Abstract
In pancreatic β cells, ABCA1, a 254 kDa membrane protein, affects cholesterol homeostasis and insulin secretion. Angiotensin II, as the main effector of the renin–angiotensin system, decreases glucose-stimulated insulin secretion (GSIS). We examined the effect of angiotensin II on ABCA1 expression in primary pancreatic islets and INS-1 cells. Angiotensin II decreased ABCA1 protein and mRNA; angiotensin II type 1 receptor (AT1R) blockade rescued this ABCA1 repression. In parallel, angiotensin II suppressed the promoter activity of ABCA1, an effect that was abrogated by PD98095, a specific inhibitor of MAPK kinase (MEK). LXR enhanced ABCA1 promoter activity, and angiotensin II decreased the nuclear abundance of LXR protein. On a chromatin immunoprecipitation assay, LXR mediated the transcription of ABCA1 by directly binding to its promoter. Mutation of the LXR binding site on the ABCA1 promoter cancelled the effect of angiotensin II. Furthermore, angiotensin II induced cholesterol accumulation and impaired GSIS; inhibition of AT1R or MEK pathway reversed these effects. In summary, our study showed that angiotensin II suppressed ABCA1 expression in pancreatic islets and INS-1 cells, indicating that angiotensin II may influence GSIS by regulating ABCA1 expression. Additional research may address therapeutic needs in diseases such as diabetes mellitus.
Keywords: liver X receptor, MAPK kinase/ERK1/2, lipotoxicity
In pancreatic β cells, lipotoxicity induced by cholesterol accumulation results in the apoptosis of β cells and impaired insulin secretion (1, 2). ABCA1 is a 254 kDa protein in membrane and serves as a key regulator in lipid exporting from cytoplasm to apolipoproteins and reverse cholesterol transport in vivo (3). Previously, it was reported that specific inactivation of the ABCA1 gene in β cells induced glucose intolerance and defective insulin secretion in mice, but normal insulin sensitivity was retained (4). Absence of ABCA1 in pancreatic islets altered cholesterol homeostasis and impaired glucose-stimulated insulin secretion (GSIS) in vitro, suggesting that cholesterol accumulation may result in dysfunction of β cells in T2D (5).
Angiotensin II (Ang II) is the central effector of the renin–angiotensin system (RAS) and is known to regulate vascular tone, blood pressure, and electrolyte homeostasis (6). Ang II type 1 (AT1) and type 2 (AT2) receptors are the main receptors involved in mediating Ang II’s effect (7). A previous study showed that Ang II significantly enhanced cholesterol accumulation by suppressing ABCA1 expression in macrophages (8), indicating the role of Ang II in the regulation of cholesterol homeostasis.
Recently, in vivo Ang II has been reported to be able to dose-dependently induce blood vessel contraction and decrease glucose-dependent insulin secretion in pancreatic islets; these effects are altered by inhibition of the AT1 receptor (AT1R) (9, 10). Interestingly, several recent large-scale clinical studies reported that, compared with other antihypertensive drugs or placebo, antagonists of angiotensin receptor or inhibitors of angiotensin-converting enzyme remarkably reduced the incidence of T2D in hypertensive patients (11). This evidence suggests the important role of Ang II in regulation of pancreatic islet function. However, the detailed mechanism in the inhibition of insulin secretion by Ang II is not completely understood. In this study, we hypothesized that Ang II might increase the cholesterol accumulation by decreasing ABCA1 expression and then induce lipotoxicity in pancreatic β cells, thus attenuating insulin synthesis.
MATERIALS AND METHODS
Cell culture
The INS-1 is a cell line originally isolated from a rat insulinoma, which is developed and propagated at the Division of Biochimie Clinique (courtesy of C. B. Wollheim, Geneva, Switzerland). In this study, experiments were performed in cells during 9 ∼35 passages, and the cells were split every 7 days. These cells were cultured in RPMI 1640 medium (SIGAMA, Tokyo, Japan) containing 11.2 mmol/l glucose and supplemented with 10% heat-inactivated FBS (Dainippon Pharmaceutical Co., Ltd., Tokyo, Japan), 50 μmol/l 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were incubated in humidified 5% CO2 at 37°C. When 80% confluent, the cells were washed twice and incubated with 0.5% FBS RPMI 1640 medium for 6 h. Then, the cells were treated with varying doses of Ang II for 24 h before harvesting as described previously (12).
Pancreatic islet isolation
Isolation of pancreatic islets from rat was performed as described previously (13). Briefly, the pancreas was digested by collagenase type V at 37°C for 15 min, and then islets were purified and collected by a 70 µm cell strainer. Finally, islets (100–200 µm) were picked and placed in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin in a 5% CO2 incubator at 37°C. Isolated islets were used for experiments in 7 d.
Western blot analysis
The 15–30 µg of proteins were separated by a 7.5% SDS-PAGE and transferred to PVDF membrane for immunoblotting. After blocking with 7.5% skim milk in PBS (pH 7.2) overnight at 4°C, the membrane was incubated with 0.1% Tween 20 in PBS (PBS-T) containing the anti-ABCA1 antibody, anti-insulin, anti-AT1 receptor, anti-scavenger receptor class B type 1 (SR-BI) (Santa Cruz Biotechnology Inc.; diluted to 1:200), anti-ABCG1 (abcam; diluted to 1:1,000) or anti-phospho- MAPK kinase, anti-MAPK kinase, anti-phospho-ERK1/2, or anti-ERK1/2 (Cell Signal Technology, 1:1,000) overnight at 4°C, or anti-GAPDH antibody (Biomol Research, Plymouth Meeting, PA; diluted to 1:5,000) for 1 h at room temperature as described previously (14). Membrane was washed three times with PBS-T for 10 min each and then incubated for 1 h at room temperature with the appropriate HRP-linked secondary antibody (DakoCytomation; diluted to 1:20,00). Membranes were again washed three times for 10 min each, and antigen–antibody complexes were visualized by ECL (GE Healthcare).
Real-time PCR
PCR was performed with a final volume of 20 µl in LightCycler (Roche, Mannheim, Germany) glass capillaries. The sequences of the forward and reverse ABCA1 primers were 5′-CCCGGCGGAGTAGAAAGG-3′ and 5′-AGGGCGATGCAAACAAAGAC-3′, respectively. Each set of PCRs included water as a negative control and five dilutions of the standard. Known amounts of DNA were then diluted to make the standards, and a regression curve of crossing points versus concentration was generated with LightCycler as described previously (15). GAPDH was used as the housekeeping standard.
Transfection of INS-1 cells and luciferase reporter gene assay
We used a construct (pABCA1-LUC) containing the ABCA1 promoter obtained using PCR and the luciferase reporter gene as previously described (16). To generate the construct of LXR binding-site mutated ABCA1 promoter, pABCA1-LUC was mutated at −57 and −52 sites with a pair of primer showed following 5′-CACAGGCTTTGACCAATAGGAACCTCTGCGCTCG-3′ and 5′-GTGTCCGAAACTGGTTATCCTTGGAGACGCGAGC-3′ (mutated nucleotides are underlined) by site-directed mutagenesis. These mutations replace residues that are critical for mediating effects of LXR via related its response sequence (TGACCG and TAACCT).
Purified promoter plasmid was transfected into INS-1 using Lipofectamine (Life Technologies, Gaithersburg, MD). Transfected cells were maintained in medium containing 10 nM Ang II for 24 h with or without pretreatment with LY294002 (LY; 10 μmol/l), PD98095 (PD; 10 μmol/l), SB203580 (SB; 1 μmol/l), H-89 (1 μmol/l), or STO609 (1 μg/ml) to separately inhibit the phosphatidylinositol 3 kinase (PI3K), MAPK kinase (MEK), p38 MAPK (p38MAPK), protein kinase A (PKA), or Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) signaling pathway for 30 min (17). To check the effect of blocking the AT1R, transfected cells were treated with 10 nM Ang II together with RNH-6270 at varying concentrations (0, 10, and 100 nM) for 24 h. Then, transfected cells were harvested, and the promoter activity of ABCA1 was measured in an aliquot of the cytoplasmic preparation. The 40 μl aliquots were taken for the luciferase assay, which was performed according to the manufacturer’s instructions (ToyoInk, Tokyo, Japan).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-ITTM kit (Active Motif) according to the manufacturer’s instructions. Chromatin was immunoprecipitated with 2 μg of either rabbit LXR antibody (Santa Cruz Biotechnology Inc) or negative control IgG. DNA was analyzed by PCR to amplify regions containing the putative LXR binding sites on rat ABCA1 promoter by using primers forward 5′-GCTTTCTGCTGAGTGACTGAACTAC-3′ and reverse 5′-GAATTACTGCTTTTTGCCGCG-3′ as described previously (18).
GSIS
Pancreatic islets or INS-1 cells treated with varying treatments were starved in Krebs–Ringer bicarbonate (KRB) buffer containing 120 mM NaCl, 5 mM KCl, 1.1 mM MgCl2, 2.5 mM CaCl2, 25 mM NaHCO3, and 0.1% BSA (pH 7.4) for 1 h. Following that, cells were incubated in KRB buffer containing 3.3 mM glucose for 1 h, and then the medium was replaced by KRB buffer supplemented with varying glucose concentrations (basal, 3.3 mM; stimulatory, 16.7 mM) together with other test regents. After 1 h incubation, the supernatant was harvested and used for insulin measurement by and ELISA kit (Shibayagi, Japan). All the incubation was performed in a 5% CO2 incubator at 37°C.
Transfection of siRNA
The siRNAs that were targeted to LXR sequences were designed as described previously (19). The siRNAs that were targeted to AT1R sequences or scramble siRNA were purchased from Santa Cruz (catalog no. sc-155992 or sc-37007). Transfection of the siRNA was performed using Lipofectamine (Life Technologies) as described previously (20).
Cholesterol content assay
To measure cellular cholesterol ester concentration, we used a colorimetric assay that utilizes reagents widely used for the measurement of cholesterol in conjunction with a random-access chemistry analyzer, ARCHITECT c8000, as described previously (21). Briefly, INS-1 cells with varying treatment were washed by PBS twice and resuspended with 800 µl of PBS. A total of 450 µl of cell suspension was treated with Triton X-100 and used for cholesterol content check by ARCHITECT c8000. The remaining 350 µl of cell suspension was used for cell-number counting. The cholesterol concentration per cell were calculated by the ratio of cholesterol content (micrograms per milliliter) to cell number (cells per milliliter).
Statistical analysis
Data were expressed as mean ± SEM. Results were analyzed by one-way ANOVA and Student’s t-test. A value of P < 0.05 was considered statistically significant. All experiments were performed at least three times.
RESULTS
Ang II decreased the expression of ABCA1 in INS-1 cells
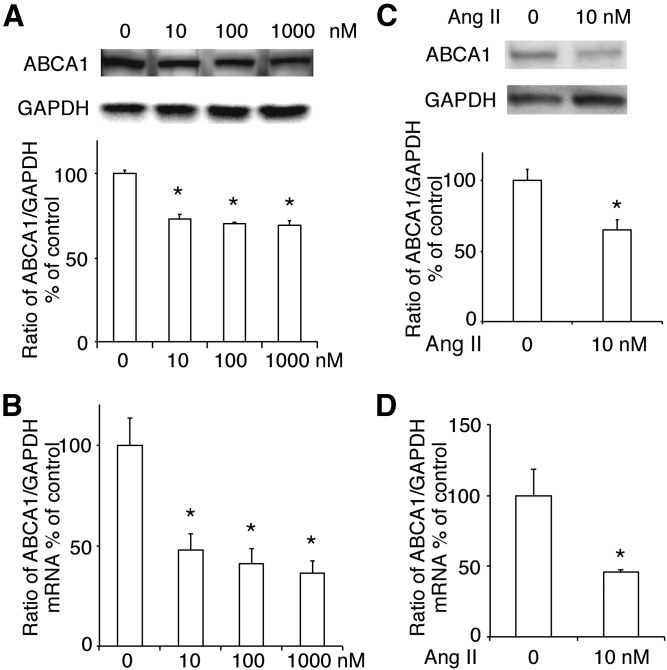
First, we isolated total protein in INS-1 cells treated with Ang II at varying concentrations (0–1,000 nM) and used Western blotting to monitor the ABCA1 expression. We found that Ang II remarkably decreased ABCA1 expression (Fig. 1A) without affecting the expression of ABCG1 and SR-BI (supplemental Fig. S1A, B) in INS-1 cells. This was confirmed by the results of real-time PCR (Fig. 1B and supplemental Fig. S1C, D). Because Ang II at a concentration of 10 nM efficiently decreased the ABCA1 expression, we used this concentration of Ang II in the following experiments. We also checked the effect of Ang II at a concentration of 10 nM in rat pancreatic islets and found that both protein and mRNA expression of ABCA1 were decreased by the treatment of Ang II (Fig. 1C, D).
Fig. 1.
The effect of Ang II on the expression of ABCA1. The abundance of ABCA1 protein (A) and mRNA (B) in INS-1 cells treated with varying concentrations (0, 10, 100, and 1,000 nM) of Ang II. C, D: The effect of Ang II at a concentration of 10 nM on the protein (C) and mRNA (D) expression of ABCA1 in rat pancreatic islets. The ratio of ABCA1 to GAPDH is shown as percent of control. A graph showing the mean ± SEM (n = 3) of separate experiments for each treatment group is included. * P < 0.05 compared with 0.
Ang II decreased the transcription of ABCA1 via the MEK/ERK signaling pathway in INS-1 cells
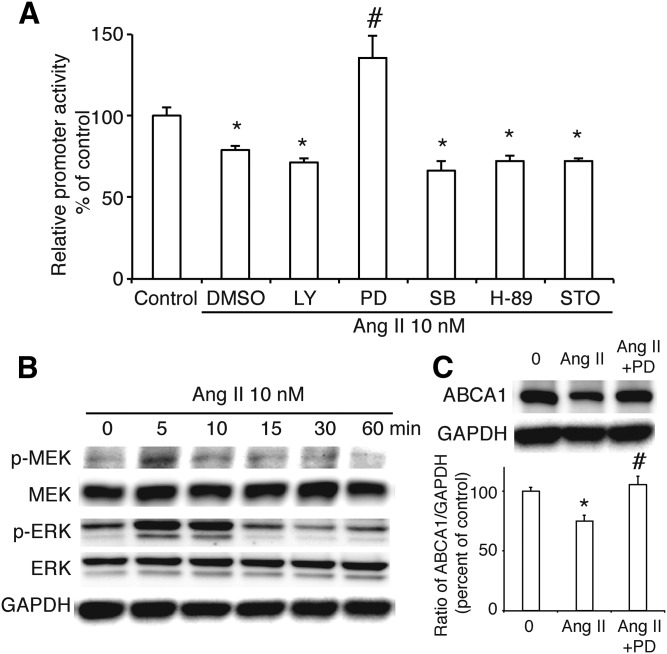
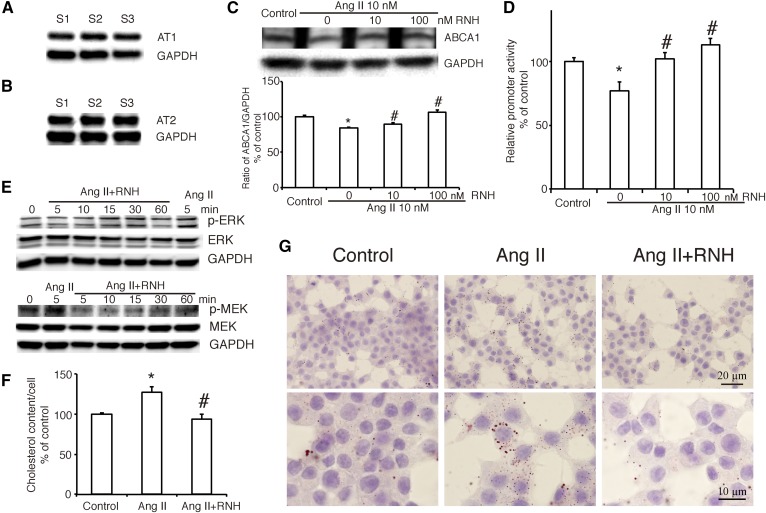
To further study the effect of Ang II on ABCA1 gene transcription, we transfected the luciferase reporter plasmid that contained the promoter region of ABCA1 into INS-1 cells and treated these cells with Ang II at a concentration of 10 nM. As shown in Fig. 2A, Ang II decreased the promoter activity of ABCA1. Next, we used several inhibitors, LY (10 μM), PD (10 μM), SB (1 μM), H-89 (1 μM), or STO609 (1 μg/ml) to separately inhibit the PI3K, MEK, p38MAPK, PKA, or CaMKK signaling pathway, and found that only inhibition of the MEK signaling pathway blockaded the effect of Ang II on ABCA1 promoter activity, suggesting that Ang II may regulate ABCA1 expression via the MEK signaling pathway. Furthermore, we treated INS-1 cells with Ang II for different times (0–60 min) to check the activation of the MEK/ERK signaling pathway. We found that there was a peak of Ser 217/221-phosphorylated MEK at 5 min after Ang II treatment and that Thr202/Tyr204-phosphorylated ERK1/2 was activated from 5 min and lasted to 10 min (Fig. 2B), confirming the previous result. At the same time, the downregulation of ABCA1 protein induced by Ang II was recovered when MEK was inhibited by PD (Fig. 2C) without affecting the expression of ABCG1 and SR-BI (supplemental Fig. S2).
Fig. 2.
Roles of Ang II on the promoter activity of ABCA1 in INS-1 cells. A: The effects of a PI3K inhibitor (LY), a MEK inhibitor (PD), a p38 MAPK inhibitor (SB), a PKA inhibitor (H-89), or CaMKK inhibitor STO609 (STO) with or without Ang II on ABCA1 promoter activity. Percentage of promoter activity is referred to control. B: Phosphorylation of MEK at Ser217/221 and ERK at Thr202/Tyr204 in INS-1 cells treated with Ang II. C: The expression of ABCA1 in INS-1 cells treated with PD and Ang II. The ratio of ABCA1 to GAPDH is shown as percent of control. A graph showing the mean ± SEM (n = 3) of separate experiments for each treatment group is included. Control, no Ang II; Ang II, Ang II at 10 nM; Ang II+PD, Ang II at a concentration of 10 nM plus PD at a concentration of 10 µM. * P < 0.05 compared with control (0); # P < 0.05 compared with DMSO plus Ang II.
Effect of Ang II on cholesterol accumulation in INS-1 cells
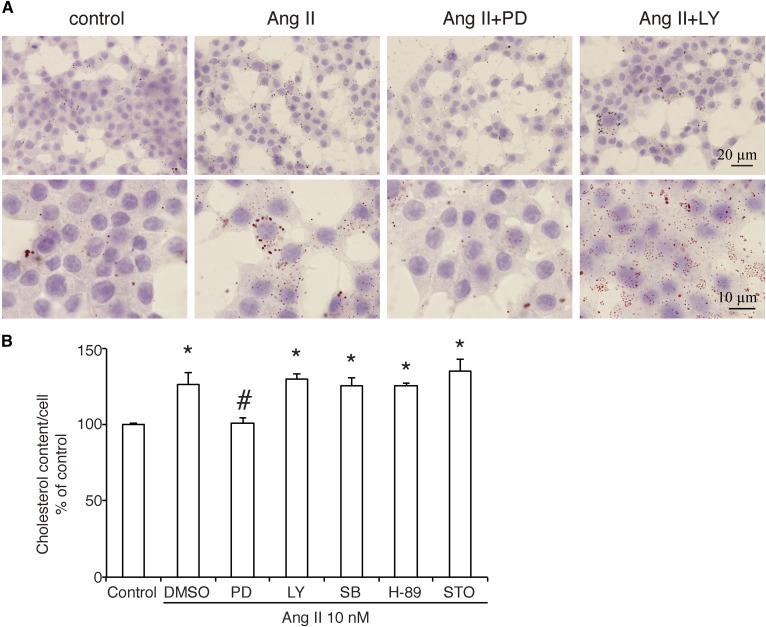
Because ABCA1 is a cholesterol transporter located in the plasma membrane, we then examined the effect of Ang II on cholesterol content in INS-1 cells. As shown by the Oil Red O stain (Fig. 3A), Ang II at a concentration of 10 nM significantly increased the number and size of lipid droplets. Statistical analysis in cholesterol content assay (Fig. 3B) showed that, although the absolute concentration is (171 ± 2.1) × 10−8 µg per cell in the control group, treatment with Ang II increased the cholesterol content to 126.8 ± 7.7% of control, and only inhibition of the MEK signaling pathway by PD altered this effect.
Fig. 3.
The effect of Ang II on the cholesterol accumulation in INS-1 cells. After 30 min preincubation in inhibitors of signaling pathways, INS-1 cells were treated by Ang II at a concentration of 10 nM for 24 h and then stained by Oil Red O or lysed to measure intracellular cholesterol ester content. A: Oil Red O stain. The images in the upper row were 200× magnification (bar = 20 µm), and those in the lower row were 400× magnification (bar = 10 µm). B: Percentage of cholesterol content per cell referred to control is shown as the mean ± SEM (n = 3) of separate experiments in the graph. The absolute cholesterol concentration in control group is (171 ± 2.1) × 10−8 µg/cell. * P < 0.05 compared with control; # P < 0.05 compared with DMSO plus Ang II.
GSIS was reduced by Ang II in INS-1 cells
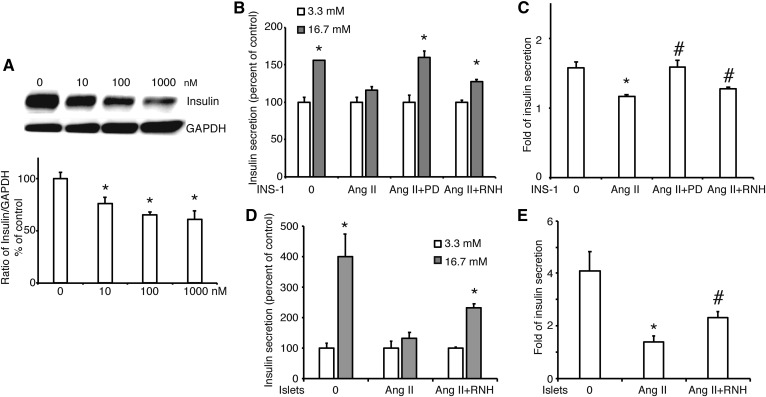
A previous study suggested that accumulation of cholesterol in pancreatic β cells resulted in impaired insulin secretion (4). Thus, we checked the abundance of insulin synthesis in INS-1 cells treated with Ang II. As shown in Fig. 4A, Ang II significantly decreased the amount of insulin in a dose-dependent manner. Following that, GSIS assay showed that high glucose (16.7 mM) increased insulin secretion to 155 ± 0.31% of basal level (3.3 mM; Fig. 4B), and the increase of insulin secretion was 1.57 ± 0.09-fold (Fig. 4C). INS-1 cells treated with Ang II were not able to release insulin response to high glucose stimulation (Fig. 4B). Compared with control, the increase of insulin secretion in Ang II-treated cells was significantly lower (Fig. 4C). However, this effect was improved when MEK was inhibited by PD or AT1R was blocked by RNH-6270. Similar results of GSIS were obtained in primary pancreatic islets from rat (Fig. 4D, E). These data demonstrated that Ang II decreased glucose-stimulated insulin release via AT1R and the MEK signaling pathway in pancreatic β cells.
Fig. 4.
The effect of Ang II on insulin synthesis and secretion in INS-1 cells. A: The abundance of insulin protein in INS-1 cells treated by Ang II at varying concentrations (0, 10, 100, and 1,000 nM). The ratio of insulin to GAPDH is shown as percent of control. B: GSIS in INS-1 cells treated with Ang II at a concentration of 10 nM, Ang II plus the MEK inhibitor PD (Ang II +PD), or Ang II plus the inhibitor of AT1R, RNH-6270 (Ang II +RNH). C: Fold of insulin secretion from low glucose (3.3 mM) to high glucose (16.7 mM) in INS-1 cells. D: GSIS in primary pancreatic islets from rat treated with Ang II at a concentration of 10 nM or Ang II plus the inhibitor of AT1R, RNH-6270 (Ang II +RNH). E: Fold of insulin secretion from low glucose (3.3 mM) to high glucose (16.7 mM) in primary pancreatic islets from rat. A graph showing the mean ± SEM (n = 3) of separate experiments for each treatment group is provided. * P < 0.05 compared with 0 (A, C, E) or compared with 3.3 mM in each group (B, D); # P < 0.05 compared with Ang II (A, C, E).
Ang II regulates ABCA1 expression via AT1R in INS-1 cells
Previous study demonstrated that both AT1R and the AT2 receptor are expressed in INS-1 cells (7). In our study, we confirmed that both of the receptors were indeed expressed in INS-1 cells (Fig. 5A, B). To investigate the mechanism in the action of Ang II on ABCA1, we used the specific inhibitors to separately blockade each receptor and found that inhibition of AT1R by RNH-6270 dose-dependently altered the effect of Ang II on the expression of ABCA1 (Fig. 5C) and its promoter activity (Fig. 5D) without affecting the expression of ABCG1 and SR-BI (supplemental Fig. S2). In contrast, inhibition of the AT2 receptor by PD123319 was not able to modify the action of Ang II on ABCA1 promoter activity (supplemental Fig. S3). This result suggested that Ang II regulates ABCA1 expression via the AT1R.
Fig. 5.
Ang II regulates the expression of ABCA1 via its receptor, AT1R in INS-1 cells. A, B: The expression of Ang II receptor AT1 (A) and AT2 (B). C: ABCA1 expression in INS-1 cells treated with or without AngII and the inhibitor of AT1R, RNH-6270 at varying concentration (0, 10, or 100 nM). The ratio of ABCA1 to GAPDH is shown as percent of control. D: The effect of RNH on Ang II-suppressed ABCA1 promoter activity. Percentage of promoter activity is referred. E: Phosphorylation of ERK and MEK in INS-1 cells treated with RNH-6270 (10 nM) and Ang II (10 nM). F, G: Cholesterol content and Oil Red O stain in INS-1 cells treated with RNH-6270 and Ang II. F: Percentage of cholesterol content per cell is referred to control. A graph showing the mean ± SEM (n = 3) of separate experiments for each treatment group is provided. G: Oil Red O stain. The images in the upper row were 200× magnification (bar = 20 µm), and those in the lower row were 400× magnification (bar = 10 µm). Ang II, Ang II at a concentration of 10 nM; RNH, RNH-6270 at a concentration of 10 nM. * P < 0.05 compared with control; # P < 0.05 compared with Ang II.
Next, we monitored the effect of AT1R inhibitor RNH-6270 on the phosphorylation of MEK and ERK1/2 and found that inhibition of AT1R abolished activation of MEK and ERK1/2 induced by Ang II at 5 min (Fig. 5E). As shown in Fig. 5F, G, inhibition of AT1R significantly improved cholesterol accumulation induced by Ang II, confirming that Ang II functions via the AT1R.
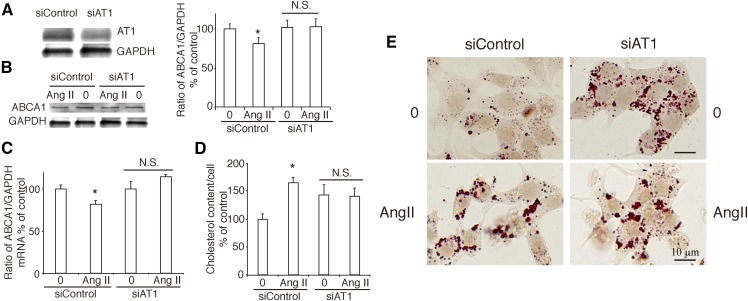
As a follow-up, we used siRNA to specifically silence the expression of AT1R in INS-1 cells (Fig. 6A) and found that the effect of Ang II on the expression of ABCA1 did not persist (Fig. 6B, C). Silence of AT1R also canceled the effect of Ang II on cholesterol accumulation (Fig. 6D, E) in INS-1 cells.
Fig. 6.
Silence of AT1R cancels the effect of Ang II on ABCA1 expression and cholesterol accumulation. A: The expression of AT1R in AT1R silenced INS-1 cells. B, C: Silence of AT1R canceled the effect of Ang II on ABCA1 expression by Western blotting (B) and real-time PCR (C). D, E: Cholesterol content and Oil Red O stain in INS-1 cells after silence of AT1R. D: Percentage of cholesterol content per cell is referred to control. A graph showing the mean ± SEM (n = 3) of separate experiments for each treatment group is provided. E: Oil Red O stain. The left line was siControl group, and the right line was siAT1 receptor group. The images were 400× magnification (bar = 10 µm). 0, no treatment; Ang II, treatment with Ang II at a concentration of 10 nM for 24 h. * P < 0.05 compared with control; # P < 0.05 compared with Ang II. N.S., no significance.
LXR is involved in the process of Ang II-regulated ABCA1 expression
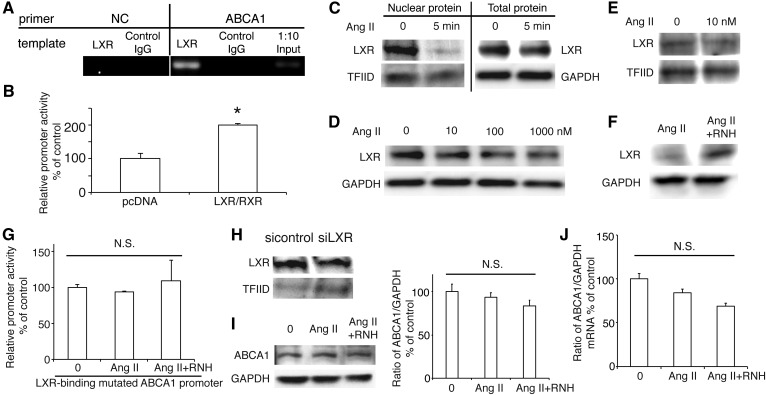
Previous study demonstrated that transcription factor LXR is able to promote ABCA1 transcription combined with the RXR in macrophages (22). In this study, we used ChIP assay to demonstrate that LXR could bind to the ABCA1 promoter region in INS-1 cells (Fig. 7A). Moreover, overexpression of LXR and RXR significantly enhanced ABCA1 promoter activity (Fig. 7B), confirming that LXR regulates ABCA1 transcription in INS-1 cells. To investigate the role of LXR in Ang II-regulated ABCA1 expression, we treated INS-1 cells with Ang II at 10 nM for 5 min and found that Ang II decreased the nuclear abundance of LXR protein without influence of total LXR expression (Fig. 7C), suggesting that Ang II regulated ABCA1 transcription through inhibiting recruitment of LXR in the nucleus. This was confirmed by the result that Ang II dose-dependently decreased the total and nuclear protein levels of LXR in INS-1 cells (Fig. 7D, E), whereas inhibition of AT1R with RNH-6270 increased the LXR expression compared with treatment with Ang II (Fig. 7F), suggesting that Ang II regulated ABCA1 transcription via transcription factor, LXR.
Fig. 7.
Roles of transcription factor LXR in Ang II-suppressed ABCA1 expression. A: Binding of LXR to ABCA1 promoter. LXR specifically immunoprecipitates ABCA1 chromatin from INS-1 cells by ChIP assay. No ChIP was detected when the chromatin was immunoprecipitated with unspecific negative control IgGs. Input was a positive control. There is no band when the templates were amplified by negative primer. B: The effect of LXR on ABCA1 promoter activity. C: The nuclear abundance of LXR protein in INS-1 cells treated with Ang II for 5 min. D, E: The expression of total LXR and nuclear LXR in INS-1 cells treated with Ang II for 24 h. F: The expression of LXR in INS-1 cells treated with Ang II and RNH6270 (RNH). G: The site-directed mutagenesis of the LXR-binding site by altering two base pairs (GATAGT to AATAGG) in ABCA1 promoter region abrogated its response to Ang II or Ang II plus RNH. H: siRNA specific to LXR effectively decreased its nuclear expression. I, J: The expression of ABCA1 protein (I) and mRNA (J) level in siLXR-transfected INS-1 cells treated with Ang II or Ang II plus RNH. Ang II, Ang II at a concentration of 10 nM; RNH, RNH-6270 at a concentration of 10 nM. * P < 0.05 compared with control (pcDNA). N.S., no significance.
To confirm this hypothesis, we mutated the LXR-binding site in the ABCA1 promoter region and found that neither Ang II nor AT1R inhibitor had any effect on this mutated promoter activity (Fig. 7G). Silencing of LXR (Fig. 7H) abolished the effects of both Ang II and the AT1R inhibitor on the protein and mRNA expression of ABCA1 (Fig. 7I, J). These findings are supportive of the idea that Ang II-regulated ABCA1 expression requires LXR.
DISCUSSION
In the present study, we found that Ang II inhibited expression of ABCA1 in the pancreatic β cell line INS-1 and isolated rat islets. A previous report indicated that GSIS was completely abolished by infusion of Ang II for 4 weeks in vivo (9). Ang II, the key effector of the RAS, mediated its effects via binding to specific receptors on the cell surface. Local pancreatic islet RAS performs multifactorial activities in the structure and function of islets, including cell proliferation, apoptosis, oxidative stress, inflammatory responses, and GSIS, and these regulatory functions are probably mediated via the AT1R (23). We confirmed that AT1R inhibitor abolished the effect of Ang II on ABCA1 expression. We also showed here that Ang II inhibited GSIS by downregulating ABCA1 expression, which may be mediated via the AT1R.
Elevation of cholesterol content in pancreatic islets, either in ob/ob mice that lack ApoE and have diabetogenic obesity or in cholesterol-overloaded β cell lines, impairs insulin secretion response to glucose (24). Consistently, a high level of cholesterol content in pancreatic islets and reduction of insulin secretion are detected in mice lacking β cell ABCA1 (4, 5), suggesting that cholesterol may directly reduce the function of β cells. In this study, we found higher cholesterol levels and lower insulin secretion after Ang II treatment (Figs. 3, 4). The important role of β-cell ABCA1 in glucose homeostasis was further demonstrated by the study that rosiglitazone increased the expression of ABCA1 in β cells (4). In addition to this report, we previously found that exendin-4, a long-acting analog of the glucagon-like peptide 1 (GLP-1), also has a stimulating effect on ABCA1 expression via CaMKK/CaMKIV signaling pathway and transcriptional factor, prolactin regulatory element-binding, which results in enhancement of insulin secretion (14, 21). These results suggested that reducing cholesterol content could protect pancreatic β cells from dysfunction.
One of the goals in this study was to study in more detail signaling pathways activated by Ang II involved in ABCA1 gene expression, which included two substrates of the MEK/ERK signaling pathway, as well as the LXR transcriptional factor. MAPKs, encoded by a group of genes with related sequences, are a family of serine/threonine protein kinases that are phosphorylated to respond to a variety of stimuli and then to modulate cell growth and differentiation (25). p44 (ERK1) and p42 (ERK2) are two isoforms of ERK and are activated by phosphorylation of threonine and tyrosine residues by MEK (26, 27). Previous studies indicated that ERK1 and ERK2 are rapidly activated by treatment with Ang II in vascular smooth muscle cells (25, 28) and in pancreatic cancer cells (29). It was also proved that Ang II induced monocyte chemoattractant protein-1, a key factor of inflammation, by the activation of MEK/ERK via AT1R in pancreatic islets and β cells (30). We examined this mechanism by using a specific inhibitor of the MEK/ERK pathway, PD, and found that Ang II-suppressed ABCA1 promoter activity occurs through an MEK/ERK-sensitive mechanism (Fig. 2). These results suggest that activation of MEK/ERK signaling by Ang II via AT1R may contribute to the role of RAS activation in the development of diabetes.
Tangier disease (TD) is a rare autosomal recessive disease caused by mutations in the ABCA1 gene. The deficiency of HDL in patients with TD leads to the accumulation of cholesterol in many tissues, such as the pancreas, spleen, tonsils, liver, gastrointestinal mucosa, lymph nodes, and peripheral nerves (31). Furthermore, it is reported that there is ABCA1 expressed in pancreatic β cells (4), suggesting that the deficiency of ABCA1 together with abnormal lipid rafts might result in the dysfunction of pancreatic β cells in patients with TD. Koseki et al. reported that four Japanese TD patients who also suffered from T2D have a significantly low value of the insulinogenic index compared with nondiabetic controls by the oral glucose tolerance test, raising the possibility that ABCA1 plays an important functional role in GSIS from β cells (32). In this study, the INS-1 cells with Ang II-suppressed ABCA1 were shown to have a decreased insulin secretion in response to high glucose concentration (shown in Fig. 4B, C).
LXRs are expressed in a variety of tissues, including kidney, brain, liver, and pancreas. It is well known that LXRs are able to regulate lipid homeostasis and metabolism, facilitate cholesterol efflux from cells, and prevent the cells from lipid toxicity and overload (33). LXR/RXR heterodimers, upon binding to LXR response elements, lead to the transcription of several genes, including ABCG1 and ABCA1 (34). A previous study also demonstrated that LXR is activated to bind to a DR4 site in the ABCA1 promoter and that this step is critical to the upregulation of ABCA1 and cholesterol efflux (22). Several transcription factors, such as activator protein 2α, zinc finger protein 202, fos-related antigen 2, and sterol regulatory element-binding protein 2, were demonstrated to negatively regulate the transcription of ABCA1 separately in THP-1 cells, HepG2 cells, and primary mouse peritoneal macrophages and hepatoma cell line (McARH7777) (35–38). Recently, Lake et al. also reported that they have identified a novel regulator in the process of LXR-mediated ABCA1 gene expression, hepatic HDL biogenesis, and macrophage cholesterol efflux (39). The reduction in the hepatic cholesterol content resulted in the upregulation of hepatic ABCA1 protein and mRNA levels, which is opposite to other cells with respect to cholesterol (40, 41). However, particularly in pancreatic β cells, little is known about the regulatory mechanisms of ABCA1 gene expression. Our results indicated that Ang II treatment decreased ABCA1 promoter activity by reducing the recruitment of LXR in the nucleus of the pancreatic cell line, which is consistent with a recent study that inhibition of ERK1/2 and activation of LXR synergistically reduce atherosclerotic lesions in ApoE-deficient mice (42). This study therefore contributes to new knowledge about the regulation of the ABCA1 gene by Ang II, suggesting that Ang II-repressed ABCA1 expression might be related to the lipotoxicity in pancreatic β cells, which may be a tissue-specific phenomenon. Further experiments will be needed to clarify the specific regulatory mechanisms.
Downregulation of ABCA1 by Ang II in pancreatic β cells raises a question that cholesterol content in INS-1 cells might be altered by Ang II treatment. Our results showed that Ang II treatment increased cholesterol content compared with the control group (Fig. 3). In addition, the MEK/ERK inhibitor PD canceled Ang II-induced cholesterol accumulation. Previous studies have already shown that there is a correlation between the elevation of cholesterol content in pancreatic islets and reduction of insulin release in ABCA1-KO mice (4, 43), suggesting that overload of cholesterol directly impairs the function of β cells. In our previous studies, we found that IGF-1 and insulin secretalogue GLP-1 decrease the cholesterol content in pancreatic β cells and also stimulate ABCA1 expression via the PI3K/Akt/FoxO1 pathway or the CaMK cascade (14, 21), resulting in enhanced insulin secretion. It is well demonstrated that the circulating RAS plays an important role in the endocrine, renal, and cardiovascular system. In addition to the circulating RAS, Ang II generating systems also persist in many tissues, including the pancreas, and it might have specific functions by paracrine/autocrine actions (30). The new data from our study suggest a novel role of the local pancreatic Ang II, and targeting Ang II could be exploded as a new therapeutic strategy to protect pancreatic islets from lipotoxicity and inhibit β-cell dysfunction in diabetes.
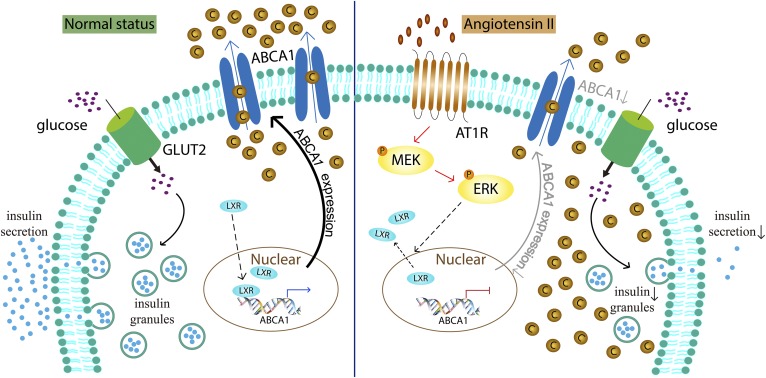
In summary, our studies showed (Fig. 8) that Ang II suppressed the expression of endogenous ABCA1 via the MEK/ERK pathway and transcription factor LXR and then induced cholesterol accumulation in primary pancreatic islets of rat and INS-1 cells, resulting in reduction of GSIS. These findings raise the possibility that Ang II may affect the function of pancreatic β cells by regulation of ABCA1 expression, which may also contribute therapeutic value in the treatment of diabetes mellitus.
Fig. 8.
Schematic diagram of the mechanisms in Ang II-induced cholesterol accumulation by downregulation of ABCA1 in pancreatic β cells. In healthy β cells (normal status shown in the left), transcription factor LXR is recruited into nuclear and induces ABCA1 expression to facilitate cholesterol out of the cell, which protects insulin secretion response to high glucose. Treatment with Ang II (right) downregulates ABCA1 expression by stimulation of the MEK/ERK pathway via AT1R and reduction of nuclear LXR, resulting in cholesterol accumulation in β cells. As a result, GSIS is impaired. C, cholesterol; P, phosphorylation of MEK or ERK.
Supplementary Material
Acknowledgments
The authors thank Ms. Yumi Miyai and Azusa Sugimoto for their technical assistance.
Footnotes
Abbreviations:
- Ang II
- angiotensin II
- AT1
- angiotensin II type 1
- AT2
- angiotensin II type 2
- AT1R
- angiotensin II type 1 receptor
- CaMKK
- Ca2+/calmodulin-dependent protein kinase kinase
- ChIP
- chromatin immunoprecipitation
- GSIS
- glucose-stimulated insulin secretion
- KRB
- Krebs-Ringer bicarbonate
- LY
- LY294002
- MEK
- MAPK kinase
- PD
- PD98095
- PI3K
- phosphatidylinositol 3 kinase
- PKA
- protein kinase A
- RAS
- renin-angiotensin system
- SB
- SB203580
- SR-BI
- scavenger receptor class B type 1
- TD
- Tangier disease
This work was funded in part by Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid 15K09415 and 16K09786 (K.M. and H.I.). The authors declare that they have no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Zhou Y. P., and Grill V.. 1995. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J. Clin. Endocrinol. Metab. 80: 1584–1590. [DOI] [PubMed] [Google Scholar]
- 2.Unger R. H. 1995. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 44: 863–870. [DOI] [PubMed] [Google Scholar]
- 3.Fielding C. J., and Fielding P. E.. 1995. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 36: 211–228. [PubMed] [Google Scholar]
- 4.Brunham L. R., Kruit J. K., Pape T. D., Timmins J. M., Reuwer A. Q., Vasanji Z., Marsh B. J., Rodrigues B., Johnson J. D., Parks J. S., et al. . 2007. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13: 340–347. [DOI] [PubMed] [Google Scholar]
- 5.Brunham L. R., Kruit J. K., Verchere C. B., and Hayden M. R.. 2008. Cholesterol in islet dysfunction and type 2 diabetes. J. Clin. Invest. 118: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touyz R. M., and Schiffrin E. L.. 2000. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 52: 639–672. [PubMed] [Google Scholar]
- 7.de Gasparo M., Catt K. J., Inagami T., Wright J. W., and Unger T.. 2000. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 52: 415–472. [PubMed] [Google Scholar]
- 8.Yang H. Y., Bian Y. F., Xiao C. S., Liang B., Zhang N., Gao F., and Yang Z. M.. 2015. Angiotensin-(1–7) stimulates cholesterol efflux from angiotensin II-treated cholesterol-loaded THP-1 macrophages through the suppression of p38 and c-Jun N-terminal kinase signaling. Mol. Med. Rep. 12: 1387–1392. [DOI] [PubMed] [Google Scholar]
- 9.Ihoriya C., Satoh M., Kuwabara A., Sasaki T., and Kashihara N.. 2014. Angiotensin II regulates islet microcirculation and insulin secretion in mice. Microcirculation. 21: 112–123. [DOI] [PubMed] [Google Scholar]
- 10.Lau T., Carlsson P. O., and Leung P. S.. 2004. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 47: 240–248. [DOI] [PubMed] [Google Scholar]
- 11.Jandeleit-Dahm K. A., Tikellis C., Reid C. M., Johnston C. I., and Cooper M. E.. 2005. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J. Hypertens. 23: 463–473. [DOI] [PubMed] [Google Scholar]
- 12.Yu X., Murao K., Sayo Y., Imachi H., Cao W. M., Ohtsuka S., Niimi M., Tokumitsu H., Inuzuka H., Wong N. C., et al. . 2004. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes. 53: 1475–1481. [DOI] [PubMed] [Google Scholar]
- 13.Li D. S., Yuan Y. H., Tu H. J., Liang Q. L., and Dai L. J.. 2009. A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4: 1649–1652. [DOI] [PubMed] [Google Scholar]
- 14.Miyai Y., Murao K., Imachi H., Li J., Nishiuchi Y., Masugata H., Iwama H., Kushida Y., Ishida T., and Haba R.. 2011. Exendin-4 regulates the expression of the ATP-binding cassette transporter A1 via transcriptional factor PREB in the pancreatic beta cell line. J. Endocrinol. Invest. 34: e268–e274. [DOI] [PubMed] [Google Scholar]
- 15.Nagao S., Murao K., Imachi H., Cao W. M., Yu X., Li J., Matsumoto K., Nishiuchi T., Ahmed R. A., Wong N. C., et al. . 2006. Platelet derived growth factor regulates ABCA1 expression in vascular smooth muscle cells. FEBS Lett. 580: 4371–4376. [DOI] [PubMed] [Google Scholar]
- 16.Nishiuchi Y., Murao K., Imachi H., Nishiuchi T., Iwama H., and Ishida T.. 2010. Transcriptional factor prolactin regulatory element-binding protein-mediated gene transcription of ABCA1 via 3′,5′-cyclic adenosine-5′-monophosphate. Atherosclerosis. 212: 418–425. [DOI] [PubMed] [Google Scholar]
- 17.Yu X., Murao K., Imachi H., Cao W. M., Li J., Matsumoto K., Nishiuchi T., Ahmed R. A., Wong N. C., Kosaka H., et al. . 2007. Regulation of scavenger receptor class BI gene expression by angiotensin II in vascular endothelial cells. Hypertension. 49: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 18.Wagner B. L., Valledor A. F., Shao G., Daige C. L., Bischoff E. D., Petrowski M., Jepsen K., Baek S. H., Heyman R. A., Rosenfeld M. G., et al. . 2003. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 23: 5780–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J. H., Park S. M., Kim O. S., Lee C. S., Woo J. H., Park S. J., Joe E. H., and Jou I.. 2009. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol. Cell. 35: 806–817. [DOI] [PubMed] [Google Scholar]
- 20.Lyu J., Imachi H., Iwama H., Zhang H. and Murao K.. 2016. Insulin-like growth factor 1 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Horm. Metab. Res. 48: 338–344. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Murao K., Imachi H., Masugata H., Iwama H., Tada S., Zhang G. X., Kobayashi R., Ishida T., and Tokumitsu H.. 2010. Exendin-4 regulates pancreatic ABCA1 transcription via CaMKK/CaMKIV pathway. J. Cell. Mol. Med. 14: 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costet P., Luo Y., Wang N., and Tall A. R.. 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275: 28240–28245. [DOI] [PubMed] [Google Scholar]
- 23.Chu K. Y., Lau T., Carlsson P. O., and Leung P. S.. 2006. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 55: 367–374. [DOI] [PubMed] [Google Scholar]
- 24.Lenzen S., and Kloppel G.. 1978. Insulin secretion and the morphological and metabolic characteristics of pancreatic islets of hyperthyroid ob/ob mice. Endocrinology. 103: 1546–1555. [DOI] [PubMed] [Google Scholar]
- 25.Duff J. L., Marrero M. B., Paxton W. G., Schieffer B., Bernstein K. E., and Berk B. C.. 1995. Angiotensin II signal transduction and the mitogen-activated protein kinase pathway. Cardiovasc. Res. 30: 511–517. [PubMed] [Google Scholar]
- 26.Crews C. M., Alessandrini A., and Erikson R. L.. 1992. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 258: 478–480. [DOI] [PubMed] [Google Scholar]
- 27.Cobb M. H., and Goldsmith E. J.. 1995. How MAP kinases are regulated. J. Biol. Chem. 270: 14843–14846. [DOI] [PubMed] [Google Scholar]
- 28.Duff J. L., Berk B. C., and Corson M. A.. 1992. Angiotensin II stimulates the pp44 and pp42 mitogen-activated protein kinases in cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 188: 257–264. [DOI] [PubMed] [Google Scholar]
- 29.Amaya K., Ohta T., Kitagawa H., Kayahara M., Takamura H., Fujimura T., Nishimura G., Shimizu K., and Miwa K.. 2004. Angiotensin II activates MAP kinase and NF-kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int. J. Oncol. 25: 849–856. [PubMed] [Google Scholar]
- 30.Chipitsyna G., Gong Q., Gray C. F., Haroon Y., Kamer E., and Arafat H. A.. 2007. Induction of monocyte chemoattractant protein-1 expression by angiotensin II in the pancreatic islets and beta-cells. Endocrinology. 148: 2198–2208. [DOI] [PubMed] [Google Scholar]
- 31.Mott S., Yu L., Marcil M., Boucher B., Rondeau C., and Genest J. Jr.. 2000. Decreased cellular cholesterol efflux is a common cause of familial hypoalphalipoproteinemia: role of the ABCA1 gene mutations. Atherosclerosis. 152: 457–468. [DOI] [PubMed] [Google Scholar]
- 32.Koseki M., Matsuyama A., Nakatani K., Inagaki M., Nakaoka H., Kawase R., Yuasa-Kawase M., Tsubakio-Yamamoto K., Masuda D., Sandoval J. C., et al. . 2009. Impaired insulin secretion in four Tangier disease patients with ABCA1 mutations. J. Atheroscler. Thromb. 16: 292–296. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C., and Dahlman-Wright K.. 2010. Liver X receptor in cholesterol metabolism. J. Endocrinol. 204: 233–240. [DOI] [PubMed] [Google Scholar]
- 34.Morel E., Demignot S., Chateau D., Chambaz J., Rousset M., and Delers F.. 2004. Lipid-dependent bidirectional traffic of apolipoprotein B in polarized enterocytes. Mol. Biol. Cell. 15: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takata Y., Chu V., Collins A. R., Lyon C. J., Wang W., Blaschke F., Bruemmer D., Caglayan E., Daley W., Higaki J., et al. . 2005. Transcriptional repression of ATP-binding cassette transporter A1 gene in macrophages: a novel atherosclerotic effect of angiotensin II. Circ. Res. 97: e88–e96. [DOI] [PubMed] [Google Scholar]
- 36.Iwamoto N., Abe-Dohmae S., Ayaori M., Tanaka N., Kusuhara M., Ohsuzu F., and Yokoyama S.. 2007. ATP-binding cassette transporter A1 gene transcription is downregulated by activator protein 2alpha. Doxazosin inhibits activator protein 2alpha and increases high-density lipoprotein biogenesis independent of alpha1-adrenoceptor blockade. Circ. Res. 101: 156–165. [DOI] [PubMed] [Google Scholar]
- 37.Porsch-Ozcurumez M., Langmann T., Heimerl S., Borsukova H., Kaminski W. E., Drobnik W., Honer C., Schumacher C., and Schmitz G.. 2001. The zinc finger protein 202 (ZNF202) is a transcriptional repressor of ATP binding cassette transporter A1 (ABCA1) and ABCG1 gene expression and a modulator of cellular lipid efflux. J. Biol. Chem. 276: 12427–12433. [DOI] [PubMed] [Google Scholar]
- 38.Tamehiro N., Shigemoto-Mogami Y., Kakeya T., Okuhira K., Suzuki K., Sato R., Nagao T., and Nishimaki-Mogami T.. 2007. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J. Biol. Chem. 282: 21090–21099. [DOI] [PubMed] [Google Scholar]
- 39.Lake N. J., Taylor R. L., Trahair H., Harikrishnan K. N., Curran J. E., Almeida M., Kulkarni H., Mukhamedova N., Hoang A., Low H., et al. . 2017. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur. Heart J. 38: 3579–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang S. L., Chen W. J., Yin K., Zhao G. J., Mo Z. C., Lv Y. C., Ouyang X. P., Yu X. H., Kuang H. J., Jiang Z. S., et al. . 2012. PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRalpha through the IGF-I-mediated signaling pathway. Atherosclerosis. 222: 344–354. [DOI] [PubMed] [Google Scholar]
- 41.Ohoka N., Okuhira K., Cui H., Wu W., Sato R., Naito M., and Nishimaki-Mogami T.. 2012. HNF4alpha increases liver-specific human ATP-binding cassette transporter A1 expression and cholesterol efflux to apolipoprotein A-I in response to cholesterol depletion. Arterioscler. Thromb. Vasc. Biol. 32: 1005–1014. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., Duan Y., Yang X., Sun L., Liu M., Wang Q., Ma X., Zhang W., Li X., Hu W., et al. . 2015. Inhibition of ERK1/2 and activation of LXR synergistically reduce atherosclerotic lesions in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 35: 948–959. [DOI] [PubMed] [Google Scholar]
- 43.de Haan W., Bhattacharjee A., Ruddle P., Kang M. H., and Hayden M. R.. 2014. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J. Lipid Res. 55: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.