Abstract
Helminth infections represent a significant public health concern resulting in devastating morbidity and economic consequences across the globe. Helminths migrate through mucosal sites causing tissue damage and the induction of type 2 immune responses. Anti-helminth protection relies on the mobilization and activation of multiple immune cells including type 2 innate lymphocytes (ILC2s), basophils, mast cells, macrophages and hematopoietic stem/progenitor cells. Further, epithelial cells and neurons have been recognized as important regulators of type 2 immunity. Collectively, these pathways stimulate host-protective responses necessary for worm expulsion and the healing of affected tissues. In this review, we focus on the innate immune pathways that regulate immunity to helminth parasites and describe how better understanding these pathways may lead to the development of new therapeutic strategies.
Keywords: Type 2 cytokines, innate immunity, mucosal immunology, helminth parasites
Type 2 inflammation promotes host-protective responses to helminths
Helminth infections constitute a serious public health concern affecting approximately one third of the global population, particularly in impoverished areas of Africa, Asia and South America[1, 2]. These high rates of infection are driven by a wide diversity of helminths that can cause human disease including but not limited to Ascaris lumbricoides (roundworm), Trichuris trichiura, Trichinella spiralis (whipworms), Ancylostoma duodenale, Necator americanus (hookworms), Strongyloides stercoralis as well as different Schistosoma and microfilariae species. Helminths often establish chronic infections resulting in anemia, malnutrition, growth impairment, cognitive deficiencies and immunopathology. Despite the implementation and effectiveness of anti-helminth medications, high reinfection rates are thought to limit their longterm impact. For example, recent studies have shown that approximately 50% of patients treated with anti-helminths will become reinfected within six months of receiving the medication[3]. In addition to humans, helminth infections are known to have devastating consequences on livestock populations. In order to combat this issue, many farmers have continuously administered anti-helminthic drugs resulting in the increased prevalence of drugresistant parasites[4]. Further, the generation of effective vaccines against helminths has been hindered due to the complexity of their life cycles, the antigenic variation of helminths at their distinct developmental stages and the multiple immunomodulatory mechanisms utilized by helminths to dampen host-protective responses[5]. Although several potential vaccine candidates against human and ruminant helminth pathogens have been described[6, 7], additional clinical studies are required to determine their effectiveness and ability to generate strong host-protective responses. Collectively, these facts highlight the urgent need for novel and more effective therapeutic strategies. In order to inform the development of new treatment options, research has focused on better understanding the interactions of various helminths with their mammalian hosts. Since studying helminth infections in human patient populations is difficult, many studies have relied on different animal models of infection including Nippostrongylus brasiliensis, Heligmosomoides polygyrus, Trichinella spiralis, Trichuris muris, Brugia malayi, Strongyloides venezuelensis and Schistosoma mansoni (Table 1). The following sections will highlight the many important discoveries these murine models have uncovered.
Table 1.
Experimental animal models of human helminth infections
| Animal model | Human infection mimicked | Form of Transmission | Route of administration | Affected compartment |
|---|---|---|---|---|
| Nippostrongylus brasiliensis |
Ascaris lumbricoides Ancylostoma duodenale Necator americanus |
Larvae | Subcutaneous | Skin, Lungs, Intestinal Tract |
| Heligmosomoides polygyrus | Larvae | Oral | Intestinal Tract | |
| Strongyloides venezuelensis | Strongyloides stercoralis | Larvae | Subcutaneous | Skin, Lungs, Intestinal Tract |
| Trichuris muris | Trichuris trichiura | Egg | Oral | Intestinal Tract |
| Trichinella spiralis | Trichinella spiralis | Larvae | Oral | Intestinal Tract, Skeletal Muscle |
| Schistosoma mansoni | Schistosoma spp. | Cercariae | Percutaneous Exposure Subcutaneous | Skin, Liver, Lungs |
| Brugia malayi | Lymphatic filariasis | Larvae | Subcutaneous | Blood, Pleural cavity |
Helminths can be transmitted to both humans and animals via the ingestion of eggs or larvae present in contaminated food or water, insect bite, or by direct penetration of immature larvae through the skin[2]. Upon infection, helminths can cause substantial tissue damage and produce excretory-secretory (ES) products (see Glossary) as they migrate through different organs, including the lungs, intestines, liver, and skin, to complete their life cycles. Collectively, helminth-induced wounding and the production of ES products are known to activate distinct hematopoietic and non-hematopoietic cells resulting in the initiation of type 2 immune responses characterized by the population expansion and activation of innate immune cells including dendritic cells (DCs), basophils, eosinophils, mast cells (MCs), ILC2s, and specialized hematopoietic stem/progenitor cells (HSPCs). These early events lead to the secretion of cytokines such as interleukin (IL)-4, IL-5, IL-9 and IL-13 that promote the polarization of alternatively activated or M2 macrophages, the activation of type 2 T helper (TH2) cells and immunoglobulin E (IgE)-producing B cells, which contribute to the development of inflammation (Figure 1). Collectively, these cellular and molecular events initiate host-protective responses such as mucus production by goblet cells, smooth muscle contraction and the proliferation of epithelial cells that stimulate helminth expulsion and promote the healing of affected tissues. In addition to these resistance mechanisms, type 2 immunity also promotes tolerogenic responses, such as the formation of granulomas, which contain parasite infection to sub-clinical levels. In summary, type 2 cytokine responses are known to be critically important in providing protection against helminth parasites.
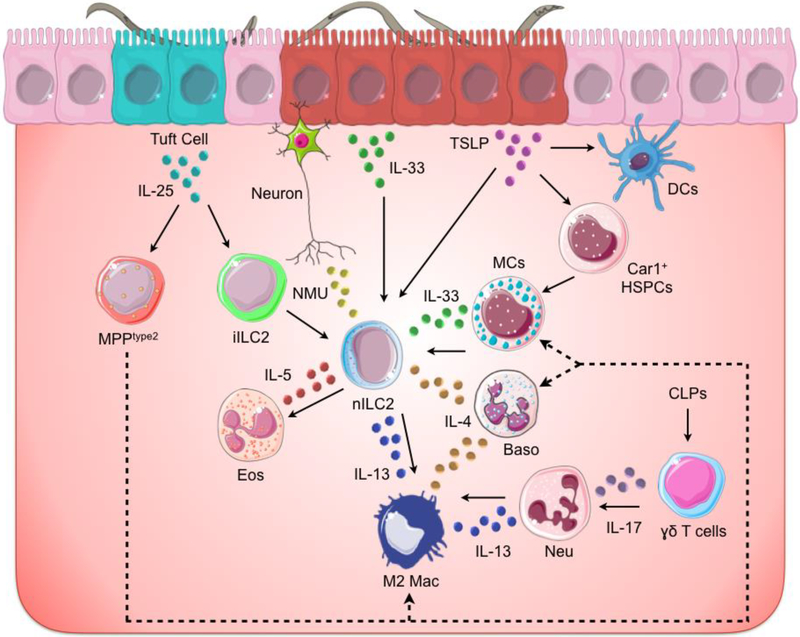
Figure 1. Initiation of anti-helminth responses at barrier surfaces.
Helminths affect barrier surfaces such as the lungs, gut and skin, causing tissue damage. In turn, epithelial cells release cytokine alarmins including IL-25, IL-33 and TSLP, which activate multiple innate immune cells including type 2 innate lymphoid cells (ILC2s), mast cells (MCs), basophils (Baso), γδ+ T cells, neutrophils (Neu) and dendritic cells (DCs). Further, cytokine alarmins induce the recruitment of hematopoietic stem/progenitor cells (HSPCs), such as type 2 multipotent progenitor cells (MPPtype2), that differentiate (dotted line) into innate immune cells and contribute to helminth-induced inflammation. In addition to epithelial cells, neurons have been recently shown to respond to helminth-derived products and initiate type 2 responses. Collectively these signals result in the secretion of type 2 cytokines including IL-4, IL-5 and IL-13. In turn, these cytokines promote host-protective responses such as the polarization of M2 macrophages (M2 mac) and the recruitment of eosinophils (Eos) resulting in the expulsion of the parasites and the healing of the affected tissues.
In contrast to helminth infections, type 2 inflammation, when uncontrolled, is also known to promote inflammation associated with allergies and asthma. Therefore, studies investigating the mechanisms that regulate allergic responses have helped to inform our understanding of helminth-induced inflammation[8]. Although the purpose of this article is to highlight recent studies describing the initiation of host-protective responses following helminth challenge, we have also highlighted some reports focused on allergic inflammation when similar aspects of type 2 immunity may be operational in the context of helminth infections. Further, since the contributions of TH2 cells and IgE-producing B lymphocytes to anti-helminth immunity are well established and have been extensively reviewed previously[9, 10], they will not be heavily covered in this article. Instead, we will focus on innate immune pathways and highlight the recently identified roles that epithelial cells and neurons[11, 12] play in promoting anti-helminth immunity. Finally, we will describe how these advances may inform new therapeutics to treat helminth infections and their associated morbidities.
More than just a barrier: epithelial cells.
Epithelial cells have important functions at mucosal sites such as the absorption of nutrients, the protection of the underlying tissues and the sensing of microbial pathogens. Further, previous studies have established that highly specialized goblet cells respond to IL-13 and secrete vast amounts of mucus, preventing the attachment of helminths to the mucosal epithelium and facilitating their expulsion. Epithelial cells are also known to produce resistin-like molecule beta (RELMβ) that binds components of the sensory apparatus of helminths and inhibits their chemotactic function in vitro[13, 14]. Moreover, paneth cell populations have been described to expand following helminth infections[15], although their precise contribution to anti-helminth immunity remains to be determined. In addition to their direct effector functions, novel studies have highlighted the pivotal role epithelial cells play as initiators of anti-helminth immunity via their ability to influence immune cell populations. Epithelial cells are reported to secrete multiple cytokine alarmins in response to helminth-induced inflammation including IL-25, IL-33 and thymic stromal lymphopoietin (TSLP)[16]. These cytokines induce the development, proliferation and activation of terminally differentiated innate cell populations (ILC2s, basophils, MCs, eosinophils and DCs) and HSPCs in the early phases of helminth infections. Further, epithelial cells secrete chemokines such as CXCL1, CXCL2 and CXCL8, as well as eotaxins that are known to attract neutrophils and eosinophils from the periphery to mucosal sites in response to inflammatory insults[17]; however, the precise contributions of epithelial-derived chemokines to anti-helminth immunity remain to be defined. The following sections will highlight the important role epithelial cell-derived alarmins play in promoting antihelminth responses.
The importance of alarmins to host-protective responses following helminth infection has been well defined. For example, IL-25 has proven to be necessary and sufficient to promote efficient worm expulsion and potent type 2 cytokine responses following N. brasiliensis, T. muris or T.spiralis infection[18–20]. IL-25 is reported to have several cellular targets, many of which are known to promote type 2 cytokine production and anti-helminth responses. For example, IL-25 has been shown to induce the accumulation of type 2 multipotent progenitor cells (MPPtype2), which promote anti-helminth immunity by differentiating into macrophages, MCs and basophils[21, 22]. Similarly, IL-25 is reported to stimulate the differentiation of intestinal ILC progenitors, defined as ‘inflammatory ILC2s’, following infection with N. brasiliensis[23, 24]. IL25-induced inflammatory ILC2s were shown to migrate into the lungs where they differentiated into mature ILC2s and contributed to the wound healing of pulmonary tissue, suggesting that IL-25 might promote type 2 responses by acting on terminally differentiated cells as well as specific HSPCs. Remarkably, intestinal epithelial tuft cells have been recently recognized as the main producers of IL-25 upon infection with N. brasiliensis and H. polygyrus. In turn, tuft cell-derived IL-25 promoted the secretion of IL-13 by ILC2s, which stimulated epithelial cell turnover in a positive feedback loop that resulted in worm clearance and tissue repair at sites of infection[14, 25]. Further, mice deficient in tuft cells exhibited delayed worm expulsion and impaired mucus production[26], demonstrating that specialized epithelial cell populations coordinate anti-helminth protection via their production of alarmins.
IL-33 is a DNA-binding protein that is actively released by the epithelial cell barrier and some hematopoietic cells (MCs and macrophages) in response to helminth-induced damage[16, 27, 28]. Once in the extracellular space, IL-33 is cleaved by MC- and neutrophil-derived proteases into bioactive forms, which signal through the T1/ST2 receptor expressed on immune cells such as ILC2s, basophils, MCs, and TH2 cells. The essential role of IL-33 in anti-helminth immunity has been illustrated by studies demonstrating that mice deficient in T1/ST2-mediated signaling have increased worm burdens and reduced type 2 responses following infection with N. brasiliensis, S. mansoni or S. venezuelensis[29–31]. Further, gain-of-function studies have shown that exogenous IL-33 enhances type 2 cytokine responses and accelerates expulsion of T. muris[32]. Collectively, these studies demonstrate that IL-33 is necessary and sufficient to promote host-protective responses to some helminth parasites. However, further studies are still required to determine the relative importance of non-hematopoietic versus hematopoietic sources of IL-33 to the development of host-protective responses following infection.
Considering the important role that IL-33 has been shown to play in promoting immunity to helminths, it is not surprising that parasites have developed mechanisms to interrupt IL-33-IL33R signaling. For example, ES products secreted by H. polygyrus have been described to inhibit type 2 inflammation by blocking the release of IL-33[33]. Size fractionation and protein analysis of ES products identified the presence of a 251 aminoacid peptide that was able to bind to active forms of human and murine IL-33 and thus was named H. polygyrus alarmin release inhibitor (HpARI)[34]. Remarkably, HpARI was demonstrated to bind to the DNA within necrotic cells, resulting in the retention of IL-33 and preventing its release. Further, it was shown that treatment with HpARI was sufficient to inhibit type 2 inflammatory responses induced by inhalation of Alternaria allergens or following N. brasiliensis infection[34], demonstrating the capacity of helminth-derived ES products to modulate inflammation by targeting this specific alarmin.
Under homeostasis, TSLP is constitutively expressed by thymic epithelial cells and functions as a growth factor for T and B lymphocytes. Like IL-25 and IL-33, epithelial cell-derived TSLP is reported to promote type 2 cytokine responses following helminth-induced tissue injury via its effects on basophils, MCs and ILC2s[16]. Additionally, TSLP is reported to regulate the polarization of TH2 cells by suppressing IL-12/23p40 production and promoting surface expression of OX40L by DC populations[16]. Further, TSLP has been shown to stimulate extramedullary hematopoiesis following infection with T. spiralis [35], demonstrating that TSLP promotes anti-helminth immunity via distinct cellular mechanisms. Importantly, TSLPR deficiency did not result in impaired type 2 cytokine responses or worm clearance following infection with S. mansoni, N. brasiliensis or H. polygyrus[36, 37] but was required for efficient expulsion of T. muris and T. spiralis[38, 39]. These interesting results suggest that the importance of TSLP may be parasite-specific. It has been suggested that the specificity of these responses may depend on whether TSLP is required to inhibit IL-12/23p40 production or whether the parasite is capable of directly inhibiting IL-12/23p40 via secretion of ES products. For example, it has been shown that antibody-mediated neutralization of IL-12/23p40 or IFNγ is sufficient to restore the clearance of T. muris in TSLPR-deficient mice[36, 38], suggesting that T. muris-induced TSLP is needed to limit detrimental type 1 responses. By contrast, TSLP may be dispensable following N. brasiliensis and H. polygyrus infection since these parasites are known to produce ES products capable of directly inhibiting IL-12/23p40 by DCs[36]. These studies further illustrate the complexity of host-parasite interactions and demonstrate the need to better understand parasite-specific responses.
In addition to cytokines, epithelial cells have also been shown to release danger-associated molecular patterns (DAMPs) such as ATP and adenosine that regulate the induction of type 2 responses. Specifically, recent studies have demonstrated that necrotic epithelial cells secrete ATP, which activates MCs via P2X7 receptor and promotes their secretion of IL-33 following H. polygyrus infection[28]. Additionally, extracellular ATP can be processed into adenosine, which signals through the A2B adenosine receptor (A2BAR) thereby promoting ILC2 and M2 macrophage activation[40, 41]. Further, A2BAR-deficiency impaired helminth expulsion following H. polygyrus infection[42], demonstrating that epithelial cells can also regulate antihelminth immunity via danger-associated signals. Future studies are required to determine the specific signaling pathways activated by ATP and adenosine to promote anti-helminth immunity.
Similar to their ability to target cytokine alarmin pathways, recent studies have also demonstrated that helminths modulate pathways activated by DAMPs to inhibit type 2 cytokine-mediated inflammation. Specifically, H. polygyrus and T. muris infections, or treatment with soluble schistosoma egg antigens were found to activate the NLRP3 inflammasome leading to the secretion of IL-1β and IL-18, a signaling cascade commonly activated by DAMPs[43–45]. In contrast to its protective role in the context of bacterial and viral infections, helminth-induced NLRP3 activation was found to inhibit type 2 immunity since NLRP3-deficient mice, IL-1β-deficient mice or mice treated with a soluble IL-1βR antagonist (Anakinra) exhibited elevated type 2 inflammatory responses that correlated with accelerated worm expulsion[43, 46]. Remarkably, it was also demonstrated that helminth-derived ES products were sufficient to induce NLRP3 activation and secretion of IL-1β and IL-18 in vitro and in vivo[43, 46], suggesting that helminths have evolved to manipulate these pathways in order to dampen host-protective responses.
A network of first responders: type 2 innate lymphoid cells (ILC2s).
Strategically positioned at mucosal surfaces including the lungs, intestines and skin, innate lymphocytes have been recently recognized as essential mediators of innate immune responses to bacterial, viral and parasitic infections[47]. According to their cytokine production as well as their expression of transcription factors, innate lymphoid cells are classified into ILC1s, ILC2s and ILC3s resembling their adaptive lymphocyte counterparts, TH1, TH2 and TH17 cells[47]. Despite lacking the expression of classical lymphoid or myeloid lineage markers, ILC2s can be identified by their expression of surface markers including CD90, CD127, ST2, CRTH2 and KLRG1[47]. Additionally, ILC2s characteristically express the TH2associated transcription factor GATA-3, which has been shown to regulate their development along with other factors such as PLZF, RORα and Id2[47]. Nonetheless, the cellular and molecular events that regulate ILC2 development remain to be fully defined. ILC2 progenitors have been identified in the fetal liver as well as adult bone marrow; however, mature tissueresident ILC2s show high proliferative potential, suggesting that in situ proliferation maintains ILC2 populations during adult life[47]. Conversely, recent studies have reported that IL-25-induced ‘inflammatory’ ILC progenitors migrate from the gastrointestinal tract into the lungs and differentiate into mature ILC2s following N. brasiliensis infection[23], suggesting that helminth-induced inflammation might promote the trafficking of ILC progenitors, which contribute to the pool of tissue-resident ILC2s[24]. Despite these advances, further studies are needed to better define the molecular mechanisms that regulate ILC2 development and trafficking between mucosal surfaces in the context of various helminth infections.
Proliferation and activation of ILC2s following helminth infection are reported to be regulated by cytokine alarmins (IL-25, IL-33 and TSLP), type 2 cytokines (IL-4 and IL-9), or by inflammatory lipid mediators such as prostaglandin D2[47, 48]. In turn, activated ILC2s secrete effector cytokines such as IL-5, IL-9 and IL-13, which promote specific anti-helminth responses including eosinophil recruitment, M2 polarization and mucus production by goblet cells[47]. In particular, ILC2-derived IL-9 has been shown to act in an autocrine manner enhancing the cytokine production and survival of ILC2s following N. brasiliensis infection thereby promoting worm expulsion and tissue repair[49, 50]. Further, ILC2s have been shown to promote wound healing via their production of amphiregulin and arginase 1 in the context of allergic inflammation and viral infection[51–53], suggesting that ILC2s may also contribute to tissue remodeling in the context of helminth infections. In addition to their innate effector mechanisms, ILC2s contribute to the establishment of adaptive immune responses to helminths. For example, it has been demonstrated that ILC2s express type 2 cytokines, MHCII and co-stimulatory molecules, all of which were required to activate T lymphocytes in vitro[54–57]. Moreover, ILC2s promote worm expulsion in a MHC-II-dependent manner when transferred into IL-13-deficient mice[54]. Likewise, ILC2-derived IL-13 has been reported to induce the production of the TH2-attracting chemokine CCL17 by DCs[56], suggesting that ILC2s contribute to long-term TH2 polarization through multiple mechanisms. Collectively, these studies demonstrate the important roles of ILC2s to host-protective responses following N. brasiliensis infection. However, little is known of their role in the context of other helminth infections. Although it has been shown that ILC2 populations expand and contribute to antihelminthic immunity following infection with H. polygyrus, T. spiralis, S. venezuelensis and schistosoma species[28, 57–60], their mechanism of action and specific contributions to protective immunity remain less well defined.
Getting to the root of the problem: neurons
The coordinated responses of epithelial cells and ILC2s highlight the important contributions of both hematopoietic and non-hematopoietic cells in promoting host-protective responses to helminths. However, recent studies have now revealed that in addition to epithelial cells, neuron-derived signals also play an important role in regulating helminth-induced inflammation. Specifically, the receptor for the neural peptide neuromedin U (NMU) was found to be exclusively expressed by ILC2s when compared to other lymphoid and myeloid cell lineages[11, 12, 61]. Remarkably, NMU stimulation was sufficient to activate ILC2s in vitro as well as in vivo and was shown to promote worm expulsion in mice infected with N. brasiliensis. Moreover, conditioned media from enteric cholinergic neurons activated with helminth-derived products was sufficient to activate ILC2s, suggesting that helminths might directly induce NMU production and thereby initiate ILC2 responses[11, 12, 61]. Further, the β2-adrenergic receptor (β2AR), which interacts with the neurotransmitter epinephrine, was also found to be expressed by ILC2s[62]. In contrast to the activating effect of NMU, however, treatment with β2AR agonists abolished ILC2 responses and impaired worm expulsion in N. brasiliensisinfected mice. Further, β2AR-deficient mice had reduced worm burdens, increased eosinophilia, and elevated mucus production[62], demonstrating that β2AR-dependent signals negatively regulate ILC2 activation.
These new studies compliment previous reports revealing that mice deficient in the muscarinic receptor M3, which binds to the neurotransmitter acetylcholine, exhibited delayed helminth expulsion and reduced type 2 responses following infection with N. brasiliensis[63, 64]. Further, CD4+ T cells were found to express the M3 muscarinic receptor and the adoptive transfer of M3 receptor-deficient T cells taken from N. brasiliensis-infected mice did not provide protection to naïve mice upon infection when compared to receptor-sufficient CD4+ T cells[63]. Remarkably, helminths secrete acetylcholinesterases, which are known to cleave and terminate the activity of acetylcholine and have been shown to inhibit type 2 inflammation[65, 66]. Collectively, these studies suggest that helminths might regulate host-protective responses by secreting factors that interfere with the cooperative actions of neurons and the immune system.
In addition to the regulation of immune responses by the central nervous system, enteroendocrine cells might also represent an additional level of regulation that links the mucosal epithelium to immune cell activation. Enteroendocrine cells (EEC) are highly specialized epithelial cells that respond to different stimuli in the mucosal lumen such as nutrients, and secrete a number of peptide hormones including cholecystokinin (CCK), somatostatin and ghrelin, as well as bioactive amines like serotonin (5-HT), that regulate distinct physiological events to enable the efficient absorption of nutrients[67]. The possible contribution of EECs was first suggested in early studies reporting a reduction in food intake by lambs infected with Trichostrongylus colubriformis and pigs infected with Ascaris suum that was reversed by the administration of a specific CCK antagonist[67, 68]. Similarly, mice infected with T. spiralis or T. muris exhibited reduced food intake rates that correlated with elevated levels of CCK and 5-HT[69–71]. Interestingly, these observations were not present in mice deficient in adaptive lymphocytes and the adoptive transfer of CD4+ T cells was sufficient to re-establish the loss of food intake as well as the secretion of CCK and 5-HT in severe combined immunodeficient (SCID) mice. Further, EECs were found to express the IL-4Rα, suggesting that EECs may respond to type 2 cytokine responses and regulate calorie intake upon helminth infection[69, 70]. Remarkably, T. spiralis-infected mice also exhibited diminished levels of the hormone leptin, which has been shown to induce hypophagia, promote type 1 responses, and suppress type 2 inflammation[71]. Moreover, T. spiralis-infected mice treated with leptin exhibited increased worm burdens that were associated with reduced levels of type 2 cytokines[71]. These exciting reports have revealed previously unappreciated regulators of helminth-induced inflammation; however, further studies are required to determine if additional neuronal- and hormone-derived signals can promote and/or inhibit type 2 cytokine-mediated inflammation. Studies of this nature are likely to identify targets with great therapeutic potential (Figure 2). Further, these studies highlight the complex relationships that exist between the mucosal epithelium and the nervous, endocrine and immune systems as well as illustrate how highly coordinated communication event regulate host-protective responses to helminth parasites.
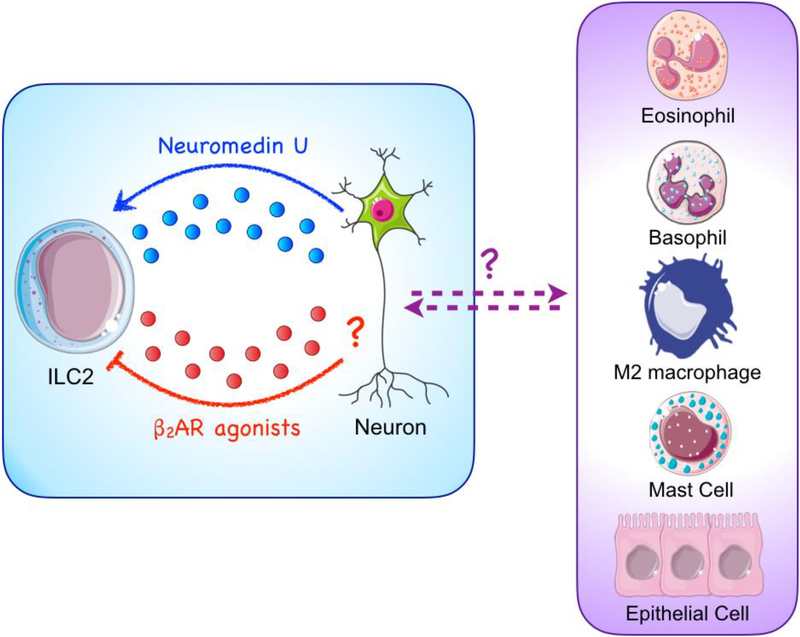
Figure 2. Neuronal regulation of type 2 responses.
Novel studies have demonstrated that neuron-derived signals modulate type 2 responses. Neuronal expression of Neuromedin U has been shown to activate ILC2s and promote anti-helminth immunity. Conversely, β2-adrenergic receptor (β2AR) agonists were shown to inhibit ILC2 responses, suggesting that neuronderived epinephrine may regulate ILC2s. Collectively, these studies suggest that neurons may positively and negatively regulate ILC2 responses. However, whether neurons and other effector cell populations engage in crosstalk that is capable of influencing helminth-induced inflammation remains to be fully defined.
Underappreciated but not forgotten: basophils
Basophils are potent cytokine-producing granulocytes that contribute to type 2 responses via their secretion of cytokines such as IL-4, IL-6 and IL-13, as well as inflammatory lipid mediators including leukotrienes and prostaglandins that activate distinct components of anti-helminth immunity such as M2 macrophages[72]. Although basophils constitute less than 1% of blood leukocytes under homeostatic conditions, peripheral basophilia is a hallmark of helminth infections, suggesting that basophils contribute to host-protective responses[72]. Further, infiltrating basophils appear to be required for granuloma formation after S. mansoni infection[73, 74], suggesting that basophils may also contribute to helminth-induced tissue remodeling. While the precise mechanisms that govern basophilia remain to be fully defined, developmental studies have reported that basophil precursor cells reside in both the bone marrow and periphery and respond to helminth-induced expression of IL-3 and/or TSLP[39, 75]. For example, basophil populations expand and activate in response to IL-3 following infection with N. brasiliensis, H. polygyrus and S. venezuelensis[75–77]. Moreover, TSLP has been reported to contribute to basophil expansion following T. spiralis and T. muris infections [39, 78]. Interestingly, models of basophil depletion have demonstrated that basophils do not appear to promote worm expulsion in the context of N. brasiliensis or H. polygyrus infections but appear to contribute to reduce parasite burdens following T. spiralis or T. muris infections[39, 75–79]. Studies have suggested that these parasite-specific differences may reflect the distinct phenotypes recognized in basophils that develop in a TSLP-rich environment[78, 80]. Specifically, it was shown that basophil precursor cells that matured in the presence of TSLP and IL-3 were more responsive to IL-33 stimulation than those matured in the presence of IL-3 alone. These data suggest that helminth parasites that promote high levels of infection-induced TSLP, in addition to IL-3, may promote a functionally distinct basophil compared to helminths that do not promote high levels of TSLP expression. Beyond being activated by infection-induced cytokines, specific helminth-derived molecules such as proteases have also been reported to directly activate basophils[81], further suggesting that the role of basophils in promoting anti-helminth immunity may be parasite-specific.
Basophils have also been recognized for their ability to assist in the optimal polarization of TH2 cells via their expression of MHC-II and secretion of IL-4[82–84]. Although MHCII-expressing basophils are recruited to the lymph nodes following helminth infection[83, 85], in vivo fluorescent imaging revealed that basophil-T cell interactions in the lymph node were short and unstable unlike those established between T cells and professional APCs[79]. Conversely, basophils established prolonged serial interactions with lung-resident T cells[79], suggesting that basophils may influence T cells differently in specific tissue environments. In addition to their interactions with adaptive lymphocytes, emerging studies have also described the complex relationship between basophils and innate lymphoid cells. For example, basophils and ILC2s have been found to be co-present in many inflamed tissues[86, 87]. Further, basophils have been shown to activate ILC2s via their production of IL-4, inducing eosinophil recruitment and allergic inflammation[86, 87]. Moreover, basophils are potent producers of prostaglandins and leukotrienes[88], which have been reported to activate ILC2s during allergic responses[48, 89, 90]. Collectively, these studies suggest that basophils and ILC2s may cooperate to promote type 2 inflammatory responses following helminth infections. However, further studies are required to better define the ability of basophils to regulate ILC2 responses and to determine whether they do so directly via cytokines production and/or indirectly by modulating helminth-induced regulatory signals of epithelial and neuronal cells.
The sections above were focused on the activation of basophils in the context of primary helminth infections. However, it is also well established that basophils express the high-affinity IgE receptor FcεR1, which can bind antigen-specific IgE and become activated via FcεR1-crosslinking upon secondary exposure to antigens [72]. Therefore, despite being dispensable for worm expulsion during primary infection, IgE-mediated activation of basophils is required for optimal TH2 polarization and protective immunity following reinfection with N. brasiliensis and H. polygyrus[77, 79, 91]. Further, depletion of basophils or deletion of FcERI on their surface resulted in diminished M2 macrophage polarization, reduced larval trapping in the skin and increased lung injury upon reinfection with N. brasiliensis[92]. In summary, basophils are capable of regulating host-protective responses following both primary and secondary exposure to helminths.
Always a contributing factor: mast cells
MCs are tissue-resident granulocytes that, similar to basophils, are capable of contributing to anti-helminth immunity via their secretion of type 2 cytokines and other effector molecules. Further, MC populations dramatically expand in peripheral tissues after multiple helminth infections[88], suggesting that MCs are important mediators of anti-helminth responses. MC progenitor cells are reported to differentiate into either mucosal MCs or connective tissue MCs, distinguishable based on the expression of mast-cell associated proteases (mcpt)[88]. Although the precise pathways governing MC development remain to be fully determined, important studies have identified a distinct lineage-committed mast cell progenitor that expands in response to infection-induced cytokines such as IL-3[93]. MC progenitors exit the bone marrow as immature cells and enter peripheral tissues where they finish their differentiation program. Importantly, stem cell factor (SCF) and its receptor c-Kit are critical for the differentiation of mature mucosal mast cells[88]. In the tissues, MCs are long-lived and exhibit in situ proliferation as well as re-granulation following stimulation[88]. MC development has been reported to be regulated by the timely expression of transcription factors such as GATA-2 and C/EBPα[88]. Additionally, recent studies have also highlighted the contribution of carbonic anhydrase (Car) enzymes to the differentiation of mucosal MCs in the context of type 2 inflammation[94]. Car1 and Car2 were specifically upregulated in bone marrow-derived MCs but not in other granulocytes such as basophils. Further, pharmacological inhibition of Car enzymes resulted in reduced MC differentiation in vitro and impaired intestinal MC responses following T.spiralis infection, suggesting that Car enzymes operate as important regulators of MC responses following infection[94].
MCs can be activated in response to cytokines including IL-3, IL-9, IL-33 and TSLP, resulting in their degranulation and rapid release of inflammatory mediators like histamine, leukotrienes and prostaglandins; cytokines such as IL-4, IL-6 and TNFα; as well as proteases including mcpt1 and mcpt2, which are well established markers of mast cell activation[88]. These effector molecules in turn induce the polarization of M2 macrophages, activate smooth muscle cell contraction, promote mucus production and increase the permeability of the intestinal epithelium leading to the expulsion of parasitic larvae[88, 105]. Additionally, it is thought that MCs might directly attack the parasites or prevent their attachment to the epithelium by their release of mcpt2 and glycosaminoglycans[95–97]. MCs can also respond to signals released following epithelial cell damage such as ATP and activate ILC2s through their secretion of prostaglandin D2[48, 98] and IL-33[28], thereby promoting anti-helminth responses. ILC2s and MCs are known to further communicate via IL-9-mediated mechanisms. Specifically, ILC2s secrete vast amounts of IL-9 following N. brasiliensis infection[99]. IL-9 is a well-known growth factor of MCs that stimulates its own production in an autocrine manner, thereby promoting MC survival and cytokine expression in the context of type 2 inflammation[100]. Perhaps most importantly, MCs have been demonstrated to stimulate the secretion of IL-25, IL-33 and TSLP by the intestinal epithelium following H. polygyrus and T. muris infection[101]. In addition to regulating the release of alarmins such as IL-33, MC-derived proteases are also capable of cleaving IL-33 into its bioactive form[102].
Not surprisingly given their infection-induced population expansion and vast array of effector functions, previous studies have identified important contributions of MCs to anti-helminth immunity. For example, studies employing KitW-sh/W/sh and the KitW/Wv animal models, which express a truncated version of c-Kit and therefore lack MC populations, suggest that MCs contribute the expulsion of H. polygyrus, S. venezuelensis, T. spiralis and T. muris[88]. However, it is important to note that KitW-sh/W/sh mice are known to have reduced intestinal peristalsis and diminished IgE levels that may also alter anti-helminth responses, therefore, more mast-cell specific studies are still required. Nonetheless, it has been shown that mcpt1-deficiency prevented worm clearance and resulted in elevated larvae colonization of skeletal muscles following T. spiralis infection[103]. Conversely, selective depletion of connective tissue MCs did not alter worm expulsion[104], suggesting that mucosal and connective tissue MCs might have distinct contributions to anti-helminth responses. In summary, like many other factors discussed above, the role of mast cells in promoting host-protective response to helminths may be situation- and parasite-specific and has been comprehensively reviewed in the following review articles that explore this topic in more depth[88, 105].
More than bystanders: eosinophils
Like basophils, eosinophil populations expand and accumulate in peripheral tissues in the context of helminth infection[106]. During homeostasis, eosinophil development occurs in the bone marrow, where eosinophil progenitors differentiate in a stage-wise manner following exposure to cytokines such as IL-3, IL-5 and GM-CSF, which induce the activation of transcription factors including GATA-1, PU.1 and C/EBPα[106]. Similarly, eosinophil development requires the proper synthesis of eosinophil-specific cationic proteins and assembly of intracellular granules[107–109], suggesting that eosinophil maturation is dependent on extrinsic and intrinsic signals. Once mature, eosinophils are reported to infiltrate non-esophageal intestinal compartments in response to eotaxin-1, which is regulated by ILC2s activated via the circadian synchronizer vasoactive intestinal peptide (VIP)[110]. Among other soluble factors, IL-5 is instrumental for eosinophil responses, since IL-5 stimulates eosinophil differentiation and extravasation from the bone marrow, enhances eosinophil responsiveness to eotaxin-1 and enhances their survival in mucosal sites during type 2 inflammation[106]. Further, IL-5Rα+ lineage committed eosinophil progenitors accumulate during allergic inflammation[111], suggesting that similar extramedullary eosinophilopoiesis may occur following helminth-induced inflammation.
Eosinophils mediate rapid and potent responses to diverse stimuli by secreting crystalline granules containing preformed cationic proteins such as eosinophil peroxidase (EPX), eosinophil derived neurotoxin (EDN), eosinophil cationic protein (ECP) and major basic protein 1 (MBP-1); cytokines including IL-4, IL-6, IL-10 and IL-13; chemokines as well as growth factors like TGFβ and VEGF[112]. Collectively, these soluble mediators activate anti-helminth mechanisms such as the polarization of M2 macrophages, the secretion of mucus and the contraction of smooth muscle cells[106]. Eosinophils release their granules via three main secretory mechanisms, namely classical exocytosis, cytolysis with granule release and piecemeal degranulation. Classic exocytosis occurs when eosinophils attach to the parasitic cuticle, inducing the fusion of granules to generate a secretory channel that releases the granule contents directly into the helminth[112]. However, this mechanism has only been observed in vitro[113] and its role in vivo requires further investigation. Conversely, eosinophils can undergo a cytolytic death pathway, which culminates in the release of DNA nets consisting of intact membrane-bound granules containing cytokine and chemokine receptors[112]. These extracellular structures have been found in tissue samples of eosinophil-driven allergic patients[114], suggesting that eosinophils might employ this mechanism to cooperate with other effector cells rather than being used to directly attack the parasite. Piecemeal degranulation refers to the selective transport and release of specific granule contents into small secretory vesicles, which are then released into the extracellular space[112]. In particular, IL-4 and IL-4Rα complexes were found to be specifically translocated to secretory vesicles upon stimulation of eosinophil with eotaxin-1[115], suggesting that piecemeal degranulation might be critical for the release of cytokines by activated eosinophils.
The contributions of eosinophils to helminth-induced protective responses also appear to be situation- and parasite-specific. For example, elevated eosinophil responses are associated with the clearance of Ascaris suum in infected pigs, an animal model for human infections with A. lumbricoides, and serum-activated eosinophils were shown to kill A. suum larvae in vitro[116, 117]. Further, eosinophil-deficient mice showed increased worm burdens following primary infections with H. polygyrus as well as B. malayi and upon secondary infection with N. brasiliensis and T. spiralis[118–121], suggesting that eosinophils contribute to anti-helminth immunity across different mammalian hosts. However, parasite expulsion was not impaired upon primary infection with N. brasiliensis, T. spiralis, T. muris or S. mansoni in mice lacking eosinophils[118, 122, 123], illustrating that the role of eosinophils to anti-helminth responses is complex. Although the mechanisms through which eosinophils operate remain to be fully defined it has been shown that eosinophils elevate their expression of MHC-II and costimulatory molecules upon T. muris infection[122], suggesting that eosinophils may regulate adaptive immune responses. Further, it has been demonstrated that eosinophil-deficient mice exhibited reduced numbers of newborn T. spiralis larvae in skeletal muscles, which correlated with diminished TH2 cell infiltration and elevated iNOS expression [124, 125]. Moreover, eosinophil-derived IL-10 and IL-4 were found to promote larval growth in the skeletal muscle by induction of regulatory T cells and suppression of STAT1-mediated inflammation[126, 127]. Collectively, these data demonstrate that eosinophils also contribute to tolerogenic responses to helminths.
License to kill: neutrophils
Although typically associated to anti-bacterial and viral immunity, neutrophils rapidly accumulate following the acute lung injury caused by N. brasiliensis infection[128]. N. brasiliensis-induced neutrophil recruitment was shown to be dependent on γδ T cell-derived IL-17A, which was produced in response to chitinase-like proteins (CLPs), such as Ym1[128, 129]. These studies correlate with previous observations demonstrating that γδ+ intraepithelial lymphocyte (IEL) populations expand following helminth infections[130, 131], although their precise contribution to anti-helminth immunity remains to be investigated. While the early cellular source(s) of CLPs has not been identified, inhibition of Ym1 resulted in elevated intestinal worm burdens as well as reduced pulmonary neutrophilia[129], suggesting that neutrophils limit larval migration in the early stages of helminth infection. Nevertheless, soluble extracts from S. stercoralis were found to induce neutrophil recruitment independent of IL-17 but dependent of CXCR2[132], exemplifying that multiple mechanisms mediate the recruitment of immune cells upon helminth infection.
Remarkably, Chen et al. demonstrated that compared to LPS-activated neutrophils, neutrophils sort-purified from the lungs of N. brasiliensis-infected mice have a distinct transcriptional signature characterized by the upregulation of il13[128]. Further, neutrophilderived IL-13 was sufficient to polarize M2 macrophages, which were shown to attach to and directly damage N. brasiliensis larvae in vitro[128]. Since neutrophils and macrophages have also been shown to surround the larvae in the lungs of mice infected with N. brasiliensis[128, 129], neutrophils and macrophages might cooperate in a manner that limits helminth migration. Similarly, neutrophils, eosinophils and macrophages appear to accumulate and cooperate to mediate parasite killing following H. polygyrus and S. stercoralis infections[133–137], suggesting that this may be a common mechanism of immunity in response to several distinct helminths. Further, neutrophil extracellular DNA traps (NETs) were found to be released upon interactions with S. stercoralis larvae and were required to mediate parasite killing in vitro[138]. NETs have previously been described to facilitate and prolong the exposure of neutrophil intracellular contents such as myeloperoxidase (MPO) to kill bacteria. Likewise, neutrophils and eosinophils were found to require MPO and MBP to kill S. stercoralis larvae in vitro and patients infected with S. stercoralis exhibited elevated serum levels of neutrophil- and eosinophil-derived granular proteins[139, 140], suggesting that NETs may act in a similar manner and extend the exposure of helminths to anti-helminth products secreted by neutrophils, eosinophils and macrophages. Collectively, these studies suggest that neutrophils promote protective immunity to helminths via several effector mechanisms.
Master of all trades: macrophages
It is well appreciated that the strong inflammatory cytokine response to helminths, characterized by the production of IL-4 and IL-13, results in the polarization of M2 macrophages, which are characterized by their expression of arg1, fizz1 (retnla), ym1 (chil3) and igf1 (Figure 3)[141–143]. However, recent studies have now demonstrated that additional factors beyond type 2 cytokines contribute to the activation of M2 macrophages following helminth infection, including ‘tissue-specific’ collectins, such as surfactant protein A (SP-A) and SP-D, the first component of the complement pathway C1q, as well as the phagocytosis of apoptotic cells. For instance, mice deficient in SP-A, one of the major components of the lipidprotein network that covers the pulmonary epithelium, exhibited delayed worm expulsion, elevated lung damage and hemorrhage as well as diminished IL-4-induced macrophage proliferation upon N. brasiliensis infection[144]. Although SP-A was not sufficient to induce M2 polarization or proliferation of alveolar macrophages in vitro, it enhanced the activation of alveolar macrophages induced by IL-4. Similarly, C1q augmented the IL-4-induced proliferation and activation of peritoneal macrophages and C1q-deficient mice had reduced macrophage responses in a model of liver fibrosis. Collectively, these studies demonstrate that SP-A and C1q function to amplify the activation of macrophages induced by IL-4 in the pulmonary and peritoneal compartments [144]. Like SP-A, mice deficient in SP-D also exhibited impaired host protective responses upon N. brasiliensis infection, which correlated with reduced M2 macrophage responses[145]. Remarkably, SP-D was found to bind to the surface of N. brasiliensis and enhance the anti-helminth activity of alveolar macrophages in vitro. Further, administration of recombinant SP-D resulted in elevated type 2 cytokine-mediated inflammation, increased M2 macrophage responses and accelerated helminth expulsion[145]. Collectively, these studies demonstrate that tissue-specific collectins contribute to host-protective responses by enhancing the anti-helminth activity of M2 macrophages. In addition to these signals, efferocytosis of apoptotic cells has been reported to promote the wound healing process following helminth infection[146]. Specifically, exposure of apoptotic neutrophils enhanced the M2 polarization of macrophages induced by IL-4. Further, ablation of tyrosine kinases involved in the recognition of apoptotic bodies, including AXL and MERTK, resulted in elevated tissue damage and hemorrhage, which correlated with reduced macrophage proliferation and M2 polarization[146]. These studies suggest that upon IL-13 stimulation by helminth-activated neutrophils, efferocytosis of apoptotic neutrophils might also contribute to the proper activation of M2 macrophages necessary to promote the tissue repair following infection.
Figure 3. Cooperative actions of innate cells activate M2 macrophages.
M2 macrophages can be induced by multiple mechanisms in the context of helminth infection. Type 2 cytokines, produced by ILC2s, basophils, mast cells, eosinophils and neutrophils contribute to M2 macrophage responses. Additionally, epithelial cells release adenosine in response to the helminth-induced damage, which further amplifies M2 macrophage activation. However, whether these signals differentially act on tissue-resident and monocyte-derived macrophages remains unknown. Further, whether neuronal-derived signals also regulate macrophage activation has not been determined.
In addition to their anti-helminth potential, M2 macrophages contribute to tissue remodeling and wound healing after helminth infection by the secretion of growth factors including TGF-β, VEGF and PDGF; as well as extracellular matrix proteins such as fibronectin, matrix metalloproteinases and the cross-linking enzyme tissue transglutamase[147]. Novel studies have also shown that M2 macrophages shift their metabolism towards lipid β-oxidation, a process that appears to be required for the protection against H. polygyrus[148]. Macrophages are required for the clearance of N. brasiliensis, H. polygyrus, S. mansoni and B. malayi[147, 149, 150]. Interestingly, depletion of macrophages resulted in impaired eosinophilia following helminth infection[151], suggesting that macrophages may contribute to eosinophil recruitment. M2 macrophages have also been shown to limit N. brasiliensis-induced pulmonary tissue injury, participate in the granuloma formation following S. mansoni infection and contribute to long-term tissue remodeling of the lung caused by N. brasiliensis[142, 149, 150, 152], demonstrating that M2 macrophages directly contribute to distinct modules of anti-helminth responses. In addition to the murine models of infection described above, human macrophages were found in lymphatic infiltrates of patients infected with microfilariae and exposure of human monocytes to microfilariae in vitro resulted in their expression of type 2associated chemokines[153, 154], suggesting that macrophages also contribute to antihelminth immunity in human patients.
Recent studies have demonstrated the profound differences between tissue-resident and monocyte-derived macrophages[155] that impact their contributions to anti-helminth responses. For instance, IL-4 promoted the expression of arg1, fizz1 and ym1 in both tissueresident and monocyte-derived macrophages. However, a more comprehensive genome-wide transcriptional analysis of these populations revealed distinct profiles in response to IL-4[156]. Further, helminth-induced IL-4 promoted the local proliferation of tissue-resident macrophages over monocyte recruitment[157], suggesting that macrophage ontogeny might determine their host-protective contributions. Conversely, Gundra et al. demonstrated that monocyte-derived macrophages adopt a transcriptional program similar to their tissue-resident counterparts in the late stages of S. mansoni infection[158], suggesting that monocyte-derived macrophages ultimately contribute to the long-term pool of tissue-resident macrophages. Remarkably, vitamins and neurotransmitters have proven to instruct tissue programming of macrophages[158, 159]; however, their contribution to anti-helminth protection requires further investigation. Collectively, these studies illustrate the complex functions of macrophages to host-protective responses following a helminth challenge. More extensive literature on this well described subject can be found here[147].
Can’t wait to grow up: hematopoietic progenitor cells
In addition to terminally differentiated cells, recent studies have demonstrated that hematopoietic progenitors accumulate at mucosal sites and contribute to type 2 inflammation[35]. For example, IL-25 was found to induce the population expansion of IL-4competent Lin–c-KitintSca-1+ cells that were transcriptionally distinct from ILC2s and termed type 2 multipotent progenitor (MPPtype2) cells[21, 22]. MPPtype2 cells gave rise to MCs, basophils and macrophages when cultured with IL-3 and were sufficient to induce the polarization of TH2 cells in vitro. Further, adoptive transfer of MPPtype2 cells restored the worm expulsion of IL-25-deficient mice infected with T. muris[21, 22], suggesting that MPPtype2 cells promote host-protective responses to helminths. Interestingly, IL-33 also induced the recruitment of MPPtype2 cells, although to a lesser extent than IL-25[21], suggesting that IL-33 might also contribute to the differentiation of type 2 innate immune cells. Similarly, CD34+c-Kit+ progenitor cells accumulated in the spleen in response to T. spiralis-induced TSLP production. Despite sharing a similar surface phenotype to bone-marrow progenitor cells, TSLP-induced progenitors had a distinct transcriptional signature enriched in pathways related to granulocyte development. Further, TLSP-induced progenitors preferentially differentiated into basophils and MCs[35]. Moreover, transfer of TSLP-induced progenitors into TSLPR-deficient mice infected with T. muris resulted in significantly increased type 2 cytokine responses and lower intestinal worm burdens. Collectively, these studies demonstrate that cytokine alarmins promote type 2 responses by activating and recruiting terminally differentiated cells as well as immature hematopoietic progenitors.
Concluding Remarks
Helminth infections remain an understudied public health concern that result in devastating health and economic consequences. The generation of anti-helminth drugs revolutionized the treatment of worm infections; however, the high rates of reinfection, the rapid generation of drug-resistant parasites as well as the lack of effective vaccination strategies urge the generation of novel therapies. Although helminths have diverse and complex life cycles, it is well established that worm infections promote host-protective type 2 responses, and as our understating of how these responses are regulated grows, it may offer us new approaches to treat these diseases. A growing body of literature has demonstrated that helminth-derived ES products and helminth-induced tissue damage rapidly activate an impressive array of both hematopoietic and non-hematopoietic cells necessary for the establishment of long-term protection. The balance of the data conveys that epithelial cells, ILC2s, basophils, MCs, eosinophils, macrophages and HSPCs work in concert to promote optimal type 2 inflammation in the contexts of primary and secondary infections. Further, recent studies have demonstrated that regulatory interactions between the nervous, endocrine and immune systems operate to promote worm expulsion and tissue repair. These studies have expanded our understanding of the coordinated actions between epithelial cells, neurons and immune cells that lead to potent host-protective responses, opening the possibility of novel therapies that focus on a systems biology approach. For example, treatment with neuromedin U resulted to increased ILC2 activation associated with accelerated worm expulsion in mice infected with N. brasiliensis[11, 12, 61]. Conversely, treatment with β2AR agonists inhibited ILC2 resulting in delayed worm clearance[62], suggesting that pharmacologic therapies that target these neuroimmune modulatory pathways may offer a novel strategy to combat helminth infections and/or their associated morbidities. Other studies have recently highlighted the possibility of boosting immunity against T. spiralis by modulating the activity of Car1 and Car2 enzymes, which are highly expressed by MCs. For instance, pharmacological inhibition of Car enzymes resulted in delayed worm clearance and specific deletion of the car1 gene led to impaired MC development[94], suggesting that pharmacological modulation of Car1 and Car2 enzymes might regulate anti-helminth immunity by altering MC responses. Similarly, studies have highlighted the critical role that metabolic alternations play in regulating immunity and inflammation. Specifically, recent studies demonstrated that M2 macrophages exhibit a change in their metabolism towards lipid β-oxidation that is required for their proper activation. Remarkably, treatment with an inhibitor of lipolysis following H. polygyrus resulted in reduced M2 responses associated with inhibited resistance to a secondary infection[148], suggesting that therapies targeting metabolic modulation of M2 macrophages might be capable of regulating worm clearance. Finally, previous reports have demonstrated the ability of helminthderived ES products to modulate immune responses in the context of helminth infections as well as allergic inflammation[33, 34, 65, 66], highlighting the possibility to target these mechanisms to promote host-protective responses. Therefore, it is imperative that we continue (see Outstanding Questions) to explore how these complex pathways promote and/or inhibit each other to best inform therapeutic strategies to treat this significant public health concern.
Highlights.
Host-protective responses to helminths include two equally important tasks: worm expulsion and wound healing.
Innate immune pathways can promote and/or inhibit the generation of host-protective responses.
Despite sharing some properties, innate cells have non-redundant anti-helminth functions.
Collectively, non-hematopoietic cells (epithelial cells and neurons) and hematopoietic immune cells regulate helminth-induced inflammation.
In addition to terminally differentiated cells, hematopoietic stem/progenitor cells promote anti-helminth immunity.
Outstanding questions.
-
➢
Do helminth-derived secretory products directly activate innate, epithelial or neuronal cells?
-
➢
Are there specialized neuronal or epithelial cell subsets that negatively regulate type 2 responses?
-
➢
Do innate immune cells contribute to distinct inflammatory responses in a helminth-specific manner?
-
➢
Can innate immune cells regulate helminth-activated epithelial- and/or neuronal-derived signals?
-
➢
How do hematopoietic and non-hematopoietic compartments collectively cooperate to promote helminth expulsion and tissue repair?
-
➢
How can the early events on helminth infection be exploited to generate therapeutic strategies to combat these diseases?
Acknowledgements:
We thank members of the Center for Immunity and Inflammation and the Siracusa lab for discussions and critical reading. We would like to thank Servier Medical Art (http://www.servier.com) for figure graphics. This work was supported by the National Institutes of Health (1R01AI123224 and 1R01AI131634–01A1 to MCS) and the New Jersey Heath Foundation.
Glossary
- Alternatively activated or M2 macrophages
macrophages activated by IL-4 or IL-13 that are associated with wound healing and anti-inflammatory responses.
- Amphiregulin
epidermal growth factor-like molecule that induces proliferation of the barrier epithelium.
- Arginase 1
enzyme that converts L-arginine to urea and L-ornithine, which is further, metabolized into proline and polyamides that drive tissue remodeling.
- Basophilia
accumulation of basophils
- Carbonic anhydrase (Car) enzymes
family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons.
- Chitinase-like proteins
secreted glycoproteins that bind that bind but do not cleave chitin, a major component of helminth cuticle.
- Co-stimulatory molecules
family of proteins expressed on the surface of antigen presenting cells that contribute to the full activation of T lymphocytes.
- Collectins
soluble C-type lectins that bind to carbohydrate motifs in the surface of microbial pathogens and facilitate their phagocytosis.
- Danger-associated molecular patterns (DAMPs)
intracellular molecules released by host-derived cells that initiate an immune response when detected in the extracellular space
- Eosinophilia
accumulation of eosinophils.
- Excretory-secretory (ES) products
soluble mediators released by helminths to modulate immune responses.
- Extramedullary hematopoiesis
development of blood cells outside of the bone marrow.
- Fibronectin
glycoprotein that contributes to tissue remodeling by binding to extracellular matrix proteins such as collagen and fibrin.
- Glycosaminoglycans
large linear polysaccharides constructed of repeating disaccharide units with the primary configurations containing an amino sugar (either GlcNAc or GalNAc) and an uronic acid (either glucuronic acid and/or iduronic acid).
- Goblet cells
intestinal or bronchial cell population secreting mucins that form the mucous layer protecting the intestinal or airway epithelia.
- Granuloma
accumulation of immune cells around a foreign substance.
- Hematopoietic stem/progenitor cells (HSPCs)
stem cells that give rise to all blood cells including erythrocytes and immune cells.
- iNOS
inducible nitric oxide synthase, an enzyme expressed by M1 macrophages and neutrophils in the context of type 1 responses.
- Intraepithelial lymphocytes (IEL)
‘innate-like’ lymphocytes residing within the epithelial layer that maintain the integrity of the mucosal epithelium and prevent the damage induced by invading pathogens.
- Leukotrienes and prostaglandins
inflammatory lipid mediators derived from arachidonic acid.
- Lipid β-oxidation
catabolic process that takes place in the mitochondria by which long-chain fatty acids are converted into acetyl-CoA that is used to generate ATP.
- Matrix metalloproteinases
calcium-dependent zinc-containing endopeptidases that degrade extracellular matrix proteins.
- Paneth cells
highly specialized epithelial cells strategically positioned at mucosal surfaces that secrete anti-microbial peptides
- STAT1
signal transducer and activator of transcription 1, transcription factor activated in the context of type 1 inflammation
- Severe Combined Immunodeficient (SCID) mice
Mice homozygous for the scid mutation that are severely deficient in functional B and T lymphocyte populations
- Tuft cells
rare chemosensory cells in the epithelial lining of the intestinal and respiratory tract
- Vasoactive intestinal peptide (VIP)
peptide hormone that causes vasodilation in the intestine
- β2-adrenergic receptor (β2AR) agonists
drugs that bind to the β2-adrenergic receptor mimicking the effects of epinephrine
- γδ T cell
T cell expressing the γδ T cell receptor that are enriched in epithelial and mucosal sites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization(2016) Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly Epidemiol Rec 91 (49–50), 585–95. [PubMed] [Google Scholar]
- 2.Jourdan PM et al. (2017) Soil-transmitted helminth infections. Lancet. [DOI] [PubMed] [Google Scholar]
- 3.Jia TW et al. (2012) Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis 6 (5), e1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlier J et al. (2014) Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol 30 (7), 361–7. [DOI] [PubMed] [Google Scholar]
- 5.McNeilly TN and Nisbet AJ (2014) Immune modulation by helminth parasites of ruminants: implications for vaccine development and host immune competence. Parasite 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan B et al. (2014) Advancing a multivalent ‘Pan-anthelmintic’ vaccine against soiltransmitted nematode infections. Expert Rev Vaccines 13 (3), 321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molehin AJ et al. (2016) Development of a schistosomiasis vaccine. Expert Review of Vaccines 15 (5), 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry EK et al. (2017) Type 2 cytokine responses: regulating immunity to helminth parasites and allergic inflammation. Curr Pharmacol Rep 3 (6), 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchery T et al. (2014) The Differentiation of CD4(+) T-Helper Cell Subsets in the Context of Helminth Parasite Infection. Front Immunol 5, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris N and Gause WC (2011) To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol 32 (2), 80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klose CSN et al. (2017) The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549 (7671), 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso V et al. (2017) Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549 (7671), 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artis D et al. (2004) RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America 101 (37), 13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Moltke J et al. (2016) Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529 (7585), 221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamal M et al. (2002) Paneth and intermediate cell hyperplasia induced in mice by helminth infections. Parasitology 125 (Pt 3), 275–81. [DOI] [PubMed] [Google Scholar]
- 16.Saenz SA et al. (2008) Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev 226, 172–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman NP et al. (2008) Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflammatory bowel diseases 14 (7), 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallon PG et al. (2006) Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203 (4), 110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angkasekwinai P et al. (2013) Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect Immun 81 (10), 3731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owyang AM et al. (2006) Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203 (4), 843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saenz SA et al. (2013) IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med 210 (9), 1823–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saenz SA et al. (2010) IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464 (7293), 1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y et al. (2015) IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol 16 (2), 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y et al. (2018) S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359 (6371), 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howitt MR et al. (2016) Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351 (6279), 1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerbe F et al. (2016) Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa S et al. (2017) Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Scientific Reports 7, 42413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimokawa C et al. (2017) Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity 46 (5), 863–874.e4. [DOI] [PubMed] [Google Scholar]
- 29.Hung L-Y et al. (2013) IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proceedings of the National Academy of Sciences of the United States of America 110 (1), 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsend MJ et al. (2000) T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 191 (6), 1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda K et al. (2012) Contribution of IL-33–activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proceedings of the National Academy of Sciences of the United States of America 109 (9), 3451–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphreys NE et al. (2008) IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol 180 (4), 2443–9. [DOI] [PubMed] [Google Scholar]
- 33.McSorley HJ et al. (2014) Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunology 7, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osbourn M et al. (2017) HpARI Protein Secreted by a Helminth Parasite Suppresses Interleukin-33. Immunity 47 (4), 739–751.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siracusa MC et al. (2013) Thymic stromal lymphopoietin-mediated extramedullary hematopoiesis promotes allergic inflammation. Immunity 39 (6), 1158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massacand JC et al. (2009) Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A 106 (33), 13968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramalingam TR et al. (2009) Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol 182 (10), 6452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor BC et al. (2009) TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 206 (3), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giacomin PR et al. (2012) Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J Immunol 189 (9), 4371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csoka B et al. (2012) Adenosine promotes alternative macrophage activation via A2A and A2B receptors. Faseb j 26 (1), 376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csoka B et al. (2018) Adenosine receptors differentially regulate type 2 cytokine production by IL-33-activated bone marrow cells, ILC2s, and macrophages. Faseb j 32 (2), 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel N et al. (2014) A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe 15 (3), 339–50. [DOI] [PubMed] [Google Scholar]
- 43.Alhallaf R et al. (2018) The NLRP3 Inflammasome Suppresses Protective Immunity to Gastrointestinal Helminth Infection. Cell Rep 23 (4), 1085–1098. [DOI] [PubMed] [Google Scholar]
- 44.Ritter M et al. (2010) Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A 107 (47), 20459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaiss MM et al. (2013) IL-1beta suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog 9 (8), e1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson BJ et al. (2015) The Schistosoma mansoni T2 ribonuclease omega-1 modulates inflammasome-dependent IL-1β secretion in macrophages. International Journal for Parasitology 45 (13), 809–813. [DOI] [PubMed] [Google Scholar]
- 47.Klose CSN and Artis D (2016) Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 17 (7), 765–774. [DOI] [PubMed] [Google Scholar]
- 48.Wojno ED et al. (2015) The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol 8 (6), 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner JE et al. (2013) IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med 210 (13), 2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohapatra A et al. (2016) Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol 9 (1), 275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monticelli LA et al. (2015) IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A 112 (34), 10762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monticelli LA et al. (2011) Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunology 12 (11), 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monticelli LA et al. (2016) Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17 (6), 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliphant CJ et al. (2014) MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41 (2), 283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maazi H et al. (2015) ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42 (3), 538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halim TY et al. (2016) Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 17 (1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelly VS et al. (2016) IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol 9 (6), 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nausch N et al. (2015) Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti-helminthic treatment. PLoS Negl Trop Dis 9 (3), e0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuda K et al. (2012) Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A 109 (9), 3451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Angkasekwinai P et al. (2017) ILC2s activated by IL-25 promote antigen-specific Th2 and Th9 functions that contribute to the control of Trichinella spiralis infection. PLoS One 12 (9), e0184684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallrapp A et al. (2017) The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549 (7672), 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moriyama S et al. (2018) beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359 (6379), 1056–1061. [DOI] [PubMed] [Google Scholar]
- 63.Darby M et al. (2015) The M3 muscarinic receptor is required for optimal adaptive immunity to helminth and bacterial infection. PLoS Pathog 11 (1), e1004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McLean LP et al. (2016) Type 3 muscarinic receptors contribute to intestinal mucosal homeostasis and clearance of Nippostrongylus brasiliensis through induction of TH2 cytokines. Am J Physiol Gastrointest Liver Physiol 311 (1), G130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussein AS et al. (2002) A distinct family of acetylcholinesterases is secreted by Nippostrongylus brasiliensis. Mol Biochem Parasitol 123 (2), 125–34. [PubMed] [Google Scholar]
- 66.Vaux R et al. (2016) Modulation of the Immune Response by Nematode Secreted Acetylcholinesterase Revealed by Heterologous Expression in Trypanosoma musculi. PLoS Pathogens 12 (11), e1005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Worthington JJ (2015) The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem Soc Trans 43 (4), 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Worthington JJ et al. (2018) Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol 11 (1), 3–20. [DOI] [PubMed] [Google Scholar]
- 69.Wang H et al. (2007) CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut 56 (7), 949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDermott JR et al. (2006) Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut 55 (4), 492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Worthington JJ et al. (2013) Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion. PLoS Pathog 9 (1), e1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siracusa MC et al. (2011) New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci 1217, 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anyan WK et al. (2013) Basophil depletion downregulates Schistosoma mansoni egginduced granuloma formation. Parasitology International 62 (6), 508–513. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz C et al. (2014) T cell-derived IL-4/IL-13 protects mice against fatal Schistosoma mansoni infection independently of basophils. J Immunol 193 (7), 3590–9. [DOI] [PubMed] [Google Scholar]
- 75.Lantz CS et al. (2008) IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab Invest 88 (11), 1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lantz CS et al. (1998) Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392 (6671), 90–3. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz C et al. (2014) Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci U S A 111 (48), E5169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siracusa MC et al. (2011) TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477 (7363), 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan BM et al. (2011) Genetic analysis of basophil function in vivo. Nat Immunol 12 (6), 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kroeger KM et al. (2009) IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol 86 (4), 769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Falcone FH et al. (2009) Antigen-driven basophil activation is indicative of early Necator americanus infection in IgE-seronegative patients. J Allergy Clin Immunol 124 (6), 1343–50 e7. [DOI] [PubMed] [Google Scholar]
- 82.Min B et al. (2004) Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med 200 (4), 507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perrigoue JG et al. (2009) MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 10 (7), 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sokol CL et al. (2009) Basophils function as antigen-presenting cells for an allergeninduced T helper type 2 response. Nature Immunology 10, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim S et al. (2010) Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol 184 (3), 1143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim BS et al. (2014) Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 193 (7), 3717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Motomura Y et al. (2014) Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 40 (5), 758–71. [DOI] [PubMed] [Google Scholar]
- 88.Voehringer D (2013) Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 13 (5), 362–75. [DOI] [PubMed] [Google Scholar]
- 89.Salimi M et al. (2017) Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lund SJ et al. (2017) Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohnmacht C et al. (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33 (3), 364–74. [DOI] [PubMed] [Google Scholar]
- 92.Obata-Ninomiya K et al. (2013) The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med 210 (12), 2583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arinobu Y et al. (2005) Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A 102 (50), 18105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Henry EK et al. (2016) Carbonic anhydrase enzymes regulate mast cell-mediated inflammation. J Exp Med 213 (9), 1663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McKean PG and Pritchard DI (1989) The action of a mast cell protease on the cuticular collagens of Necator americanus. Parasite Immunol 11 (3), 293–7. [DOI] [PubMed] [Google Scholar]
- 96.Maruyama H et al. (2000) A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J Immunol 164 (7), 3749–54. [DOI] [PubMed] [Google Scholar]
- 97.Onah DN and Nawa Y (2004) Mucosal mast cell-derived chondroitin sulphate levels in and worm expulsion from FcRgamma-knockout mice following oral challenge with Strongyloides venezuelensis. J Vet Sci 5 (3), 221–6. [PubMed] [Google Scholar]
- 98.Lewis RA et al. (1982) Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol 129 (4), 1627–31. [PubMed] [Google Scholar]
- 99.Licona-Limon P et al. (2013) Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity 39 (4), 744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noelle RJ and Nowak EC (2010) Cellular sources and immune functions of interleukin-9. Nature Reviews Immunology 10, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hepworth MR et al. (2012) Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A 109 (17), 6644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lefrancais E et al. (2014) Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A 111 (43), 15502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knight PA et al. (2000) Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med 192 (12), 1849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reitz M et al. (2016) Mucosal mast cells are indispensable for the timely termination of Strongyloides ratti infection. Mucosal Immunology 10, 481. [DOI] [PubMed] [Google Scholar]
- 105.Vukman KV et al. (2016) Mast cells: new therapeutic target in helminth immune modulation. Parasite Immunol 38 (1), 45–52. [DOI] [PubMed] [Google Scholar]
- 106.Huang L and Appleton JA Eosinophils in Helminth Infection: Defenders and Dupes. Trends in Parasitology 32 (10), 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bettigole SE et al. (2015) The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol 16 (8), 829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doyle AD et al. (2013) Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood 122 (5), 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matthews SP et al. (2016) Cystatin F Ensures Eosinophil Survival by Regulating Granule Biogenesis. Immunity 44 (4), 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nussbaum JC et al. (2013) Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502 (7470), 245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Southam DS et al. (2005) Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J Allergy Clin Immunol 115 (1), 95–102. [DOI] [PubMed] [Google Scholar]
- 112.Weller PF and Spencer LA (2017) Functions of tissue-resident eosinophils. Nat Rev Immunol 17 (12), 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scepek S et al. (1994) Compound exocytosis and cumulative degranulation by eosinophils and their role in parasite killing. Parasitol Today 10 (7), 276–8. [DOI] [PubMed] [Google Scholar]
- 114.Saffari H et al. (2014) Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol 133 (6), 1728–34 e1. [DOI] [PubMed] [Google Scholar]
- 115.Spencer LA et al. (2006) Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A 103 (9), 3333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masure D et al. (2013) The Intestinal Expulsion of the Roundworm Ascaris suum Is Associated with Eosinophils, Intra-Epithelial T Cells and Decreased Intestinal Transit Time. PLOS Neglected Tropical Diseases 7 (12), e2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masure D et al. (2013) A Role for Eosinophils in the Intestinal Immunity against Infective Ascaris suum Larvae. PLOS Neglected Tropical Diseases 7 (3), e2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knott ML et al. (2007) Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol 37 (12), 136778. [DOI] [PubMed] [Google Scholar]
- 119.Huang L et al. (2015) Eosinophils mediate protective immunity against secondary nematode infection. J Immunol 194 (1), 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hewitson JP et al. (2015) Concerted activity of IgG1 antibodies and IL-4/IL-25-dependent effector cells trap helminth larvae in the tissues following vaccination with defined secreted antigens, providing sterile immunity to challenge infection. PLoS Pathog 11 (3), e1004676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cadman ET et al. (2014) Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog 10 (3), e1003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Svensson M et al. (2011) Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunol 33 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Swartz JM et al. (2006) Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 108 (7), 2420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fabre V et al. (2009) Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol 182 (3), 1577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gebreselassie NG et al. (2012) Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol 188 (1), 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang L et al. (2014) Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol 193 (8), 4178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang L et al. (2015) Eosinophils and IL-4 Support Nematode Growth Coincident with an Innate Response to Tissue Injury. PLoS Pathog 11 (12), e1005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen F et al. (2014) Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 15 (10), 938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sutherland TE et al. (2014) Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol 15 (12), 1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Little MC et al. (2005) The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. J Immunol 175 (10), 6713–22. [DOI] [PubMed] [Google Scholar]
- 131.Bozic F et al. (2000) Analysis of intestinal intraepithelial lymphocyte populations in experimental Trichinella spiralis infection of mice. Folia Parasitol (Praha) 47 (1), 55–9. [PubMed] [Google Scholar]
- 132.O’Connell AE et al. (2011) Soluble extract from the nematode Strongyloides stercoralis induces CXCR2 dependent/IL-17 independent neutrophil recruitment. Microbes Infect 13 (6), 536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anthony RM et al. (2006) Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature medicine 12 (8), 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morimoto M et al. (2004) Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol 172 (4), 2424–30. [DOI] [PubMed] [Google Scholar]
- 135.Liu Z et al. (2004) Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev 201, 57–74. [DOI] [PubMed] [Google Scholar]
- 136.Bonne-Annee S et al. (2013) Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun 81 (9), 3346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Galioto AM et al. (2006) Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice. Infect Immun 74 (10), 5730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bonne-Annee S et al. (2014) Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect 16 (6), 502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Connell AE et al. (2011) Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun 79 (7), 2770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rajamanickam A et al. (2018) Elevated Systemic Levels of Eosinophil, Neutrophil, and Mast Cell Granular Proteins in Strongyloides Stercoralis Infection that Diminish following Treatment. Front Immunol 9, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reece JJ et al. (2006) Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun 74 (9), 4970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reece JJ et al. (2008) Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun 76 (8), 3511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siracusa MC et al. (2008) Dynamics of lung macrophage activation in response to helminth infection. J Leukoc Biol 84 (6), 1422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Minutti CM et al. (2017) Local amplifiers of IL-4Rα–mediated macrophage activation promote repair in lung and liver. Science 356 (6342), 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thawer S et al. (2016) Surfactant Protein-D Is Essential for Immunity to Helminth Infection. PLoS Pathog 12 (2), e1005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bosurgi L et al. (2017) Macrophage function in tissue repair and remodeling requires IL4 or IL-13 with apoptotic cells. Science 356 (6342), 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Dyken SJ and Locksley RM (2013) Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 31, 317–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang SC et al. (2014) Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15 (9), 846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borthwick LA et al. (2016) Macrophages are critical to the maintenance of IL-13dependent lung inflammation and fibrosis. Mucosal Immunol 9 (1), 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen F et al. (2012) An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18 (2), 260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Voehringer D et al. (2007) Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol 81 (6), 1434–44. [DOI] [PubMed] [Google Scholar]
- 152.Marsland BJ et al. (2008) Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur J Immunol 38 (2), 479–88. [DOI] [PubMed] [Google Scholar]
- 153.Semnani RT et al. (2011) Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect Immun 79 (10), 3957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parkhouse RM et al. (1985) Human macrophages and T-lymphocyte subsets infiltrating nodules of Onchocerca volvulus. Clin Exp Immunol 62 (1), 13–8. [PMC free article] [PubMed] [Google Scholar]
- 155.Lavin Y et al. (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159 (6), 1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gundra UM et al. (2014) Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123 (20), e110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jenkins SJ et al. (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332 (6035), 1284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gundra UM et al. (2017) Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat Immunol 18 (6), 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gabanyi I et al. (2016) Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 164 (3), 378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]