Abstract
Evidence suggests lower nadir testosterone levels during the first year of androgen deprivation therapy improve advanced prostate cancer clinical outcomes. We evaluated pivotal trials for subcutaneously administered leuprolide acetate (1-, 3-, 4-, and 6-month doses) to determine nadir testosterone levels. Pooled analysis showed 99%, 97%, and 91% of patients reached nadir testosterone ≤20, ≤10, and ≤5 ng/dL respectively (median ≤3 ng/dL). Across all available categories, $88% of patients reached nadir testosterone ≤5 ng/dL, and <3% experienced a microsurge. Achievement and maintenance of low nadir testosterone levels may improve progression-free survival and time to onset of castrate-resistant prostate cancer.
Keywords: Prostate cancer, Leuprolide acetate, Nadir testosterone, Androgen deprivation therapy, LHRH agonist
Prostate cancer (PCa) is the second most common cancer afflicting men in the United States, with approximately 13% of men receiving this diagnosis during their lifetime.1 PCa is an androgendependent neoplasm and proliferates in the presence of testosterone (T).2 Suppression of T can inhibit the growth of cancer and is the underlying concept for androgen deprivation therapy (ADT) based on the original research conducted by Huggins and Hodges in 1941.3 Luteinizing hormone-releasing hormone (LHRH) agonists such as leuprolide acetate (LA) are the most commonly used drugs for ADT with the objective of reducing T to castrate levels.
In the United States, the biochemical and regulatory definition of castration is T >50 ng/dL, based on the sensitivity of the assays available when ADT was first developed 50 years ago.2,4 With the advent of improved assays, T now can be measured to much lower limits of quantification (LOQ) of >3 ng/dL.5 T levels of 20 to 32 ng/dL during ADT are associated with a delay in onset of castrate-resistant PCa (CRPC) and lower risk of death for patients compared with those with higher T levels,2,6 suggesting the lowest possible serum T should be the objective of ADT. Consequently, in the 2012 Bethesda consensus published by a group of US experts, a 20 ng/dL threshold for serum T during ADT in patients with advanced PCa was recommended, although American Urological Association (AUA) and National Comprehensive Cancer Network (NCCN) guidelines have not adopted this recommendation.7 In 2014, the European Association of Urology (EAU) updated its guidelines for the treatment of PCa to redefine target level of T during ADT to >20 ng/dL.8 Nadir serum T, the lowest value during ADT, has been associated with delay in disease progression and improved survival.9,10 However, although data on nadir T levels have been reported with some therapies (ie, abiraterone acetate with ADT),11-13 no data have been published on nadir T levels achieved with LHRH agonist monotherapy. To address this gap in knowledge, a secondary evaluation was conducted by pooling patients from four pivotal trials of LA injected subcutaneously (SC) (SC-LA; ELIGARD®, leuprolide acetate for injectable suspension; Tolmar Pharmaceuticals, Inc., Fort Collins, CO). The LA formulations are composed of a unique, biodegradable, dual polymerbased, extended-release delivery system. SC-LA is available in 1-, 3-, 4-, and 6-month doses that form solid implants upon interaction with SC fluid and subsequently slowly release LA.14,15 SC-LA maintains mean serum LA levels between 0.1 and 1.0 ng/mL and produces consistent T suppression to ≤20 ng/dL.16,17
Materials and Methods
Study Design
Data were pooled from four prospective, open-label, fixed-dose clinical trials in patients with advanced PCa.14-17 Briefly, patients aged 40 to 86 years with PCa and no prior use of ADT received one of four formulations of SC-LA: (1) 7.5 mg every 28 days for 24 weeks (1-month formulation; n 5 120); (2) 22.5 mg every 84 days for 24 weeks (3-month formulation; n 5 117); (3) 30 mg every 112 days for 32 weeks (4-month formulation; n 5 90); and (4) 45 mg every 168 days for 48 weeks (6-month formulation; n 5 111) in accordance with the manufacturer’s instructions. The primary endpoint was serum T and secondary endpoints included serum LH, bone pain, urinary symptoms, and World Health Organization (WHO) performance status. Serum T concentrations were measured at screening, baseline, 2, 4, and 8 hours after dosing, days 1, 2, 3, and 7, and then every week until the next dose, following which the sampling schedule was repeated until the end of each study.
Assessments
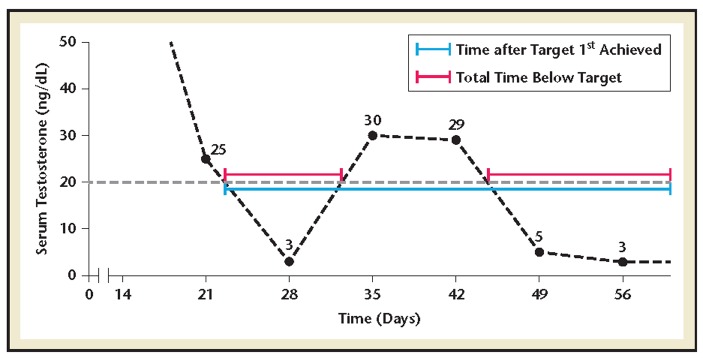
Serum T was measured by radioimmunoassay with an LOQ of 3 ng/dL; values below this level were recorded as ≤3 ng/dL. Nadir T was defined as the lowest value observed during treatment. Serum T and nadir T concentrations were summarized using descriptive statistics. Microsurges were identified as absolute increases in T of <25 ng/dL within 4 weeks after administration of a second dose. The onset of T suppression and the proportion of time serum T remained below 50 ng/dL, 20 ng/dL, and 10 ng/dL levels were calculated by extrapolating the point at which the T level first crossed the target, calculating the total time T levels remained below the target, and then dividing this by the total time after the target was first achieved (Figure 1).
Figure 1.
(A) Median time to testosterone ≤10, ≤20, or ≤50 ng/dL. (B) Proportion of time testosterone levels maintained ≤10, ≤20, or ≤50 ng/dL.
Results
Patient Demographics and Baseline Characteristics
There were 120, 117, 90, and 111 patients in the 1-, 3-, 4-, and 6-month trials, respectively. Baseline characteristics and demographics were similar between study cohorts and mean baseline serum T concentrations ranged from 361 to 386 ng/dL (Table 1).
Table 1.
Patient Demographics and Baseline Characteristics
| SC-LA Formulated With a Biodegradable, Dual Polymer-based, Extended-release Delivery System Dose Groups | ||||
|---|---|---|---|---|
| 1 Month, 7.5 mg (n5120) | 3 Month, 22.5 mg (n5117) | 4 Month, 30 mg (n590) | 6 Month, 45 mg (n5111) | |
| Mean age (range) | 72.8 (52-85) | 73.1 (46-85) | 73.5 (53-84) | 73.2 (50-86) |
| Age, years, n (%) | ||||
| 40-49 | 0 | 1 (0.9) | 0 | 0 |
| 50-59 | 8 (6.7) | 6 (5.1) | 6 (6.7) | 6 (5.4) |
| 60-69 | 28 (23.3) | 27 (23.1) | 20 (22.2) | 25 (22.5) |
| 70-79 | 60 (50.0) | 52 (44.4) | 42 (46.7) | 55 (49.6) |
| 80-89 | 24 (20.0) | 31 (26.5) | 22 (24.4) | 25 (22.5) |
| Height, in, mean (range) | 69.0 (62-75) | 68.2 (55-74) | 69.0 (60-78) | 68.9 (62-76) |
| Weight, lbs, mean (range) | 185.3 (126-287) | 186.1 (130-296) | 196.5 (133-313) | 190.1 (109-321) |
| Race, n (%) | ||||
| White | 92 (76.7) | 93 (79.5) | 71 (78.9) | 84 (75.7) |
| Black | 15 (12.5) | 13 (11.1) | 10 (11.1) | 19 (17.1) |
| Hispanic | 13 (10.8) | 7 (6.0) | 8 (8.9) | 6 (5.4) |
| Asian | 0 | 3 (2.6) | 0 | 1 (0.9) |
| Other | 0 | 1 (0.9) | 1 (1.1) | 1 (0.9) |
SC-LA, subcutaneously administered leuprolide acetate.
Onset and Maintenance of Testosterone Suppression
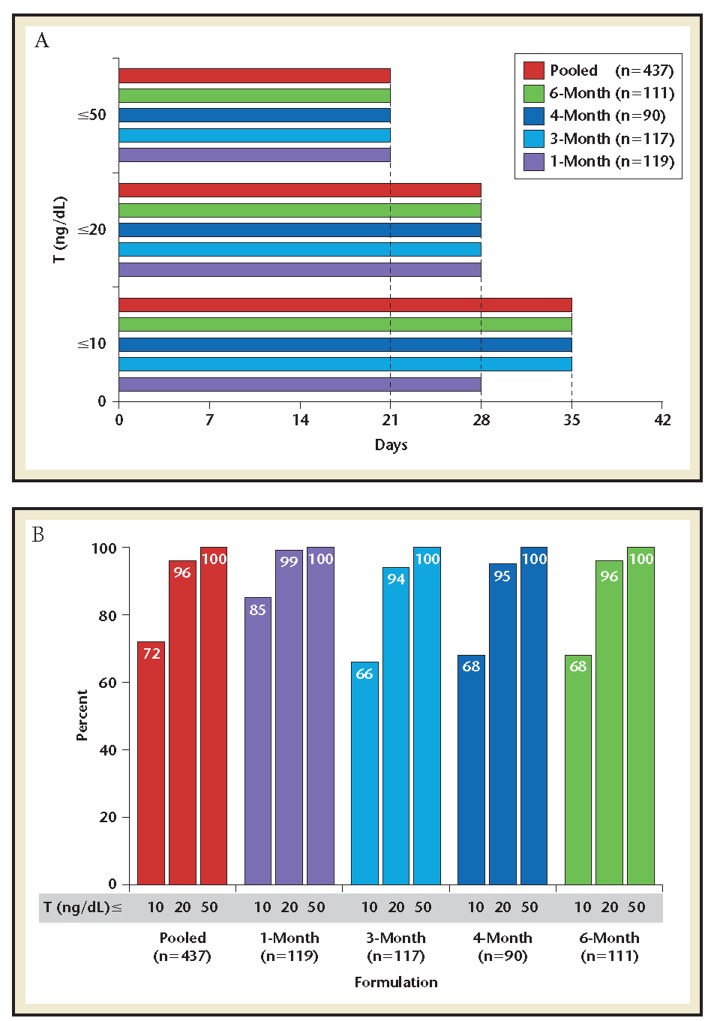
In the pooled population, median onsets of T ≤50, ≤20, and ≤10 ng/dL were 21, 28, and 35 days, respectively (Figure 2A). These durations were the same in each trial, except for the 1-month formulation where the median onset of T ≤10 ng/dL was 7 days earlier at 28 days. The mean proportions of time T suppression was maintained below each target were 100% for T ≤50 ng/dL, 94% to 99% for T ≤20 ng/dL, and 66% to 85% for T ≤10 ng/dL (Figure 2B).
Figure 2.
Proportion of time testosterone below suppression target* (illustrative for T ≤20 ng/dL). *Proportion of time below target calculated by dividing the total time below target by the time after target first achieved.
Nadir Testosterone
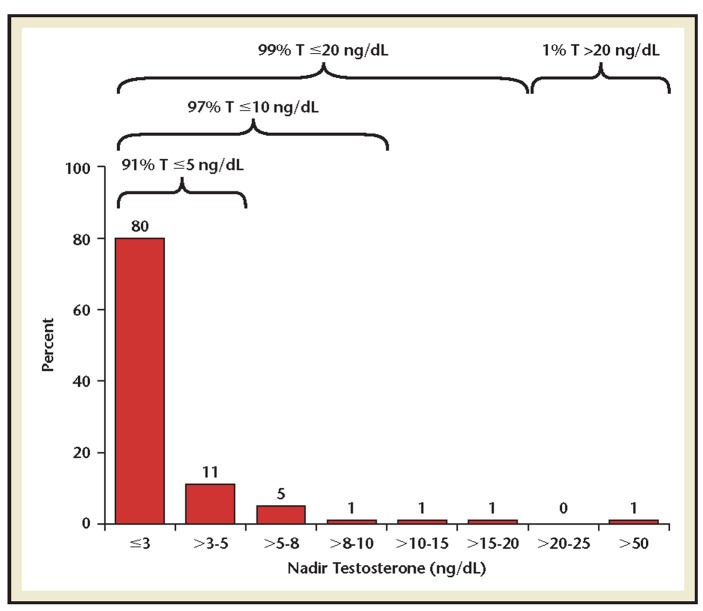
Pooled analysis showed 99%, 97%, and 91% of patients reached nadir T ≤20 ng/dL, ≤10 ng/dL, and ≤5 ng/dL, respectively, with 80% achieving a nadir T below the LOQ of 3 ng/dL (Figure 3). When comparing each formulation, 93%, 88%, 90%, and 93% of patients who received the 1-, 3-, 4-, and 6-month doses, respectively, reached a nadir T ≤5 ng/dL.
Figure 3.
Proportion of patients by nadir testosterone level achieved over study duration (n = 437).
Testosterone Microsurges
Microsurge in T occurred in 0.9% to 3.4% (pooled 1.9%) of patients across the four studies after the second dose (Table 2). Of the eight patients who experienced microsurge, six maintained T below 50 ng/dL and two patients exceeded it.
Table 2.
Incidence of Microsurges Within 4 Weeks After Second Dose
| Formulationa | Number of Patients Experiencing Microsurge With Peak | ||||
|---|---|---|---|---|---|
| ≤50 ng/dL | <50 ng/dL | ||||
| M1 month (n5113) | 1 | 0 | |||
| 3 month (n5115) | 2 | 0 | |||
| 4 month (n588) | 1 | 2 | |||
| 6 month (n5108) | 2 | 0 | |||
aIncluding only patients who achieved castration levels of testosterone by second dose.
Safety
The safety profiles in the pivotal trials have been previously described and, as expected, were consistent with known effects of LHRH agonist therapy.14–17
Discussion
Treatment of advanced PCa patients with SC-LA resulted in reliable T suppression to ≤50 ng/dL by 3 weeks, ≤20 ng/dL by 4 weeks, and ≤10 ng/dL by 5 weeks. Nadir T levels were below the LOQ of 3 ng/dL in 80% and ≤5 ng/dL in 91% of the pooled patient population. SC-LA maintained consistently low T levels, with 100%, 94% to 99%, and 66% to 85% of the treatment durations having T levels below 50, 20, and 10 ng/dL, respectively, across the four formulations. The incidence of microsurge was 1.9%, which is lower than reported for intramuscular-LA (4%) in a phase 3 study.18,19 SC-LA produced profound T suppression to levels below historic targets and those achieved by surgical castration. This T suppression profile may extend time to disease progression and improve patient survival.9 In some patients, SC-LA suppresses T to the very low levels reported with the new androgen pathway inhibitor drugs for treatment of advanced PCa, such as abiraterone acetate (alone or combined with ADT).20,21 Abiraterone (a CYP17A1 inhibitor) with ADT suppresses T levels to near zero.22 Enzalutamide and apalutamide, also with ADT, impact the androgen signaling pathway by blocking the activity of androgens at the AR within the cells.23 With the recent approvals of abiraterone in metastatic castrate-sensitive PCa and apalutamide in non-metastatic CRPC, androgen pathway inhibitors now have indications across a wider spectrum of disease, drive overall T signaling to near zero, and improve survival and other endpoints.11,12,22,23 The publications of studies for these drugs do not disclose the magnitude of T reductions achieved in patients who were randomized to the control arms that received ADT alone. These data would be informative and might identify differences in T suppression between various forms of ADT and their impact on clinical endpoints.
Achieving very low nadir T levels during the first year of ADT may be prognostic for improved cancerspecific survival.9 It would be informative to understand how effective ADT drugs are in achieving these very low levels by consistently measuring T throughout treatment. The results of our analyses confirm the efficacy of SC-LA in achieving and maintaining T levels below 20 ng/dL, and reaching nadir T ≤3 ng/dL in 80% of patients, providing confidence that patients receiving SC-LA can achieve and maintain very low T levels. Potential limitations of these analyses are the assessment of nadir T was not a primary objective of the studies and there may be analytical issues associated with pooling of data from multiple studies. However, these are mitigated as the studies had almost identical designs and objectives, including consistent assessment of T levels and use of a single, central laboratory for the assays.
In conclusion, SC-LA provides consistent, stable, and durable T suppression to levels far below those previously defined as adequate for medical castration in PCa patients. With the increasing understanding of the relevance of nadir T levels during ADT, greater consideration should be given to the drug and formulation selected for initiating ADT based on the available data on T suppression. Future studies should assess if there are clinical benefits of achieving very low nadir T levels and compare T suppression levels between various drugs and formulations.
The authors thank Jocelyn Hybiske, PhD, a consultant at Xelay Acumen, for editorial support. The study was originally sponsored and funded by Tolmar, Inc.
All authors approved the paper in its current form. CMP, PT, JR, JH, DMB, SA, and SE contributed to the overall concept for the paper and participated in writing and editing of the manuscript.
CMP has held consulting or advisory roles for Tolmar, Janssen, Medivation, Bayer, and Dendreon. PT has held a consulting or advisory role for Tolmar. JR has held consulting or advisory roles for Tolmar, Ferring, Bayer, Astellas, Sanofi, Janssen, and GenomeDx. JH has held a consulting or advisory role for Tolmar. DMB and SA are employees of Tolmar Pharmaceuticals, Inc. SE has held consulting or advisory roles for Tolmar, Janssen, and Medivation.
References
- 1.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648–651. doi: 10.1111/j.1464-410X.2009.08814.x. [DOI] [PubMed] [Google Scholar]
- 3.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. AACR. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 4.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–1024. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 5.van der Sluis TM, Bui HN, Meuleman EJ, et al. Lower testosterone levels with luteinizing hormone-releasing hormone agonist therapy than with surgical castration: new insights attained by mass spectrometry. J Urol. 2012;187:1601–1606. doi: 10.1016/j.juro.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 6.Dason S, Allard CB, Wang JG, et al. Intermittent androgen deprivation therapy for prostate cancer: translating randomized controlled trials into clinical practice. Can J Urol. 2014;21(2 Supp 1):28–36. [PubMed] [Google Scholar]
- 7.Djavan B, Eastham J, Gomella L, et al. Testosterone in prostate cancer: the Bethesda consensus. BJU Int. 2012;110:344–352. doi: 10.1111/j.1464-410X.2011.10719.x. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, O’Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33:1151–1156. doi: 10.1200/JCO.2014.58.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamada S, Sakamoto S, Ando K, et al. Nadir testosterone after long-term followup predicts prognosis in patients with prostate cancer treated with combined androgen blockade. J Urol. 2015;194:1264–1270. doi: 10.1016/j.juro.2015.03.120. [DOI] [PubMed] [Google Scholar]
- 11.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 13.Crawford ED, Shore ND, Petrylak DP, et al. Abiraterone acetate and prednisone in chemotherapynaive prostate cancer patients: rationale, evidence and clinical utility. Ther Adv Med Oncol. 2017;9:319–333. doi: 10.1177/1758834017698644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Marreno R, Chu FM, Gleason D, et al. A sixmonth, open-label study assessing a new formulation of leuprolide 7.5 mg for suppression of testosterone in patients with prostate cancer. Clin Ther. 2002;24:1902–1914. doi: 10.1016/s0149-2918(02)80087-x. [DOI] [PubMed] [Google Scholar]
- 15.Chu FM, Jayson M, Dineen MK, et al. A clinical study of 22.5 mg. La-2550: a new subcutaneous depot delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2002;168:1199–1203. doi: 10.1016/S0022-5347(05)64625-3. [DOI] [PubMed] [Google Scholar]
- 16.Sartor O, Dineen MK, Perez-Marreno R, et al. An eight-month clinical study of LA-2575 30.0 mg: a new 4-month, subcutaneous delivery system for leuprolide acetate in the treatment of prostate cancer. Urology. 2003;62:319–323. doi: 10.1016/s0090-4295(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 17.Crawford ED, Sartor O, Chu F, et al. A 12-month clinical study of LA-2585 (45.0 mg): a new 6-month subcutaneous delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2006;175:533–536. doi: 10.1016/S0022-5347(05)00161-8. [DOI] [PubMed] [Google Scholar]
- 18.Shore ND. Experience with degarelix in the treatment of prostate cancer. Ther Adv Urol. 2013;5:11–24. doi: 10.1177/1756287212461048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 20.Taplin ME, Montgomery B, Logothetis CJ, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32:3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shore ND, Chu F, Moul J, et al. Polymer-delivered subcutaneous leuprolide acetate formulations achieve and maintain castrate concentrations of testosterone in four open-label studies in patients with advanced prostate cancer. BJU Int. 2017;119:239–244. doi: 10.1111/bju.13482. [DOI] [PubMed] [Google Scholar]
- 22.Hussain M, Fizazi K, Saad F, et al. PROSPER: safety and efficacy study of enzalutamide in patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) Presented at GU-ASCO; February 8-10, 2018 San Francisco, CA.
- 23.Small JE, Saad F, Chowdhury S, et al. SPARTAN, a phase 3 double-blind, randomized study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC) Presented at GU-ASCO; February 8-10, 2018, San Francisco, CA. [Google Scholar]