Abstract
High-grade gliomas harbor abundant myeloid cells that suppress anti-tumor immunity and support tumor growth. Targeting transcription factors, such as NF-κB p50, that mediate suppressive myeloid M2 polarization may prove therapeutic. GL261-Luc glioblastoma cells were inoculated into wild-type and p50−/− mice, followed by analysis of tumor growth, survival, tumor myeloid cells, and T cells. The absence of host p50 slows tumor growth and enables regression in 30% of recipients, leading to prolonged survival. Tumors developing in p50−/− mice possess a greater concentration of tumor-infiltrating myeloid cells (TIMs) than those in wild-type mice. TIMs are predominantly F4/80hi macrophages which, along with tumor-associated microglia, express increased pro-inflammatory M1 and reduced immune-suppressive M2 markers. In p50−/− mice, total tumor CD4 T cells are threefold more abundant, whereas CD8 T-cell numbers are unchanged, and both produce increased IFNγ and Granzyme B. Naïve splenic p50−/− CD8 T cells manifest increased activation, whereas naïve p50−/− and WT CD4 T cells show similar Th1, Th2, and Th17 polarization. Antibody targeting CD4, but not CD8, fully obviates the p50−/− survival advantage. Combined CD4 and CD8 T-cell depletion reverses myeloid M2 polarization in wild-type hosts, without affecting myeloid M1 polarization in p50−/− hosts. Finally, gliomas grow similarly in p50(f/f) and p50(f/f);Lysozyme-Cre mice, the latter having reduced p50 specifically in myeloid cells and tumor microglia. Thus, high-grade glioma T cells play a key role in directing M2 polarization of tumor myeloid cells, and reducing NF-κB p50 in both tumor myeloid cells and T cells may contribute to glioma therapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2184-2) contains supplementary material, which is available to authorized users.
Keywords: Glioblastoma, Macrophages, NF-κB p50, T cells, Immunotherapy
Introduction
Glioblastoma (GBM) is a uniformly fatal brain tumor. The microenvironment of high-grade gliomas contains an abundance of myeloid cells, which comprise as much as 40% of the tumor [1, 2]. Glioma myeloid cells broadly fall into two categories: (1) brain-resident microglia, which derive from embryonic macrophages and are present in the healthy brain and (2) tumor-infiltrating myeloid cells (TIM) that are derived from blood monocytes and granulocytes and are recruited to the brain under pathological conditions [3, 4].
Blood monocytes can mature into tumor-associated macrophages (TAMs); in addition, TIM can retain a more immature phenotype and be classified further into granulocytic or monocytic MDSC [5–9]. Gliomas produce cytokines such as CSF1 that polarize TAMs and microglia towards the immune-suppressive, tumor-supportive M2, as opposed to the pro-inflammatory M1 phenotype [1, 10]. M2 TAM also support tumor growth by producing proangiogenic, proliferative, and invasive factors.
Due to their abundance and ability to influence T-cell function, glioma myeloid cells represent a potential therapeutic target. Supporting the utility of tumor myeloid reprogramming, inhibiting the tyrosine kinase activity of the CSF1 receptor alters TAM in a PDGF;Cdkn2a−/− murine glioma model by reducing M2 and increasing M1 markers, leading to prolonged survival. The same agent slows growth of human GBM in NOD/SCID mice [11]. However, gliomas secrete additional cytokines capable of inducing M2 polarization, including GM-CSF and IL-4, ultimately enabling outgrowth of CSF1R inhibitor-resistant gliomas [12, 13].
CSF1, GM-CSF, and IL-4 signaling may converge on common transcription factors to favor M2 gene expression. Transcription factors contributing to M2 macrophage polarization include NF-κB p50 (p50), C/EBPβ, KLF4, STAT6, PPARγ, and RORC1 [14–23]. Melanoma and fibrosarcoma cells grow more slowly in p50−/− compared with wild-type (WT) hosts, and also in WT hosts transplanted with p50−/− compared with WT marrow [15], but detailed characterization of the cellular mechanisms responsible for slowed tumor growth is lacking. We add to the existing literature by investigating the contribution of host p50 to the growth of glioma, a tumor that arises in a unique microenvironment. We find that intracranial GL261 glioma cells grow more slowly in p50−/− mice and exhibit striking tumor regression in a subset of these mice, with prolonged survival. We have characterized changes in the glioma immune microenvironment associated with this dramatic phenotype and demonstrate not only macrophage and microglia reprogramming, but also a previously unappreciated role for T cells in directing this process. In addition, we show that glioma growth is unimpaired in a novel mouse model lacking p50 specifically in macrophages, activated monocytes, and granulocytes. We conclude that targeting NF-κB p50 in T cells, or in both T cells and myeloid cells, may provide an effective glioma immunotherapy.
Materials and methods
Tumor inoculation, imaging, and survival analysis
GL261-Luc cells [24] were grown in DMEM with 10% FBS. They were inoculated intra-cranially into the striatum as described [25]. For tumor imaging, mice were injected with luciferin (100 μg/g i.p.) in PBS; after 10 min the mice were anesthetized using isofluorane-O2 gas and imaged using the Spectrum (Perkin Elmer) in vivo imaging system (IVIS). To deplete CD4 or CD8 T cells, WT and p50−/− mice were administered rat-anti-CD4 or CD8 antibodies (Bio-X-Cell) i.p.
Tumor myeloid and T-cell isolation
Mice anesthetized with ketamine and xylazine were perfused with ice-cold PBS at 7 mL/min for 8 min via their exposed left ventricle using a syringe pump. Brains were removed from euthanized mice and placed in calcium/magnesium-free HBSS. Enzymatic cell dissociation was accomplished using Neural Tissue Dissociation Kit P (Miltenyi), following the protocol for the Octo Dissociator, program 37C_ABDK. HBSS with 1.26 mM CaCl2, 0.5 mM MgCl2, and 0.4 mM MgSO4 was then added, followed by passage through a 40 μm cell strainer and centrifugation at 300×g for 5 min. The pellet was resuspended in 7 mL 30% isotonic Percoll in PBS and centrifuged at room temperature for 10 min at 700×g. The top myelin layer and Percoll were aspirated, and the cell pellet was washed with MACS buffer (Miltenyi). Cells were then either stained for flow cytometry (FC), or separated into CD11b+ and CD11b− or CD3+ and CD3− cell fractions using CD11b or CD3 positive selection kits and LS columns (Miltenyi).
Tumor myeloid and T-cell subset and activation analyses
All antibody staining was preceded by 15 min of 1:50 FcγR block in FC buffer, on ice. Extracellular antibodies were then added to FC buffer containing FcγR block, and incubated for 45 min on ice. Intracellular staining was accomplished after surface staining using the Foxp3 staining kit (eBioscience). Myeloid subsets were stained with anti-CD11b-FITC, anti-Ly6C-AF700, anti-MR-PE-Cy7, anti-CD11c-PE/Dazzle594, anti-Ly6G-BV605 (BioLegend), anti-MHCII-eFluor450 (eBioscience), and anti-F4/80-APC (BioRad). To evaluate Tregs, cells were stained with anti-CD3-AF488, anti-CD4-APC, anti-CD25-PerCP-Cy5.5 (BioLegend), and anti-Foxp3-PE (BD Pharmingen). To assess T-cell activation, total tumor cells were incubated for 4 h at 37 °C in a 5% CO2 incubator with Protein Transport Inhibitor Cocktail containing brefeldin A and monensin, or with Cell Stimulation Cocktail containing protein transport inhibitors and PMA/ionomycin (eBioscience). Cells were then stained with anti-CD3-AF488, anti-CD4-PE, and anti-CD8-PerCP-Cy5.5 followed by intracellular stain with anti-IFNγ-APC (BioLegend). In addition, 1E5 CD3+ cells were stimulated with 4E4 CD3/CD28 Dynabeads (ThermoFisher) for 3 days, followed by staining using anti-CD3-PerCP-Cy5.5, anti-CD8-BV650, anti-CD4-BV605, anti-IFNγ-APC, anti-TNFα-BV421 (BioLegend), and anti-GranzymeB-PE (eBioscience).
Naïve splenic T-cell analysis
To obtain naïve CD4 T cells, spleens were passed through a cell strainer, subjected to red cell lysis, and selected using a CD8 positive selection kit (Miltenyi). CD8− cells were then subjected to negative selection using a naïve CD4 T-cell isolation kit (Miltenyi). The cells bound to the column included APC. To obtain naïve CD8 T cells, splenocytes were subjected to selection using the Pan T-cell isolation kit II (Miltenyi), yielding CD3+ T cells and the bound CD3− fraction that includes APCs. The CD3+ cells were then further processed using a naïve CD8α T-cell isolation kit (Miltenyi). Naïve T cells were combined with irradiated (3000 cGy) APCs and cultured with different cytokine and antibody combinations to favor lineage polarization, plus anti-CD3 antibody, followed by PMA/ionomycin stimulation and FC analysis [26].
Bone-marrow-derived myeloid cells and peritoneal macrophages
To obtain bone-marrow-derived macrophages (BMDM), marrow was cultured on bacterial dishes with DMEM, 10% heat-inactivated FBS, and CSF1 (20 ng/ml) for 7 days, followed by stimulation of adherent cells with IL-4 (20 ng/ml) or IFNγ (20 ng/ml) or vehicle for 24 h. To isolate peritoneal macrophages, 10 ml of ice-cold HBSS was inserted into the peritoneal cavity and removed. Cells were resuspended in DMEM with 10% heat-inactivated FBS and transferred to a tissue culture plate overnight.
RNA, DNA, and western blot analyses
RNA was isolated using NucleoSpin RNA II (Machery-Nagel). First-strand cDNA was prepared using AMV reverse transcriptase (Promega) and oligodT. Quantitative real-time PCR (qRT-PCR) was carried out using Lo-Rox SYBR Green (Alkali Scientific). Primers are listed (Supplementary Table S1). Genomic DNA from p50(f/f) and p50(f/f);Lys-Cre BMDM was PCR amplified using intron 3 primer 5′-CACAAGTGTGTGGAGGCTGAAGG-3′ and intron 4 primer 5′-ACTTTCCTACTGTTTCCCCAGAGCC-3′, followed by agarose gel electrophoresis and ethidium bromide staining. Total cellular proteins prepared in Laemmli sample buffer were subjected to Western blotting using p50 (13586, Cell Signaling) and β-actin (AC-15, Sigma) antibodies.
Data analysis
Survival curves were compared using the log rank test. Tumor bioluminescence, myeloid and T-cell subsets, and RNA expression values were compared using the Student t test. Means and SEs are shown.
Results
Absence of NF-κB p50 slows GL261 glioma growth and prolongs survival
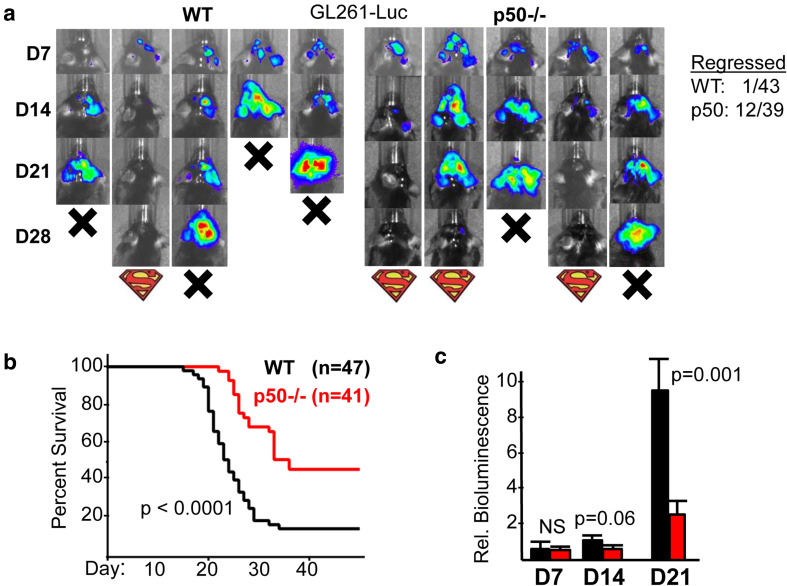
GL261 cells, derived from a B6 mouse by chemical mutagenesis, are widely used as a glioma model. These cells closely mimic human GBM angiogenesis and invasiveness and contain abundant M2 TAMs [24]. WT or p50−/− mice were implanted with GL261-Luc cells and assessed weekly by IVIS to track tumor growth. Representative images for WT and p50−/− recipients are shown (Fig. 1a, left). We found that 12 of the 39 tumors in p50−/− hosts that were detectable on day 14 subsequently regressed, as illustrated by the second p50−/− mouse. In contrast, only 1 of 43 such tumors in WT mice regressed (Fig. 1a, right). p50−/− tumor recipients had significantly prolonged survival compared with WT recipients (Fig. 1b), with a trend toward reduced tumor size on day 14 and a significant, > threefold reduction in tumor size on day 21 (Fig. 1c). The day 21 data under-estimates the difference in tumor sizes, as 11 WT recipients died prior to day 21 imaging.
Fig. 1.
Glioma growth is slowed in mice lacking NF-κB p50. a Representative IVIS images on days 7, 14, and 21 after GL261-Luc inoculation into WT or p50−/− recipients (left). Mice dead on the indicated days are denoted as X, and those that showed prolonged survival with the Superman logo. Also shown (right) are the numbers of WT and p50−/− mice that were IVIS+ on day 14 and later demonstrated tumor regression. b Survival curves of WT vs p50−/− mice inoculated on day 0 with GL261-Luc cells, with total number of mice in each group and Log Rank p values shown. c Relative IVIS bioluminescence of tumors on days 7, 14, and 21 from the same mice used for survival analysis. Mean, SE, and p values are shown. NS Not significant
Characterization of glioma-associated myeloid cells
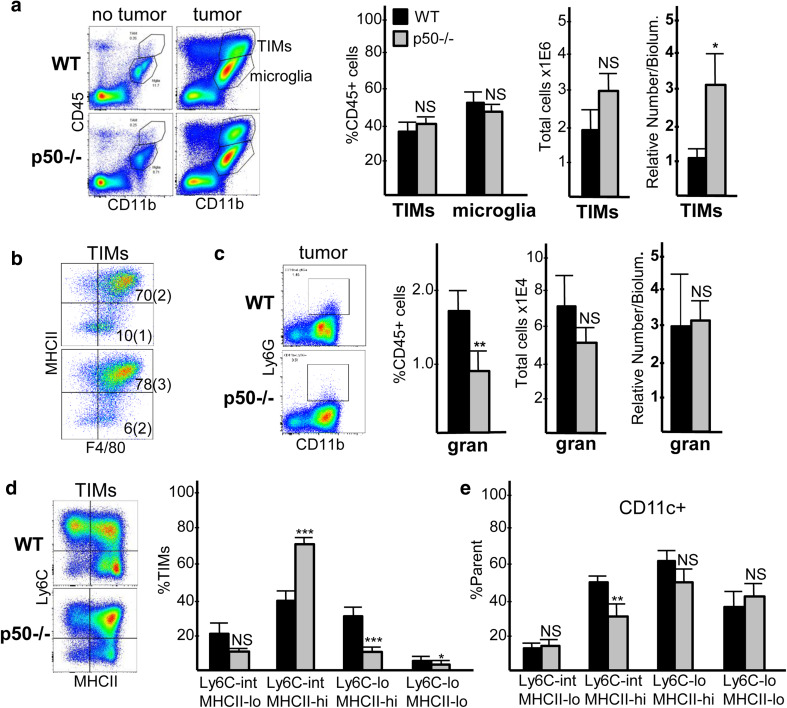
Previous studies demonstrate that tumors harbor F4/80hiLy6Cint/loMHCIIhi/lo macrophages, F4/80loLy6ChiMHCIIlo monocytes, and Ly6G+ granulocytes, the latter two subsets potentially representing monocytic or granulocytic MDSCs [27–29]. We sought to characterize GL261 glioma-associated myeloid cells. CD11b is a pan-myeloid marker. Microglia can be partially resolved from other myeloid populations by their low/intermediate expression of CD45 [27, 30]. FC demonstrates abundant CD45intCD11bint microglia in healthy tumor-free brains, but very few CD45hiCD11bhi cells (less than 0.5% of total cells and 8% of CD45+ cells, on average, n = 3, not shown). In WT and p50−/− tumor-bearing mice, we observe a large increase in CD45hiCD11bhi cells, which we denote tumor-infiltrating myeloid cells or TIMs (Fig. 2a, left). TIMs comprise about 40%, and microglia 45%, of CD45+ cells in both WT and p50−/− tumor-bearing brains (Fig. 2a, center), with CD45+ cells representing ~ 50% of total cells (not shown). Since substantial TIM accumulation only occurs in the presence of tumor but not in healthy brain, we approximated TIM density by dividing the total TIM number by tumor bioluminescence for each mouse, revealing a ~ threefold increase in TIM density in p50−/− hosts (Fig. 2a, right). 80–84% of TIMs are F4/80hi, and 70–78% are MHCIIhi (Fig. 2b). Ly6G+ cells represent only 1–2% of CD45+ cells (Fig. 2c). Ly6C and MHCII staining has previously been used to subdivide TIM subsets, with Ly6CintMHCIIhi cells correlating with reduced tumor growth [28, 29, 31]. As we perfused mice with PBS prior to brain isolation, we detect few tumor-associated Ly6Chi monocytes (Supplementary Fig. 1a), but do find Ly6Cint and Ly6Clo cells with varying expression of MHCII, as seen in a representative FC plot (Fig. 2d, left); these uniformly express the F4/80 macrophage marker (Supplementary Fig. 1b). When cells are gated into four quadrants as Ly6CintMHCIIlo, Ly6CintMHCIIhi, Ly6CloMHCIIhi, and Ly6CloMHCIIlo, we find nearly a twofold increase in the frequency of Ly6CintMHCIIhi TIMs in p50−/− compared to WT tumor-bearing mice, making this the most predominant TIM population (Fig. 2d, right). When normalized to tumor size using bioluminescence, we observe a fourfold increase in the density of Ly6CintMHCIIhi TIMs, and no change in the density of Ly6CloMHCIIhi TIMs, in p50−/− mice (not shown). In a rat glioma model, CD11chi macrophages potentiated orthotopic tumor growth [32]. Consistent with reduced tumor growth, CD11c expression was decreased in the predominant Ly6CintMHCIIhi TIM subset, but was unchanged in the other three Ly6C;MHCII populations in p50−/− hosts (Fig. 2e).
Fig. 2.
Characterization of glioma myeloid cells. a Representative CD11b;CD45 FC data for brain cells from control and tumor-bearing mice isolated on day 14 after GL261-Luc inoculation, with TIM and microglia populations indicated (left). TIMs and microglia were quantified as a percent of CD45+ cells, and TIMs were also quantified as total number per tumor or by their numbers per bioluminescence upon IVIS imaging, in relative units, as a measure of tumor size (right, mean and SE from ten determinations). b Representative MHCII;F4/80 FC data for TIMs, with mean indicated (SE in parenthesis) for the percent of cells in the upper right and lower right quadrants. c Representative Ly6G;CD11b plots within tumor CD45+ gate (left). Ly6G+ cells were quantified as percent of CD45+ cells, total number per tumor, and by the number per bioluminescence unit (right, mean and SE from three determinations). d Representative Ly6C;MHCII FC plots within the TIM gate (left). The indicated Ly6C;MHCII subsets were quantified as percent of TIMs (right, mean and SE from ten determinations). e Percent of each Ly6C;MHCII subset expressing CD11c (mean and SE from four determinations)
Absence of NF-κB p50 favors a pro-inflammatory M1 TIM phenotype
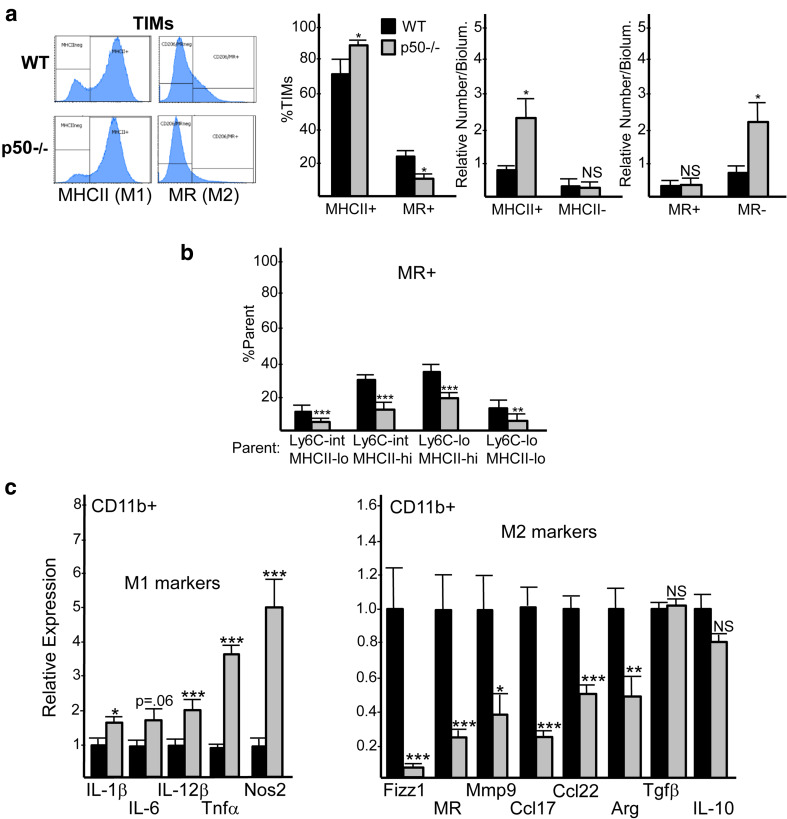
The frequency of TIMs expressing MHCII was increased and of those expressing the M2 marker mannose receptor (MR) decreased in p50−/− compared with WT tumor recipients (Fig. 3a, left). The density of MHCIIhi TIMs and MR− TIMs were both increased in p50−/− mice (Fig. 3a, right). We further found that MR expression was decreased in all four p50−/− TIM Ly6C;MHCII subsets (Fig. 3b).
Fig. 3.
Glioma myeloid cells have increased M1 markers and decreased M2 markers and express less CD11c in p50−/− recipients. a Representative FC plots for expression of MHCII and MR among CD11bhiCD45hi TIMs are shown. MHCII+, MHCII−, MR+, and MR− cells were quantified as percent of TIMs or as the relative number per bioluminescence unit (mean and SE from ten determinations). b MR+ or CD11c+ cells were quantified as the percent of cells within each Ly6C;MHCII quadrant (mean and SE from ten determinations). c RNAs prepared from tumor CD11b+ cells on day 14 after GL261-Luc inoculation were subjected to qRT-PCR analysis for indicated M1 and M2 markers. The relative expression of each mRNA, normalized to cyclophilin A, is shown for WT or p50−/− recipients, with expression in WT mice set to 1.0. Mean and SE are shown for 16 mice total in each group, combined from three separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001
We also conducted FC on glioma CD45intCD11bint microglia. On average, 71% of WT and 94% of p50−/− microglia were F4/80hi (Supplementary Fig. 2a). The proportion of WT vs p50−/− microglia expressing MHCII was increased (27 vs 56%, p = 0.001) and the proportion expressing MR decreased (14 vs 11%, p = 0.05); and only 3% of WT or p50−/− microglia expressed CD11c (Supplementary Fig. 2b).
To evaluate additional polarization markers, we isolated tumor CD11b+ cells 14–16 days after GL261-Luc inoculation and evaluated RNA from these cells by qRT-PCR (Fig. 3c). In p50−/− CD11b+ cells, the M1 macrophage markers IL-1𝛽, IL-12𝛽, Tnf𝛽, and Nos2 were increased, with a trend towards increased IL-6; the M2 markers Fizz1, MR, Mmp9, Ccl17, Ccl22, and Arg were reduced. M2-associated Tgf𝛽 and IL-10 were not significantly different. Collectively, these FC and qRT-PCR data indicate that p50−/− TIMs and microglia are reprogrammed towards an M1 phenotype. As there is some overlap in tumor sizes at day 14, we were able to directly compare WT and p50−/− TIM in a several tumors of the same size. Compared to WT TIM, the p50−/− TIM maintain increased M1 polarization and increased frequency of their Ly6CintMHCIIhi subset, indicating that these changes are not merely a consequence of smaller tumor size (Supplementary Fig. 3).
Increased tumor T-cell activation in glioma-bearing NF-κB p50−/− mice
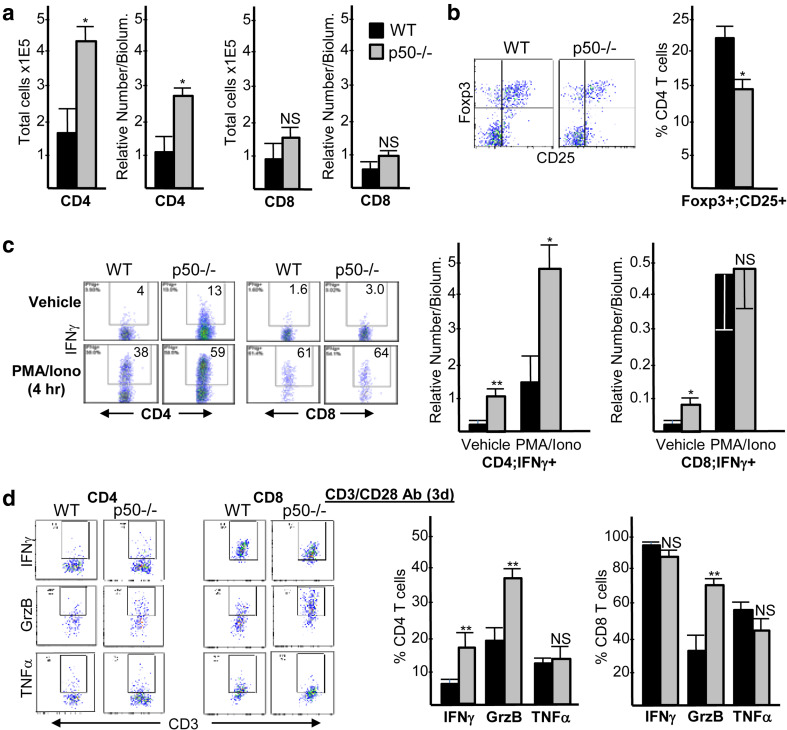
Glioma-bearing WT and p50−/− brains were analyzed by FC for total CD4 and CD8 tumor-infiltrating lymphocytes (TIL) (Fig. 4a, left). CD4 TIL were increased ~ threefold in tumors forming in p50−/− mice, both in absolute number and density. CD8 TIL numbers were not significantly changed (Fig. 4a, right). CD4 TIL were the predominant tumor T cell present, being ~ twofold greater than CD8 TIL in WT mice, and ~ threefold greater than CD8 TIL in p50−/− mice. Of note, in the absence of tumor there were only 10,400 CD4 and 450 CD8 T cells per brain, on average (n = 3, not shown). CD25+Foxp3+ Tregs were reduced ~ 1.5-fold, as a percent of total CD4 TIL, in p50−/− recipients (Fig. 4b); however, due to the overall increase in the total number of CD4 TIL in p50−/− mice, total numbers of Tregs were unchanged and Treg density was increased in the absence of p50 (Supplementary Fig. 4a).
Fig. 4.
Gliomas in p50−/− hosts have increased total and activated CD4 T cells and, to a lesser extent, increased activated CD8 T cells. a CD4 or CD8 cells were quantified as total number per tumor and the relative number per tumor bioluminescence 14 days after inoculation of GL261-Luc into WT or p50−/− mice (mean and SE from four determinations). b Representative Foxp3;CD25 FC plots within the CD3+CD4+ T-cell subset (left) and quantification of Foxp3+CD25+ Tregs as a percent of CD4 cells (right, mean and SE from four determinations) are shown. c Representative CD4;IFNγ and CD8;IFNγ FC plots, within the CD3+ T-cell gate, are shown 4 h after exposure to vehicle or PMA/ionomycin (left). CD4+IFNγ+ or CD8+IFNγ+ cells were quantified as the relative number per bioluminescence unit (right, mean and SE from four determinations). d Representative CD3;IFNγ, CD3;Granzyme B (GrzB), and CD3;TNFα FC plots, within CD4+ or CD8+ gates, are shown 3 days after stimulation of isolated CD3+ tumor cells with Dynabeads containing CD3 and CD28 antibodies (left). IFNγ+, GrzB+, and TNFα+ cells were quantified as percent of CD4+ or CD8+ cells (right, mean and SE from five determinations)
Intracellular IFNγ expression, a marker of T-cell activation, was assessed in TIL after 4 h exposure to PMA/ionomycin or vehicle (Fig. 4c). In p50−/− mice, IFNγ expression was increased in vehicle-treated CD4 and CD8 TIL, as well as in stimulated CD4 TIL. Notably, the density of vehicle-treated IFNγ-expressing CD4 TIL was 10-fold higher than that of CD8 TIL in p50−/− recipients, and ~ threefold greater in absolute number. Designating IFNγ-expressing T cells as T effectors, the p50−/− Treg:CD4 effector ratio was reduced 10-fold in vehicle-treated and threefold in PMA/ionomycin-treated cells as compared to WT ratios. The p50−/− Treg:CD8 effector ratio was reduced twofold in vehicle-treated cells and was slightly increased in PMA/ionomycin-treated cells (Supplementary Fig. 4b). Intracellular IFNγ Granzyme B, and TNFα expression were also assessed in TIL after 3 days of ex vivo stimulation with CD3/CD28 antibodies (Fig. 4d). p50−/− CD4 TIL had increased IFNγ and Granzyme B, and p50−/− CD8 TIL also had increased Granzyme B. Thus, compared with WT mice, tumors from p50−/− mice possess elevated numbers of CD4 TIL that exhibit a more activated, anti-tumor phenotype at baseline and upon stimulation. CD8 TIL are also more activated in p50−/− mice at baseline, albeit at lower numbers.
To evaluate the cell-intrinsic impact of p50 deletion in T cells, we isolated naïve splenic CD4 T cells or CD8 T cells from WT and p50−/− mice and cultured them under conditions that favor differentiation into several CD4 subsets or the CD8 Tc1 subset. Evaluation of intracellular IFNγ, Foxp3, and IL-17 expression indicated that p50−/− CD4 T cells possess no defect in their ability to become Th1, Treg, or Th17 cells. IL-4 secretion was unchanged in p50−/− CD4 T cells differentiated into the Th2 subset (Supplementary Fig. 5a). In contrast, CD8 Tc1 formation was increased in the absence of p50, indicated by elevated proportions of CD8 T cells expressing IFNγ, Granzyme B, and TNFα (Supplementary Fig. 5b). Lack of increased IFNγ or TNFα in tumor CD8 p50−/− T cells cultured with CD3/CD28 may reflect their alteration by the tumor or limitations of this ex vivo assay.
T-cell depletion eliminates the survival benefit of p50−/− and reverses M2 polarization in WT hosts
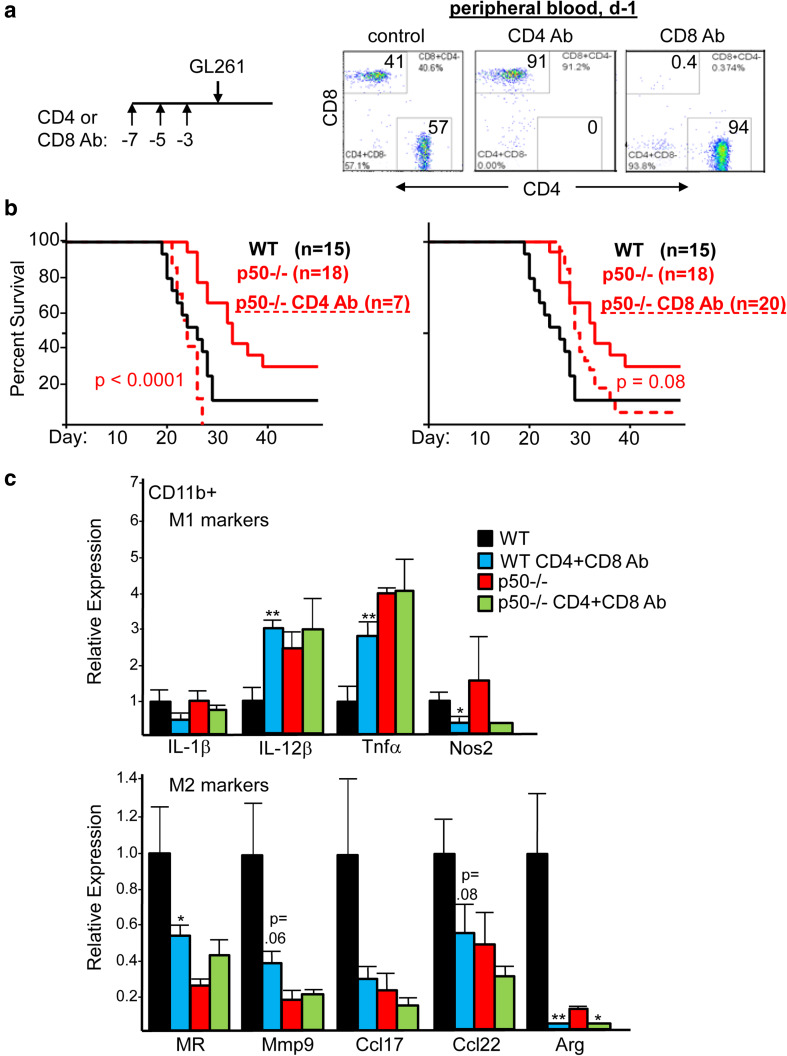
To remove T cells, mice received three doses of CD4 or CD8 antibody 7, 5, and 3 days prior to GL261-Luc implantation; depletion was confirmed by analysis of peripheral blood 1 day prior to implantation (Fig. 5a). CD4 antibody fully obviated the survival advantage of p50−/− mice, whereas CD8 antibody reduced, but did not eliminate, this advantage (Fig. 5b). CD8 T cells recovered to 3% of peripheral blood cells by day 19, on average, compared to 18% at baseline (not shown), potentially reducing the effect of CD8 depletion on survival. Neither CD4 nor CD8 antibody affected the survival of WT mice (Supplementary Fig. 6).
Fig. 5.
Survival advantage of p50−/− mice is eliminated by depletion of CD4 T cells and partially eliminated by CD8 depletion, and combined CD4 and CD8 depletion reverses WT tumor myeloid M2 polarization. a WT and p50−/− mice received 200 µg CD4 or CD8 antibody (Ab) on days 7, 5, and 3, followed by GL261-Luc cell inoculation on d0, as diagrammed (left). Representative CD4;CD8 FC plots demonstrating efficient depletion of CD4 or CD8 T cells from the peripheral blood 1 day prior to GL261 inoculation (right). b Survival curves for glioma-bearing WT mice, p50−/− mice, or p50−/− mice pre-treated with CD4 or CD8 Ab. The number of mice in each group and Log Rank p values comparing the control and Ab-treated p50−/− groups are shown. The untreated WT and p50−/− mice in this experiment are a subset of those presented in Fig. 1 and were inoculated concurrently with those exposed to the T-cell-depleting Abs. c WT and p50−/− mice received both CD4 and CD8 antibody (Ab), or no Ab, on days -7, -5, and -3, followed by GL261-Luc cell inoculation on d0 and an additional CD4/CD8 Ab injection on d8. CD11b+ cells isolated on day 14 were subjected to qRT-PCR analysis for indicated mRNAs (mean and SE from three determinations)
To evaluate TIM polarization in the absence of T cells, we pre-depleted CD4 and CD8 cells, inoculated mice with GL261-Luc, and then isolated brain CD11b+ cells on day 14. We evaluated M1 and M2 markers by qRT-PCR, focusing on those markers that differed without depletion (Fig. 5c). Strikingly, T-cell depletion in WT mice increased IL-12𝛽 and TNF𝛼 (M1) and decreased MR, Mmp9, Ccl17, Ccl22, and Arg (M2) to levels seen in untreated p50−/− mice. In contrast, T-cell depletion of p50−/− mice had little effect on these mRNAs, with the exception of Arg. T-cell depletion did not increase the expression of IL-1𝛽 or Nos2 in WT mice. The lack of prolonged survival in WT mice depleted of CD4 or CD8 T cells suggests that the resulting M2-to-M1 shift in TIM is not as strong as that resulting from p50 gene deletion.
Deletion of neutrophil/macrophage NF-κB p50 does not slow glioma growth
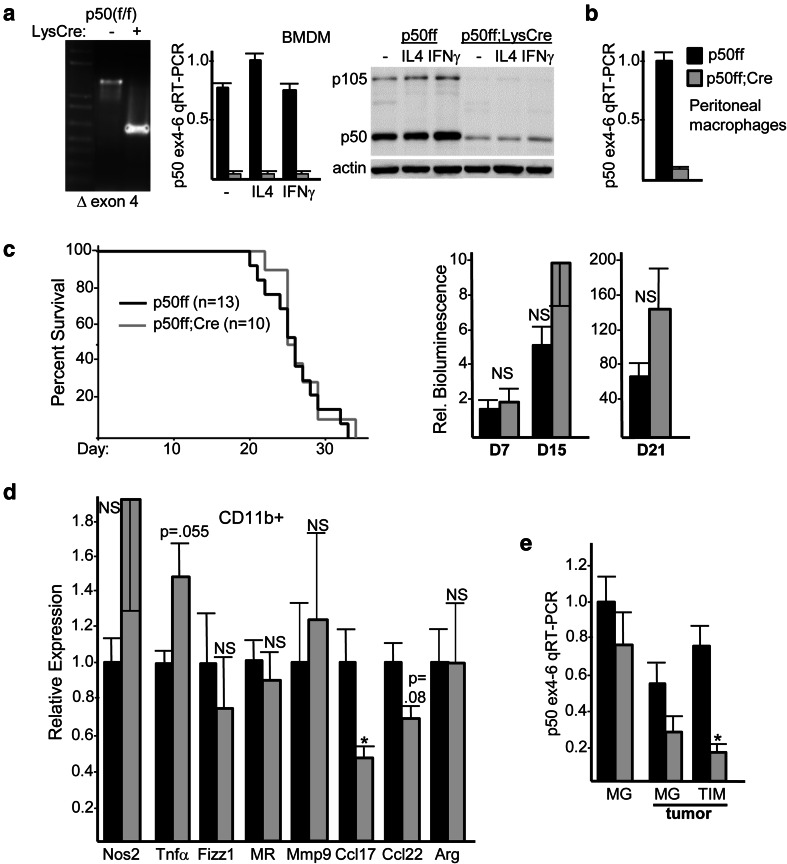
p50(flacZ/+) mice, harboring a floxed p50 exon 4 with an intron 3 lacZ cassette flanked by frt sites, were bred with ROSA26-FLPo and then Lysozyme-Cre to generate p50(f/f) and p50(f/f);Lys-Cre mice. Lys-Cre deletes floxed alleles in activated monocytes, macrophages, and granulocytes [33]. Efficient Lys-Cre-mediated deletion of exon 4 was evident upon PCR of BMDM genomic DNA (Fig. 6a, left), resulting in markedly reduced p50 RNA and protein in BMDM at baseline and after stimulation with IL-4 or IFNγ (Fig. 6a, center and right). The p105 precursor of p50 was also reduced. Efficient deletion was also evident in peritoneal macrophages (Fig. 6b). Survival of p50(f/f) or p50(f/f);Lys-Cre mice inoculated with GL261-Luc glioma cells was essentially identical, with tumor growth also unaffected by reduced myeloid p50 (Fig. 6c). Analysis of tumor CD11b+ cell RNA reveals a trend towards increased Nos2 and TNF𝛼 (M1), as well as reduced Ccl17 with a trend towards reduced Ccl22 (M2); in contrast to p50−/− tumor hosts, Fizz1, MR, Mmp9, and Arg (M2) were not reduced in p50(f/f);Lys-Cre mice (Fig. 6d). Lack of slowed tumor growth and prolonged survival in p50(f/f);Lys-Cre mice may in part reflect poor p50 exon 4 deletion in microglia. Microglial deletion of exon 4 was evaluated by analysis of RNA in brain CD11b+ cells from tumor-free mice, and also in sorted CD11bintCD45int cells from tumor-bearing mice, in comparison to CD11bhiCD45hi TIMs (Fig. 6e). p50 RNA was decreased 80% in TIMs, only 20% in naïve microglia, and 50% in tumor-associated microglia, the latter potentially an underestimate due to inclusion of non-tumor microglia.
Fig. 6.
Glioma growth is not slowed in mice lacking myeloid NF-κB p50. a PCR analysis of genomic DNA from p50(f/f) and p50(f/f);Lys-Cre mice (left). RNA isolated from BMDM under basal conditions in CSF1 or after stimulation for 24 h with IL-4 (M2 inducer) or IFNγ (M1 inducer) was analyzed by qRT-PCR using p50 exon 4 and exon 6 primers (center, mean and SE from three determinations); total cellular proteins from the same cultures were analyzed by Western blotting for p50 and β-actin (right). b Peritoneal macrophages from p50(f/f) and p50(f/f);Lys-Cre mice were analyzed for p50 RNA by exon 4–6 qRT-PCR (mean and SE from three determinations). c Survival curves of p50(f/f) vs p50(f/f);Lys-Cre mice inoculated on day 0 with GL261-Luc cells, with total number of mice in each group and Log Rank p values shown (left). Relative IVIS bioluminescence of tumors on days 7, 15, and 21 from the same mice used for survival analysis (right). d RNAs prepared from tumor CD11b+ cells on day 14 after GL261-Luc inoculation were subjected to qRT-PCR analysis for indicated M1 and M2 markers. The relative expression of each mRNA, normalized to cyclophilin A, is shown, with expression in p50(f/f) mice set to 1.0. Mean and SE are shown for three mice per group. e p50 RNA expressed was evaluated by exon 4–6 qRT-PCR in microglia (MG) from tumor-free mice and in MG and TIMs sorted from tumors 14 days after GL261 inoculation (mean and SE from three determinations)
Discussion
GL261 gliomas grew substantially slower in p50−/− compared with WT hosts. Tumor regression was evident after day 14 in 30% of p50−/− recipients, suggesting effective immune surveillance resulting in prolonged survival. Of note, GL261 cells carry a high mutational burden and express > 1700 neoepitopes [34].
Gliomas in WT and p50−/− mice contained a large population of CD45hiCD11bhi cells. Both TAMs and MDSC have been identified in tumors and support tumor growth. MDSC are immature myeloid cells that can be either granulocytic (Ly6Ghi) or monocytic (Ly6Chi), both of which are characterized by low or absent F4/80 and MHCII [7, 35]. We found that the majority of CD45hiCD11bhi cells were F4/80hi TAMs in both WT and p50−/− mice, and only a small proportion expressed Ly6G. Although we observed a large proportion of p50−/− TIMs expressing intermediate Ly6C, these cells co-expressed high levels of both F4/80 and MHCII, ruling out the possibility that they are suppressive monocytic MDSC. Thus, the glioma-infiltrating myeloid cells in this model are predominantly TAMs and microglia.
When normalized to tumor bioluminescence, we found a threefold increase in TIM density in p50−/− mice. Evaluation of surface markers confirmed that p50−/− TIMs express less MR and more MHCII, consistent with the partial M2-to-M1 phenotypic shift found via mRNA analysis of CD11b+ cells. Interestingly, a similar increase in MHCII and decrease in MR was seen in CD45intCD11bint microglia. The Ly6CintMHCIIhi TIM subset was increased in p50−/− mice which, together with M2-to-M1 reprogramming, correlates with reduced growth of gliomas and other tumors [1, 11, 31]. TNFα- and Nos2-producing inflammatory dendritic cells (Tip-DCs) have recently been identified within the Ly6CintMHCIIhi population [31]. Although p50−/− CD11b+ cells have increased Tnfα and Nos2 mRNA expression, we observe reduced CD11c within the Ly6CintMHCIIhi subset of p50−/− TIMs, which is inconsistent with a Tip-DC identity. Reduced CD11c expression in tumor-bearing p50−/− mice is consistent with the finding that F4/80hiCD11chi TAMs increase glioma growth upon intracranial co-inoculation into NOD/SCID mice [32]. Supporting the idea that myeloid NF-κB activation in the absence of p50 contributes to glioma control, inhibition of myeloid-specific PI3Kγ induces TAMs to adopt an M1 phenotype, with increased NF-κB p65 activity and slowed growth of several murine carcinoma lines [36].
Potentially as a consequence of the observed changes in tumor myeloid cells, we discovered a marked increase in total and IFNγ-expressing CD4 TIL in p50−/− mice, which outnumbered total and activated CD8 TIL threefold in absolute number, and 10-fold in density. Stimulation with PMA/ionomycin increased IFNγ expression in a greater proportion of CD4 TIL from p50−/− mice than WT mice. Upon stimulation for 3 days ex vivo using CD3/CD28 antibodies, we detected increased Granzyme B in CD8 TIL, and increased Granzyme B, IFNγ, and TNFα in CD4 TIL from p50−/− hosts. We detected no change in Tgfb mRNA expression in p50−/− CD11b+ cells, which correlates with unaltered total numbers of tumor Tregs. However, when increased numbers of IFNγ+ CD4 T cells are taken into account, we find a 10-fold decrease in the ratio of Treg:CD4 effectors in tumors from p50−/− mice. We find a similar trend, albeit a lower twofold change, in the Treg:CD8 effector ratio. Collectively, our T-cell data suggest a greater relevance of CD4 T-cell activation than CD8 T-cell activation for reducing GL261 glioma growth and prolonging survival in the absence of p50. The crucial role of CD4 T cells in controlling brain tumor growth has been seen in the GL261 model upon immune checkpoint blockade and in a medulloblastoma model upon tumor Cdk5 kinase loss that results in reduced tumor PD-L1 [37, 38]. In the clinical setting, low CD4 counts have been correlated with increased tumor growth and earlier death in glioma patients [39, 40].
The contribution of CD4 and CD8 T cells to slowed tumor growth in p50−/− hosts was evident from the complete ablation of survival advantage upon CD4 T-cell depletion, and from partial ablation of survival advantage upon CD8 T-cell depletion. CD8 T-cell activation was at least in part cell-intrinsic, as cultured naïve splenic p50−/− CD8 T cells gave rise to increase Tc1 cells. Although ex vivo naïve splenic p50−/− CD4 T-cell skewing to Th1, Th2, Th17, or Treg phenotypes was equivalent to that of WT cells, we cannot rule out a cell-intrinsic effect of p50 deletion on tumor CD4 Th1 activation in vivo.
Two additional findings support a role for T-cell-intrinsic changes in the absence of p50 contributing to slowed glioma growth in p50−/− mice. First, combined CD4 and CD8 T-cell ablation largely reversed tumor myeloid M2 polarization in WT hosts without affecting tumor myeloid M1 polarization in p50−/− hosts. To our knowledge, these data reveal a largely unappreciated role for T cells in maintaining tumor-supportive TAM M2 polarization. The M1 markers IL-1𝛽 and Nos2 were not increased in WT hosts upon T-cell depletion, suggesting that their increase in p50−/− tumor myeloid cells may result from a myeloid cell-intrinsic change. Second, glioma growth was not slowed in p50(f/f);Lys-Cre compared with p50(f/f) tumor recipients. Future experiments will utilize additional Cre transgenes, e.g., CD4-Cre, CD8α-Cre, CD11c-Cre, or combinations, to further dissect the relevant cell types.
Collectively, our findings suggest that targeting NF-κB p50 in both tumor T cells and myeloid cells has the potential to contribute to glioma therapy. Although targeting the p50 protein may be problematic, using a p50 siRNA directed to these cells in vivo represents a viable therapeutic option. Depleting p50 in T cells may both obviate their ability to induce tumor myeloid M2 polarization and directly favor their activation by tumor antigens, whereas depleting myeloid p50 may directly enable their adoption of an anti-tumor M1 phenotype. Importantly, inactivation of the NF-κB p50 transcription factor could potentially avoid resistance mechanisms that arise when upstream, cytoplasmic signaling pathways are targeted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- B6
C57BL/6
- BMDM
Bone-marrow-derived macrophage
- FC
Flow cytometry
- GBM
Glioblastoma
- IVIS
In vivo imaging system
- MR
Mannose receptor
- p50
NF-κB p50
- PBS
Phosphate-buffered saline
- qRT-PCR
Quantitative real-time PCR
- TAM
Tumor-associated macrophage
- TIM
Tumor-infiltrating myeloid cell
- WT
Wild type
Author contributions
TB, AM, RS, DJB, and SH-B conducted experiments. CGD, ML, and ADF guided the conduct of experiments. TB and ADF wrote the manuscript.
Funding
This study was supported by grants from Alex’s Lemonade Stand Foundation, Hyundai Hope on Wheels, the Allegheny Health Network-Johns Hopkins Cancer Research Fund, the National Institutes of Health (T32 CA60441 and P30 CA006973), and by the Giant Food Children’s Cancer Research Fund.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Animal source
WT C57BL/6 (B6) mice were obtained from Charles River Laboratories, Nfkb1(p50)−/−, Lys-Cre, and ROSA26-FLPo mice from Jackson Laboratory (6097, 4781, 12930), and p50(flacZ/+) mice from the Knockout Mouse Project (CSD29053).
Cell line authentication
GL261-Luc cells were obtained from Perkin Elmer, who had authenticated it as C57BL/6 in origin by mouse STR analysis.
References
- 1.Kennedy BC, Showers CR, Anderson DE, Anderson L, Canoll P, Bruce JN, Anderson SC. Tumor-associated macrophages in glioma: friend or foe? J Oncol. 2013;2013:486912. doi: 10.1155/2013/486912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shand FH, Ueha S, Otsuji M, Koid SS, Shichino S, Tsukui T, Kosugi-Kanaya M, Abe J, Tomura M, Ziogas J, Matsushima K. Tracking intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci USA. 2014;111:7771–7776. doi: 10.1073/pnas.1402914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J, Vinnakota K, Kettenmann H, Kotulska K, Grajkowska W, Kaminska B. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230:310–321. doi: 10.1002/path.4192. [DOI] [PubMed] [Google Scholar]
- 13.Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, Holland EC, Sutton JC, Joyce JA. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018. doi: 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, Ulevitch RJ. Regulation of an essential innate immune response by the p50 subunit of NF-κB. J Clin Invest. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-kB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 16.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kB. Proc Natl Acad Sci USA. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kono Y, Kawakami S, Higuchi Y, Yamashita F, Hashida M. In vitro evaluation of inhibitory effect of nuclear factor-κB activity by small interfering RNA on pro-tumor characteristics of M2-like macrophages. Biol Pharm Bull. 2014;37:137–144. doi: 10.1248/bpb.b13-00659. [DOI] [PubMed] [Google Scholar]
- 18.Rackov G, Hernandez-Jimenez E, Shokri R, Carmona-Rodríguez L, Mañes S, Álvarez-Mon M, López-Collazo E, Martínez-A C, Balomenos D. p21 mediates macrophage reprogramming through regulation of p50–p50 NF-κB and IFN-β. J Clin Invest. 2016;126:3089–3103. doi: 10.1172/JCI83404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:1135–1144. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 23.Strauss L, Sangaletti S, Consonni FM, Szebeni G, Morlacchi S, Totaro MG, Porta C, Anselmo A, Tartari S, Doni A, Zitelli F, Tripodo C, Colombo MP, Sica A. RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell. 2015;28:253–269. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Newcomb EW, Zagzag D. The murine GL261 glioma experimental model to assess novel brain tumor treatments. In: Van Meir EG, editor. CNS cancer, models, markers, prognostic factors, targets, and therapeutic approaches. New York: Humana Press; 2009. pp. 228–241. [Google Scholar]
- 25.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produces long term survivors in mice with intracranial glioma. Int J Radiat Oncol Biol Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty S, Reynolds JM. Mouse naïve CD4+ T cell isolation and in vitro differentiation into T cell subsets. J Vis Exp. 2015;98:52739. doi: 10.3791/52739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–961. doi: 10.1097/00006123-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 28.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 29.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, Sund M, Brage SE, Fuxe J, Rolny C, Li F, Ravetch JV, Karlsson MC. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 30.Muller A, Brandenburg S, Turkowski K, Muller S, Vajkoczy Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer. 2015;137:278–288. doi: 10.1002/ijc.29379. [DOI] [PubMed] [Google Scholar]
- 31.Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AH, Ugel S, Sasso MS, Qualls JE, Kratochvill F, Zanovello P, Molon B, Ries CH, Runza V, Hoves S, Bilocq AM, Bindea G, Mazza EM, Bicciato S, Galon J, Murray PJ, Bronte V. T cell cancer therapy requires CD40-40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell. 2016;30:377–390. doi: 10.1016/j.ccell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokubu Y, Tabu K, Muramatsu N, Wang W, Murota Y, Nobuhisa I, Jinushi M, Taga T. Induction of protumoral CD11c(high) macrophages by glioma cancer stem cells through GM-CSF. Genes Cells. 2016;21:241–251. doi: 10.1111/gtc.12333. [DOI] [PubMed] [Google Scholar]
- 33.Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johanns TM, Ward JP, Miller CA, Wilson C, Kobayashi DK, Bender D, Fu Y, Alexandrov A, Mardis ER, Artyomov MN, Schreiber RD, Dunn GP. Endogenous neoantigen-specific CD8 T cells identified in two glioblastoma models using a cancer immunogenomics approach. Cancer Immunol Res. 2016;4:1007–1015. doi: 10.1158/2326-6066.CIR-16-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Nagaraj S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EE, Varner JA. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539:437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, Nicholas S, Kellett M, Ruzevick J, Jackson C, Albesiano E, Durham NM, Ye X, Tran PT, Tyler B, Wong JW, Brem H, Pardoll DM, Drake CG, Lim M. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, Huang AY, Petrosiute A. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353:399–403. doi: 10.1126/science.aae0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, Luznik L, Drake CG. Sustained CD4+ T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3:e27357. doi: 10.4161/onci.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, NABTT. CNS Consortium Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.