Abstract
Yeung and Prakriya highlight new research showing that STIM1 must bind to all six Orai1 subunits to effectively activate the channel.
Most animal cells exhibit a conserved mechanism for Ca2+ entry, termed store-operated Ca2+ entry (SOCE), that is activated by depletion of endoplasmic reticulum (ER) Ca2+ stores in response to stimulation of cell surface receptors coupled to G proteins or tyrosine kinases (Prakriya and Lewis, 2015). Following the drop in ER Ca2+ concentration, the ER membrane protein, STIM1 (Stromal Interaction Molecule 1), which functions as the ER lumen Ca2+ sensor, oligomerizes and migrates to the ER–plasma membrane junctions, where it binds to and gates store-operated Orai1 channels (Fig. 1 A). The ensuing sustained Ca2+ influx is critical for driving various cellular processes such as proliferation, migration, exocytosis, and cytoskeletal rearrangement (Prakriya and Lewis, 2015). However, the mechanism by which STIM1 binds to and activates Orai1, as well as the stoichiometry of this interaction, are unclear. In this issue of the Journal of General Physiology, Yen and Lewis investigate this question and find that STIM1 must bind to all six Orai1 subunits to effectively activate the channel.
Figure 1.
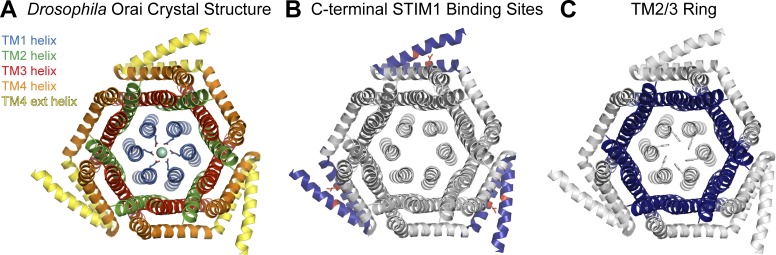
Structural features of the Orai channel. (A) Top down view of the crystal structure of Drosophila Orai (PDB ID: 4HKR), showing a hexameric channel with concentric layers of transmembrane domains (TMs) surrounding the pore-lining TM1 helices (Hou et al., 2012). TMs 1–4 and the C-terminal extensions are shown in blue, green, red, orange, and yellow, respectively. (B) The peripheral STIM1 binding sites formed by pairs of neighboring C-terminal extensions are highlighted in blue, with residue I316 (human Orai1 L273) shown in red sticks. (C) TMs 2 and 3, colored in blue, form an interlocked ring situated in between TM1 and TM4. This cage of helices may play a key role in enforcing cooperativity in transmitting the STIM1 binding signal from the C termini of different Orai1 subunits to the pore. Residue F171 (human Orai1 F99), which forms the dynamically regulated part of the hydrophobic gate in the pore, is depicted in gray sticks.
In addition to its dependence on ER Ca2+ stores, a key feature of Orai1 is its exceptional Ca2+ selectivity (PCa/PNa > 1,000; Hoth and Penner, 1993; Prakriya et al., 2006), which allows conduction of Ca2+ but not Na+ ions, thereby permitting Orai1 channels to stimulate downstream signaling pathways without triggering cellular depolarization. A second unique feature is their extremely small unitary conductance (Zweifach and Lewis, 1993; Prakriya and Lewis, 2006), which allows the channels to produce spatially restricted and tightly controlled local Ca2+ signals important for conferring functional specificity (Rizzuto and Pozzan, 2006; Clapham, 2007; Courjaret and Machaca, 2014). Interestingly, unlike most other channels, gating and ion selectivity are dynamically coupled in Orai1 channels such that STIM1 binding controls gate opening and also imparts Ca2+ selectivity to the pore (McNally et al., 2012). Where and how STIM1 binds to Orai1, however, is still up for debate. Although there is a putative STIM1 binding site at the N terminus that has been detected in studies using fragments of the Orai1 protein (Park et al., 2009; Zhou et al., 2010), the most well-established STIM1 binding site is at the Orai1 C terminus (Fig. 1 B; Li et al., 2007; Muik et al., 2008; Navarro-Borelly et al., 2008; Frischauf et al., 2009; Park et al., 2009; McNally et al., 2013; Stathopulos et al., 2013; Zheng et al., 2013; Palty et al., 2015; Tirado-Lee et al., 2015), where pairs of neighboring C-terminal tails form coiled-coil interactions with each other to create the trimer-of-dimers arrangement in this hexameric channel (Fig. 1 A; Hou et al., 2012).
The question remains, however: How many STIM1 molecules bind to each channel, and how does each Orai1 binding site regulate channel activation? One can view Orai1 as a ligand-gated channel that is activated by an intracellular ligand, STIM1, which binds to the active site at the Orai1 C terminus (Li et al., 2007; Muik et al., 2008; Navarro-Borelly et al., 2008; Frischauf et al., 2009; Park et al., 2009; Tirado-Lee et al., 2015). In contrast to the study of many other ligand-gated channels, however, accurate titration of the agonist at each Orai1 channel in a live-cell setting remains a major challenge. Most of the common pharmacological tools used to recruit STIM1 to the channel, such as thapsigargin and ionomycin, cause irreversible ER Ca2+ store depletion, leading to complete channel activation with no easy way to calculate or control how many STIM1 proteins are bound to each Orai1 subunit.
To overcome these experimental limitations, several innovative approaches were used previously to assess the dependence of Orai1 activation on the number of bound STIM1 molecules. In one approach, Hoover and Lewis (2011) overexpressed varying amounts of each protein and found a steep nonlinear relationship between STIM1/Orai1 ratio and current amplitudes. Current amplitudes dropped off dramatically when fewer than two STIM1 molecules per Orai1 subunit were present in the STIM1–Orai1 punctae (Hoover and Lewis, 2011). However, this study could not directly measure whether the STIM1 molecules were, in fact, bound to Orai1 channels. Using a very different approach, Li et al. (2011) showed that currents arising from an Orai1 construct that was directly tethered to two minimal activation domains of STIM1 (termed Orai1-SS) were larger than those from Orai1 monomers with only one tethered STIM1 domain (Orai1-S). Moreover, in contrast to Orai1-S currents, Orai1-SS currents could not be further augmented by independently coexpressing the SS domain, suggesting that attachment of the two S domains evokes maximal channel activation. Another study used a constitutively conducting Orai1 mutant with a leaky channel gate (Orai1 V102C; McNally et al., 2012) to show that increasing the STIM1/Orai1 ratio boosted not only channel currents, but also Ca2+ selectivity, such that Orai1-SS channels were significantly more Ca2+ selective than Orai1-S channels. Collectively, these studies demonstrate the strong nonlinearity of channel activation as well as the dynamic coupling of gating with ion selectivity. However, how individual ligand binding sites on Orai1 contribute to channel activation and ion selectivity was not examined in any of these studies and has remained largely unknown until now.
In this issue of The Journal of General Physiology, Yen and Lewis (2018) addressed this question by introducing a mutation in Orai1 concatemers to control the number of sites on each Orai1 channel available for STIM1 interaction. The L273D mutation abrogates interaction between STIM1 and the Orai1 C terminus in monomeric Orai1 and has been a widely used tool to eliminate STIM1 binding to the channel (Li et al., 2011). Previous work using tetrameric concatemers revealed that a single L273D mutation eliminated ∼50% of the overall current, much more than would be expected (25%) if each monomer contributed equally to channel gating (Li et al., 2011). However, interpretation of these earlier results was complicated by the assumption that Orai1 channels are made of four subunits, not six as we now know (Hou et al., 2012; Cai et al., 2016; Yen et al., 2016).
In the new study, Yen and Lewis (2018) first examined the amount of STIM1–Orai1 interaction in the context of Orai1 dimers. As expected, WT–WT Orai1 dimers interacted with STIM1, while L273D–L273D dimers did not (Yen and Lewis, 2018). Surprisingly, however, L273D displayed a detectable amount of STIM1 binding when placed next to a WT subunit, suggesting that the neighboring WT subunit is able to enhance STIM1 binding to the L273D subunit (Yen and Lewis, 2018). The finding that STIM1 interacts with neighboring Orai1 subunits interdependently implies that STIM1 likely binds to pairs of C termini. Notably, despite having 83% of the FRET level of WT–WT channels, WT-L273D channels did not conduct detectable currents, revealing that, although mutating three out of six subunits only modestly affects STIM1 binding, it completely abolishes STIM1-mediated gating (Yen and Lewis, 2018). This striking result provides strong support for previous inferences (Hoover and Lewis, 2011; Li et al., 2011) of a highly nonlinear relationship between STIM1 binding and channel activation.
To more directly examine how individual Orai1 subunits contribute to gating, Yen and Lewis (2018) used hexameric concatemers so that the binding site at each subunit could be manipulated by introducing L273D mutations into individual protomers. Intriguingly, Orai1 hexamers with just a single L273D mutation produced whole-cell currents that were only 35% of the amplitude of those observed in WT hexamers (Yen and Lewis, 2018). When mutations were introduced into multiple subunits within the hexamer, the current declined exponentially with each additional L273D subunit reducing the current by 64% (Yen and Lewis, 2018). Although exactly how much STIM1 binding is lost in aggregate with each L273D mutation was not determined, this result directly demonstrates the extreme nonlinearity of Orai1 gating by STIM1 and reaffirms the conclusion that STIM1 binding to each of the six Orai1 C termini is required for full channel function.
Next, Yen and Lewis (2018) sought to understand the biophysical basis of the dramatic current reduction in channels containing a single L273D subunit. Whole-cell currents are the product of the number of available channels (N), the unitary conductance of each channel (i), and the open probability (Po). Because the unitary Ca2+ conductance of Orai channels—only several femtosiemens (Zweifach and Lewis, 1993; Prakriya and Lewis, 2006)—is too small for conventional single-channel recording methods, they conducted nonstationary noise analysis to estimate the Po of channels with an introduced L273D subunit versus those composed of all WT subunits. Yen and Lewis (2018) took advantage of the fact that, like many other Ca2+ channels, Orai1 channels conduct monovalent ions such as Na+ in the absence of divalent ions. This Na+ current is blocked by the addition of μM amounts of Ca2+, which produces transient fluctuations in the whole-cell current. The current variance is then mapped against the mean current amplitude to generate estimates of N, i, and Po (Prakriya and Lewis, 2006).
This analysis revealed several unexpected and remarkable changes in hexamers with a single L273D subunit. First, there appeared to be only a small decrease in the measured Po , which, at first glance, could not explain the substantial reduction in whole-cell current. However, an intrinsic limitation of the noise analysis method used in studies of Orai1 is that it only registers channels that flicker over the sampling window, which is typically ∼200 ms. Therefore, although the number of channels at the membrane were not different for the two constructs when measured by fluorescence-activated cell sorting, N was dramatically reduced for the L273D-containing channels because many channels in this group remained silent throughout the experiment. In fact, taking into the account the channels that did not open at all, Yen and Lewis (2018) concluded that Po was reduced by ∼90% when only one L273D subunit was included in the six-subunit concatemer. This central result underscores the vital importance of all six intact ligand binding sites for gating (Yen and Lewis, 2018). Even more interestingly, channels containing the single L273D subunit displayed diminished ion selectivity, observed by a reduction of Ca2+ affinity at the selectivity filter, and reduced selectivity for Na+ relative to Cs+ under divalent-free conditions (Yen and Lewis, 2018). These results highlight the importance of STIM1 binding to all six subunits for not only increasing the Po but for conferring the exquisite Ca2+ selectivity observed in native Orai1 currents.
Overall, this study, which clearly demonstrates the robust nonlinear dependence of Orai1 pore opening and Ca2+ selectivity on STIM1 binding, provides a new framework for understanding the channel activation process. Yen and Lewis (2018) reasoned that their noise analysis results are incompatible with a simple closed–open two-state model. Instead, they imply that the majority of channels in the 1xL273D mutant are in one of multiple silent closed states that cannot equilibrate to an open state in the sampling window. Importantly, their results put previous measurements of Orai1 channel Po in a new light, because the high apparent Po measured using 200-ms sampling windows is not unique to the experiments in this study. In fact, almost all previous measurements of Orai channel Po have yielded values in the 0.7–0.8 range (Prakriya and Lewis, 2006; Kilch et al., 2013; Yamashita and Prakriya, 2014; Mullins et al., 2016). This is remarkable considering the different unitary conductances across different mutant backgrounds (Mullins et al., 2016) and the different ways of activating the channel, either by STIM1 (Prakriya and Lewis, 2006; Kilch et al., 2013; Yamashita and Prakriya, 2014) or, in the case of Orai3 channels, by the Ca2+ release-activated Ca2+ channel modulator 2-APB (Yamashita and Prakriya, 2014). This phenomenon can be explained by reasoning that noise analysis of Orai channels predominantly captures the last transition to the maximally open state, independently of how it arrives at this final step (whether with five versus six STIM1-bound Orai1 subunits [Yen and Lewis, 2018] or via STIM1 versus 2-APB for Orai3 channels [Yamashita and Prakriya, 2014]). Moreover, because the different Orai variants in these studies have different ion selectivities and unitary conductances, the last transition is also apparently independent of the final pore configuration in the open state. The tight coupling of gating and selectivity seen in STIM1-gated channels must therefore occur at a step before the final opening transition. Collectively, their results suggest that, in a physiological setting when Orai1 channels open in a stepwise manner after STIM1 binding (Prakriya and Lewis, 2006), the measured transitions are mostly between the closed, poorly ion-selective, high conductance five-subunit STIM1 bound state and the ligand-saturated low conductance, Ca2+-selective active state.
The consistently high measurements of active channel Po across various experiments suggests that the allosteric mechanisms that drive the final step of pore opening are fundamentally similar in nature and hints at topological features intrinsic to the channel that must underlie this cooperativity. What could be the molecular basis of this cooperativity? A recent study has proposed that STIM1-mediated pore opening involves pore helix rotation that reorients the selectivity filter while opening a hydrophobic gate (Yamashita et al., 2017). The pore helices are encased by a ring of interwoven transmembrane helices, TMs 2–3, which are in turn surrounded by the peripheral TM4 regions where STIM1 binds (Fig. 1; Hou et al., 2012). Thus, one possibility is that the nonrotated state of some pore helices may account for the dramatic drop in Po in subliganded channels. Alternatively, the cooperative nature of channel activation could arise from the closely packed interlocked TM2–3 helices, which have been proposed to form a rigid ring that relays gating information from the peripheral STIM1 binding sites to the central pore helices (Fig. 1 C; Yeung et al., 2018). This ring could diffuse the effects of individual ligand binding sites and serve as a logical “AND” gate that keeps the channel stabilized in the closed state until all six subunits are bound to STIM1 and only then collectively rearrange the pore helices into the activated conformation.
Many biological molecules with multiple ligand binding sites exhibit pronounced nonlinearity between binding and activation that is essential for their physiological functions (Perutz, 1989). Because Ca2+ is a ubiquitous secondary signaling molecule (Clapham, 2007), cooperativity in the Orai1 activation process may serve to minimize “false positive” channel openings and activation of downstream pathways when the channels are not fully engaged with STIM1. Do other STIM–Orai isoform combinations also exhibit this type of exquisite cooperativity, and what implications does this have for the regulation of SOCE in different cell types? These are just a few of the countless exciting questions that remain to be answered. Nevertheless, the elegant work of Yen and Lewis (2018), demonstrating the remarkable nonlinearity between STIM1 binding and Orai1 channel Po, represents an important advance in our understanding of how Orai1 channels open.
Acknowledgments
The authors declare no competing financial interests.
Eduardo Ríos served as editor.
References
- Cai X., Zhou Y., Nwokonko R.M., Loktionova N.A., Wang X., Xin P., Trebak M., Wang Y., and Gill D.L.. 2016. The Orai1 Store-operated Calcium Channel Functions as a Hexamer. J. Biol. Chem. 291:25764–25775. 10.1074/jbc.M116.758813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. 2007. Calcium signaling. Cell. 131:1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Courjaret R., and Machaca K.. 2014. Mid-range Ca2+ signalling mediated by functional coupling between store-operated Ca2+ entry and IP3-dependent Ca2+ release. Nat. Commun. 5:3916 10.1038/ncomms4916 [DOI] [PubMed] [Google Scholar]
- Frischauf I., Muik M., Derler I., Bergsmann J., Fahrner M., Schindl R., Groschner K., and Romanin C.. 2009. Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1-3 channels by a STIM1 coiled-coil mutant. J. Biol. Chem. 284:21696–21706. 10.1074/jbc.M109.018408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover P.J., and Lewis R.S.. 2011. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. USA. 108:13299–13304. 10.1073/pnas.1101664108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., and Penner R.. 1993. Calcium release-activated calcium current in rat mast cells. J. Physiol. 465:359–386. 10.1113/jphysiol.1993.sp019681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Pedi L., Diver M.M., and Long S.B.. 2012. Crystal structure of the calcium release-activated calcium channel Orai. Science. 338:1308–1313. 10.1126/science.1228757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilch T., Alansary D., Peglow M., Dörr K., Rychkov G., Rieger H., Peinelt C., and Niemeyer B.A.. 2013. Mutations of the Ca2+-sensing stromal interaction molecule STIM1 regulate Ca2+ influx by altered oligomerization of STIM1 and by destabilization of the Ca2+ channel Orai1. J. Biol. Chem. 288:1653–1664. 10.1074/jbc.M112.417246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lu J., Xu P., Xie X., Chen L., and Xu T.. 2007. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem. 282:29448–29456. 10.1074/jbc.M703573200 [DOI] [PubMed] [Google Scholar]
- Li Z., Liu L., Deng Y., Ji W., Du W., Xu P., Chen L., and Xu T.. 2011. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 21:305–315. 10.1038/cr.2010.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally B.A., Somasundaram A., Yamashita M., and Prakriya M.. 2012. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 482:241–245. 10.1038/nature10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally B.A., Somasundaram A., Jairaman A., Yamashita M., and Prakriya M.. 2013. The C- and N-terminal STIM1 binding sites on Orai1 are required for both trapping and gating CRAC channels. J. Physiol. 591:2833–2850. 10.1113/jphysiol.2012.250456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P., Schindl R., Hesch C., Polzinger B., Fritsch R., et al. . 2008. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem. 283:8014–8022. 10.1074/jbc.M708898200 [DOI] [PubMed] [Google Scholar]
- Mullins F.M., Yen M., and Lewis R.S.. 2016. Orai1 pore residues control CRAC channel inactivation independently of calmodulin. J. Gen. Physiol. 147:137–152. 10.1085/jgp.201511437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Borelly L., Somasundaram A., Yamashita M., Ren D., Miller R.J., and Prakriya M.. 2008. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 586:5383–5401. 10.1113/jphysiol.2008.162503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R., Stanley C., and Isacoff E.Y.. 2015. Critical role for Orai1 C-terminal domain and TM4 in CRAC channel gating. Cell Res. 25:963–980. 10.1038/cr.2015.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.Y., Hoover P.J., Mullins F.M., Bachhawat P., Covington E.D., Raunser S., Walz T., Garcia K.C., Dolmetsch R.E., and Lewis R.S.. 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 136:876–890. 10.1016/j.cell.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M.F. 1989. Mechanisms of cooperativity and allosteric regulation in proteins. Q. Rev. Biophys. 22:139–237. 10.1017/S0033583500003826 [DOI] [PubMed] [Google Scholar]
- Prakriya M., and Lewis R.S.. 2006. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J. Gen. Physiol. 128:373–386. 10.1085/jgp.200609588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M., and Lewis R.S.. 2015. Store-Operated Calcium Channels. Physiol. Rev. 95:1383–1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., and Hogan P.G.. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature. 443:230–233. 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., and Pozzan T.. 2006. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86:369–408. 10.1152/physrev.00004.2005 [DOI] [PubMed] [Google Scholar]
- Stathopulos P.B., Schindl R., Fahrner M., Zheng L., Gasmi-Seabrook G.M., Muik M., Romanin C., and Ikura M.. 2013. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat. Commun. 4:2963 10.1038/ncomms3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado-Lee L., Yamashita M., and Prakriya M.. 2015. Conformational Changes in the Orai1 C-Terminus Evoked by STIM1 Binding. PLoS One. 10:e0128622 10.1371/journal.pone.0128622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., and Prakriya M.. 2014. Divergence of Ca(2+) selectivity and equilibrium Ca(2+) blockade in a Ca(2+) release-activated Ca(2+) channel. J. Gen. Physiol. 143:325–343. 10.1085/jgp.201311108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Yeung P.S., Ing C.E., McNally B.A., Pomès R., and Prakriya M.. 2017. STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate. Nat. Commun. 8:14512 10.1038/ncomms14512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M., and Lewis R.S.. 2018. Physiological CRAC channel activation and pore properties require STIM1 binding to all six Orai1 subunits. J. Gen. Physiol.:jgp.201711985 10.1085/jgp.201711985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M., Lokteva L.A., and Lewis R.S.. 2016. Functional Analysis of Orai1 Concatemers Supports a Hexameric Stoichiometry for the CRAC Channel. Biophys. J. 111:1897–1907. 10.1016/j.bpj.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung P.S., Yamashita M., Ing C.E., Pomès R., Freymann D.M., and Prakriya M.. 2018. Mapping the functional anatomy of Orai1 transmembrane domains for CRAC channel gating. Proc. Natl. Acad. Sci. USA. 115:E5193–E5202. 10.1073/pnas.1718373115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Zhou M.H., Hu C., Kuo E., Peng X., Hu J., Kuo L., and Zhang S.L.. 2013. Differential roles of the C and N termini of Orai1 protein in interacting with stromal interaction molecule 1 (STIM1) for Ca2+ release-activated Ca2+ (CRAC) channel activation. J. Biol. Chem. 288:11263–11272. 10.1074/jbc.M113.450254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Meraner P., Kwon H.T., Machnes D., Oh-hora M., Zimmer J., Huang Y., Stura A., Rao A., and Hogan P.G.. 2010. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat. Struct. Mol. Biol. 17:112–116. 10.1038/nsmb.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A., and Lewis R.S.. 1993. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA. 90:6295–6299. 10.1073/pnas.90.13.6295 [DOI] [PMC free article] [PubMed] [Google Scholar]