Abstract
Trihydroxyoctadecenoic acids (TriHOMEs) are linoleic acid-derived oxylipins with potential physiological relevance in inflammatory processes as well as in maintaining an intact skin barrier. Due to the high number of possible TriHOME isomers with only subtle differences in their physicochemical properties, the stereochemical analysis is challenging and usually involves a series of laborious analytical procedures. We herein report a straightforward analytical workflow that includes reversed-phase ultra-HPLC-MS/MS for rapid quantification of 9,10,13- and 9,12,13-TriHOME diastereomers and a chiral LC-MS method capable of resolving all sixteen 9,10,13-TriHOME and 9,12,13-TriHOME regio- and stereoisomers. We characterized the workflow (accuracy, 98–120%; precision, coefficient of variation ≤6.1%; limit of detection, 90–98 fg on column; linearity, R2 = 0.998) and used it for stereochemical profiling of TriHOMEs in bronchoalveolar lavage fluid (BALF) of individuals with chronic obstructive pulmonary disease (COPD). All TriHOME isomers were increased in the BALF of COPD patients relative to that of smokers (P ≤ 0.06). In both COPD patients and smokers with normal lung function, TriHOMEs with the 13(S) configuration were enantiomerically enriched relative to the corresponding 13(R) isomers, suggesting at least partial enzymatic control of TriHOME synthesis. This method will be useful for understanding the synthetic sources of these compounds and for elucidating disease mechanisms.
Keywords: lipid mediators, fatty acid/biosynthesis, fatty acid/oxidation, inflammation, methods/high-performance liquid chromatography, liquid chromatography-tandem mass spectrometry, trihydroxy linoleates, chiral chromatography, fatty acid biosynthesis
Oxylipins are oxygenated PUFAs that can regulate a diverse set of homeostatic and inflammatory processes (1). They are derived from fatty acids, including arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and linoleic acid, and are formed by both nonenzymatic and enzymatic processes (2–4). The most extensively studied group of oxylipins are the eicosanoids (derived from the 20-carbon PUFA, arachidonic acid), which have been demonstrated to be important signaling molecules in the regulation of inflammatory processes (1). Despite the fact that linoleic acid (an 18-carbon chain fatty acid) is the most abundant PUFA in humans (5, 6), linoleic acid-derived oxylipins have been studied to a much lesser extent than the eicosanoids.
A major end product of the oxidation of linoleic acid is the trihydroxyoctadecenoic acids (TriHOMEs) (7, 8). The most well-described TriHOME regioisomers are 9,10,13-TriHOME and 9,12,13-TriHOME; however, other trihydroxy-linoleates, such as 9,10,11-TriHOME and 11,12,13-TriHOME, have also been reported (9, 10). Both 9,10,13-TriHOME and 9,12,13-TriHOME were first described in 1970 by Graveland (11), who showed that these species can be formed as lipoxygenase-catalyzed products of linoleic acid in flour-water suspensions. Since then, they have been reported in potato leaves (9, 12), onion bulbs (13), and mechanically deboned meat (14), as well as in beer (15, 16), where they were suggested to contribute to the characteristic bitter taste. In addition, 9,12,13-TriHOME was described as a potentially useful adjuvant for influenza vaccine formulations (17, 18). In contrast to many of the other oxylipin species whose physiological significance has been extensively studied, little is known about the physiological relevance of TriHOMEs. Recently, a potential physiological role of TriHOMEs was reported by Brash and coworkers who suggested that TriHOMEs play an essential part in the skin barrier function of the human epidermis (19, 20). Furthermore, TriHOME levels have been found to be dysregulated in asthma and chronic obstructive pulmonary disease (COPD) (21–24). There is a significant interest in determining the stereochemistry of the observed TriHOME profile, which can provide insight into both the route of synthesis as well as the potential pathophysiological implications. For example, one putative route for the biosynthesis of the TriHOMEs is via 15-lipoxygenase (15-LOX) activity to form one of the hydroxy species. The activity of 15-LOX exclusively produces the S-isomer as opposed to autoxidation, which results in a racemic mixture (25).
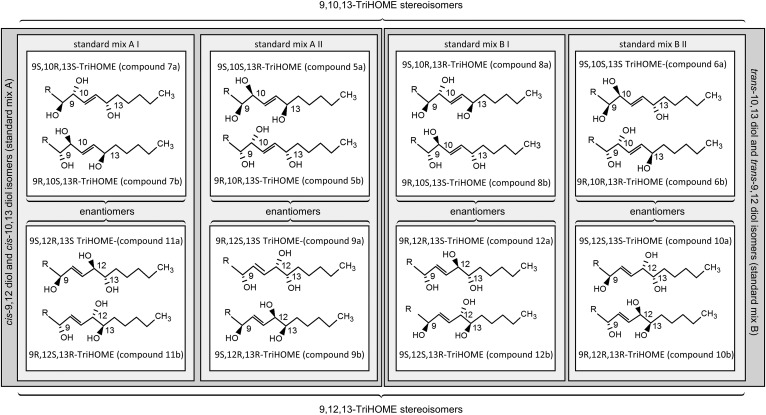
One particular challenge in studying the TriHOMEs is that 9,10,13-TriHOME and 9,12,13-TriHOME each contain three chiral centers, and therefore 16 isomers (eight enantiomer pairs) are possible (Fig. 1). This large number of possible isomers and their similar physicochemical properties make characterization of the absolute stereochemistry of TriHOMEs challenging and laborious (8, 19, 26). Previous approaches to assign the regio- and stereochemistry of TriHOMEs have employed derivatization followed by a series of analytical techniques, including normal-phase HPLC, chiral HPLC, GC-MS, NMR, and circular dichroism analysis (8, 17–20, 26). In the current study, we describe a straightforward chromatographic workflow that enables characterization and quantification of the eight 9,10,13-TriHOMEs and the eight 9,12,13-TriHOMEs based on synthesized racemic TriHOME standard mixes and standards with defined regio- and stereochemistry. The workflow first employs rapid quantification of the 9,10,13-TriHOME and 9,12,13-TriHOME diastereomers by reversed-phase ultra-HPLC (UHPLC)-MS/MS, followed by chiral HPLC-MS/MS analysis to resolve all 16 TriHOME isomers in a single chromatographic procedure. The developed workflow was thoroughly characterized and used to elucidate differences in the TriHOME isomer profile in bronchoalveolar lavage fluid (BALF) of female COPD patients compared with healthy individuals.
Fig. 1.
Overview of the analyzed TriHOME isomers. The numbering of the TriHOME isomers (5a/b–12a/b) corresponds to the system proposed when their structures were originally reported (8). InChIKeys are provided in supplementary Table S2. R = (CH2)7COOCH3.
MATERIALS AND METHODS
Chemicals and reagents
MS-grade methanol, isopropanol, acetonitrile, and acetic acid were obtained from Fisher Scientific (Waltham, MA). Glycerol and triphenylphosphine dibromide were purchased from Sigma-Aldrich (St. Louis, MO). Disodium phosphate and citric acid monohydrate were obtained from Merck (Darmstadt, Germany). PBS was purchased from Thermo Fisher Scientific (Pittsburgh, PA), Milli-Q ultrapure deionized water was used in all experiments (Millipore Corp., Billerica, MA). Sodium hydroxide, diethyl-ether, hydrochloric acid, and vanadium oxyacetylacetonate were purchased from VWR (Spånga, Sweden). Dichloromethane, hexane, and ethyl acetate were obtained from Skandinaviska Genetec (Västra Frölunda, Sweden). Diazomethane was prepared in-house (with special permit). Ethanol was purchased from Kemetyl (Haninge, Sweden). Linoleic acid was obtained from NuChek (Elysian, MN). The standards, 9(S),10(S),13(S)-TriHOME (compound 6a) and 9(S),12(S),13(S)-TriHOME (compound 10a) were purchased from Larodan (Solna, Sweden). Internal standard (IS) stock solution, TriHOME standard stock solutions, and calibration curve standards were dissolved in methanol and stored at −80°C.
Preparation of racemic TriHOME standard mixtures
The preparation of racemic TriHOME standard mixtures containing cis-10,13-diol and cis-9,12-diol isomers (TriHOME mix A; compounds 5a/b, 7a/b, 9a/b, and 11a/b) and TriHOME standard mixtures of trans-10,13-diol and trans-9,12-diol isomers (TriHOME mix B; compounds 6a/b, 8a/b, 10a/b, and 12a/b) was performed as described earlier (8). The numbering system of the TriHOME isomers is based upon earlier work and is maintained herein for consistency, as described in Fig. 1 (8). In brief, linoleic acid (200 mg) was autoxidized by refluxing in 40% ethanol under a stream of oxygen gas for 47 h. Extraction with ethyl acetate gave a product that was treated with diazomethane and subjected to silica gel open column chromatography. Material eluted with ethyl acetate was subjected to preparative TLC using ethyl acetate as the mobile phase. In agreement with previous work (8), two major bands appeared, i.e., TriHOME mix A [retention factor (Rf) = 0.48, containing the methyl esters of TriHOME compounds 5a/b, 7a/b, 9a/b, and 11a/b] and TriHOME mix B (Rf = 0.59, containing the methyl esters of TriHOME compounds 6a/b, 8a/b, 10a/b, and 12a/b). Methyl esters of compound mix A and compound mix B were further separated into erythro and threo isomers by preparative normal-phase HPLC using a Nucleosil 50-5 column (250 × 4.6 mm; Macherey-Nagel, Düren, Germany) and a solvent system of 2-propanol-hexane (5:95, v/v) at a flow rate of 2 ml/min. This provided mixtures of the methyl esters of the erythro isomers, 7a/b + 11a/b (retention volume, 49 ml) and 8a/b + 12a/b (retention volume, 45 ml), and of the threo isomers, 5a/b + 9a/b (retention volume, 56 ml) and 6a/b + 10 a/b (retention volume, 51 ml). The corresponding free acids were obtained by treatment with 0.2 M NaOH in 80% aqueous methanol at 23°C for 15 h. Their identities were confirmed by analysis of the Me3Si/methyl ester derivatives by GC-MS, as described previously (8). The concentrations of individual TriHOME diastereomers were determined by preparing adequate dilutions of the standard mixtures in 84% methanol and measuring the concentration in quadruplicates, as described in the section, Quantification of TriHOME diastereomers by reversed-phase UHPLC-MS/MS analysis.
Synthesis of TriHOME isomers 5a, 7a, 8a, 9a, 11a, and 12a
TriHOME isomers with defined regio- and stereochemistry were prepared as described in detail previously (8, 27). Briefly, the lipoxygenase-derived hydroperoxides, 9(S)-HpODE and 13(S)-HpODE, were converted into α,β-epoxy alcohols by treatment with vanadium oxyacetylacetonate. These were subjected to acid hydrolysis and fractionated by TLC into the desired TriHOME. As an example, the preparation of TriHOME compound 10a [9(S),12(S),13(S)-TriHOME, pinellic acid) is described in detail. The 13(S)-HpODE (131 mg) was converted into its methyl ester and added to 150 ml of hexane saturated with vanadium oxyacetylacetonate (about 30 μM). After 60 min at 23°C, extraction with diethyl ether was performed and the product subjected to preparative normal-phase HPLC (four injections of about 35 mg per injection) using a Nucleosil 50-7 column (250 × 10 mm; Macherey-Nagel) and a solvent system of 2-propanol-hexane (1:99, v/v) at a flow rate of 4 ml/min. The erythro and threo isomers of the epoxy alcohol methyl trans-11,12-epoxy-13(S)-hydroxy-9(Z)-octadecenoate (of which 9,12,13-TriHOME is a major hydrolysis product) eluted with effluent volumes of 52 and 76 ml, respectively, and the threo isomer [methyl 11(R),12(R)-epoxy-13(S)-hydroxy-9(Z)-octadecenoate] was collected. The material was dissolved in methanol (3 ml) and water (100 ml) was added. Hydrolysis was initiated by addition of 10 drops of 2 M hydrochloric acid. After 20 min at 23°C, the initially cloudy solution had cleared completely and the product was recovered by extraction with ethyl acetate. Preparative TLC (eluent: ethyl acetate) furnished the methyl esters of TriHOMEs 9a (Rf = 0.48) and 10a (Rf = 0.59). The further purification of the methyl ester of 10a by normal-phase HPLC and the subsequent saponification were carried out as described for the racemic TriHOMEs in the section, Preparation of racemic TriHOME standard mixtures. The absolute configuration of the allylic alcohol group present in each “a” isomer was determined by steric analysis of (−)-methoxycarbonyl derivatives using authentic references as described previously (28). The concentrations of prepared standards were determined by preparing adequate dilutions in 84% methanol and measuring the concentrations in quadruplicates as described in the section, Quantification of TriHOME diastereomers by reversed-phase UHPLC-MS/MS analysis.
Synthesis of stable isotope-labeled TriHOME (compound 13C3-10a)
The [11,12,13-13C3]linoleic acid (used as starting material for preparation of IS compound 13C3-10a) was prepared starting with [13C3]propargyl alcohol using acetylenic coupling protocols followed by semihydrogenation. Briefly [13C3]propargyl alcohol [250 mg; purchased from Cambridge Isotope Laboratories (Tewksbury, MA)] was converted into its tetrahydropyranyloxy derivative, which was treated with butyllithium and coupled to 1-bromopentane. The resulting tetrahydropyranyloxy derivative of [1,2,3-13C3]2-octyn-1-ol was converted into 1-bromo-2-octyne by treatment with triphenylphosphine dibromide in dichloromethane. Following purification on a silica gel column, the labeled 1-bromo-2-octyne was submitted to CuI-promoted coupling (29) to methyl 9-decynoate. The acetylenic ester was semi-hydrogenated by stirring under hydrogen gas in the presence of P-2 nickel (30). Purification by reversed-phase HPLC gave the methyl ester as a colorless oil (373 mg; yield, 30%). The free acid was obtained following alkaline hydrolysis and a second purification by reversed-phase HPLC. The isotopic composition was 96.6% 13C3-labeled, 2.6% 13C2-labeled, 0.7% 13C1-labeled, and 0.1% unlabeled molecules. The [11,12,13-13C3]9(S),12(S),13(S) TriHOME (compound 13C3-10a) was prepared from 13C-labeled linoleic acid for use as IS. Synthesis was performed as described for unlabeled compound 10a, but using [11,12,13-13C3]13(S)-HpODE obtained from [11,12,13-13C3]linoleic acid as the starting material. The isotopic composition was 96.9% 13C3-labeled, 2.5% 13C2-labeled, 0.5% 13C1-labeled, and 0.1% unlabeled molecules.
Solid phase extraction of BALF
BALF samples (800 μl) were spiked with 10 μl of isotopically labeled IS and diluted with 200 μl of extraction buffer (citric acid/Na2HPO4, pH 5.6). Automated solid phase extraction was performed using an Extrahera liquid handling system (Biotage, Uppsala, Sweden) and Evolute Express ABN SPE cartridges (cartridge capacity: 60 mg, 3 ml sample volume; Biotage). SPE cartridges were conditioned with 2.5 ml methanol followed by equilibration using 2.5 ml of water. After equilibration, the samples were loaded onto the cartridges and washed with 2 ml of wash buffer (10% methanol). Analytes were eluted in 2.5 ml methanol. After elution, 30 μl of trap solvent (30% glycerol in methanol) were added to each extract, and methanol was evaporated using a TurboVAP LV evaporation system (Biotage) under a gentle stream of nitrogen gas. After evaporation samples were reconstituted in 70 μl of methanol/water (6:1, v/v) and filtered by centrifugation for 2 min at 12,000 g using 0.1 μm of polyvinylidene fluoride membrane spin filters (Merck Millipore, Billerica, MA).
Quantification of TriHOME diastereomers by reversed-phase UHPLC-MS/MS analysis
All TriHOME analyses were performed on a Waters Acquity UPLC coupled to a Waters Xevo TQ-XS triple quadrupole mass spectrometer (Waters, Milford, MA). Chromatographic separation of four 9,10,13-TriHOME and four 9,12,13-TriHOME diastereomers was achieved using a reversed-phase Acquity UPLC BEH C18 column (2.1 × 150 mm, 1.7 μm particle size; Waters). The mobile phases consisted of: 0.1% acetic acid in water (mobile phase A) and 5% methanol in acetonitrile (mobile phase B). Compounds were separated for 12.5 min by isocratic elution with 30% mobile phase B. After 12.5 min, the column was washed with 100% mobile phase B for 2 min followed by re-equilibration with 30% mobile phase B for another 2 min (total run time: 16.5 min). Separations were performed at a flow rate of 0.3 ml/min and the column oven temperature was set to 40°C. Mass spectrometric detection was performed in negative ionization mode using multiple reaction monitoring mode applying the following transitions: 9,10,13-TriHOME, m/z 329.1 → 139.0; collision energy (CE), 21 eV; 9,12,13-TriHOME, m/z 329.1 → 211.0; CE, 25 eV; [11,12,13-13C3] 9(S),12(S),13(S)-TriHOME (used as IS), m/z 332.1 → 213.0; CE, 25 eV. For all transitions, a cone voltage of 37 was applied. For TriHOME quantification, a 9-point calibration curve (concentration range: 0.023–150 ng/ml) was prepared from a stock solution containing 9(S),10(S),13(S)-TriHOME (compound 6a) and 9(S),12(S),13(S)-TriHOME (compound 10a) by making serial 1:3 dilutions. Each calibration level (50 μl) was mixed with 10 μl of IS and 10 μl of water. The injection volume of calibration standards as well as of extracted samples was 7.5 μl. Analyte concentrations were determined from relative peak areas (analyte peak area/IS peak area).
Chiral analysis of TriHOMEs by LC-MS/MS
For chiral analysis of the TriHOME isomers, the same LC-MS/MS system as described in the section, Quantification of TriHOME diastereomers by reversed-phase UHPLC-MS/MS analysis, was used. Chiral separation of eight 9,10,13-TriHOME and eight 9,12,13-TriHOME stereoisomers was achieved using a Chiralpak AD-RH column (2.1 × 150 mm, 3 μm particle size; Daicel, Illkirch, France). The mobile phases consisted of 0.1% acetic acid in water (mobile phase A) and 20% isopropanol in acetonitrile (mobile phase B). Compounds were separated for 97 min by isocratic elution with 35% mobile phase B. After 97 min, the column was washed with 100% mobile phase B for 5 min followed by re-equilibration with 35% mobile phase B for another 5 min (total run time: 107 min). Separations were performed at a flow rate of 0.1 ml/min and at a column temperature of 35°C. The injection volume of extracted samples was 20 μl. Mass spectrometric detection was performed in negative ionization mode using multiple reaction monitoring applying the settings described in the section, Quantification of TriHOME diastereomers by reversed-phase UHPLC-MS/MS analysis. Enantiomeric excess (ee) was calculated using the formula:
where a and b are the peak areas of the respective enantiomers.
Performance characterization of the reversed-phase UHPLC-MS/MS method
Proper performance of the developed analytical workflow was demonstrated by evaluating intra-day and inter-day accuracy, intra-day and inter-day precision, recovery, linearity, limit of detection (LOD), and limit of quantification (LOQ) of the method. Nine-point calibration curves were prepared (concentration range: 0.023–150 ng/ml) as described in the section, Quantification of TriHOME diastereomers by reversed-phase UHPLC-MS/MS analysis, and linearity was determined applying 1/x weighted least squares linear regression analysis. For inter-day and intra-day accuracy and precision determinations, as well as for recovery calculations, sample matrix (20% extraction buffer in PBS) was spiked with TriHOME mix A and TriHOME mix B standards at two different concentrations [quality control (QC) high: 3.3–5.4 ng/ml; QC low: 0.033–0.054 ng/ml). IS (10 μl) was added to each QC sample and samples were extracted as described in the section, Solid phase extraction of BALF. Both QC high and QC low samples were prepared in triplicates on three different days. After analysis, intra-day (n = 3) accuracy and inter-day (n = 3) accuracy were calculated as: average determined concentration/nominal concentration × 100. Intra-day precision and inter-day precision were defined as coefficient of variation (CV = standard deviation/mean) of measured triplicates. TriHOME recovery was determined by comparing IS peak areas after extraction and IS peak areas of nonextracted solutions with the same IS concentration (n = 5). LOD values (signal/noise = 3) and LOQ (signal/noise = 10) were estimated from signal/noise values obtained from the lowest measured calibration curve standard.
Performance characterization of chiral LC-MS/MS method
The ability of the chiral LC-MS/MS method to measure the ee of the individual TriHOME enantiomers was investigated by spiking compounds 5a, 6a, 7a, 8a, 9a, 10a, 11a, and 12a (enantiopure compounds) at four different concentrations (no spike, 0 ng/ml; low spike, 0.5 ng/ml; medium spike, 2 ng/ml; and high spike, 4 ng/ml) into solutions containing TriHOME mix A/TriHOME mix B (2.54–4.20 ng/ml of racemic mixtures). The ee error percent was calculated by comparing nominal ee with measured ee (ee error = nominal ee – measured ee).
Analysis of TriHOMEs in BALF of COPD patients
BALF samples were obtained from the Karolinska Clinical and Systems Medicine Investigations of Smoking-related COPD (COSMIC) study (www.clinicaltrials.gov/ct2/show/NCT02627872). Detailed information on the study was previously published (21, 31). Briefly, COSMIC is a three-group study designed to perform clinical and molecular characterizations of COPD patients as well as of healthy smokers and healthy never-smokers. For the current study, only the female subset of the study was analyzed. BALF from 20 healthy never-smokers, 19 smokers with normal lung function, and 17 COPD patients with mild-to-moderate disease [Global Initiative for Chronic Obstructive Lung Disease stage I-II/A-B, forced expiratory volume in 1 s (FEV1) 51–97% of predicted value; FEV1/forced vital capacity (FVC <0.7)] were analyzed. In order to minimize the confounding effect of smoking in COPD, COPD patients that were ex-smokers (6 individuals) were analyzed separately from the smoking COPD patient population (11 individuals). Prior to the measurements, samples were randomized and the analysis was performed blinded. The study was approved by the Stockholm Regional Ethical Board (COSMIC cohort: No. 2006/959-31/1; validation cohort: No. 2005/733-31/1-4) and participants provided their informed written consent.
RESULTS
Method development and characterization for 9,10,13-TriHOME and 9,12,13-TriHOME diastereomer quantification
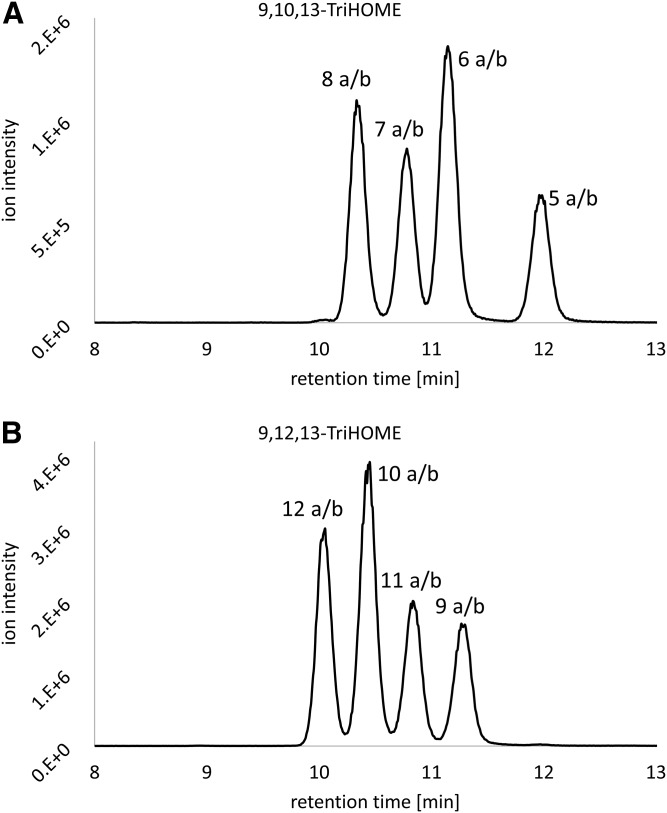
The regioisomers, 9,10,13-TriHOME and 9,12,13-TriHOME (as well as the isotopically labeled IS), could be distinguished based upon unique MS/MS transitions (supplemental Fig. S1). A reversed-phase UHPLC-MS/MS method was developed to quantify the four 9,10,13-TriHOME and four 9,12,13-TriHOME enantiomer pairs. To investigate whether the various TriHOME isomers show differences with regard to ionization efficiency, calibration curves of the different isomer standards were injected separately and the obtained calibration curve slopes were compared. Because no major differences in curve slopes were obtained, it was concluded that all TriHOME isomers possess similar ionization efficiencies (data not shown). The method was optimized with regard to mobile phase composition and flow rate, column temperature, and sample solvent composition. Despite the fact that the analytes have only minimal differences with regard to their hydrophobicity, the high separation power of UHPLC enabled baseline separation of the four 9,10,13 TriHOME and four 9,12,13 TriHOME enantiomer pairs within <12.5 min. Based upon individual injections of the TriHOME standard mixes, AI, AII, BI, and BII, the peaks were assigned to the corresponding TriHOME isomer as indicated in Fig. 2 and supplemental Fig. S2. Subsequently, the developed reversed-phase UHPLC-MS/MS method was characterized with respect to intra-day and inter-day accuracy, intra-day and inter-day precision, recovery, linearity, LOD, and LOQ. An overview of generated analytical figures of merit is shown in Table 1.
Fig. 2.
Reversed-phase UHPLC-MS/MS chromatogram of a solution containing TriHOME standard mix A and standard mix B resolving the four 9,10,13-TriHOME enantiomer pairs and the four 9,12,13-TriHOME enantiomer pairs. Peaks were assigned to the corresponding isomers based upon individual injections of standard mixes AI, AII, BI, and BII. A: Chromatogram of 9,10,13-TriHOME, MS/MS transition: m/z 329.1 → 139.0. B: Chromatogram of 9,12,13-TriHOME, MS/MS transition: m/z 329.1 → 211.0. Compound nomenclature is provided in Fig. 1.
TABLE 1.
Analytical figures of merit of the reversed-phase UHPLC-MS/MS method
| Compounda | Intra-day Accuracy [(%) ± Precision (CV)] | Inter-day Accuracy [(%) ± Precision (CV)] | LOD/LOQ (fg on Column) | Linearity | Recovery (%) | ||
| QC low | QC high | QC low | QC high | ||||
| 5 | 118 ± 2.2 | 108 ± 0.7 | 119 ± 1.6 | 114 ± 4.7 | 90/300 | R2: 0.998 | — |
| 6 | 104 ± 1.0 | 92 ± 3.9 | 108 ± 3.6 | 96 ± 4.6 | 90/300 | R2: 0.998 | — |
| 7 | 98 ± 0.8 | 84 ± 0.7 | 101 ± 3.2 | 88 ± 4.0 | 90/300 | R2: 0.998 | — |
| 8 | 117 ± 1.7 | 102 ± 3.2 | 120 ± 2.3 | 108 ± 4.7 | 90/300 | R2: 0.998 | — |
| 9 | 107 ± 1.2 | 94 ± 0.6 | 106 ± 2.5 | 100 ± 4.8 | 98/320 | R2: 0.998 | — |
| 10 | 102 ± 3.6 | 88 ± 2.5 | 106 ± 3.4 | 92 ± 4.0 | 98/320 | R2: 0.998 | — |
| 11 | 110 ± 2.4 | 91 ± 1.1 | 111 ± 3.6 | 98 ± 6.1 | 98/320 | R2: 0.998 | — |
| 12 | 115 ± 2.1 | 103 ± 3.1 | 118 ± 1.9 | 108 ± 4.3 | 98/320 | R2: 0.998 | — |
| IS | — | — | 83 | ||||
See Fig. 1 for compound structure and nomenclature.
Development and characterization of a chiral LC-MS method for identification of TriHOME enantiomers
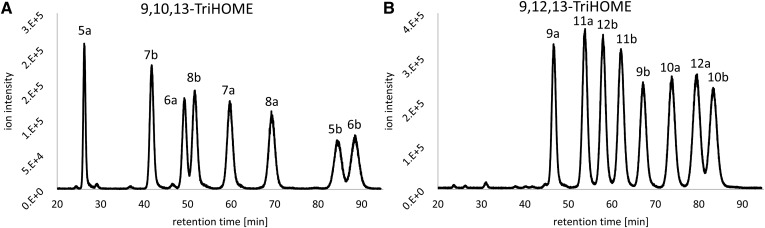
The reversed-phase UHPLC-MS/MS method was able to successfully quantify all four 9,10,13-TriHOME and four 9,12,13-TriHOME enantiomer pairs. However, enantiomers generally possess equal hydrophobicity and are consequently indistinguishable in reversed-phase chromatography setups. Therefore, an additional chiral LC-MS method was developed in order to distinguish the TriHOME enantiomers. After optimization of column type, column temperature, mobile phase composition, and flow rate, all eight 9,10,13-TriHOME and eight 9,12,13-TriHOME isomers could be separated (Fig. 3). In the optimization process, both 3 μm and 5 μm particle size chiral columns were evaluated, with the smaller particle size showing improved resolving power. This was particularly important, as the late-eluting 9,10,13-TriHOME compounds, 5b [9(R),10(R),13(S)-TriHOME] and 6b [9(R),10(R),13(R)-TriHOME], resolved poorly on a 5 μm particle size column (data not shown), but were separated satisfactorily using the same chromatographic conditions on a 3 μm particle size column (Fig. 3). After optimization of chromatographic conditions, peaks were unequivocally assigned by injections of compound mixes AI (7a/b and 11a/b), AII (5a/b and 9a/b), BI (8a/b and 12a/b), and BII (6a/b and 10 a/b) followed by injections of pure enantiomers 5a–12a.
Fig. 3.
Chiral LC-MS resolution of a mix of TriHOME standard mix A and standard mix B containing all eight 9,10,13-TriHOME and eight 9,12,13-TriHOME isomers. Peaks were assigned to the corresponding isomer based upon individual injections of standard mixes AI, AII, BI, and BII (each containing one enantiomer pair of 9,10,13-TriHOME and 9,12,13-TriHOME) followed by injections of pure enantiomers 5a–12a. A: Chromatogram of 9,10,13-TriHOME isomers, MS/MS transition: m/z 329.1 → 139.0. B: Chromatogram of 9,12,13-TriHOME isomers, MS/MS transition: m/z 329.1 → 211.0.
The chiral method was developed to determine the ee and the relative abundances of the different TriHOME isomers (for absolute quantification, the faster reversed-phase UHPLC-MS/MS method was used). Because the method used isocratic elution, it could be expected that the measured peak areas would linearly increase with increasing amount of analyte. To confirm this assumption, the ee accuracy was determined by spiking pure enantiomers 5a–12a at four different concentrations into solutions containing TriHOME mix A and TriHOME mix B (racemic mixtures). Subsequently, nominal ee was compared with measured ee. As shown in Table 2, good accuracy at all spiked concentrations with a maximum ee error of 11 percent points was measured.
TABLE 2.
Accuracy of the chiral LC-MS/MS method for ee determination
| Compounda | Nominal ee (%) | Measured ee (%) | ee (error%)b | |||||||||
| Nonec | Low | Med | High | None | Low | Med | High | None | Low | Med | High | |
| 5 | 0 | 13 | 37 | 54 | 6 | 21 | 43 | 64 | −6 | −8 | −6 | −10 |
| 6 | 0 | 13 | 38 | 55 | 1 | 20 | 43 | 61 | −1 | −7 | −5 | −6 |
| 7 | 0 | 11 | 32 | 49 | −2 | 12 | 34 | 55 | 2 | −1 | −2 | −6 |
| 8 | 0 | 13 | 38 | 55 | 0 | 15 | 36 | 59 | 0 | −2 | 2 | −4 |
| 9 | 0 | 16 | 44 | 61 | 3 | 25 | 50 | 72 | −3 | −9 | −6 | −11 |
| 10 | 0 | 16 | 44 | 61 | 2 | 22 | 47 | 66 | −2 | −6 | −3 | −5 |
| 11 | 0 | 13 | 38 | 55 | 3 | 21 | 44 | 66 | −3 | −8 | −6 | −11 |
| 12 | 0 | 16 | 43 | 60 | −2 | 17 | 39 | 59 | 2 | −1 | 4 | 1 |
Different levels of pure enantiomers 5a–12a (no spike, 0 ng/ml; low spike, 0.5 ng/ml; medium spike, 2 ng/ml; high spike, 4 ng/ml) were spiked into solutions containing TriHOME mix A and TriHOME mix B (2.54–4.20 ng/ml of racemic mixtures).
See Fig. 1 for compound structure and nomenclature.
The ee error% = nominal ee – measured ee.
None = no spike.
Determination of TriHOME diastereomer concentrations in BALF of COPD patients
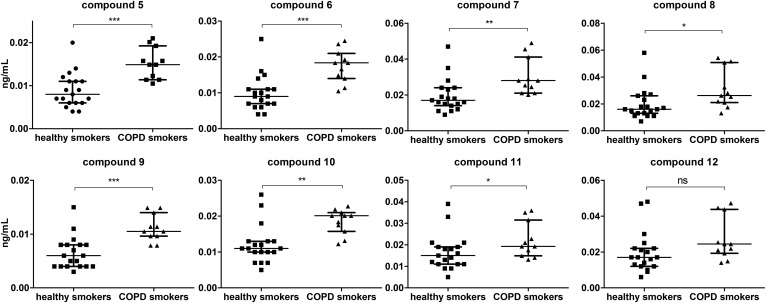
The developed reversed-phase UHPLC-MS/MS method was applied to compare TriHOME diastereomer concentrations in BALF of females from a COPD clinical study. Ion chromatograms of 9,10,13-TriHOME and 9,12,13-TriHOME diastereomers from a representative BALF sample are shown in supplemental Fig. S3. In order to minimize the confounding effect of smoking, healthy nonsmokers and COPD patients that are ex-smokers were analyzed separately from healthy smokers and smoking COPD patients (supplemental Table S1). All four 9,10,13-TriHOME and four 9,12,13-TriHOME enantiomer pairs were elevated in smoking COPD females compared with healthy individuals, with compounds 5–11 reaching statistical significance (Fig. 4).
Fig. 4.
The concentration in BALF of the 9,10,13-TriHOME and 9,12,13-TriHOME diastereomers of female smokers with normal lung function versus smoking female COPD patients. Values are shown as the median with interquartile range. Significance was tested by a nonparametric Mann-Whitney test, with significance levels: ns, not significant (P = 0.06); *P < 0.05; **P < 0.01; ***P < 0.001.
Chiral analysis of TriHOMEs in BALF of COPD patients
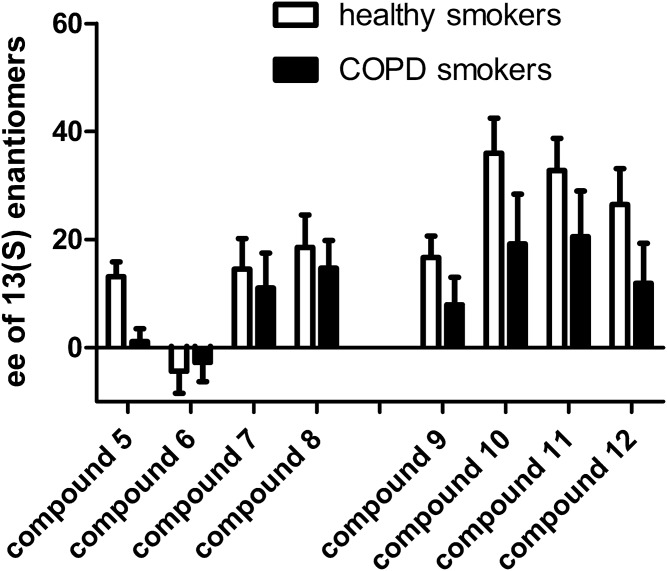
BALF samples from female smokers with normal lung function or with COPD were subsequently analyzed on the chiral LC-MS platform in order to determine the relative abundance of all eight 9,10,13-TriHOME and eight 9,12,13-TriHOME stereoisomers. Ion chromatograms of 9,10,13-TriHOME and 9,12,13-TriHOME stereoisomers from a representative BALF sample are shown in supplemental Fig. S4. The relative abundance of 9,10,13-TriHOME stereoisomers varied between 8% (compound 5a) and 18% (compound 8b) in the healthy control group. For 9,12,13-TriHOME stereoisomers, relative abundance ranged from 6% (compound 9b) to 21% (compound 12a). None of the relative isomer abundances were significantly altered in female COPD patients compared with females with normal lung function (supplemental Fig. S5). The ee determinations furthermore revealed that TriHOMEs with (S) configuration at C-13 were in excess relative to TriHOMEs with (R) configuration, except for compound 6 (for which the ee was essentially zero). Although the pattern was observed in COPD patients as well as in smokers with normal lung function, it appeared for all compounds to be more pronounced in healthy smokers (Fig. 5).
Fig. 5.
Average ee of TriHOMEs with (S) configuration at carbon 13 in BALF of female smokers with normal lung function and smoking female COPD patients. Error bars indicate 95% confidence intervals (healthy smokers, n = 19; COPD smokers, n = 11).
DISCUSSION
Linoleic acid is now the most highly consumed fat in the Western diet (32), which has led to a concomitant interest in the oxylipin products of linoleic acid (32–35). The TriHOMEs in particular have experienced an increasing degree of attention due to their potential significance in human physiology. They have been hypothesized to play an important part in maintaining the human skin water barrier [9(R),10(S),13(R)-TriHOME, compound 7b (19, 20)] and were found to be dysregulated in several recent studies in respiratory diseases (21–23).
A major limitation in the majority of studies concerned with TriHOMEs to date has been the difficulty to distinguish the individual TriHOME stereoisomers. Previous studies either did not resolve stereoisomerism of the TriHOMEs (instead reported only absolute amounts of 9,10,13-TriHOME and 9,12,13-TriHOME) or had to apply a series of analytical techniques (including normal-phase and chiral chromatography, GC-MS, NMR, and circular dichroism) in order to unequivocally assign the stereoisomeric configuration of TriHOMEs. We have therefore developed a straightforward chromatographic workflow that makes use of the combined analytical power of state of the art reversed-phase UHPLC, chiral chromatography, and MS/MS data acquisition to characterize and quantify all eight 9,10,13-TriHOME and eight 9,12,13-TriHOME isomers. Based on synthesized racemic standards and standards with defined regio- and stereochemistry, all separated TriHOME peaks could be assigned to the corresponding isomer. To the best of our knowledge, this is the first method that is capable of measuring all 16 TriHOME isomers in a single chromatographic run.
Compounds 6a [9(S),10(S),13(S)-TriHOME] and 10a [9(S),12(S),13(S)-TriHOME, the most prominent TriHOME isomer from plant sources (9, 16, 36)] were commercially available; however, it was necessary to synthesize the remaining TriHOME isomer standards in order to be able to assign isomers to the corresponding peaks. We synthesized enantiopure standards of compounds 5a, 7a, 8a, 9a, 11a, and 12a and prepared standard mixes containing racemic mixtures of enantiomer pairs (compound mixes A, B, AI, AII, BI, and BII; Fig. 1). On the reversed-phase column, the elution order followed a previously published pattern that identified TriHOMEs with threo configuration of C-9 and C-10 (for 9,10,13-TriHOMEs) or C-12 and C-13 (for 9,12,13-TriHOMEs) as being less polar than the corresponding erythro isomers (8). In contrast, there was no discernable pattern for the elution order for the TriHOME isomers on the chiral column.
A limitation of the current chromatographic workflow is the long run time of the chiral separation method. While slow elution of compounds was necessary to obtain satisfactory separation, the throughput of the method is limited. An additional drawback of the long chromatographic cycle time is reduced sensitivity as signal/noise decreases with increasing peak width and elution time. Future work should therefore focus on the development of chromatographic methods with reduced elution time. Due to recent progress in supercritical fluid chromatography systems, this technique is potentially promising for chiral TriHOME analysis and could be a worthwhile approach for future studies. In addition, the development of chiral columns with sub 2 μm particle size would increase the efficiency of the separation of the individual isomers and thereby also reduce the required chromatographic cycle time (37, 38).
The applicability of the analytical workflow was demonstrated by analyzing BALF samples from female smokers with or without COPD. Previous analysis of the same samples identified both 9,10,13-TriHOME and 9,12,13-TriHOME as being elevated in female COPD patients compared with smokers with normal lung function (21). However, the applied method was unable to resolve the TriHOME stereoisomers, but instead reported overall concentrations of 9,10,13-TriHOMEs and 9,12,13-TriHOMEs. Those samples were reanalyzed with the current workflow to investigate in detail which TriHOME isomers were responsible for the observed overall increase of TriHOMEs in COPD patients. The results confirmed the previously published findings of elevated TriHOME levels in COPD, with all four 9,10,13-TriHOME and all four 9,12,13-TriHOME enantiomer pairs increasing (Fig. 4). Further analysis of the samples with the chiral LC-MS method revealed consistent ee of TriHOME compounds with the 13(S) configuration over isomers with the 13(R) configuration (Fig. 5). This observation was true for both smokers with normal lung function as well as for COPD patients; however, in COPD patients, this excess was slightly reduced for all compounds, implying a higher rate of autoxidation. A plausible explanation for this observation is that with the onset of COPD, immune cells become activated and release increased amounts of reactive oxygen species (39), which results in increased production of TriHOMEs by autoxidation. In addition, the hypoxia associated with COPD could also increase the oxidative stress and result in increased autoxidation of linoleic acid. While the biosynthetic pathways of TriHOMEs are still uncertain, the ee of 13(S) compounds suggests the involvement of 15-LOX activity in the biosynthesis of TriHOMEs. The enzyme 15-LOX is well-known to enantioselectively hydroxylate linoleic acid at the C-13 position (40, 41), producing only the S-isomer. It is also possible that the observed enantioselectivity could be due to dietary sources because multiple plants produce TriHOMEs enzymatically (9, 12, 13).
In conclusion, the current work describes for the first time a straightforward workflow to identify and characterize all 9,10,13-TriHOME and 9,12,13-TriHOME isomers. Although the method was characterized for the analysis of TriHOMEs in BALF, its application can most likely be extended to other biological fluids. Application of this method to individuals with COPD suggests that TriHOME synthesis is complex and involves both enzymatic and nonenzymatic synthetic routes. Accordingly, this method will not only be useful for understanding the synthetic sources of these compounds, but also for elucidating disease mechanisms. Given the increased consumption of linoleic acid in the diet, there is a need to understand the potential physiological effects of the different TriHOME isomers produced. The developed method can be applied to investigate the biosynthesis of TriHOMEs as well as to increase our understanding of their physiological relevance.
Supplementary Material
Footnotes
Abbreviations:
- BALF
- bronchoalveolar lavage fluid
- CE
- collision energy
- COPD
- chronic obstructive pulmonary disease
- CV
- coefficient of variation
- ee
- enantiomeric excess
- FEV1
- forced expiratory volume in 1 s
- FVC
- forced vital capacity
- IS
- internal standard
- LOD
- limit of detection
- LOQ
- limit of quantification
- 15-LOX
- 15-lipoxygenase
- QC
- quality control
- Rf
- retention factor
- TriHOME
- trihydroxyoctadecenoic acid
- UHPLC
- ultra-HPLC
This work was supported by Swedish Heart-Lung Foundation Grant 20170734, Swedish Research Council Grants 2016-02798, 2017-01142, and 2016-01209, the Stockholm County Council, the Swedish Association for Chest Physicians, the Karolinska Institutet, and the Centre for Allergy Research Highlights Asthma Markers of Phenotype (ChAMP) consortium, which is funded by the Swedish Foundation for Strategic Research, the Karolinska Institutet, AstraZeneca and Science for Life Laboratory Joint Research Collaboration, and the Vårdal Foundation. C.E.W. was supported by Swedish Heart-Lung Foundation Grant 20170603.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Dennis E. A., and Norris P. C.. 2015. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostermann A. I., and Schebb N. H.. 2017. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 8: 2355–2367. [DOI] [PubMed] [Google Scholar]
- 3.Murakami M. 2011. Lipid mediators in life science. Exp. Anim. 60: 7–20. [DOI] [PubMed] [Google Scholar]
- 4.Jahn U., Galano J. M., and Durand T.. 2008. Beyond prostaglandins–chemistry and biology of cyclic oxygenated metabolites formed by free-radical pathways from polyunsaturated fatty acids. Angew. Chem. Int. Ed. Engl. 47: 5894–5955. [DOI] [PubMed] [Google Scholar]
- 5.Gabbs M., Leng S., Devassy J. G., Monirujjaman M., and Aukema H. M.. 2015. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 6: 513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennebelle M., Zhang Z., Metherel A. H., Kitson A. P., Otoki Y., Richardson C. E., Yang J., Lee K. S. S., Hammock B. D., Zhang L., et al. 2017. Linoleic acid participates in the response to ischemic brain injury through oxidized metabolites that regulate neurotransmission. Sci. Rep. 7: 4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuchida M., Miura T., and Miyaki K.. 1972. Identification of trihydroxy-octadecenoates derived from UV irradiated or autoxidized sesame oil and methyl linoleate and mechanism of their formation. J. Jpn. Oil Chem. Soc. 21: 269–274. [Google Scholar]
- 8.Hamberg M. 1991. Regio-and stereochemical analysis of trihydroxyoctadecenoic acids derived from linoleic acid 9-and 13-hydroperoxides. Lipids. 26: 407–415. [DOI] [PubMed] [Google Scholar]
- 9.Hamberg M. 1999. An epoxy alcohol synthase pathway in higher plants: biosynthesis of antifungal trihydroxy oxylipins in leaves of potato. Lipids. 34: 1131–1142. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J-H., Wen W., Hu Y-S., Tang Y., and Yu R-M.. 2012. Hydroxyl octadecenoic acids biosynthesized by crown galls of Panax quinquefolium induced by artermisinic acid. Zhong Yao Cai. 35: 869–872. [PubMed] [Google Scholar]
- 11.Graveland A. 1970. Enzymatic oxidations of linoleic acid and glycerol-1-monolinoleate in doughs and flour-water suspensions. J. Am. Oil Chem. Soc. 47: 352–361. [Google Scholar]
- 12.Göbel C., Feussner I., Hamberg M., and Rosahl S.. 2002. Oxylipin profiling in pathogen-infected potato leaves. Biochim. Biophys. Acta. 1584: 55–64. [DOI] [PubMed] [Google Scholar]
- 13.Ustünes L., Claeys M., Laekeman G., Herman A. G., Vlietinck A. J., and Özer A.. 1985. Isolation and identification of two isomeric trihydroxy octadecenoic acids with prostaglandin E-like activity from onion bulbs. Prostaglandins. 29: 847–865. [DOI] [PubMed] [Google Scholar]
- 14.Püssa T., Raudsepp P., Toomik P., Pällin R., Mäeorg U., Kuusik S., Soidla R., and Rei M.. 2009. A study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J. Food Compos. Anal. 22: 307–314. [Google Scholar]
- 15.Garbe L-A., Hübke H., and Tressl R.. 2005. Enantioselective formation pathway of a trihydroxy fatty acid during mashing. J. Am. Soc. Brew. Chem. 63: 157–162. [Google Scholar]
- 16.Hamberg M. 1991. Trihydroxyoctadecenoic acids in beer: qualitative and quantitative analysis. J. Agric. Food Chem. 39: 1568–1572. [Google Scholar]
- 17.Shirahata T., Sunazuka T., Yoshida K., Yamamoto D., Harigaya Y., Kuwajima I., Nagai T., Kiyohara H., Yamada H., and Ōmura S.. 2006. Total synthesis, elucidation of absolute stereochemistry, and adjuvant activity of trihydroxy fatty acids. Tetrahedron. 62: 9483–9496. [Google Scholar]
- 18.Shirahata T., Sunazuka T., Yoshida K., Yamamoto D., Harigaya Y., Nagai T., Kiyohara H., Yamada H., Kuwajima I., and Ōmura S.. 2003. Total synthesis and adjuvant activity of all stereoisomers of pinellic acid. Bioorg. Med. Chem. Lett. 13: 937–941. [DOI] [PubMed] [Google Scholar]
- 19.Chiba T., Thomas C. P., Calcutt M. W., Boeglin W. E., O’Donnell V. B., and Brash A. R.. 2016. The precise structures and stereochemistry of trihydroxy-linoleates esterified in human and porcine epidermis and their significance in skin barrier function: implication of an epoxide hydrolase in the transformations of linoleate. J. Biol. Chem. 291: 14540–14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanashi H., Boeglin W. E., Morisseau C., Davis R. W., Sulikowski G. A., Hammock B. D., and Brash A. R.. 2018. Catalytic activities of mammalian epoxide hydrolases with cis and trans fatty acid epoxides relevant to skin barrier function. J. Lipid Res. 59: 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balgoma D., Yang M., Sjödin M., Snowden S., Karimi R., Levänen B., Merikallio H., Kaarteenaho R., Palmberg L., Larsson K., et al. 2016. Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD. Eur. Respir. J. 47: 1645–1656. [DOI] [PubMed] [Google Scholar]
- 22.Nording M. L., Yang J., Hegedus C. M., Bhushan A., Kenyon N. J., Davis C. E., and Hammock B. D.. 2010. Endogenous levels of five fatty acid metabolites in exhaled breath condensate to monitor asthma by high-performance liquid chromatography: electrospray tandem mass spectrometry. IEEE Sens. J. 10: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundström S. L., Yang J., Källberg H. J., Thunberg S., Gafvelin G., Haeggström J. Z., Grönneberg R., Grunewald J., van Hage M., Hammock B. D., et al. 2012. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS One. 7: e33780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundström S. L., Levänen B., Nording M., Klepczynska-Nyström A., Sköld M., Haeggström J. Z., Grunewald J., Svartengren M., Hammock B. D., Larsson B-M., et al. 2011. Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS One. 6: e23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haeggström J. Z., and Funk C. D.. 2011. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 111: 5866–5898. [DOI] [PubMed] [Google Scholar]
- 26.Thomas C. P., Boeglin W. E., Garcia-Diaz Y., O’Donnell V. B., and Brash A. R.. 2013. Steric analysis of epoxyalcohol and trihydroxy derivatives of 9-hydroperoxy-linoleic acid from hematin and enzymatic synthesis. Chem. Phys. Lipids. 167–168: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamberg M. 1987. Vanadium-catalyzed transformation of 13 (S)-hydroperoxy-9 (Z), 11 (E)-octadecadienoic acid: structural studies on epoxy alcohols and trihydroxy acids. Chem. Phys. Lipids. 43: 55–67. [Google Scholar]
- 28.Hamberg M. 1971. Steric analysis of hydroperoxides formed by lipoxygenase oxygenation of linoleic acid. Anal. Biochem. 43: 515–526. [DOI] [PubMed] [Google Scholar]
- 29.Caruso T., and Spinella A.. 2003. Cs2CO3 Promoted coupling reactions for the preparation of skipped diynes. Tetrahedron. 59: 7787–7790. [Google Scholar]
- 30.Brown C. A., and Ahuja V. K.. 1973. Catalytic hydrogenation. VI. Reaction of sodium borohydride with nickel salts in ethanol solution. P-2 Nickel, a highly convenient, new, selective hydrogenation catalyst with great sensitivity to substrate structure. J. Org. Chem. 38: 2226–2230. [Google Scholar]
- 31.Forsslund H., Mikko M., Karimi R., Grunewald J., Wheelock Å. M., Wahlström J., and Sköld C. M.. 2014. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest. 145: 711–722. [DOI] [PubMed] [Google Scholar]
- 32.Choque B., Catheline D., Rioux V., and Legrand P.. 2014. Linoleic acid: between doubts and certainties. Biochimie. 96: 14–21. [DOI] [PubMed] [Google Scholar]
- 33.Vangaveti V. N., Jansen H., Kennedy R. L., and Malabu U. H.. 2016. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 785: 70–76. [DOI] [PubMed] [Google Scholar]
- 34.Ramsden C. E., Domenichiello A. F., Yuan Z. X., Sapio M. R., Keyes G. S., Mishra S. K., Gross J. R., Majchrzak-Hong S., Zamora D., Horowitz M. S., et al. 2017. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci. Signal. 10: eaal5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moghaddam M. F., Grant D. F., Cheek J. M., Greene J. F., Williamson K. C., and Hammock B. D.. 1997. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 3: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato T., Yamaguchi Y., Abe N., Uyehara T., Namai T., Kodama M., and Shiobara Y.. 1985. Structure and synthesis of unsaturaded trihydroxy c18 fatty: acids in rice plants suffering from rice blast disease. Tetrahedron Lett. 26: 2357–2360. [Google Scholar]
- 37.Lemasson E., Bertin S., and West C.. 2016. Use and practice of achiral and chiral supercritical fluid chromatography in pharmaceutical analysis and purification. J. Sep. Sci. 39: 212–233. [DOI] [PubMed] [Google Scholar]
- 38.Ciogli A., Ismail O. H., Mazzoccanti G., Villani C., and Gasparrini F.. 2018. Enantioselective ultra high performance liquid and supercritical fluid chromatography: the race to the shortest chromatogram. J. Sep. Sci. 41: 1307–1318. [DOI] [PubMed] [Google Scholar]
- 39.Babior B. M., Kipnes R. S., and Curnutte J. T.. 1973. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soberman R. J., Harper T. W., Betteridge D., Lewis R. A., and Austen K. F.. 1985. Characterization and separation of the arachidonic acid 5-lipoxygenase and linoleic acid omega-6 lipoxygenase (arachidonic acid 15-lipoxygenase) of human polymorphonuclear leukocytes. J. Biol. Chem. 260: 4508–4515. [PubMed] [Google Scholar]
- 41.Wecksler A. T., Kenyon V., Deschamps J. D., and Holman T. R.. 2008. Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation. Biochemistry. 47: 7364–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.