Abstract
Overexpression and/or mutations of the receptor tyrosine kinase (RTK) subfamilies, such as epidermal growth factor receptors (EGFRs) and vascular endothelial growth factor receptors (VEGFRs), are closely associated with tumor cell growth, differentiation, proliferation, apoptosis, and cellular invasiveness. Monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs) specifically inhibiting these RTKs have shown remarkable success in improving patient survival in many cancer types. However, poor response and even drug resistance inevitably occur. In this setting, the ability to detect and visualize RTKs with noninvasive diagnostic tools will greatly refine clinical treatment strategies for cancer patients, facilitate precise response prediction, and improve drug development. Positron emission tomography (PET) agents using targeted radioactively-labeled antibodies have been developed to visualize tumor RTKs and are changing clinical decisions for certain cancer types. In the present review, we primarily focus on PET imaging of RTKs using radiolabeled antibodies with an emphasis on the clinical applications of these immunoPET probes.
Keywords: Cancer, immunoPET, receptor tyrosine kinases (RTKs), molecular imaging, response prediction, personalized medicine
Introduction
Due to their complex heterogeneity and tendency to spread throughout the body, treating cancers is very challenging. Over the past quarter century, progress in cancer biology has led to the discovery and identification of receptor tyrosine kinases (RTKs) which control many fundamental cell behaviors and drive tumor initiation, maintenance, and progression (1). These discoveries have fueled the development of effective targeted therapeutics such as monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs), shifting cancer patients’ care from traditional empirical treatments to an era of personalized treatment.
Human RTKs contain 20 subfamilies and only approximately half of the RTK families are well-understood (2). RTKs are anchored in the cytoplasmic membrane, and gain-of-function mutations and/or overexpression of these RTKs are closely related to growth and proliferation in malignant tissues (2). Of them, vascular endothelial growth factor A and VEGFRs have implicated roles in tumor angiogenesis, and visualization of VEGFR expression in vivo with a radiopharmaceutical may be a viable clinical option for imaging angiogenesis (3). The epidermal growth factor receptor (EGFR) belongs to another family of RTKs and includes three other members (erbB2/HER-2, erbB3/HER-3, and erbB4/HER-4). We previously showed that HER-kinase-targeted imaging agents would enable maximum benefit (i.e., patient stratification, therapeutic response monitoring and new drug development) in cancer patient management (4). c-Met, another RTK for hepatocyte growth factor (HGF), has been found overexpressed or aberrantly activated in a variety of cancers (5).
Although mAbs targeting the above-mentioned RTKs have become a standard of care for patients with certain mutation-positive tumors, the efficacy of the currently-approved mAbs as single agents is very limited; therefore, synergistic regimens containing conventional chemotherapeutic agents and the mAbs are applied (6). In addition, drug resistance almost invariably occurs in cancer patients treated with these therapeutic antibodies. Traditionally, biopsy and immunohistochemistry are performed to determine the RTK status of cancer tissues and to guide subsequent treatment. However, spatial expression levels of RTKs can vary over time and among lesions (1), indicating the urgent need for novel noninvasive approaches to visualize RTKs’ plasticity throughout the whole body. Moreover, noninvasive methods that can select patients who will potentially benefit from combinational therapy and predict treatment response will greatly optimize management strategies for cancer patients.
In recent years, molecular imaging has rapidly developed and has been widely used in the clinical setting and in preclinical arenas (7–9). Single photon emission computed tomography (SPECT) and positron emission tomography (PET) are radionuclide molecular imaging techniques that enable noninvasive evaluation of biochemical changes and expression of molecular targets within living subjects. Although a SPECT probe, 111In-labeled trastuzumab, has been used in clinical trials to detect HER2-positive metastatic breast cancer (10), the sensitivity of SPECT is several orders of magnitude lower than PET (11). In comparison, PET imaging is of high sensitivity and can be used to investigate physiological and molecular mechanisms of human diseases (11). PET imaging of RTKs with radiolabeled mAbs, denoted as immunoPET, may provide a noninvasive method for assessing the dynamics of RTKs, selecting patients for personalized treatment, predicting response to RTK inhibition therapy, and facilitating drug development (7).
Most PET imaging clinical trials have been focused on using radiolabeled FDA-approved antibodies (12), such as trastuzumab for breast cancer and esophagogastric adenocarcinoma (EGA) (13–17), cetuximab for colorectal and lung cancer (18–20), and bevacizumab for several indications (21–26). PET imaging using the positron emitters 64Cu (t1/2, 12.7 h) and 89Zr (t1/2, 78.4 h) for antibody labeling has been extensively studied over the last decade (8,27). 89Zr has a longer half-life, which generally matches the serum half-life of most mAbs and therefore is suitable for antibody imaging (28,29). A straightforward strategy for generating RTK-specific PET radiotracers has mostly, but not entirely, involved the radiolabeling of therapeutic mAbs. Antibody fragments, nanobodies, and smaller molecule inhibitors have also been investigated for in vivo visualization of RTKs. With shorter biological half-lives, these probes are well suited for fast imaging protocols when labeled with rapidly-decaying radioisotopes such as 68Ga (t1/2, 68 min) and 18F (t1/2, 110 min). Our review will focus mainly on the current research in the field of antibody-based immunoPET probes and highlight potential clinical implications of immunoPET in visualizing RTKs and in guiding clinical decisions (Figure 1).
Figure 1.
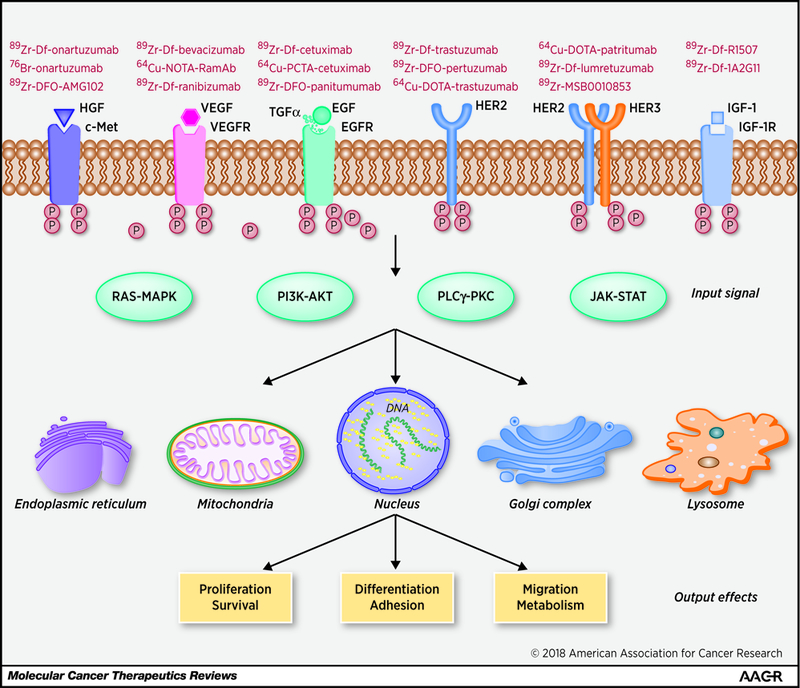
Receptor tyrosine kinases (RTKs) are deregulated in most human cancers and control many fundamental cell behaviors by regulating biochemical signals. Representative antibody-based positron emission tomography (PET) probes targeting corresponding RTKs (or pathways) are shown. MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3-kinase; JAK, Janus kinase; PKC, protein kinase C; PLCγ, phospholipase C‑γ; STAT, signal transducer and activator of transcription.
PET imaging of the HGF/c-Met pathway
Dysregulation of HGF/c-Met signaling is implicated in a number of malignancies (30), and synergistic effects of EGF and HGF were noted in non-small cell lung cancer (NSCLC) (31). Moreover, c-Met amplification can drive HER3-dependent PI3K signaling, thereby mediating NSCLC resistance to EGFR inhibitors (32,33). Currently, c-Met targeted therapy involves small molecule inhibitors (crizotinib, capmatinib, etc.) (31,34), and antibodies (emibetuzumab, ficlatuzumab, rilotuzumab, onartuzumab, etc.) (35,36). Phase II trials in patients with advanced NSCLC demonstrated that the addition of onartuzumab to the EGFR inhibitor erlotinib resulted in an improvement in progression-free and overall survival for c-Met-positive patients (37); however, a Phase III study failed to observe the additive therapeutic effect of onartuzumab to erlotinib (38). Despite these disappointing results, suppressing the HGF/c-Met pathway may still have an anti-tumor effect in other primary tumors and/or overcome the resistance of molecularly targeted therapies (30,39). Noninvasive PET visualization of c-Met dynamics could therefore potentially facilitate patient stratification and guide c-Met-directed therapies.
Initial attempts to develop nuclear medicine imaging probes for detecting c-Met expression used murine mAbs (40,41). Based on these studies, taking the step to evaluate immunoPET probes using humanized or fully human anti-c-Met mAbs is relatively straightforward. Using the humanized therapeutic mAb onartuzumab which inhibits HGF binding and therefore the HGF/MET signaling pathway (35), Jagoda et al. initially synthesized 89Zr-DFO-onartuzumab and 76Br-onartuzumab, and found that the former probe exhibited higher uptake and tumor-to-muscle ratios in MKN-45 gastric carcinoma models (42). Li et al. developed c-Met-targeting bivalent cys-diabodies and radiolabeled one of the candidates with 89Zr. The authors found that uptake of 89Zr-labeled H2 cys-diabody was higher in the gefitinib-resistant NSCLC model than in the low c-MET-expressing NSCLC model, indicating that immunoPET could be used to assess c-MET expression levels for both therapeutic and diagnostic purposes (43). Pool et al. then reported that the readily-translatable probe 89Zr-Df-onartuzumab could effectively discriminate erlotinib-induced c-Met upregulation in NSCLC models (44). This study herein highlighted the potential value of 89Zr-Df-onartuzumab PET in assessing c-Met upregulation-mediated erlotinib resistance in patients with NSCLC. More importantly, c-Met-directed treatment using NVP-AUY-922, a heat shock protein 90 (HSP90) inhibitor (45), could reduce 89Zr-Df-onartuzumab uptake in HCC827 models (Figure 2A). Rilotumumab (AMG102) is a HGF-binding antibody and can bind to and neutralize HGF, thus preventing its binding to c-Met. However, a Phase III trial failed to observe the therapeutic effects of rilotumumab in patients with c-Met-positive gastric or gastro-oesophageal adenocarcinoma (46). Previously, there were no tools to noninvasively determine the levels of HGF present in the local tumor microenvironment. To this end, 89Zr-DFO-AMG102 was developed by Price et al, and this probe could selectively accumulate in tumors with high levels of HGF protein (Figure 2B, C) (47).
Figure 2.

Representative c-Met and VEGF pathway-specific PET probes. (A) 89Zr-Df-onartuzumab is a c-Met targeting probe. While in vehicle-treated HCC827 xenografts uptake of 89Zr-Df-onartuzumab did not differ before treatment and after treatment (left), the corresponding uptake of the radiotracer in the NVP-AUY-922-treated mice decreased more than 30% after treatment (right), indicating that 89Zr-Df-onartuzumab PET may provide a powerful tool for visualizing c-Met dynamics. (B) 89Zr-DFO-AMG102 is another HGF/c-Met signaling pathway-specific PET probe. PET maximum intensity projection (MIP) images in HGF and MET double-positive U87MG tumor models at 24 h and 120 h after injection of 89Zr-DFO-AMG102. (C) Corresponding MIP images in MKN45 tumor models which had negative HGF and positive MET in the local tumor microenvironment. These results imply that 89Zr-DFO-AMG102 may act as a companion diagnostic tool for selection of patients more likely to respond to any HGF-targeted therapy. (D) 89Zr-Df-bevacizumab PET MIP image before everolimus treatment showed multiple local and distant metastases from a left kidney tumor. 89Zr-Df-bevacizumab PET demonstrated that tumor uptake decreased significantly after 6 weeks of treatment using everolimus. (E) In vivo performance of 89Zr-Df-bevacizumab PET in the diagnosis and localization of diffuse intrinsic pontine glioma. Gadolinium-enhanced T1 MR images (upper panel) showed the enhanced primary pontine glioma and metastatic gliomas (white arrows) in the right ventricular trigone and cervicomedullary junction, and all these glioma lesions were clearly visualized by 89Zr-Df-bevacizumab PET (lower panel). Panels reproduced with permission from ref. 26, 47, 59, © SNMMI and ref. 44, © Springer.
Our team produced a recombinant human HGF and labeled the agent with 64Cu. PET imaging revealed specific and prominent uptake of the tracer in c-Met–positive U87MG tumors but significantly lower uptake in c-Met-negative MDA-MB-231 tumors (48). One concern for these tracers based on the HGF ligand is their potential to stimulate tumor growth by activating c-Met (48). Burggraaf et al. initially developed a fluorescently-labeled peptide (GE-137) for optical imaging of c-Met, and this probe showed high affinity for human c-Met in 15 subjects with high risk of colorectal cancer (49). In addition to optical imaging in assessing c-Met (49,50), 18F-AH113804 is a peptide-based c-Met-specific PET imaging probe which has been used to visualize locoregional recurrence of breast cancer in a clinical trial (51).
These results suggest that HGF/c-Met specific PET imaging may correctly identify patients most likely to benefit from c-Met-targeted therapies or from EGFR inhibition therapy, as it has been reported that c-Met is implicated in acquired resistance to EGFR inhibitors in NSCLC (31,33).
PET Imaging of VEGF/VEGFR pathway
The VEGF/VEGFR signaling pathway plays an important role in the regulation of angiogenesis. VEGFs bind to three associated transmembrane RTKs known as VEGFR-1, VEGFR-2, and VEGFR-3. Among them, VEGFR-2 is a key receptor involved in the development of blood vasculature and is an attractive target for antiangiogenic tumor therapy (52). Approved antiangiogenic drugs such as bevacizumab, lenvatinib, sorafenib, sunitinib, and pazopanib target this pathway and are associated with modest survival advantages in certain kinds of cancers (53). Bevacizumab and ramucirumab are IgG1 antibodies directed against vascular endothelial growth factor A (VEGF-A) and VEGFR2, respectively. To date, clinical PET studies using 89Zr-Df-bevacizumab were performed in patients with breast cancer, neuroendocrine tumors, renal cell carcinoma (RCC), NSCLC, and glioma (21,22,26,54,55).
Based on fundamental preclinical studies (3,9,56,57), in a clinical feasibility study Gaykema et al. demonstrated that 89Zr-Df-bevacizumab could be used to detect primary breast cancer (22). Another pilot clinical study in patients with metastatic renal cell carcinoma demonstrated that 89Zr-Df-bevacizumab PET visualized renal tumor lesions with striking heterogeneity between lesions, therefore reflecting differences in vascular characteristics (24). Everolimus, an inhibitor of mammalian target of rapamycin (mTOR), also inhibits VEGF-A expression, but there are not reliable biomarkers to predict efficacy of everolimus in patients with metastatic RCC at the moment. van Es et al. investigated the value of 89Zr-Df-bevacizumab as a tool to identify everolimus efficacy in thirteen patients with metastatic RCC and reported that everolimus decreased 89Zr-Df-bevacizumab tumor uptake in ten patients who received continuous everolimus treatment and achieved stable disease at 3 months (Figure 2D) (26). Jansen et al. reported that 89Zr-Df-bevacizumab had poor uptake in mice bearing diffuse intrinsic pontine glioma (DIPG) (23), implying that treatment with bevacizumab in DIPG patients is justified only after 89Zr-Df-bevacizumab immunoPET demonstrates positive VEGF expression in tumor tissue. In spite of this disappointing preclinical data, the same team further reported that, in children with DIPG, five of seven primary tumors showed focal 89Zr-Df-bevacizumab uptake while no significant uptake was seen in the healthy brain (58). A further study by Veldhuijzen van Zanten et al. reported that 89Zr-Df-bevacizumab uptake was correlated with microvascular proliferation in a patient with DIPG and highlighted that 89Zr-Df-bevacizumab PET could be used to delineate intra-lesional heterogeneity (Figure 2E) (59). These results indicate that 89Zr-Df-bevacizumab immunoPET is feasible in children with DIPG and may help select patients with the greatest chance of benefit from bevacizumab treatment. Notably, 89Zr-Df-bevacizumab could also offer a tool to refine clinical management of patients with von Hippel-Lindau disease (25), and neuroendocrine tumors (55). From a clinical perspective, a patient’s baseline plasma VEGF-A level is an independent prognostic factor for certain cancer types (60), and resistance to antiangiogenic therapies ultimately leads to patients’ relapse and poor survival (53,61). Therefore, it would be significant to develop personalized therapeutic strategies through immunoPET imaging of angiogenesis biomarkers that might help select proper patients and reflect the response of tumor cells to antiangiogenic drugs.
Ranibizumab is a mAb Fab derivative of bevacizumab and has a higher affinity for all soluble and matrix-bound human VEGF-A isoforms than bevacizumab (62). Nagengast et al. initially created 89Zr-Df-ranibizumab and reported that VEGF PET imaging using 89Zr-Df-ranibizumab allowed serial analysis of angiogenic changes in ovarian tumor models following treatment with the kinase inhibitor sunitinib (63). In addition, several studies have been performed to image tumor vasculature by visualizing VEGFR-2. Meyer and coauthors engineered and radiolabeled single-chain VEGF and further validated the feasibility of imaging VEGFR-1 and VEGFR-2 in an orthotopic murine tumor model (64). We synthesized and characterized a ramucirumab-based PET imaging agent, 64Cu-NOTA-RamAb, for mapping VEGFR-2 expression in vivo (65), and found that this technology could visualize VEGFR-2 in nude mice bearing HCC4006 and A549 NSCLC tumor models. Based on our results, a study from Eric et al. further suggested that a three-time-point method could be used to assess the in vivo performance of 64Cu-NOTA-RamAb (66).
PET imaging of HER2
The human epidermal growth factor receptor 2 (HER2) transmembrane oncoprotein is overexpressed in many human tumors, and several HER2-targeting agents have entered clinical practice (4). However, despite this success, responses to these antibodies and small molecules have been hampered by resistance or poor responses. In this setting, molecular PET imaging techniques can help select proper patients for subsequent therapy and elucidate the underlying resistance mechanisms (4,67–69).
Trastuzumab was the first clinically-approved anti-HER2 antibody for breast cancer patients. In preclinical studies, trastuzumab has been used as a targeting agent to map HER2 expression and distribution either by PET imaging or by SPECT imaging (4,13,70,71). In addition, 89Zr-Df-trastuzumab PET, rather than 18F-FDG PET, successfully assessed the pharmacodynamic effects of afatinib (an EGFR-HER2 dual inhibitor) in HER2-positive gastric cancer models (Figure 3A, B) (72). In clinical practice, HER2 status has been determined by immunohistochemistry and fluorescence in situ hybridization has been validated to predict the efficacy of the HER2-targeting antibody-drug conjugate trastuzumab emtansine. Since SPECT imaging using 111In-trastuzumab discovered new HER2-positive lesions in 13 of 15 patients with breast cancer (10), Dijkers et al. developed 89Zr-Df-trastuzumab (13), and reported that PET imaging using this probe in breast cancer patients was able to detect both previously-known metastatic lesions and lesions which had been previously undetected (73). Recently, 89Zr-Df-trastuzumab PET has been investigated as a useful tool to predict response in breast cancer patients treated with trastuzumab emtansine, and to improve the understanding of tumor heterogeneity in breast cancers (14). Indeed, the heterogeneity within and across tumor lesions in a single patient or among different patients is increasingly being recognized, with significant therapeutic implications (74). In support of this concept, Ulaner et al. demonstrated that 89Zr-DFO-trastuzumab PET/CT detected unsuspected HER2-positive metastases in patients with HER2-negative primary breast cancer (15,75). It is quite difficult for conventional biopsy techniques, which generally obtain samples from a limited number of lesions, to identify these unique cohorts. This exciting finding may facilitate better management of HER2-negative breast cancer patients, as about 10%−15% of patients with HER2-negative primary breast cancer may still benefit from HER2-targeted therapy (76). Furthermore, early changes in 89Zr-Df-trastuzumab uptake in breast cancer metastases following treatment with heat shock protein 90 inhibitor NVP-AUY922 correlated with changes of lesion size measured on CT images (77). In addition to 89Zr-labeled trastuzumab, 64Cu-DOTA-trastuzumab is another alternative for optimizing trastuzumab treatment (16).
Figure 3.

Representative HER2-, EGFR- and HER3- targeting PET probes and their potential clinical applications. (A) 89Zr-Df-trastuzumab PET specifically imaged HER2-positive NCI-N87 tumors (red circle). On the contrary, 18F-FDG and 18F-FLT PET nonspecifically detected both the HER2-positive NCI-N87 tumor (red circle) and HER2-negative MKN-74 tumor (black circle). (B) Afatinib therapy in HER2-positive xenograft models reduced uptake of 89Zr-Df-trastuzumab in a time-dependent manner, justifying the potential value of 89Zr-trastuzumab PET in measuring the pharmacodynamic effects of afatinib in the clinical setting. (C) 64Cu-PCTA-cetuximab PET imaging clearly visualized EGFR-positive TE-8 xenograft models (white dotted circle). (D) 177Lu-cetuximab radioimmunotherapy, rather than saline or cetuximab treatment, markedly reduced 18F-FDG uptake in TE-8 models. (E) 89Zr-Df-lumretuzumab is a HER3 specific immunoPET probe. PET/CT scanning performed 4 d after injection of 89Zr-lumretuzumab detected one lung metastasis (white arrow) from ampullary cancer. Panels reproduced with permission from ref. 72, 99, © SNMMI and ref. 115, © AACR.
HER2 is overexpressed in EGA and trastuzumab has been approved by the FDA to treat EGA patients with positive HER2 expression (78). Considering the fact that only a subset of patients with HER2-positive EGA respond to trastuzumab (79), O’Donoghue et al. studied the value of 89Zr-DFO-trastuzumab in assessing HER2 status in primary and metastatic EGA (17), and reported that this imaging agent detected local and metastatic lesions in 80% of the imaged patients. This study indicated that 89Zr-DFO-trastuzumab PET has superior advantages over single-site biopsy, because this noninvasive imaging technology can assess variation in levels of HER2 and target engagement in both the primary and metastatic tumor lesions simultaneously. Notably, bifunctional chelators have certain impact on the in vitro stability and in vivo performance of 89Zr-labeled trastuzumab and antibody-drug conjugates (80).
Pertuzumab is another HER2-targeting monoclonal antibody approved by the FDA after a survival benefit was achieved for patients with HER2-positive metastatic breast cancer. Importantly, pertuzumab binds to a HER2 binding site distinct from that of trastuzumab, augmenting the binding and treatment efficacy of each other (81). Thus, it is of great importance to noninvasively detect the biodistribution of pertuzumab-specific binding sites when synergistic regimens containing trastuzumab and pertuzumab are given for breast cancer patients. In this setting, Marquez et al. conducted an in vivo study in breast cancer models using 89Zr-DFO-pertuzumab and observed significant tracer uptake in MDA-MB-231 xenografts (82). More importantly, pretargeting strategies using unlabeled trastuzumab could further enhance 89Zr-DFO-pertuzumab accumulation in tumors. Combination treatments with trastuzumab and pertuzumab have also shown enhanced antitumor effects in xenograft models of human ovarian cancer (83), and the addition of pertuzumab to chemotherapy in patients with platinum-resistant ovarian carcinoma demonstrated favorable trends in progression-free survival (84). Based these preclinical and clinical data, our group synthesized 64Cu-NOTA-pertuzumab and broadened the application of the probe to detect both subcutaneous and orthotopic ovarian cancer models (85). These solid data demonstrated that 64Cu-NOTA-pertuzumab/89Zr-DFO-pertuzumab are effective tools for imaging HER2 expression. More recently, a first-in-human study in patients with HER2-positive breast cancer showed that 89Zr-DFO-pertuzumab PET/CT was safe and demonstrated optimal imaging 5–8 days post-administration. Additionally, 89Zr-DFO-pertuzumab PET/CT was able to image multiple sites of HER2-positive malignancy including HER2-positive brain metastases (86). Further clinical studies are still needed to validate the efficacies of these two probes, especially in improving patient stratification.
Compared with intact antibodies, antibody fragments and single-domain antibodies (sdAbs) have superior imaging characteristics, such as rapid clearance, high target-to-background ratios, reduced radiation dose, and engineered sites for site-specific conjugation (87,88). However, compared with clinically-available antibodies, the immune responses, safety profiles, and efficacy of these novel sdAbs in humans are largely unknown. Positron emitter-labeled nanobodies as probes for evaluating HER2 status have been reported (89–92). One strength of such probes, for example, [18F]RL-I-5F7 and [18F]RL-I-2Rs15d (89,90), is their ability to rapidly cross the intact blood-brain barrier and detect brain metastases from breast cancers one hour after injection of the tracers. In comparison, the best time points for detecting brain metastases using either 89Zr-Df-trastuzumab or 89Zr-DFO-pertuzumab were 4–5 days for the former tracer and 5–8 days for the latter following tracer administration (73,86). Another strength is the higher tumor-to-blood and tumor-to-muscle ratios which will result in higher contrast PET imaging (89,93). A phase I study from Keyaerts et al. showed PET imaging using a 68Ga-HER2-nanobody is a safe procedure and tracer accumulation in HER2-positive breast carcinoma metastases is high which warrants further assessment in a phase II trial (94).
PET imaging of EGFR (HER1)
EGFR and its relatives (i.e., ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4) are well-known oncogenic drivers in several types of cancers such as lung cancer, breast cancer, and glioblastoma. EGFR was among the first RTKs for which the ligand binding mechanism was studied (95). Inhibitors for this specific RTK, including antibody therapeutics (e.g., cetuximab and panitumumab) and small-molecule TKIs (e.g., erlotinib, gefitinib, lapatinib and osimertinib), have been the most successful examples of molecularly-targeted therapies in cancers (95,96).
Cetuximab is a chimeric EGFR-specific IgG1 mAb and functions by preventing ligand activation and receptor dimerization (97). It was shown in a phase I first-in-human study that 89Zr-Df-cetuximab was safe and well-tolerated (20). PET imaging using 89Zr-labeled cetuximab may also select NSCLC patients, head and neck cancer patients, and colorectal cancer patients who will potentially benefit from cetuximab treatment (18,19,98). In recent years, theranostic approaches using mAbs have represented a rapidly expanding component of cancer treatments (12). Song et al. prepared 64Cu/177Lu-labeled versions of cetuximab and assessed the theranostic efficacy in an esophageal squamous cell carcinoma model (99). The authors elaborately found that 64Cu-PCTA-cetuximab immunoPET evaluated EGFR expression levels in tumors, and 177Lu-cetuximab radioimmunotherapy effectively inhibited tumor growth much more thoroughly than that of cetuximab alone (Figure 3C, D). Thus, the pair of 64Cu/177Lu-cetuximab may provide a tailored treatment strategy for EGFR-positive patients by immunoPET imaging of EGFR expression and by selective therapeutic radiation delivery.
Panitumumab is a fully humanized mAb that binds to EGFR and is FDA-approved for use in receptor-expressing colorectal cancers without KRAS mutations (100). Wei et al. synthesized 89Zr-DFO-panitumumab and this probe accumulated in various subcutaneous athymic nude female xenograft models (101). Lindenberg et al. then calculated the maximum dosing for effective imaging with 89Zr-DFO-panitumumab in three patients, and results from this study indicated that injection of approximately 1 mCi (37 MBq) of 89Zr-DFO-panitumumab intravenously was safe and detected lung metastases from colorectal cancer, but imaging of the included three patients failed to demonstrate significant radiotracer accumulation in tumors for all time points and failed to detect tumors in the liver due to physiologic hepatic excretion of the probe (102). Future studies may add unlabeled panitumumab to optimize the radiotracer’s uptake in tumors (103). More recently, imgatuzumab (GA201), a novel humanized anti-EGFR IgG1 isotype mAb, has been found to efficiently inhibit EGFR pathway activation in head and neck cancer patients and may serve as a potential probe for visualizing EGFR-expressing tumors after radiolabeling with isotopes (104,105).
Repebody, a novel non-antibody protein scaffold (106), has also been engineered to monitor EGFR expression (107,108). One advantage of repebody-based PET imaging probes is their small molecular weight (~30 kDa), which may enable early visualization of various EGFR-expressing solid tumors as early as 1 h after injection of the probes. Furthermore, due to superior circulation clearance and tissue penetration, those probes could be labeled with positron emitters with shorter half-lives, such as 64Cu (t1/2, 12.7 h) and 18F (t1/2, 110 min) (109).
As we previously reviewed (110), PET imaging using bispecific peptide- and antibody-based heterodimers may demonstrate higher targeting efficacy and specificity than their monospecific peers. We generated a bispecific immunoconjugate (denoted as Bs-F(ab)2) by linking a cetuximab Fab and a TRC105 Fab (targeting CD105), and confirmed that 64Cu-NOTA-Bs-F(ab)2 had a synergistic improvement on both affinity and specificity in visualizing small U87MG tumor nodules (<5 mm in diameter) (111). Similar results were reported by Kwon et al. who developed an immunoconjugate targeting HER2 and EGFR and verified its diagnostic efficacy in breast cancer models (112), and by Mahmood et al. who prepared PET probes specific to EGFR and HER3 and monitored resistance to phosphatidylinositol-3 kinase (PI3K) and protein Kinase B (PKB, also known as Akt) inhibitors using breast cancer models as well (66).
PET imaging of HER3
Unlike other members of the family, HER3 has relatively weak kinase activity but still can form highly activated heterodimers with EGFR and HER2. Activating mutations in HER3 have been identified in multiple cancer types and therefore HER3 is now being examined as a direct therapeutic target (113). Preclinical and clinical PET imaging has also been developed using anti-HER3 antibodies, such as lumretuzumab (114,115), patritumab (116), and mAb3481 (117), or using peptides (118), and affibodies (119–121). In a pilot clinical trial which included eleven participants, Lockhart et al. reported than although administration of 64Cu-DOTA-patritumab is safe, the diagnostic efficacy was limited in solid tumors (116). Comparatively, 89Zr-Df-lumretuzumab PET could visualize intra- and inter- patient tumor heterogeneity and target accessibility, and the most optimal PET conditions were found to be 4 and 7 days after administration of 89Zr-Df-lumretuzumab with 100 mg cold antibody (Figure 3E) (115). In the absence of side effects of lumretuzumab in treating HER3-expressing solid tumors (122), 89Zr-Df-lumretuzumab may have potential value in guiding lumretuzumab treatment of patients with HER3-positive solid tumors. More recently, Warnders et al. constructed a HER3 signaling specific probe-89Zr-MSB0010853, and reported that 89Zr-MSB0010853 PET could detect HER3-overexpressing H441 NSCLC cancer models in vivo (123). Although development of therapeutic HER3 antibodies is still in an early phase (122,124), these preliminary results shed light on the feasibility of noninvasive HER3-specific PET imaging for mapping HER3 expression and distribution of HER3 antibodies.
PET imaging of other RTKs
Apart from the above RTKs, there are 17 more RTK subfamilies and nearly half of these RTK families are poorly understood (2). One example is the platelet-derived growth factor receptor-alpha (PDGFR-α), which has been reported to be involved in tumor angiogenesis and maintenance of several cancer types including thyroid cancer (125–127). Importantly, PDGFR-α is associated with lymph node metastases in papillary thyroid carcinoma (PTC) (127). Michael et al. described a PDGFRα-specific immunoPET probe - 64Cu-NOTA-D13C6 - for PTC and demonstrated that PET imaging using this agent could delineate PDGFRα expression in PTC models in vivo and could therefore potentially serve as a novel and promising radiotracer for imaging PDGFRα positive metastasis from PTC (128). Another example is the insulin-like growth factor-1 receptor (IGF-1R), which has a well-established role in malignant tumors and has become a promising target for cancer therapy and imaging (129,130). As R1507 is a mAb directed against IGF-1R, Heskamp et al. developed 111In-R1507 and 89Zr-Df-R1507 and reported that both of the tracers could be applied to noninvasively determine IGF-1R expression in vivo in breast cancer xenografts (131). Our group also developed an IGF-1R-specific PET probe (denoted as 89Zr-DFO-1A2G11) and demonstrated highly specific uptake of the tracer in IGF-1R positive pancreatic tumor models (132), implying the potential value of this probe in identifying patients that may benefit from anti-IGF-1R therapy.
In addition, RTKs are important drug targets, and several small molecule inhibitors have been developed and approved by the FDA in addition to mAbs. Actually, development of radiolabeled TKIs and their analogues is under preclinical and clinical translational research (133), and various radiolabeled TKIs have been developed to image tumors in both preclinical and clinical studies for several RTKs, such as EGFR (134,135), HER2 (136), VEGFR (137), tropomyosin receptor kinase (Trk) (138), stem cell growth factor receptor (SCFR) (139), and IGF-1R (129,140,141).
Conclusion and future perspectives
Currently, the role of spatial deregulation of RTKs in tumorigenesis may be vastly underappreciated, and the development of high-resolution immunoPET techniques for detecting RTK activity in normal and tumor tissues will be essential for studying the role of spatial RTK patterning in the heterogeneous tumor environment. In this state-of-the-art review, we presented typical examples of deregulated RTKs in cancers and summarized strategies to prepare noninvasive PET imaging probes specific for these targets. The current review demonstrates that noninvasive immunoPET could accurately delineate RTK status within a tumor or across multiple tumors within a patient. Visualization of RTK phenotype using immunoPET could facilitate selection of patients for targeted therapies and response assessment/prediction. In addition, a greater understanding of the spatial expression of RTKs with the help of immunoPET could affect ongoing efforts to develop therapeutic drugs targeting RTKs.
In 2017, the development of antibody therapeutics proceeded at a fast pace, and this is expected to continue in the coming years. Several RTK-targeting therapeutic antibodies (such as margetuximab, (vic-)trastuzumab duocarmazine, and DS-8201 for HER2, and depatuxizumab mafodotin for EGFR) are undergoing evaluation in late-stage clinical studies of patients with cancer (142). For mAb-based immunoPET probes, 89Zr is utilized in clinical trials much more extensively than any other radiometals. DFO and DFO-based bifunctional chelators are the most commonly-employed chelators for 89Zr-radiolabeling (143), and there are two simple protocols that can be followed to do 89Zr radiolabeling (144,145). However, DFO is not an optimal chelator for 89Zr coordination because of 89Zr transchelation, which will cause high bone uptake of 89Zr. This was exemplified by a recent study by Vugts et al., in which the authors reported that, when compared to DFO, the bifunctional isothiocyanate variant of desferrioxamine (denoted as octadentate DFO*) was advantageous for 89Zr labeling because 89Zr-DFO*-trastuzumab had enhanced stability in in vitro studies and superior performance in in vivo studies over 89Zr-DFO-trastuzumab (80). Therefore, there is still room for future studies to optimize radiolabeling strategies when developing 89Zr-immunoconjugates (143). With the advent and maturation of click chemistry (146), future studies may further harness this powerful method to synthesize and assess antibody-based PET imaging probes with improved in vivo performance. In addition to singly-targeted imaging probes, antibody-based heterodimers or dual-targeting probes may have higher targeting efficacy and superior specificity than their corresponding monospecific peers (110,147).
Generally, adequate tissue penetration ability, which is inversely proportional to the size of an imaging probe, and high signal-to-noise ratio, which is closely related to the circulation of a probe, are two important properties of an immunoPET probe. sdAbs, also known as nanobodies or heavy chain–only antibodies bearing a variable region (VHH), hit the sweet spot and have emerged as substitutes for their full-size counterparts in diagnostic or therapeutic applications (87). Enzymatic methods such as sortase have been used to append metal chelators to enable labeling sdAbs with 64Cu or 89Zr (148,149). Since a first-in-human trial demonstrated the success of 68Ga-HER2-Nanobody in a clinical setting (94), and it is easier for immunoPET probes derived from sdAbs to traverse the blood brain barrier (90), future studies may further design noninvasive means to detect RTKs through the use of sdAbs (150).
In the development of antibody-based diagnostic, therapeutic or theranostic probes, we should pay attention to the fact that the immunodeficient strains of animals we use in preclinical studies may impact the in vivo fate and performance of the investigated immunoPET probes. For example, a recent study reported that immunoPET radiotracers had inefficient tumor targeting and high off-target binding to the spleen in highly immunodeficient mouse models (151). This was consistent with a previous study which reported that antibody-drug conjugates had limited antitumor activity in NSG mice (152). In this setting, pretargeting strategies may also be harnessed for optimizing both the imaging quality and therapy effects in the coming future (151,153,154).
Besides assessing RTKs and selecting proper patients for subsequent molecularly-targeted therapy using either small molecules or antibodies, noninvasive imaging of RTKs could facilitate image-guided radionuclide therapy. Actually, therapy of B-cell lymphomas with radiolabeled mAbs has produced impressive clinical results since two radiolabeled anti-CD20 antibodies, 131I-tositumomab and 90Y-ibritumomab tiuxetan, were approved by FDA more than a decade ago (155,156). RTKs are ideal candidates for investigating and performing radioimmunotherapy (157,158). Therefore, radiolabeled antibodies or antibody fragments targeting markers included in the current review and other potential neoantigens may provide another therapeutic option for patients with cancer in the era of molecularly-targeted therapy and precision medicine.
We firmly believe that immunoPET will allow for better cancer patient management in the clinical setting with the fast development of this field. Considering most data with immunoPET probes has been generated in preclinical stages or in small groups of patients, further studies are still needed to push clinical translation of some of these promising probes and to confirm the value of clinically-reported probes.
Acknowledgement
This work was partially sponsored by the Ph.D. Innovation Fund of Shanghai Jiao Tong University School of Medicine (No. BXJ201736) and the China Scholarship Council (No. 201706230067) to W. Wei, the Shanghai Key Discipline of Medical Imaging (No. 2017ZZ02005) to Q.Y. Luo, the American Cancer Society (125246-RSG-13–099-01-CCE) and the National Institutes of Health (P30CA014520) to W. Cai. E. B. Ehlerding was partially sponsored by the National Institutes of Health (T32CA009206, and T32GM008505).
Footnotes
Conflict of interest
The authors declare no competing finanical interest.
References
- 1.Casaletto JB, McClatchey AI. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat Rev Cancer 2012;12:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2010;141:1117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006;47:2048–56. [PubMed] [Google Scholar]
- 4.Cai W, Niu G, Chen X. Multimodality imaging of the HER-kinase axis in cancer. Eur J Nucl Med Mol Imaging 2008;35:186–208. [DOI] [PubMed] [Google Scholar]
- 5.Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res 2006;12:3657–60. [DOI] [PubMed] [Google Scholar]
- 6.Regad T Targeting RTK Signaling Pathways in Cancer. Cancers (Basel) 2015;7:1758–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCabe KE, Wu AM. Positive progress in immunoPET--not just a coincidence. Cancer Biother Radiopharm 2010;25:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aluicio-Sarduy E, Ellison PA, Barnhart TE, Cai W, Nickles RJ, Engle JW. PET radiometals for antibody labeling. J Labelled Comp Radiopharm 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med 2008;49 Suppl 2:113S–28S. [DOI] [PubMed] [Google Scholar]
- 10.Perik PJ, Lub-De Hooge MN, Gietema JA, van der Graaf WT, de Korte MA, Jonkman S, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2006;24:2276–82. [DOI] [PubMed] [Google Scholar]
- 11.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 2012;92:897–965. [DOI] [PubMed] [Google Scholar]
- 12.Moek KL, Giesen D, Kok IC, de Groot DJA, Jalving M, Fehrmann RSN, et al. Theranostics Using Antibodies and Antibody-Related Therapeutics. J Nucl Med 2017;58:83S–90S. [DOI] [PubMed] [Google Scholar]
- 13.Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med 2009;50:974–81. [DOI] [PubMed] [Google Scholar]
- 14.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol 2016;27:619–24. [DOI] [PubMed] [Google Scholar]
- 15.Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin Nucl Med 2017;42:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortimer JE, Bading JR, Park JM, Frankel PH, Carroll MI, Tran TT, et al. Tumor Uptake of (64)Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J Nucl Med 2018;59:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donoghue JA, Lewis JS, Pandit-Taskar N, Fleming SE, Schoder H, Larson SM, et al. Pharmacokinetics, Biodistribution, and Radiation Dosimetry for (89)Zr-Trastuzumab in Patients with Esophagogastric Cancer. J Nucl Med 2018;59:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menke-van der Houven van Oordt CW, Gootjes EC, Huisman MC, Vugts DJ, Roth C, Luik AM, et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015;6:30384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Even AJ, Hamming-Vrieze O, van Elmpt W, Winnepenninckx VJ, Heukelom J, Tesselaar ME, et al. Quantitative assessment of Zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: a theragnostic approach. Oncotarget 2017;8:3870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Loon J, Even AJG, Aerts H, Ollers M, Hoebers F, van Elmpt W, et al. PET imaging of zirconium-89 labelled cetuximab: A phase I trial in patients with head and neck and lung cancer. Radiother Oncol 2017;122:267–73. [DOI] [PubMed] [Google Scholar]
- 21.Bahce I, Huisman MC, Verwer EE, Ooijevaar R, Boutkourt F, Vugts DJ, et al. Pilot study of (89)Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res 2014;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaykema SB, Brouwers AH, Lub-de Hooge MN, Pleijhuis RG, Timmer-Bosscha H, Pot L, et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J Nucl Med 2013;54:1014–8. [DOI] [PubMed] [Google Scholar]
- 23.Jansen MH, Lagerweij T, Sewing AC, Vugts DJ, van Vuurden DG, Molthoff CF, et al. Bevacizumab Targeting Diffuse Intrinsic Pontine Glioma: Results of 89Zr-Bevacizumab PET Imaging in Brain Tumor Models. Mol Cancer Ther 2016;15:2166–74. [DOI] [PubMed] [Google Scholar]
- 24.Oosting SF, Brouwers AH, van Es SC, Nagengast WB, Oude Munnink TH, Lub-de Hooge MN, et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J Nucl Med 2015;56:63–9. [DOI] [PubMed] [Google Scholar]
- 25.Oosting SF, van Asselt SJ, Brouwers AH, Bongaerts AH, Steinberg JD, de Jong JR, et al. 89Zr-Bevacizumab PET Visualizes Disease Manifestations in Patients with von Hippel-Lindau Disease. J Nucl Med 2016;57:1244–50. [DOI] [PubMed] [Google Scholar]
- 26.van Es SC, Brouwers AH, Mahesh SVK, Leliveld-Kors AM, de Jong IJ, Lub-de Hooge MN, et al. (89)Zr-Bevacizumab PET: Potential Early Indicator of Everolimus Efficacy in Patients with Metastatic Renal Cell Carcinoma. J Nucl Med 2017;58:905–10. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Hong H, Cai W. PET tracers based on Zirconium-89. Curr Radiopharm 2011;4:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez R, Sun H, England CG, Valdovinos HF, Ehlerding EB, Barnhart TE, et al. CD146-targeted immunoPET and NIRF Imaging of Hepatocellular Carcinoma with a Dual-Labeled Monoclonal Antibody. Theranostics 2016;6:1918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.England CG, Jiang D, Ehlerding EB, Rekoske BT, Ellison PA, Hernandez R, et al. (89)Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur J Nucl Med Mol Imaging 2018;45:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol 2011;3:S21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salgia R MET in Lung Cancer: Biomarker Selection Based on Scientific Rationale. Mol Cancer Ther 2017;16:555–65. [DOI] [PubMed] [Google Scholar]
- 32.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039–43. [DOI] [PubMed] [Google Scholar]
- 33.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 2010;17:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson ML, Yu HA, Hart EM, Weitner BB, Rademaker AW, Patel JD, et al. Phase I/II Study of HSP90 Inhibitor AUY922 and Erlotinib for EGFR-Mutant Lung Cancer With Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. J Clin Oncol 2015;33:1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A 2013;110:E2987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolfo C, Van Der Steen N, Pauwels P, Cappuzzo F. Onartuzumab in lung cancer: the fall of Icarus? Expert Rev Anticancer Ther 2015;15:487–9. [DOI] [PubMed] [Google Scholar]
- 37.Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr., Blumenschein GR Jr., et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Mocci S, Phan S, et al. Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J Clin Oncol 2017;35:412–20. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer 2018;17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay RV, Cao B, Skinner RS, Su Y, Zhao P, Gustafson MF, et al. Nuclear imaging of Met-expressing human and canine cancer xenografts with radiolabeled monoclonal antibodies (MetSeek). Clin Cancer Res 2005;11:7064s–9s. [DOI] [PubMed] [Google Scholar]
- 41.Perk LR, Stigter-van Walsum M, Visser GW, Kloet RW, Vosjan MJ, Leemans CR, et al. Quantitative PET imaging of Met-expressing human cancer xenografts with 89Zr-labelled monoclonal antibody DN30. Eur J Nucl Med Mol Imaging 2008;35:1857–67. [DOI] [PubMed] [Google Scholar]
- 42.Jagoda EM, Lang L, Bhadrasetty V, Histed S, Williams M, Kramer-Marek G, et al. Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab. J Nucl Med 2012;53:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li K, Tavare R, Zettlitz KA, Mumenthaler SM, Mallick P, Zhou Y, et al. Anti-MET immunoPET for non-small cell lung cancer using novel fully human antibody fragments. Mol Cancer Ther 2014;13:2607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pool M, Terwisscha van Scheltinga AGT, Kol A, Giesen D, de Vries EGE, Lub-de Hooge MN. (89)Zr-Onartuzumab PET imaging of c-MET receptor dynamics. Eur J Nucl Med Mol Imaging 2017;44:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res 2008;68:2850–60. [DOI] [PubMed] [Google Scholar]
- 46.Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price EW, Carnazza KE, Carlin SD, Cho A, Edwards KJ, Sevak KK, et al. (89)Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection. J Nucl Med 2017;58:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo H, Hong H, Slater MR, Graves SA, Shi S, Yang Y, et al. PET of c-Met in Cancer with (6)(4)Cu-Labeled Hepatocyte Growth Factor. J Nucl Med 2015;56:758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burggraaf J, Kamerling IM, Gordon PB, Schrier L, de Kam ML, Kales AJ, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med 2015;21:955–61. [DOI] [PubMed] [Google Scholar]
- 50.Esfahani SA, Heidari P, Kim SA, Ogino S, Mahmood U. Optical Imaging of Mesenchymal Epithelial Transition Factor (MET) for Enhanced Detection and Characterization of Primary and Metastatic Hepatic Tumors. Theranostics 2016;6:2028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arulappu A, Battle M, Eisenblaetter M, McRobbie G, Khan I, Monypenny J, et al. c-Met PET Imaging Detects Early-Stage Locoregional Recurrence of Basal-Like Breast Cancer. J Nucl Med 2016;57:765–70. [DOI] [PubMed] [Google Scholar]
- 52.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873–87. [DOI] [PubMed] [Google Scholar]
- 53.Simon T, Gagliano T, Giamas G. Direct Effects of Anti-Angiogenic Therapies on Tumor Cells: VEGF Signaling. Trends Mol Med 2017;23:282–92. [DOI] [PubMed] [Google Scholar]
- 54.Bahce I, Yaqub M, Smit EF, Lammertsma AA, van Dongen GA, Hendrikse NH. Personalizing NSCLC therapy by characterizing tumors using TKI-PET and immuno-PET. Lung Cancer 2017;107:1–13. [DOI] [PubMed] [Google Scholar]
- 55.van Asselt SJ, Oosting SF, Brouwers AH, Bongaerts AH, de Jong JR, Lub-de Hooge MN, et al. Everolimus Reduces (89)Zr-Bevacizumab Tumor Uptake in Patients with Neuroendocrine Tumors. J Nucl Med 2014;55:1087–92. [DOI] [PubMed] [Google Scholar]
- 56.Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, et al. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med 2007;48:1313–9. [DOI] [PubMed] [Google Scholar]
- 57.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther 2006;5:2624–33. [DOI] [PubMed] [Google Scholar]
- 58.Jansen MH, Veldhuijzen van Zanten SEM, van Vuurden DG, Huisman MC, Vugts DJ, Hoekstra OS, et al. Molecular Drug Imaging: (89)Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J Nucl Med 2017;58:711–6. [DOI] [PubMed] [Google Scholar]
- 59.Veldhuijzen van Zanten SEM, Sewing ACP, van Lingen A, Hoekstra OS, Wesseling P, Meel MH, et al. Multiregional Tumor Drug-Uptake Imaging by PET and Microvascular Morphology in End-Stage Diffuse Intrinsic Pontine Glioma. J Nucl Med 2018;59:612–5. [DOI] [PubMed] [Google Scholar]
- 60.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009;27:3312–8. [DOI] [PubMed] [Google Scholar]
- 61.Bueno MJ, Mouron S, Quintela-Fandino M. Personalising and targeting antiangiogenic resistance: a complex and multifactorial approach. Br J Cancer 2017;116:1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dedania VS, Bakri SJ. Current perspectives on ranibizumab. Clin Ophthalmol 2015;9:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagengast WB, Lub-de Hooge MN, Oosting SF, den Dunnen WF, Warnders FJ, Brouwers AH, et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res 2011;71:143–53. [DOI] [PubMed] [Google Scholar]
- 64.Meyer JP, Edwards KJ, Kozlowski P, Backer MV, Backer JM, Lewis JS. Selective Imaging of VEGFR-1 and VEGFR-2 Using 89Zr-Labeled Single-Chain VEGF Mutants. J Nucl Med 2016;57:1811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo H, England CG, Graves SA, Sun H, Liu G, Nickles RJ, et al. PET Imaging of VEGFR-2 Expression in Lung Cancer with 64Cu-Labeled Ramucirumab. J Nucl Med 2016;57:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laffon E, Marthan R. A three-time-point method for assessing kinetic parameters of (64)Cu-labeled Ramucirumab trapping in VEGFR-2 positive lung tumors. Phys Med 2017;43:1–5. [DOI] [PubMed] [Google Scholar]
- 67.Wimana Z, Gebhart G, Guiot T, Vanderlinden B, Larsimont D, Doumont G, et al. N-Acetylcysteine breaks resistance to trastuzumab caused by MUC4 overexpression in human HER2 positive BC-bearing nude mice monitored by (89)Zr-Trastuzumab and (18)F-FDG PET imaging. Oncotarget 2017;8:56185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gebhart G, Flamen P, De Vries EG, Jhaveri K, Wimana Z. Imaging Diagnostic and Therapeutic Targets: Human Epidermal Growth Factor Receptor 2. J Nucl Med 2016;57 Suppl 1:81S–8S. [DOI] [PubMed] [Google Scholar]
- 69.Pereira PMR, Abma L, Henry KE, Lewis JS. Imaging of human epidermal growth factor receptors for patient selection and response monitoring - From PET imaging and beyond. Cancer Lett 2018;419:139–51. [DOI] [PubMed] [Google Scholar]
- 70.J NT, Pandya DN, Pailloux SL, Ogasawara A, Vanderbilt AN, Gill HS, et al. Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) Based Macrocyclic Chelator for (89)Zr(4+) and Its Use for ImmunoPET Imaging of HER2 Positive Model of Ovarian Carcinoma in Mice. Theranostics 2016;6:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma T, Sun X, Cui L, Gao L, Wu Y, Liu H, et al. Molecular imaging reveals trastuzumab-induced epidermal growth factor receptor downregulation in vivo. J Nucl Med 2014;55:1002–7. [DOI] [PubMed] [Google Scholar]
- 72.Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med 2013;54:936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010;87:586–92. [DOI] [PubMed] [Google Scholar]
- 74.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015;27:15–26. [DOI] [PubMed] [Google Scholar]
- 75.Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J Nucl Med 2016;57:1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 2008;358:1409–11. [DOI] [PubMed] [Google Scholar]
- 77.Gaykema SB, Schroder CP, Vitfell-Rasmussen J, Chua S, Oude Munnink TH, Brouwers AH, et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res 2014;20:3945–54. [DOI] [PubMed] [Google Scholar]
- 78.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- 79.Ock CY, Lee KW, Kim JW, Kim JS, Kim TY, Lee KH, et al. Optimal Patient Selection for Trastuzumab Treatment in HER2-Positive Advanced Gastric Cancer. Clin Cancer Res 2015;21:2520–9. [DOI] [PubMed] [Google Scholar]
- 80.Vugts DJ, Klaver C, Sewing C, Poot AJ, Adamzek K, Huegli S, et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for (89)Zr-immuno-PET. Eur J Nucl Med Mol Imaging 2017;44:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lua WH, Gan SK, Lane DP, Verma CS. A search for synergy in the binding kinetics of Trastuzumab and Pertuzumab whole and F(ab) to Her2. NPJ Breast Cancer 2015;1:15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marquez BV, Ikotun OF, Zheleznyak A, Wright B, Hari-Raj A, Pierce RA, et al. Evaluation of (89)Zr-pertuzumab in Breast cancer xenografts. Mol Pharm 2014;11:3988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faratian D, Zweemer AJ, Nagumo Y, Sims AH, Muir M, Dodds M, et al. Trastuzumab and pertuzumab produce changes in morphology and estrogen receptor signaling in ovarian cancer xenografts revealing new treatment strategies. Clin Cancer Res 2011;17:4451–61. [DOI] [PubMed] [Google Scholar]
- 84.Kurzeder C, Bover I, Marme F, Rau J, Pautier P, Colombo N, et al. Double-Blind, Placebo-Controlled, Randomized Phase III Trial Evaluating Pertuzumab Combined With Chemotherapy for Low Tumor Human Epidermal Growth Factor Receptor 3 mRNA-Expressing Platinum-Resistant Ovarian Cancer (PENELOPE). J Clin Oncol 2016;34:2516–25. [DOI] [PubMed] [Google Scholar]
- 85.Jiang D, Im HJ, Sun H, Valdovinos HF, England CG, Ehlerding EB, et al. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur J Nucl Med Mol Imaging 2017;44:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ulaner GA, Lyashchenko SK, Riedl C, Ruan S, Zanzonico PB, Lake D, et al. First-in-human HER2-targeted imaging using (89)Zr-pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J Nucl Med 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakravarty R, Goel S, Cai W. Nanobody: the “magic bullet” for molecular imaging? Theranostics 2014;4:386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu AM. Engineered antibodies for molecular imaging of cancer. Methods 2014;65:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaidyanathan G, McDougald D, Choi J, Koumarianou E, Weitzel D, Osada T, et al. Preclinical Evaluation of 18F-Labeled Anti-HER2 Nanobody Conjugates for Imaging HER2 Receptor Expression by Immuno-PET. J Nucl Med 2016;57:967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Z, Vaidyanathan G, McDougald D, Kang CM, Balyasnikova I, Devoogdt N, et al. Fluorine-18 Labeling of the HER2-Targeting Single-Domain Antibody 2Rs15d Using a Residualizing Label and Preclinical Evaluation. Mol Imaging Biol 2017;19:867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam K, Chan C, Reilly RM. Development and preclinical studies of (64)Cu-NOTA-pertuzumab F(ab’)2 for imaging changes in tumor HER2 expression associated with response to trastuzumab by PET/CT. MAbs 2017;9:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cleeren F, Lecina J, Ahamed M, Raes G, Devoogdt N, Caveliers V, et al. Al(18)F-Labeling Of Heat-Sensitive Biomolecules for Positron Emission Tomography Imaging. Theranostics 2017;7:2924–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xavier C, Blykers A, Vaneycken I, D’Huyvetter M, Heemskerk J, Lahoutte T, et al. (18)F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl Med Biol 2016;43:247–52. [DOI] [PubMed] [Google Scholar]
- 94.Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J Nucl Med 2016;57:27–33. [DOI] [PubMed] [Google Scholar]
- 95.Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol 2014;6:a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 2014;24:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010;10:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Dijk LK, Yim CB, Franssen GM, Kaanders JH, Rajander J, Solin O, et al. PET of EGFR with (64) Cu-cetuximab-F(ab’)2 in mice with head and neck squamous cell carcinoma xenografts. Contrast Media Mol Imaging 2016;11:65–70. [DOI] [PubMed] [Google Scholar]
- 99.Song IH, Lee TS, Park YS, Lee JS, Lee BC, Moon BS, et al. Immuno-PET Imaging and Radioimmunotherapy of 64Cu-/177Lu-Labeled Anti-EGFR Antibody in Esophageal Squamous Cell Carcinoma Model. J Nucl Med 2016;57:1105–11. [DOI] [PubMed] [Google Scholar]
- 100.Ciombor KK, Bekaii-Saab T. A Comprehensive Review of Sequencing and Combination Strategies of Targeted Agents in Metastatic Colorectal Cancer. Oncologist 2018;23:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei L, Shi J, Afari G, Bhattacharyya S. Preparation of clinical-grade (89) Zr-panitumumab as a positron emission tomography biomarker for evaluating epidermal growth factor receptor-targeted therapy. J Labelled Comp Radiopharm 2014;57:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lindenberg L, Adler S, Turkbey IB, Mertan F, Ton A, Do K, et al. Dosimetry and first human experience with (89)Zr-panitumumab. Am J Nucl Med Mol Imaging 2017;7:195–203. [PMC free article] [PubMed] [Google Scholar]
- 103.Nayak TK, Garmestani K, Milenic DE, Brechbiel MW. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J Nucl Med 2012;53:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Temam S, Spicer J, Farzaneh F, Soria JC, Oppenheim D, McGurk M, et al. An exploratory, open-label, randomized, multicenter study to investigate the pharmacodynamics of a glycoengineered antibody (imgatuzumab) and cetuximab in patients with operable head and neck squamous cell carcinoma. Ann Oncol 2017;28:2827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pool M, Kol A, Lub-de Hooge MN, Gerdes CA, de Jong S, de Vries EG, et al. Extracellular domain shedding influences specific tumor uptake and organ distribution of the EGFR PET tracer 89Zr-imgatuzumab. Oncotarget 2016;7:68111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee SC, Park K, Han J, Lee JJ, Kim HJ, Hong S, et al. Design of a binding scaffold based on variable lymphocyte receptors of jawless vertebrates by module engineering. Proc Natl Acad Sci U S A 2012;109:3299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pyo A, Yun M, Kim HS, Kim TY, Lee JJ, Kim JY, et al. (64)Cu-Labeled Repebody Molecules for Imaging of Epidermal Growth Factor Receptor-Expressing Tumors. J Nucl Med 2018;59:340–6. [DOI] [PubMed] [Google Scholar]
- 108.Yun M, Kim DY, Lee JJ, Kim HS, Kim HS, Pyo A, et al. A High-Affinity Repebody for Molecular Imaging of EGFR-Expressing Malignant Tumors. Theranostics 2017;7:2620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goux M, Becker G, Gorre H, Dammicco S, Desselle A, Egrise D, et al. Nanofitin as a New Molecular-Imaging Agent for the Diagnosis of Epidermal Growth Factor Receptor Over-Expressing Tumors. Bioconjug Chem 2017;28:2361–71. [DOI] [PubMed] [Google Scholar]
- 110.Ehlerding EB, Sun L, Lan X, Zeng D, Cai W. Dual-Targeted Molecular Imaging of Cancer. J Nucl Med 2018;59:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo H, Hernandez R, Hong H, Graves SA, Yang Y, England CG, et al. Noninvasive brain cancer imaging with a bispecific antibody fragment, generated via click chemistry. Proc Natl Acad Sci U S A 2015;112:12806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwon LY, Scollard DA, Reilly RM. (64)Cu-Labeled Trastuzumab Fab-PEG24-EGF Radioimmunoconjugates Bispecific for HER2 and EGFR: Pharmacokinetics, Biodistribution, and Tumor Imaging by PET in Comparison to Monospecific Agents. Mol Pharm 2017;14:492–501. [DOI] [PubMed] [Google Scholar]
- 113.Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res 2014;20:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Terwisscha van Scheltinga AG, Lub-de Hooge MN, Abiraj K, Schroder CP, Pot L, Bossenmaier B, et al. ImmunoPET and biodistribution with human epidermal growth factor receptor 3 targeting antibody (8)(9)Zr-RG7116. MAbs 2014;6:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bensch F, Lamberts LE, Smeenk MM, Jorritsma-Smit A, Lub-de Hooge MN, Terwisscha van Scheltinga AGT, et al. (89)Zr-Lumretuzumab PET Imaging before and during HER3 Antibody Lumretuzumab Treatment in Patients with Solid Tumors. Clin Cancer Res 2017;23:6128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lockhart AC, Liu Y, Dehdashti F, Laforest R, Picus J, Frye J, et al. Phase 1 Evaluation of [(64)Cu]DOTA-Patritumab to Assess Dosimetry, Apparent Receptor Occupancy, and Safety in Subjects with Advanced Solid Tumors. Mol Imaging Biol 2016;18:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pool M, Kol A, de Jong S, de Vries EGE, Lub-de Hooge MN, Terwisscha van Scheltinga AGT. (89)Zr-mAb3481 PET for HER3 tumor status assessment during lapatinib treatment. MAbs 2017;9:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Larimer BM, Phelan N, Wehrenberg-Klee E, Mahmood U. Phage Display Selection, In Vitro Characterization, and Correlative PET Imaging of a Novel HER3 Peptide. Mol Imaging Biol 2018;20:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Da Pieve C, Allott L, Martins CD, Vardon A, Ciobota DM, Kramer-Marek G, et al. Efficient [(18)F]AlF Radiolabeling of ZHER3:8698 Affibody Molecule for Imaging of HER3 Positive Tumors. Bioconjug Chem 2016;27:1839–49. [DOI] [PubMed] [Google Scholar]
- 120.Rosestedt M, Andersson KG, Mitran B, Tolmachev V, Lofblom J, Orlova A, et al. Affibody-mediated PET imaging of HER3 expression in malignant tumours. Sci Rep 2015;5:15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Orlova A, Malm M, Rosestedt M, Varasteh Z, Andersson K, Selvaraju RK, et al. Imaging of HER3-expressing xenografts in mice using a (99m)Tc(CO) 3-HEHEHE-Z HER3:08699 affibody molecule. Eur J Nucl Med Mol Imaging 2014;41:1450–9. [DOI] [PubMed] [Google Scholar]
- 122.Meulendijks D, Jacob W, Martinez-Garcia M, Taus A, Lolkema MP, Voest EE, et al. First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3 Monoclonal Antibody, in Patients with Metastatic or Advanced HER3-Positive Solid Tumors. Clin Cancer Res 2016;22:877–85. [DOI] [PubMed] [Google Scholar]
- 123.Warnders FJ, Terwisscha van Scheltinga AGT, Knuehl C, van Roy M, de Vries EFJ, Kosterink JGW, et al. Human Epidermal Growth Factor Receptor 3-Specific Tumor Uptake and Biodistribution of (89)Zr-MSB0010853 Visualized by Real-Time and Noninvasive PET Imaging. J Nucl Med 2017;58:1210–5. [DOI] [PubMed] [Google Scholar]
- 124.Juric D, Dienstmann R, Cervantes A, Hidalgo M, Messersmith W, Blumenschein GR Jr., et al. Safety and Pharmacokinetics/Pharmacodynamics of the First-in-Class Dual Action HER3/EGFR Antibody MEHD7945A in Locally Advanced or Metastatic Epithelial Tumors. Clin Cancer Res 2015;21:2462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oseini AM, Roberts LR. PDGFRalpha: a new therapeutic target in the treatment of hepatocellular carcinoma? Expert Opin Ther Targets 2009;13:443–54. [DOI] [PubMed] [Google Scholar]
- 126.Lopez-Campistrous A, Adewuyi EE, Benesch MGK, Ko YM, Lai R, Thiesen A, et al. PDGFRalpha Regulates Follicular Cell Differentiation Driving Treatment Resistance and Disease Recurrence in Papillary Thyroid Cancer. EBioMedicine 2016;12:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J, Wang P, Dykstra M, Gelebart P, Williams D, Ingham R, et al. Platelet-derived growth factor receptor-alpha promotes lymphatic metastases in papillary thyroid cancer. J Pathol 2012;228:241–50. [DOI] [PubMed] [Google Scholar]
- 128.Wagner M, Wuest M, Hamann I, Lopez-Campistrous A, McMullen TPW, Wuest F. Molecular imaging of platelet-derived growth factor receptor-alpha (PDGFRalpha) in papillary thyroid cancer using immuno-PET. Nucl Med Biol 2018;58:51–8. [DOI] [PubMed] [Google Scholar]
- 129.Sun Y, Sun X, Shen B. Molecular Imaging of IGF-1R in Cancer. Mol Imaging 2017;16:1536012117736648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orlova A, Hofstrom C, Strand J, Varasteh Z, Sandstrom M, Andersson K, et al. [99mTc(CO)3]+-(HE)3-ZIGF1R:4551, a new Affibody conjugate for visualization of insulin-like growth factor-1 receptor expression in malignant tumours. Eur J Nucl Med Mol Imaging 2013;40:439–49. [DOI] [PubMed] [Google Scholar]
- 131.Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, Franssen GM, Versleijen-Jonkers YM, Oyen WJ, et al. ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J Nucl Med 2010;51:1565–72. [DOI] [PubMed] [Google Scholar]
- 132.England CG, Kamkaew A, Im HJ, Valdovinos HF, Sun H, Hernandez R, et al. ImmunoPET Imaging of Insulin-Like Growth Factor 1 Receptor in a Subcutaneous Mouse Model of Pancreatic Cancer. Mol Pharm 2016;13:1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tolmachev V, Stone-Elander S, Orlova A. Radiolabelled receptor-tyrosine-kinase targeting drugs for patient stratification and monitoring of therapy response: prospects and pitfalls. Lancet Oncol 2010;11:992–1000. [DOI] [PubMed] [Google Scholar]
- 134.Bahce I, Smit EF, Lubberink M, van der Veldt AA, Yaqub M, Windhorst AD, et al. Development of [(11)C]erlotinib positron emission tomography for in vivo evaluation of EGF receptor mutational status. Clin Cancer Res 2013;19:183–93. [DOI] [PubMed] [Google Scholar]
- 135.Yaqub M, Bahce I, Voorhoeve C, Schuit RC, Windhorst AD, Hoekstra OS, et al. Quantitative and Simplified Analysis of 11C-Erlotinib Studies. J Nucl Med 2016;57:861–6. [DOI] [PubMed] [Google Scholar]
- 136.Henry KE, Ulaner GA, Lewis JS. Human Epidermal Growth Factor Receptor 2-Targeted PET/Single- Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis. PET Clin 2017;12:269–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Slobbe P, Poot AJ, Haumann R, Schuit RC, Windhorst AD, van Dongen GA. Two anti-angiogenic TKI-PET tracers, [(11)C]axitinib and [(11)C]nintedanib: Radiosynthesis, in vivo metabolism and initial biodistribution studies in rodents. Nucl Med Biol 2016;43:612–24. [DOI] [PubMed] [Google Scholar]
- 138.Bernard-Gauthier V, Mahringer A, Vesnaver M, Fricker G, Schirrmacher R. Design and synthesis of a fluorinated quinazoline-based type-II Trk inhibitor as a scaffold for PET radiotracer development. Bioorg Med Chem Lett 2017;27:2771–5. [DOI] [PubMed] [Google Scholar]
- 139.Peng Z, Maxwell DS, Sun D, Bhanu Prasad BA, Pal A, Wang S, et al. Imatinib analogs as potential agents for PET imaging of Bcr-Abl and c-KIT expression at a kinase level. Bioorg Med Chem 2014;22:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su X, Cheng K, Liu Y, Hu X, Meng S, Cheng Z. PET imaging of insulin-like growth factor type 1 receptor expression with a 64Cu-labeled Affibody molecule. Amino Acids 2015;47:1409–19. [DOI] [PubMed] [Google Scholar]
- 141.Majo VJ, Arango V, Simpson NR, Prabhakaran J, Kassir SA, Underwood MD, et al. Synthesis and in vitro evaluation of [18F]BMS-754807: a potential PET ligand for IGF-1R. Bioorg Med Chem Lett 2013;23:4191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs 2018;10:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heskamp S, Raave R, Boerman O, Rijpkema M, Goncalves V, Denat F. (89)Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art (89)Zr Radiochemistry. Bioconjug Chem 2017;28:2211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GA. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med 2003;44:1271–81. [PubMed] [Google Scholar]
- 145.Perk LR, Vosjan MJ, Visser GW, Budde M, Jurek P, Kiefer GE, et al. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur J Nucl Med Mol Imaging 2010;37:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meyer JP, Adumeau P, Lewis JS, Zeglis BM. Click Chemistry and Radiochemistry: The First 10 Years. Bioconjug Chem 2016;27:2791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo H, Hong H, Yang SP, Cai W. Design and applications of bispecific heterodimers: molecular imaging and beyond. Mol Pharm 2014;11:1750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Popp MW, Antos JM, Grotenbreg GM, Spooner E, Ploegh HL. Sortagging: a versatile method for protein labeling. Nat Chem Biol 2007;3:707–8. [DOI] [PubMed] [Google Scholar]
- 149.Massa S, Vikani N, Betti C, Ballet S, Vanderhaegen S, Steyaert J, et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: a versatile strategy for multiple molecular imaging modalities. Contrast Media Mol Imaging 2016;11:328–39. [DOI] [PubMed] [Google Scholar]
- 150.Ingram JR, Schmidt FI, Ploegh HL. Exploiting Nanobodies’ Singular Traits. Annu Rev Immunol 2018 [DOI] [PubMed] [Google Scholar]
- 151.Sharma SK, Chow A, Monette S, Vivier D, Pourat J, Edwards KJ, et al. Fc-mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models. Cancer Res 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stefan N, Gebleux R, Waldmeier L, Hell T, Escher M, Wolter FI, et al. Highly Potent, Anthracycline-based Antibody-Drug Conjugates Generated by Enzymatic, Site-specific Conjugation. Mol Cancer Ther 2017;16:879–92. [DOI] [PubMed] [Google Scholar]
- 153.Kraeber-Bodere F, Rousseau C, Bodet-Milin C, Frampas E, Faivre-Chauvet A, Rauscher A, et al. A pretargeting system for tumor PET imaging and radioimmunotherapy. Front Pharmacol 2015;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keinanen O, Fung K, Pourat J, Jallinoja V, Vivier D, Pillarsetty NK, et al. Pretargeting of internalizing trastuzumab and cetuximab with a (18)F-tetrazine tracer in xenograft models. EJNMMI Res 2017;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.England CG, Rui L, Cai W. Lymphoma: current status of clinical and preclinical imaging with radiolabeled antibodies. Eur J Nucl Med Mol Imaging 2017;44:517–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Green DJ, Press OW. Whither Radioimmunotherapy: To Be or Not To Be? Cancer Res 2017;77:2191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Levy A, Nigro G, Sansonetti PJ, Deutsch E. Candidate immune biomarkers for radioimmunotherapy. Biochim Biophys Acta 2017;1868:58–68. [DOI] [PubMed] [Google Scholar]
- 158.Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 2013;19:5182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


