastic simulation model was developed from derived probabilities, reimbursements, and costs, and analyzed by Monte Carlo simulation.
Results:
Reimbursement to the facility for distal radius ORIF cases ranged from $1,102.20 to $7,393.86, with an average of $3,824.56. Per case operating costs to the facility ranged from $1,250 to $7,270, with an average of $2,817.42. In the US, variations in implant cost 25% above or below the mean translates to annual operating profits realized by facilities ranging from a loss of $57,047,720 to profits of $55,189,729. On average, per case operating costs for distal radius fractures need to be less than $2956 for facilities to realize a per case profit.
Conclusion:
Value based purchasing is by necessity becoming integrated into clinical decision making by orthopaedic surgeons. Variations of 25% around the mean per case operating cost can vary facility operating margins by $112,237,450 annually. Arming the orthopaedic surgeon with the realities of the cost of implant selection in the operative management of distal radius fractures will lead to better value based decision making, substantial cost savings to the US hospital system, and ultimately payers and patients.
Key Words: Cost, Distal radius fracture, Economic analysis, Implants, Wrist
Introduction
Distal radius fractures represent one of the most common injuries to the upper extremity. The incidence of distal radius fractures is becoming an increasing societal fracture burden, in part due to an aging population (1, 2). There are a number of effective management options for these fractures, including non-operative management, percutaneous pinning, open reduction and internal fixation (ORIF) with dorsal or volar plates, bridge plating, and external fixation (3-5). Over the past two decades, advances in implant design has led to a shift in management towards ORIF with volar plates (6).
While there are a number of different specific implant choices for surgical treatment of distal radius fractures, there is limited evidence based guidance on the optimal implant choice (6-8). As such, selection of implants is often determined by surgeon preference or availability. Although no single volar plate that has emerged to demonstrate superior outcomes, there are significant differences in implant cost associated with the variety of option. These economic differences may not be appreciated either by surgeons using the implants or the hospitals and surgical centers where the implants are used, which in turn absorb any incremental associated costs.
Despite the relatively high volume utilization of these implants, we are not aware of any previous studies evaluating the cost to the provider of distal radius fracture locked volar plating. The goal of this study is to characterize the economic implications of implant selection in the surgical management of distal radius fractures.
Materials and Methods
Data
A retrospective review of facility billing records at a mid-size community outpatient surgical center was conducted for codes utilized in the open treatment of fractures of the distal radius (Common Procedural Terminology codes 25607, 25608, and 25609) between January 1, 2014 and June 30, 2014. Institutional Review Board approval was obtained for the deidentified review of billing records. The per case reimbursement collected from the third party payer by the facility was tabulated, as well as per case implant cost paid out by the facility to manufacturers for all implants utilized in each case. Construct costs included all hardware implanted during the procedure, including plates, screws, clips, or pins. Facility non-physician fee, non-implant fixed and variable costs were estimated at $900 per case using an estimate of $20/minute of operating room time, with an operative time of 45 minutes. These estimates were derived from previous published data, and it is assumed to account for all non-implant associated costs incurred by the facility (9).
Population distributions of the at risk patient population were derived from the US Census 2012 Population Estimates (10). Age and sex specific incidence of distal radius fracture were obtained from a large scale epidemiological study of distal radius fracture, and based on the same study the rate of operative management was approximated to 75% of cases (2).
Model/Analysis
To evaluate the cohort of distal radius ORIF cases, we utilized a decision tree model approach with stochastic Monte Carlo simulation analysis. Decision modeling has been increasingly utilized in orthopaedic care, and has been recognized as a means of guiding clinical care and economic policy (11-13). This technique allows an investigator to structure a decision tree model that describes the possibilities, course, and outcomes of a clinical scenario, progressing from incidence at the left side of the model through management to final outcomes at the right side of the model – at each branch of the tree a random event occurs, and based on probabilities and outcomes built into the model a given pathway is taken. A Monte Carlo simulation allows individual theoretical patients to be followed across the decision tree, incorporating chance and variability – the simulation is then repeated over and over again to represent a population sample.
In this study, the decision model was built from derived probabilities from the literature and costs from our financial review. The model simulates age and sex specific rates of distal radius fracture and decision for surgery. For those patients simulated to undergo surgery, the model calculates individual patient specific reimbursement to the facility based on age (i.e. Medicare for cases in patients over 65 and private reimbursement for patients under 65), as well as implant cost per case sampled from distributions built from our economic data. The simulation is iterated 100,000 times, and subsequently projected to the at risk US population. Model analysis was performed using TreeAge Pro 2015 (TreeAge Software, Williamstown, MA).
A sensitivity analysis was conducted by varying private reimbursements (keeping Medicare rates consistent) as well as implant costs each across a range 25% above and below the base case estimate.
Results
Our billings and accounts review identified 52 distal radius ORIF charges and implant purchases over the study period. Forty-two case reimbursements originated from private insurers (80.7%), of which thirteen different plans were represented, while ten case reimbursements were administered by Medicare (19.2%). The payments made by the facility for the costs of implants from five different manufacturers averaged $1,917 (range $350 - $6,370). The average facility reimbursement averaged $3,825 (range $1,102.20 - $7,394). When estimated fixed costs were combined with implant costs to calculate the operating expense, the average per case cost to the facility was $2,817 (range $1,250 to $7,270) [Figure 1]. The difference between facility reimbursement and per case operating expenses (facility profits) revealed an average per case operating profit of $1,007 ranging from a loss of $4,144 to a profit of $5,746.
Figure 1.
Per Case Costs to and Reimbursements to Facility for Distal Radius ORIF from 1/1/14 to 6/1/14
Our decision tree simulation model estimated that utilization of an average cost implant would result in approximately $25,086,806 (95% C.I. $24,544,564 to $25,629,415) in facility operating profits from distal radius fracture ORIF annually in the United States. Given the average private and Medicare reimbursement, the breakeven maximum implant cost to facilities that would prevent an operating loss is $2955.90 per case per construct [Figure 2].
Figure 2.
Per Case Profit by Implant Cost
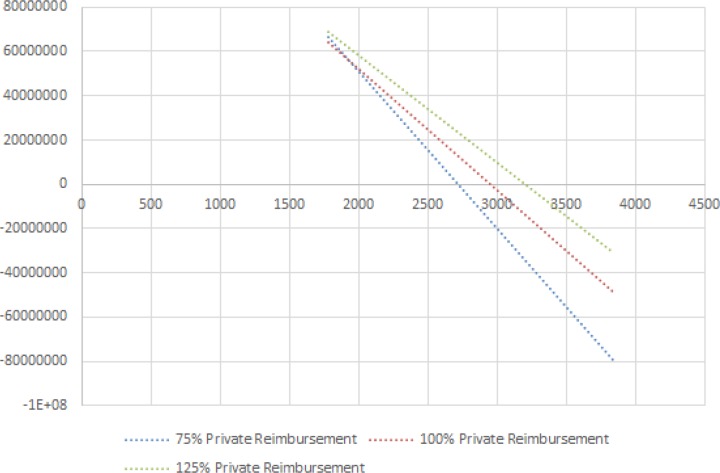
Our sensitivity analysis demonstrates that profit realized by a facility is more sensitive to incremental changes in implant cost than reimbursement rate [Figure 3]. Furthermore, profit is more sensitive to changes in reimbursement at higher implant costs.
Figure 3.
Sensitivity Analysis Varying Implant Cost and Private Reimbursement
Discussion
Distal radius fractures ORIF with locked volar plating is a common procedure performed in the United States where no single implant has emerged as clinically dominant. Our model suggests that differences in implant cost can have substantial impact on operating margins. The variation in operating profits with utilization of implants costing 25% above and below the mean for distal radius constructs can translate to a range of $112,237,250 (-$57,047,720 to $55,189,729) realized by facilities annually in the US. Furthermore, our sensitivity analysis suggests that operating profits are more influenced by the cost of implants than discounts in private reimbursement; this is like secondary to the large fraction of patients requiring distal radius ORIF with Medicare as their primary third-party payer.
With ongoing efforts to improve value in orthopaedics, there is increasing attention on the costs associated with delivery of care. When an optimal evidence based management option presents itself it generally should be favored within reason. However, in situations where clinical equivalence or true uncertainty exists, economics can, and should, begin to play a role in decision making. Most economic studies in health care take the perspective of the patient/third-party payer, which in many cases is appropriate in evaluating societal economic burdens. The health care industry, though, is a multifaceted and multilayered environment where other players have greater stakes in different interests and perspectives. In general, implant costs are absorbed by facilities and hospitals. These costs are recuperated by facilities with facility reimbursement fees. Therefore, any savings generated from implant purchasing may be directly realized by facilities because positive operating margins are inversely related to implant cost.
Our study has several limitations. First, our implant cost estimates and facility reimbursements are based off the experience of a single center in a single region, and may not be directly generalizable to all regions. However, we selected a community surgical center as the foundation of the analysis as this likely represents a midrange estimate of reimbursements and implant costs, with most centers nationally falling within our sensitivity analysis and along our projections. We noted that our dataset demonstrated a high percentage of private third-party payers, however our simulation model was adjusted for estimated age specific incidence and payer case-mix, and model outcomes are likely an accurate representation of the population in practice. Secondly, individual implant costs as well as reimbursements demonstrate fluidity over time. While our analysis may be valid at this time, significant price shifts in the future may potentially increase or decrease the variability and impact of implant choice. Third, our study takes the liberty of grouping all operative distal radius fractures together. While we felt this is acceptable in most cases, there may be some situations where the requirement for specific hardware drives cost one direction or another – however, the magnitude of this likely is small and would not make appreciable differences in the results.
In the universal effort to contain rising healthcare costs, value based purchasing are by necessity becoming integrated into clinical decision making by orthopaedic surgeons in the US. Particularly with shifts toward bundling of payments, both surgeons and facilities will become integrally linked and responsible for management measures. Arming the orthopaedic surgeon with the realities of the cost of implant selection in the operative management of distal radius fractures will lead to better value based decision making, substantial cost savings to the US hospital system, and ultimately payers and patients.
References
- 1.Beharrie AW, Beredjiklian PK, Bozentka DJ. Functional outcomes after open reduction and internal fixation for treatment of displaced distal radius fractures in patients over 60 years of age. J Orthop Trauma. 2004;18(10):680–6. doi: 10.1097/00005131-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Wilcke MK, Hammarberg H, Adolphson PY. Epidemiology and changed surgical treatment methods for fractures of the distal radius: a registry analysis of 42,583 patients in Stockholm County, Sweden, 2004–2010. Acta Orthop. 2013;84(3):292–6. doi: 10.3109/17453674.2013.792035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bales JG, Stern PJ. Treatment strategies of distal radius fractures. Hand Clin. 2012;28(2):177–84. doi: 10.1016/j.hcl.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Koval K, Haidukewych GJ, Service B, Zirgibel BJ. Controversies in the management of distal radius fractures. J Am Acad Orthop Surg. 2014;22(9):566–75. doi: 10.5435/JAAOS-22-09-566. [DOI] [PubMed] [Google Scholar]
- 5.Bhat SB, Ilyas AM. Economic analysis of bisphosphonate use after distal radius fracture for prevention of hip fracture. Arch Bone Jt Surg. 2017;5(6):380–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Downing ND, Karantana A. A revolution in the management of fractures of the distal radius? J Bone Joint Surg Br. 2008;90(10):1271–5. doi: 10.1302/0301-620X.90B10.21293. [DOI] [PubMed] [Google Scholar]
- 7.Chung KC, Petruska EA. Treatment of unstable distal radial fractures with the volar locking plating system Surgical technique. J Bone Joint Surg Am. 2007;89(Suppl 2):256–66. doi: 10.2106/JBJS.G.00283. [DOI] [PubMed] [Google Scholar]
- 8.Chung KC, Watt AJ, Kotsis SV, Margaliot Z, Haase SC, Kim HM. Treatment of unstable distal radial fractures with the volar locking plating system. J Bone Joint Surg Am. 2006;88(12):2687–94. doi: 10.2106/JBJS.E.01298. [DOI] [PubMed] [Google Scholar]
- 9.Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233–6. doi: 10.1016/j.jclinane.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.2012 population estimates; generated by Suneel Bhat Using American Fact Finder. United States Census Bureau; 2014. Available at: URL: http://factfinder2.census.gov ; 2014. [Google Scholar]
- 11.Mather RC, Garrett WE, Cole BJ, Hussey K, Bolognesi MP, Lassiter T, et al. Cost-effectiveness analysis of the diagnosis of meniscus tears. Am J Sports Med. 2015;43(1):128–37. doi: 10.1177/0363546514557937. [DOI] [PubMed] [Google Scholar]
- 12.Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325–34. doi: 10.1016/j.jse.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 13.Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912–8. doi: 10.1016/j.jhsa.2011.09.039. [DOI] [PubMed] [Google Scholar]