Abstract
Glutathione peroxidase 1 (GPX1) is a selenium-dependent enzyme that reduces intracellular hydrogen peroxide and lipid peroxides. While past research explored regulations of gene expression and biochemical function of this selenoperoxidase, GPX1 has recently been implicated in the onset and development of chronic diseases. Clinical data have shown associations of human GPX1 gene variants with elevated risks of diabetes. Knockout and overexpression of Gpx1 in mice may induce types 1 and 2 diabetes-like phenotypes, respectively. This review assembles the latest advances in this new field of selenium biology, and attempts to postulate signal and molecular mechanisms mediating the role of GPX1 in glucose and lipid metabolism-related diseases. Potential therapies by harnessing the beneficial effects of this ubiquitous redox-modulating enzyme are briefly discussed.
Keywords: Diabetes, Glucose, Glutathione peroxidase 1, Lipid, Selenium
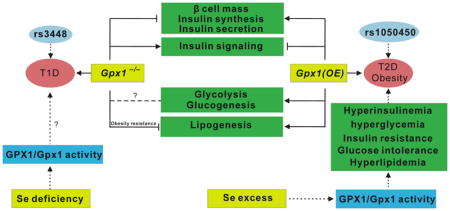
Graphical Abstract
Introduction
Diabetes resulted in a total of 1.6 million deaths in 2015 [1], and is projected to be the seventh leading cause of death by 2030 [2]. The prevalence of diabetes is rapidly rising not only in developed countries but also in middle- and low-income nations [3]. Overdosing or deprivation of dietary selenium (Se) is associated with increased risks of type 2 diabetes (T2D), following a U-shaped curve [4–6]. Although nutritional essentiality of Se and cellular glutathione peroxidase 1 (GPX1) were both identified in 1957 [7–9], GPX1 had not been known until 1972 as the very first selenoprotein and selenoperoxidase to help link these two important discoveries [7, 10–12]. However, a virtually exclusive focus on the redox-modulating functions of GPX1 and the “undoubted” belief in its benefit, similar to that of other antioxidants, to insulin sensitivity and function have made the novel finding of T2D-like phenotypes in the Gpx1-overexpressing mice initially counterintuitive [13–16]. Nevertheless, that metabolic paradox has prompted interests in potential roles of the redox enzymes such as GPX1 in glucose and lipid metabolism [10, 17–19]. Subsequently, a new research field has been created during the past decade or so [20–23] to explore the role and mechanism of GPX1 in regulating insulin synthesis, secretion, and sensitivity, glucose homeostasis, lipogenesis, and lipolysis and in the onset and progression of diabetes.
Diet-mediated GPX1 expression on glucose and lipid metabolism
Selenium deficiency
Dietary Se deficiency decreased GPX1 gene and protein expression in different tissues of several mammalian species [24–35]. While the deficiency did not affect body weights of mice [36], it decreased blood glucose concentration and hepatic concentrations of total cholesterol (TC), triglyceride (TG), and nonesterified free fatty acid (NEFA) in 5-month old mice [13, 15, 37], compared with the Se-adequate controls. Dietary Se deficiency decreased hepatic mRNA abundances of lipogenesis-related genes such as cytochrome P450, family 7, subfamily a, polypeptide 1 (Cyp7a1), sterol regulatory element binding transcription factor 1a (Srebp1a) and 2 (Srebp2), and hepatic activities of glucokinase (Gk) and phosphoenolpyruvate carboxykinase (Pepck) in the muscle of mice [14, 15, 37]. Meanwhile, dietary Se deficiency enhanced pancreatic islet mRNA abundances of catalase (Cat), transcription factor C-fos (Cfos), hepatic nuclear factor 4, alpha (Hnf4α), forkhead box o1 (Foxo1), glucokinase (Gk1), insulin 1 (Ins1), and transformation related protein 53 (Trp53) in the 5-month old mice. In rats, dietary Se deficiency decreased Gpx activity in erythrocytes of dams on day 19 of gestation and in the liver of dams on day 14 postpartum, but elevated mRNA abundances of insulin receptor substrate 2 (Irs2) in the liver of dams on day 14 postpartum. In pigs, dietary Se deficiency did not affect plasma glucose or insulin concentration, but decreased plasma concentration of TC [34, 38].
Because broiler chicks are fast growing and susceptible to dietary Se deficiency [25, 26, 39], and also contain much higher blood glucose concentrations than mammalian species, they may serve as a unique model to study roles of Se and GPX1 in glucose and insulin metabolism. Feeding chicks an Se-deficient diet for 15 weeks decreased TC and TG, but elevated insulin and glucose concentrations in their plasma [39]. While the Se deficiency enhanced mRNA abundances of forkhead box a 2 (FOXA2), glucagon (GCG), and insulin receptor substrate 1 (IRS1) in the liver [39], it decreased transcript numbers of 16 insulin-related genes in three tissues. These genes include IRS2, insulin (INS), pancreatic and duodenal homeobox factor 1 (PDX1), protein tyrosine phosphatase, non-receptor type 1 (PTPN1), and solute carrier family 2, facilitated glucose transporter member 2 (SLC2A2) in the liver; AKT serine/threonine kinase 1 (AKT1), B-Raf proto-oncogene, serine/threonine kinase (BRAF), FOXO1, FOXA2, insulin receptor (INSR), IRS1, IRS2, INS, neuronal differentiation 1 (NEUROD1), PTPN1, phosphoinositide 3-kinase (PI3K), SLC2A2, and uncoupling protein (UCP) in the muscle, and AKT1, FOXA2, Hnf1 homeobox a (HNF1A), HNF4α, INSR, and PDX1 in the pancreas. In summary, dietary Se deficiency dys-regulated glucose homeostasis and altered expression of many insulin- and lipogenesis-related genes in the liver, muscle, and pancreas of both mammalian and avian species.
Selenium supranutrition
Rats
Compared with those fed 0.3 mg Se/kg diet [33], dams of rats fed 3.0 mg Se/kg diet had greater Gpx activities in the erythrocytes on day 19 of gestation and in the liver on day 14 postpartum. Supranutritional Se induced hyperinsulinemia, insulin resistance, and glucose intolerance in the dams at late gestation and/or day 14 postpartum as well as in the offspring at the age of 112 days old. These impairments concurred with decreased transcript and/or protein levels of insulin signaling proteins in the liver and muscle of dams and/or pups. Compared with the 0.3 mg Se/kg diet, the 3.0 mg Se/kg diet resulted in 50% decreases in transcripts of Akt2, Insr, and Irs1 and 36% decrease in the transcript of Foxo1 in the liver of the offspring. The decreased hepatic transcripts of Insr and Akt2 were verified by approximately 60% decreases in the respective proteins Insr and Akt. Although the transcripts of these genes in the muscle was not significantly altered by the high-Se diet, the treatment decreased the expression of Irs2 and phosphatidylglycerol phospholipase (Pgc1) in the muscle of dams on day 14 postpartum. Meanwhile, Foxo1 expression was decreased by both Se depletion and supranutrition.
Pigs
Compared with those fed 0.3 mg Se/kg diet [40], pigs fed 3.0 mg Se/kg diet had GPX activities in the liver and muscle enhanced by 21 and 57%, respectively. However, there were no significant differences in the transcript levels of GPX1 in the two tissues between the two diets. Pigs fed 1.0 mg Se/kg had 23–28% lower plasma TG and(or) TC concentrations than did those fed 0.3 mg Se/kg. Pigs fed 3.0 mg Se/kg diet had doubled plasma insulin concentration at week 11 than pigs fed 0.3 mg Se/kg diet. Their TC and TG concentrations in the adipose tissue were 2.4-fold and 41% greater, respectively, than those fed 0.3 mg Se/kg. Likewise, hepatic concentrations of TC, TG, and NEFA in pigs fed 3.0 mg Se/kg diet were 40%, 2.3-fold, and 63% greater, respectively, than those fed 0.3 mg Se/kg. However, no such differences in the lipid profiles of the muscle tissue were seen between these two levels of dietary Se. Compared with those fed the 0.3 mg Se/kg diet, pig fed 3.0 mg Se/kg diet showed up-regulations of SREBP1 (59%) and fatty acid synthase (FASN) (doubled) in the liver and peroxisome proliferator-activated receptor gamma (PPARG) and TRP53 (42–48%) in the muscle, and down-regulations of CYP7A1 (88%) in the liver and ACC1 (51%) and FASN (57%) in the muscle, respectively.
Chicks
In broiler chicks [41], a high Se (3.0 mg Se/kg) diet elevated plasma GPX activity by 37% at week 4 and muscle GPX activities by about 1.8-, 2.2- and 2.8-fold at week 2, 4, and 6, respectively, compared with the 0.3 mg Se/kg diet. Meanwhile, the high Se diet resulted in 38% higher GPX activity in the pancreas compared with that in the 0.3 mg Se/kg group at week 2 [41]. Broilers fed 3.0 mg Se/kg exhibited a lower fasting plasma glucose concentration, but higher plasma insulin concentration compared with those fed the 0.3 mg Se/kg at week 2. Plasma concentrations of TC and TG were also higher in broilers fed 3.0 mg Se/kg than those fed 0.3 mg Se/kg. The 3.0 mg Se/kg diet increased muscle transcripts of FOXO1, HNF4α, IRS2, and PI3K, hepatic transcripts of GCG, HNF4α, and SLC2A2, and pancreatic transcripts of HNF4α, and IRS2 at week 6. In contrast, the 3.0 mg Se/kg diet downregulated insulin signaling-related genes of AKT1, FOXA2, INS, PI3K, and UCP in the pancreas and AKT1, GCG, and INSR in the muscle at the same time. Meanwhile, hepatic transcripts of GCG were elevated by the Se supranutrition and deficiency in broiler chicks. Pancreatic transcripts of AKT1, and FOXA2, muscle transcripts of AKT1, and INSR in the chicks were downregulated by the Se supranutrition and deficiency in the same direction. In contrast, muscle transcripts of FOXO1 and IRS2, hepatic transcript of SLC2A2, and pancreatic transcript of HNF4α were affected by the Se supranutrition and deficiency in opposite ways. Organic sources of Se from 2-hydroxy-4-methylselenobutanoic acid and Se-enriched yeast seemed to be more effective in restoring hepatic GPX1 transcript and GPX activity in tissues than sodium selenite in broiler chicks [42]. However, differences of these Se forms in affecting glucose and lipid metabolism remain unclear.
Humans
Blood or plasma Se, instead of GPX(1) activity, has often been measure to assess body Se status in human population studies. A recent review [43] indicated that five out of eight cross-sectional studies had shown positive associations between serum/plasma Se and T2D or fasting circulating glucose. Among the five randomized controlled trials (RCTs) with Se supplementation, three trials, including the well-known Se and Vitamin E Cancer Prevention Trial [44], showed no effect, one showed lower fasting serum insulin and homeostasis model assessment of insulin resistance, and only one, the Nutritional Prevention of Cancer study conducted in the dermatology outpatients, showed an increased incidence of T2D [45]. But, Algotar et al. [46]failed to observe the same positive effect of Se on diabetes prevention at a later time. The Selenium and Celecoxib Trial for the prevention of colorectal adenoma recurrence suggested that Se supplementation might increase the risk of T2D in older participants following removal of adenomas [47]. A recent case control study reported that high serum Se concentrations were associated with increased risks for diabetes mellitus, independent of central obesity and insulin resistance [48].
Higher risks of dyslipidemia were associated with higher circulating Se concentrations in the observation trials from all the three times of National Health and Nutrition Examination Survey conducted between 1988–2012 in US [49–51], as well as from the populations of Lebanon [52], Taiwan [53], Britain [54], Finland [55], and Spain [56, 57]. But results from the Se supplementation trials were inconsistent, similar to those for the glucose metabolism. Supplementing Se at 100 μg/day for 6 months increased the cord blood concentrations of TG in a small group of pregnant women (n = 34 for supplementation vs 32 for placebo) [58]. While supplementing antioxidants including Se increased for > 7 years the risk of dyslipidemia in women [59]. However, supplementing Se to the Britain [60] and Chinese [61] adults improved their blood lipid profiles. In addition, the 1988–94 US survey showed that higher levels of serum Apo B and Apo A1 were associated with the highest vs. the lowest serum Se concentrations [49]. Serum lipoprotein (A) concentrations were positively correlated with Se in a 140 adult male population [62]. Notably, those who performed the Spanish trial [56] supported a hypothesis that regulations of blood Se and lipid profiles shared common pathways. Overall, results on the associations of Se with glucose and lipid metabolism from the human studies are inconsistent or even conflicting. Large randomized controlled trials are needed to confirm the pro-diabetic or pro-dyslipidemic potential of excessive Se and GPX1.
Fat, Vitamin E, and other factors
High-fat intake
Although GPX1 mRNA, protein, and activity were highly responsive to dietary Se changes in different tissues of various species [25, 29, 30, 33–35, 42, 63], its mRNA was not changed by a high-fat diet in the heart, hypothalamus, kidney, liver, muscle, pancreas, perirenal adipose tissue (PAT), pituitary, subcutaneous adipose tissue (SAT), or thyroid [64, 65] of pigs. However, several other selenoprotein genes including DIO2, SELENOI, SELENOS, SELENOV, and TXNRD1 in the thyroid; SELENOF in the liver; SELENOO in the kidney; GPX4, GPX6, DIO1, and SELENOV in the muscle; GPX4 and SELENOM in the pituitary; and GPX3 in the hypothalamus were up-regulated by the high fat diet in pigs. Meanwhile, DIO1, SELENOH, SELENOI, SELENOK, SELENOM, SELENOW, and TXNRD1 in the pancreas; SELENOH, SELENOI, and TXNRD1 in the hypothalamus; SELENOI, SELENOM, and MSRB1 in the subcutaneous fat; SELENOH, SELENOK, SELENOP, SELENOV, and SELENOW in the perirenal fat; GPX3, GPX6, DIO3, and SELENOV in the liver; SELENOI, and TXNRD1 in the pituitary; SELENOM in the kidney were down-regulated by the high fat diet in these pigs.
Compared with the control, pigs fed the high fat diet had greater concentrations of serum TG, TC, low density lipoprotein and NEFA [64, 65]. The high fat diet up-regulated 5 lipogenesis-related genes in 3 tissues. These genes included agouti signaling protein (ASIP), agouti related protein (AGRP), and resistin (RETN) in the skeletal muscle; uncoupling protein 3 (UCP3) in the thyroid; and uncoupling protein 2 (UCP2) in the pituitary. In contrast, 11 genes were downregulated in 6 tissues. These changes included AGRP in the liver, kidney, and PAT; leptin receptor (LEPR) in the liver, kidney, and SAT; adiponectin receptor 2 (ADIPOR2) in the kidney, PAT, and SAT; and fatty acid binding protein 4 (FABP4) in the PAT, pituitary, and hypothalamus. In addition, adiponectin (ADIPOQ) was downregulated in the PAT and SAT. Genes that were affected in single tissues include ASIP, fatty acid binding protein 3 (FABP3), RETN, and UCP2 in the liver; fatty acid binding protein 1 (FABP1) in the PAT; and FASN in the SAT. Apparently, these high fat diet–mediated changes of lipogenesis-related gene expression were not directly related to GPX1 mRNA or activity.
Vitamin E
Vitamin E has a close relationship with Se in regulating lipid metabolism [44, 66, 67]. The observation that diabetes leads to high concentrations of organic peroxides, and cholesterol, and the fact that vitamin E protects fatty acids from oxidation or peroxidation implicate it as a possible inhibitor of diabetogenesis [68]. In fact, supplementing the diabetic group with vitamin E and Se for 5 weeks led to a significant increase in GPX activities in plasma [69] and decreases in plasma concentrations of malondialdehyde (MDA) and oxidized low-density lipoprotein (LDL) [69]. In contrast, vitamin E supplementation (all-rac-α-tocopheryl acetate at 50 mg/kg) to a vitamin E deficient diet for chicks led to ~30–50% decreases in transcripts of GPX1, SELENOI, TXNRD1, and TXNRD2 in the liver [25], despite no effect on the liver GPX1 activity. Notably, the same treatment in another study did not affect GPX1 protein, but decreased GPX4 protein and transcripts of SELENOF and SELENOW in the muscle of Se-adequate chicks [26]. The same treatment also elevated transcript of muscle SELENOM, regardless of the Se status of chicks.
Feeding young adult mice high levels of dietary vitamin E (all-rac-α-tocopheryl acetate at 750 or 7,500 mg/kg) could not replace the protection by Gpx1 against the paraquat-induced lethality [70], although hepatic Gpx activities were elevated by ~40% in the Gpx1−/− mice and by 17~24% the wild-type mice compared with those fed 0 or 75 mg of all-rac-α-tocopheryl acetate//kg. Contrary to the chick results, hepatic Gpx4 activities in both Gpx1−/− and wild-type mice showed dose-dependent increases (36 and 48%, respectively) in response to the increases in dietary vitamin E supplementation. Apparently, supplemental dietary vitamin E at the nutrient requirement and higher levels could affect the transcript, protein, and activity of several selenoproteins including Gpx4. However, its impacts may be species, tissue, and selenoprotein-dependent, and the functional implication for the interaction of Se and vitamin E remains unclear. Recent studies have illustrated the involvement of Gpx4 in the ferroptotic cell death that entails cellular iron accumulation and lipid peroxidation, and Gpx4−/− cells could be rescued from the cell death by vitamin E [71].
Other factors
Zinc finger protein 143 (ZNF143) transcription factor mediated cell survival through upregulation of the GPX1 activity at the mitochondrial respiratory dysfunction [72]. A novel upregulation of Gpx1 by knockout of regenerating islet-derived 3 beta (Reg3β) aggravated acetaminophen-induced hepatic protein nitration, while the knockout enhanced Gpx1 activity via selenocysteine lyase upregulation [73]. Skeletal muscle Gpx1 expression in mice was altered by both exercise and dyslipidemia through changes in DNA methylation [74].
Genetically-altered GPX1 expression on glucose and lipid metabolism
Gpx1 knockout
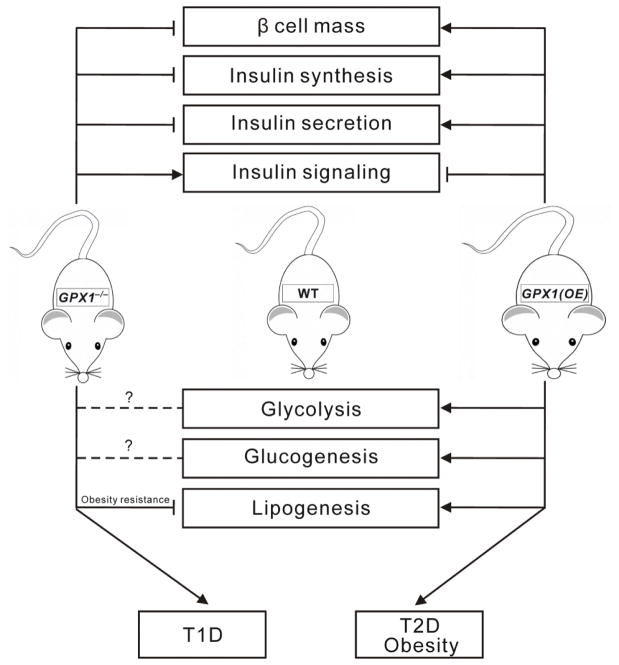
Compared with wild type mice, Gpx1−/− mice had lower pancreatic β-cell mass, hypoinsulinemia, mild hyperglycemia, and impaired ATP production and glucose-stimulated insulin secretion(GSIS) in islet [16] (Figure 1). The molecular mechanism was associated with decreased Pdx1 and elevated Ucp2 in pancreas [16]. Knockout of Gpx1, contrary to the overexpression of Gpx1, improved insulin sensitivity [16]. This was because appropriate amount of intracellular reactive oxygen species (ROS) is important to control the activity of protein phosphatases. Consistently, the improved insulin sensitivity in the Gpx1−/− mice were associated with elevated intracellular hydroperoxides and phosphorylation of p53 and p38-AMP-activated protein kinase (Mapk) in the islets and enhanced PI3K/Akt signaling and glucose uptake in the muscle. Meanwhile, Gpx1−/− mice were protected from the high-fat diet-induced insulin resistance by a mechanism related to enhanced oxidation of the PI3K antagonist phosphatase and tensin homolog (Pten) [75].
Figure 1.
Roles of glutathione peroxidase 1 (Gpx1) in insulin physiology, glucose, lipid, and protein metabolism. Overexpression of Gpx1 [Gpx1(OE)] induced hypertrophy of β cells, hyperinsulinemia, hyper secretion of insulin, hyperglycemia, hyperlipidemia, insulin resistance, and obesity. In contrast, knockout of Gpx1 (Gpx1−/−) led to hypotrophy of β-cells, hypoinsulinemia, hyposecretion of insulin, and elevated insulin sensitivity. Lines ending with arrows, activation or increase; Lines ending with cross bars, inhibition or decrease; Dash with question mark, unknown function; T1D, type 1 diabetes; T2D, type 2 diabetes;
Gpx1−/− mice showed decreased expression of gluconeogenic genes such as glucose-6- phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (Pepck), increased glucose uptake by white gastric and diaphragm skeletal muscles through membrane docking of glucose transporter 4 (Glut4) upon Akt substrate of 160 kDa (As160) phosphorylation on Thr642 [pAS160(Thr642)], and enhanced insulin-induced oxidation β fibroblast cells [75]. In line with the elevated PI3K/Akt signaling in the Gpx1−/− muscle, the phosphorylation (Thr642) of the AS160, a Rab GTPase that regulates Glut4 docking on the plasma membrane for glucose uptake, was increased, whereas glycogen synthase Ser-640/641 phosphorylation was reduced (a consequence of Akt phosphorylating and inhibiting glycogen synthase kinase 3). No significant change in insulin-induced PI3K/Akt signaling was seen in the liver or adipose tissue of the Gpx1−/− mice. In another study [76], Gpx1−/− mice fed an obesogenic high-fat diet for 12 weeks exhibited systemic oxidative stress and hyperglycemia, but had unaltered whole body insulin sensitivity, improved hepatic insulin signaling, and decreased whole body glucose production, hepatic steatosis and damage, plasma insulin, and glucose stimulated insulin secretion. The attenuated insulin secretion was associated with the decreased islet β cell Pdx1 and insulin production, elevated pancreatic Ptp (protein tyrosine phosphatase) oxidation, and accelerated Y701 phosphorylation of signal transducer and activator of transcription 1 (Stat1).
In hepatocyte-specific Gpx1 knockout mice [77], insulin induced a combined change in the liver via the PI3K/Akt2 pathway in the postprandial state: decreased transcription of gluconeogenic genes of Pck1 and G6pc and increased transcription of Gk1 and other genes that promote glycogen storage or glycolysis. This combination coordinately repressed hepatic glucose production and prevented postprandial hyperglycaemia. In the fasting state, expression of G6pc and Pck1 was also decreased in the liver of these mice, accompanied by a 7.2-fold increase in the Gk1 transcript. Because Gk catalyzes the conversion of glucose to G6P and serves as the first step of glycolysis or glycogen synthesis, hepatic glycogen storage was elevated in these mice. In addition, the expression of pyruvate dehydrogenase kinase 4 (Pdk4) was decreased, whereas hepatocyte basal and insulin-induced H2O2 generations were exacerbated by the Gpx1 deficiency. Moreover, the insulin-induced phosphorylation of insulin receptor-Y1162/Y1163 and Akt-S473 was enhanced. These results were consistent with that the GPX1 deficiency repressed hepatic glucose production and promoted glucose storage and utilization without altering lipogenesis [18].
Gpx1 overexpression
GPX1 overproduction may be beneficial if diabetes or obese are developed. However, excessive GPX1 activity is actually deleterious to normal metabolism. Figure 1 illustrates molecular and biochemical mechanisms for the T2D-like phenotypes induced by Gpx1 overexpression [Gpx1(OE)] in mice [13, 15, 37, 78]. The over-produced Gpx1 activity in the pancreatic islets enhanced β cell mass and insulin synthesis and secretion via modulations of key genes and proteins at the epigenetic, transcript, and/or protein levels. These effects led to hypersecretion of insulin and hyperinsulinemia. Meanwhile, Gpx1 overexpression also impaired insulin responsiveness in the liver and muscle and disturbed lipogenesis, glycolysis, and gluconeogenesis in these tissues. The attenuated phosphorylations of Insr and Akt in both liver and muscle after insulin stimulation were associated with over-quenching intracellular ROS that are required for inhibiting protein phosphatases [13, 15, 78, 79]. This subsequently contributed to insulin resistance in these mice. Dietary Se deficiency actually improved the T2D-like phenotypes in the Gpx1(OE) mice [37]. The improvement was mediated by reversing gene expression of key factors involved in insulin synthesis and secretion (Beta2, Cfos, Foxa2, Pregluc, Ins1, Trp53, and Sur1) to the wild type levels. Dietary Se deficiency also downregulated hepatic gene expression of two rate-limiting enzymes for lipogenesis (Acc1 and Gk1), and lowered activities of hepatic Gk and muscle Pepck in these mice.
As discussed above, elevated GPX1 activity was associated with excessive dietary Se intakes and insulin resistance in various species [33, 34, 40, 41]. Because the Gpx1 overexpression induced insulin resistance via diminishing intracellular ROS, elevating other antioxidant enzymes or antioxidants may cause similar problems [68, 80]. Overall, the development of T2D-like phenotypes in the Gpx1(OE) mice offers a unique model for the study of redox control and insulin resistance [81, 82].
GPX1 on high-fat diet/diabetic-related atherosclerosis
Recent clinical studies have suggested a major protective role for GPX1 against atherosclerosis [83–85]. Lack of functional Gpx1 accelerated diabetes-associated atherosclerosis via upregulation of pro-inflammatory and pro-fibrotic pathway in ApoE-deficient mice [86]. However, a specific deficiency in Gpx1 did not cause changes in biomarkers of oxidative damage or increased atherosclerosis in a murine model with the high fat diet-induced atherogenesis [87]. Thus, effects of Gpx1 and high fat diet in the presence and absence of ApoE deficiency were different. In ApoE-deficient mice, deficiency of Gpx1 accelerated the progression of atherosclerosis [88]. Likewise, lack of Gpx1 accelerated atherosclerosis and upregulated proatherogenic pathways in diabetic ApoE/Gpx1 double-knockout mice, thereby establishing Gpx1 as an important therapeutic target [89, 90]. Ebselen reduced atherosclerotic lesions in most regions of the diabetic ApoE-deficient aorta, except for the aortic sinus, suggesting its effectiveness as a potential antiatherogenic therapy of diabetic-macrovascular disease. Ebselen might elicit its effect via modulation of transcription factors such as NF-κB and AP-1 [89, 90].
Atherosclerotic lesions within the aortic sinus region, as well as arch, thoracic, and abdominal lesions, were significantly increased in diabetic ApoE/Gpx1 double-knockout mice aortas compared with diabetic ApoE-deficient mice aortas [86]. This was associated with increased staining for smooth muscle cells (SMCs) and macrophages, consistent with increased SMC migration and macrophage infiltration. Furthermore, a range of molecules implicated in the progression and development of atherosclerosis, including vascular cell adhesion molecule-1(VCAM1), vascular endothelial growth factor and connective tissue growth factor, cytokines, growth factors, and receptors for advanced glycation end products, were increased by the absence of Gpx1 [86]. Furthermore, plasmalogen enrichment via batyl alcohol supplementation attenuated atherosclerosis in ApoE and ApoE/Gpx1 double deficient mice, with a greater effect in the latter group [91]. Plasmalogen enrichment may represent a viable therapeutic strategy to prevent atherosclerosis and reduce cardiovascular disease risk, particularly under conditions of elevated oxidative stress and inflammation [91].
Subcellular location of GPX1 and its interaction with other GPX enzymes
Immunogold ultrastructural staining showed that GPX1 exists not only in cytosol but also in the mitochondria and nucleus [20, 92]. Cytosol GPX1 overexpression reversed the tumor cell growth inhibition caused by manganese-dependent superoxide dismutase overexpression, altered intracellular GSH, GSSG, and ROS [92], and attenuated degradation of the inhibitory subunit a of NF-κB [92]. The GPX1 gene codes for both the cytosolic and mitochondrial forms of the enzyme [93, 94]. Liver is highly dependent on GPX1 for its mitochondrial antioxidant defenses [95]. Knockout or overexpression of Gpx1 did not produce significant changes in the other forms of GPX, implying an independent expression of these selenoperoxidases [96, 97]. The relationship between Gpx1 and steroidogenesis was confirmed by the immunocytochemical localization of the enzyme in the rat adrenal cortical cells [22], and both cytosol- and mitochondrial-Gpx1 were modified by lipoperoxidative damage in those cells. It seemed that the pattern of Gpx1 staining was a sensitive and specific indicator of oxidative damage in the cells [98, 99].
Expression of GPX2 is mainly in the gastrointestinal epithelium, but is also localized in the epithelium lining of the lung, bladder, and breast[100]. This enzyme has been detected only in cytosol. Knockout of Gpx2 in mice induced an increase in Gpx1 expression that could only compensate partially for the loss of Gpx2 in the colon [101]. The Gpx2−/− mice were susceptible to allergic airway inflammation [102]. A double knockout of Gpx1 and Gpx2 produced a worse impairment of the intestinal integrity, than the single knockout, resulting in spontaneous developments of ileocolitis [103] and colon cancer [104]. Both symptoms could be efficiently prevented by bringing back one allele of Gpx2 but not Gpx1 [105].
As the only extracellular isoform of the GPX family, GPX3 is detectable in the plasma and in extracellular body fluids such as chamber water of the eye, thyroid colloid lumen, and amniotic fluid [106]. Liver Se concentration and cytosolic GPX activity were not altered by the knockout of Gpx3 [107]. Three forms of GPX4 proteins are expressed by the Gpx4 gene: a long form (lGPX4), a short form (sGPX4), and a nuclear form (nGPX4) [108]. The lGPX4 has a mitochondrial signal at the N terminus, and is believed to be targeted to mitochondria [109]. The sGPX4 protein is synthesized using the second translation start codon and is believed to be the non-mitochondrial GPX4 protein found in cytoplasm, nucleus, and microsome. The nGPX4 protein is encoded from an alternative first exon called exon Ib and is expressed mainly in sperm nuclei. nGPX4 proteins are dispensable for both somatic functions and fertility [110]. Likewise, there was no change in the expression of GPXs in the liver of 1 day old Gpx4-deficient mice [111]. Moreover, GPX6 has been found only in the olfactory epithelium [112].
Polymorphisms of human GPX1 on glucose and lipid metabolism
There are a number of recognized single nucleotide polymorphisms (SNP) of GPX1 associated with obesity and insulin resistance in humans (Table 1). A well-known missense mutation of rs1050450 (C to T substitution) results in the substitution of leucine for proline at codon 198 (or 200) of the GPX1 protein [85, 113]. A few studies have shown the leucine allele to be associated with outcomes of oxidative stress, central obesity and insulin resistance, with some sex-related differences [114, 115]. Male T allele (leucine) carriers had a higher metabolic syndrome prevalence, with higher waist-hip ratios, serum TG and insulin, homeostasis model assessment of β cell function, and systolic and diastolic blood pressures [115, 116]. Female T allele carriers showed higher body fat mass, serum insulin, and homeostasis model assessment of insulin resistance [114]. Nutritional supplementation of Se from Brazil nuts was associated with higher DNA damage in the leucine carriers [114]. The GPX1 Pro200Leu polymorphism (rs1050450) was associated with morbid obesity, independently of the presence of prediabetes or diabetes in women from central Mexico [117]. Carriers of the T allele also had higher levels of lipoperoxides and MDA in LDLs [118].
Table 1.
Genetic variants in human GPX1 associated with diabetes/obesity-related phenotypes
| Analyzed variant(dbSNP) | Other designation | Metabolic phenotype | Reference |
|---|---|---|---|
| rs1050450 | 594C/T, Pro198Leu | The CT/TT genotype had higher waist-hip ratios, triacylglycerol concentrations, homeostasis model assessment for β-cell function, and systolic and diastolic blood pressures in men | [115] |
| The CT/TT genotype had higher body fat mass, insulin and HOMA-IR in women | [115] | ||
| Leu carriers had higher lipoperoxides and MDA in LDL | [118] | ||
| Leu carriers showed higher DNA damage after Se supplementation | [114] | ||
| Leu carriers had higher lipoperoxides and MDA in LDL, lower GPX activity | [113] | ||
| Erythrocyte GPX activity was lowered with the T allele dose | [113] | ||
| Pro198Leu | The variant T allele was associated with a higher risk of developing diabetic peripheral neuropathy | [116] | |
| Ala5/Ala6+ Pro198Leu | Ala6/198Leu polymorphism had a 40% decrease in GPX1 activity | [85] | |
| -602A/G+2C/T | 25% decrease in transcriptional activity | [85] | |
| Pro200Leu | linked to morbid obesity in central Mexican women | [117] | |
| rs8179169 | Arg5Pro | Had an effect on erythrocyte Se, with lower concentrations in individuals with the GC genotype | [132] |
| rs3448 | XT/CC | The CT/TT allele was associated with higher plasma concentrations of isoprostane and advanced oxidation protein products | [21] |
GPX1, glutathione peroxidase 1; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, Low-density lipoprotein; MDA, malondialdehyde; Se, selenium.
A combination of Pro198Leu SNP (rs1050450) with the copy number variant Ala5/Ala6 at codon 7–11 decreased the activity of the enzyme by 40% in vitro [85]. The same study demonstrated that the combination of two other SNPs (-602A/G and 2C/T) decreased the transcriptional activity of GPX1 by 25% [85]. These data suggest that the T allele was associated with lower GPX activity and a possible higher oxidative stress status, aggravating the obesity-associated phenotypes. The genotype distribution of GPX1 Pro198Leu variant in the Chinese population (the frequency of T allele is 14%) was different from that in the Swedes (the frequency of T allele is 9.0%) [119, 120]. Pro198Leu polymorphism of GPX1 raised the risk of T2D in Han Chinese of Shanghai. The T allele was a risk factor of T2D but not of diabetic coronary heart disease [120]. Another GPX1 variant, T-allele of rs3448, was associated with kidney complications in T1D patients [21], which was consistent with the implication of GPX1 in the protection against renal oxidative stress in those patients [121].
Conclusion and perspective
Overall, this review highlights the dual role of GPX1 in glucose and lipid metabolism and the related human health implications. As the most abundant isoform of the GPX family, GPX1 exerts its impacts via regulating gene expression, protein function, and enzyme activities of key factors involved in both macro- and micro-nutrient metabolism [97, 122, 123]. The combined effects of the Gpx1 overexpression [14, 15, 20, 44, 124] in the insulin-producing and insulin-responsive tissues lead to metabolic phenotypes similar to T2D [37, 78, 79]. Meanwhile, the T1D-like phenotypes in the Gpx1−/− mice [16, 75] seem to be reciprocal. These two extremes underscore the importance to maintain an appropriate expression and activity of this selenoperoxidase for controlling redox balance and glucose and lipid metabolism [13, 17, 97, 124–126]. Excessive ROS accumulation, due to Gpx1 deficiency, inhibits gene expression or protein production of key transcriptional factors like Pdx1, leading to lowered islet β cell mass, insulin synthesis, and insulin secretion [37, 78]. However, the physiological level of ROS is essential to control protein phosphatase activity for insulin signaling. Overly diminishing intracellular ROS by Gpx1 overexpression desensitizes insulin signaling [4, 21, 77, 123]. Along with the chronic hyperinsulinemia resultant from the dysregulated islet β cell mass, insulin synthesis, and insulin secretion, this desensitization leads to insulin resistance in the Gpx1(OE) mice [13, 79].
Illustrating the associations of GPX1 polymorphisms with risks of diabetes and obesity in different populations [113, 115–118] highlights GPX1 as a novel, key regulator of insulin physiology and energy metabolism. Diabetic patients with decreased GPX1 function, due to GPX1 polymorphism, had an increased risk for cardiovascular diseases [83–85]. The deficiency of GPX1 accelerated diabetic atherosclerosis in the ApoE-knockout mice [86], and the acceleration concurred with an increased nitrotyrosine formation and transcriptional changes of inflammatory and profibrotic factors [86]. Ebselen was shown to reduce diabetes-associated atherosclerosis [89]. This well-known GPX1 mimic has also been successfully used to decrease oxidative injuries [89, 127], to prevent noise-induced hearing loss [128], and reduce neurotoxicity in a variety of animal models in which GPX1 deficiency caused opposite effects [129].
Ebselen has been shown to improve GSIS in islets of Gpx1−/− mice [127]. The rescue results from a coordinated transcriptional regulation of four key GSIS regulators via the PGC-1α-mediated signaling pathway, and supports the notion that excessive ROS inhibits GSIS [130] and ebselen removes this inhibition by acting as a GPX mimetic to scavenge the elevated intracellular H2O2 due to the lack of Gpx1. Likewise, the SOD mimic, copper diisopropylsalicylate, that catalyzes H2O2 production from superoxide, also rescued the defected GSIS in the superoxide dismutase-1 (Sod1) knockout mouse pancreatic islets. This suggested that the lack or blocking of enzymatic production of H2O2 from superoxide in the Sod1−/− islets impaired GSIS. Because an adequate amount of H2O2 is required to initiate GSIS [131], the Sod1-deficient islets might not produce sufficient H2O2 or have appropriate ratios of H2O2 to superoxide to support GSIS. Thus, the SOD mimic treatment could have rescued GSIS by restoring H2O2 generation. However, this H2O2 restoring notion could not explain the positive effect of ebselen (supposed to decrease H2O2) on GSIS in the Sod1-deficent islets. Meanwhile, the SOD mimic affected the gene expression of the Pgc-1α pathway in a different or just opposite way from that of ebselen in the same type of islets (double knockouts of Gpx1 and Sod1) [127]. These findings not only have illustrated that GPX1 and SOD1, as two important intracellular antioxidant enzymes, function distinguishably in regulating insulin secretion, but also underscored the ROS concentration- or redox balance-dependent effects of their mimics. This complexity highlights the necessities and opportunities of discretional applications of various antioxidant enzyme mimics in treating insulin-related disorders [15].
Highlights.
Supranutrition of Se is associated with hyperglycemia and hyperinsulinemia
Knockout of Gpx1 induces metabolic changes similar to type 1 diabetes
Overexpression of Gpx1 produces type 2 diabetes-like phenotypes
Human GPX1 polymorphism links to risks of diabetes and obesity
Acknowledgments
The research in the authors’ laboratory was supported in part by a grant from the Major International (Regional) Joint Research Program of the Natural Science Foundation of China (No. 31320103920), the 111 Project from the Education Ministry of China (No. B18053), and a grant of NIH DK 53018 (to XL).
Abbreviations used
- ACC1
acetylcoenzyme A carboxylase 1
- ASIP
agouti signaling protein
- BETA2
transcription factor Beta 2
- CYP7a1
cytochrome P450, family 7, subfamily a, polypeptide 1
- cFOS
transcription factor C-fos
- FASN
fatty acid synthase
- FOXA22
forkhead box protein A2
- GK1
glucokinase
- INSR
insulin receptor
- GPX1
glutathione peroxidase 1
- GPX1(OE)
GPX1 overexpression
- GSIS
glucose-stimulated insulin secretion
- LDL
low-density lipoprotein
- NEFA
nonesterified free fatty acid
- PAT
perirenal adipose tissue
- PDX1
pancreatic and duodenal homeobox 1
- PEPCK
phosphoenolpyruvate carboxykinase
- ROS
reactive oxygen species
- SAT
subcutaneous adipose tissue
- Se
selenium
- SLC2A2
solute carrier family 2 member 2
- SNPs
single nucleotide polymorphisms
- SREBPs
sterol regulatory element-binding proteins
- SUR1
sulfonylurea receptor 1
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TC
total cholesterol, TG, triglyceride
- Trp53
transformation related protein 53
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D. Prevalence and ethnic pattern of diabetes and prediabetes in china in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. Plos Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou B, Lu Y, Hajifathalian K, Bentham J, Cesare MD, Danaei G, Bixby H, Cowan MJ, Ali MK, Taddei C. Worldwide trends in diabetes since 1980: pooled analysis of 751 population-based measurement studies with over 4.4 million participants. Lancet. 2016;387(1):513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa-Wong AN, Berry MJ, Seale LA. Selenium and metabolic disorders: an emphasis on type 2 diabetes risk. Nutrients. 2016;8:80. doi: 10.3390/nu8020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayman M. Selenium and human health. Lancet. 2012;379(1):256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 6.Sárközy M, Szũcs G, Pipicz M, Zvara Á, ÉK, Fekete V, Szũcs C, Bárkányi J, Csonka C, Puskás LG. The effect of a preparation of minerals, vitamins and trace elements on the cardiac gene expression pattern in male diabetic rats. Cardiovasc Diabetol. 2015;14:85. doi: 10.1186/s12933-015-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz K. Development and status of experimental work on factor 3-selenium. Fed Proc. 1961;20:189–197. [PubMed] [Google Scholar]
- 9.Schwarz K, Foltzs CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. Nutr Rev. 1999;15:255. [PubMed] [Google Scholar]
- 10.Mills GC. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957;229:189–197. [PubMed] [Google Scholar]
- 11.Moriarty PM, Reddy CC, Maquat LE. Selenium deficiency reduces the abundance of mRNA for Se-dependent glutathione peroxidase 1 by a UGA-dependent mechanism likely to be nonsense codon-mediated decay of cytoplasmic mRNA. Mol Cell Biol. 1998;18:2932–2939. doi: 10.1128/mcb.18.5.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25:465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei XG, Cheng WH, Mcclung JP. Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 2007;27:41–61. doi: 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- 14.Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on βcells and diabetes. Antioxid Redox Signal. 2011;14:489–503. doi: 10.1089/ars.2010.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR, Holmgren A, Arnér ES. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev. 2016;96:307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, Lei XG. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 2011;14:391–401. doi: 10.1089/ars.2010.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crack PJ, Cimdins K, Ali U, Hertzog PJ, Iannello RC. Lack of glutathione peroxidase-1 exacerbates abeta-mediated neurotoxicity in cortical neurons. J Neural Transm. 2006;113:645–657. doi: 10.1007/s00702-005-0352-y. [DOI] [PubMed] [Google Scholar]
- 18.de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273:22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 19.Zhu JH, Mcclung JP, Zhang X, Aregullin M, Chen C, Gonzalez FJ, Kim TW, Lei XG. Comparative impacts of knockouts of two antioxidant enzymes on acetaminophen-induced hepatotoxicity in mice. Exp Biol Med (Maywood) 2009;234:1477–1483. doi: 10.3181/0904-RM-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammedi K, Patente TA, Bellili-Muñoz N, Driss F, Le NH, Fumeron F, Roussel R, Hadjadj S, Corrêa-Giannella ML, Marre M. Glutathione peroxidase-1 gene (GPX1) variants, oxidative stress and risk of kidney complications in people with type 1 diabetes. Metabolism. 2016;65:12–19. doi: 10.1016/j.metabol.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Murakoshi M, Osamura RY. Immunolocalization of glutathione-peroxidase (GPX1) in the rat adrenal cortex: correlation between steroidogenesis and lipid peroxidation. Acta Histochem Cytochem. 2017;50:57–61. doi: 10.1267/ahc.17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Huang K, Lei XG. Selenium and diabetes--evidence from animal studies. Free Radic Biol Med. 2013;65:1548–1556. doi: 10.1016/j.freeradbiomed.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker RD, Baker SS, Larosa K, Whitney C, Newburger PE. Selenium regulation of glutathione peroxidase in human hepatoma cell line hep3B. Arch Biochem Biophys. 1993;304:53–57. doi: 10.1006/abbi.1993.1320. [DOI] [PubMed] [Google Scholar]
- 25.Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG. The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr. 2011;141:1605–1610. doi: 10.3945/jn.111.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang JQ, Ren FZ, Jiang YY, Xiao C, Lei XG. Selenoproteins protect against avian nutritional muscular dystrophy by metabolizing peroxides and regulating redox/apoptotic signaling. Free Radic Biol Med. 2015;83:129–138. doi: 10.1016/j.freeradbiomed.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Saedi MS, Smith CG, Frampton J, Chambers I, Harrison PR, Sunde RA. Effect of selenium status on mRNA levels for glutathione peroxidase in rat liver. Biochem Biophys Res Commun. 1988;153:855–861. doi: 10.1016/s0006-291x(88)81174-4. [DOI] [PubMed] [Google Scholar]
- 28.Sunde RA, Hadley KB. Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med (Maywood) 2010;235(2):3–31. doi: 10.1258/ebm.2009.009262. [DOI] [PubMed] [Google Scholar]
- 29.Sunde RA, Raines AM. Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv Nutr. 2011;2:138–150. doi: 10.3945/an.110.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29:329–338. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunde RA, Saedi MS, Knight SAB, Smith CG, Evenson JK. Regulation of expression of glutathione peroxidase by selenium. In: Wendel A, editor. Selenium in Biology and Medicine. Springer; Berlin Heidelberg: 1989. [Google Scholar]
- 32.Sunde RA, Thompson BM, Palm MD, Lweiss S, Thompson KM, Jacqueline A. Selenium regulation of selenium-dependent glutathione peroxidases in animals and transfected CHO cells. Biomed Environ Sci. 1997;10(z1):346–355. [PubMed] [Google Scholar]
- 33.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radical Biol Med. 2012;52:1335–1342. doi: 10.1016/j.freeradbiomed.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr. 2009;139:1061–1066. doi: 10.3945/jn.109.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou JC, Zheng S, Mo J, Liang X, Xu Y, Zhang H, Gong C, Liu XL, Lei XG. Dietary selenium deficiency or excess reduces sperm quality and testicular mRNA abundance of nuclear glutathione peroxidase 4 in rats. J Nutr. 2017;147:1947–1953. doi: 10.3945/jn.117.252544. [DOI] [PubMed] [Google Scholar]
- 36.Cao L, Zhang L, Zeng H, Wu RT, Wu TL, Cheng WH. Analyses of selenotranscriptomes and selenium concentrations in response to dietary selenium deficiency and age reveal common and distinct patterns by tissue and sex in telomere-dysfunctional mice. J Nutr. 2017;147:1858–1866. doi: 10.3945/jn.117.247775. [DOI] [PubMed] [Google Scholar]
- 37.Yan X, Pepper M, Vatamaniuk M, Roneker C, Li L, Lei XG. Dietary selenium deficiency partially rescues type 2 diabetes-like phenotypes of glutathione peroxidase-1-overexpressing male mice. J Nutr. 2012;142(1):975–1982. doi: 10.3945/jn.112.164764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Zhao H, Zhang Q, Tang J, Li K, Xia XJ, Wang KN, Li K, Lei XG. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J Nutr. 2012;142:1410–1416. doi: 10.3945/jn.112.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Wang L, Tang J, Jia G, Liu G, Chen X, Cai J, Shang H, Zhao H. Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. Plos One. 2017;12:e0182079. doi: 10.1371/journal.pone.0182079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Xin GL. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr. 2016;146:1625–1633. doi: 10.3945/jn.116.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Tang J, Xu J, Gang J, Liu G, Chen X, Cai J, Shang H, Hua Z. Supranutritional dietary selenium induced hyperinsulinmia and dyslipidemia via affected expression of selenoprotein genes and insulin signal-related genes in broiler. Rsc Adv. 2016;6(8):4990–84998. [Google Scholar]
- 42.Zhao L, Sun LH, Huang JQ, Briens M, Qi DS, Xu SW, Lei XG. A novel organic selenium compound exerts unique regulation of selenium speciation, selenogenome, and selenoproteins in broiler chicks. J Nutr. 2017;147(7):89–797. doi: 10.3945/jn.116.247338. [DOI] [PubMed] [Google Scholar]
- 43.Rayman MP, Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radical Biol Med. 2013;65:1557–1564. doi: 10.1016/j.freeradbiomed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Klein E, Thompson IJ, Tangen C, Crowley J, Lucia M, Goodman P, Minasian L, Ford L, Parnes H, Gaziano J. Vitamin E and the risk of prostate cancer: updated results of the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 46.Algotar AM, Hsu CH, Singh P, Stratton SP. Selenium supplementation has no effect on serum glucose levels in men at high risk of prostate cancer. J Diabetes. 2013;5:465–470. doi: 10.1111/1753-0407.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, Wang F, Bhattacharyya A, Hsu CH, Chow HHS, Ahnen DJ. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. 2016;108:djw152. doi: 10.1093/jnci/djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu CW, Chang HH, Yang KC, Chia-Sheng K, Long-Teng L, Huang KC. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res Care. 2016;4:e000253. doi: 10.1136/bmjdrc-2016-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bleys J, Navasacien A, Stranges S, Menke A, Rd ME, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr. 2008;88:416–423. doi: 10.1093/ajcn/88.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen K, Werner M, Malecki K. Serum selenium and lipid levels: associations observed in the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Environ Res. 2015;140:76–84. doi: 10.1016/j.envres.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Laclaustra M, Stranges S, Navasacien A, Ordovas JM, Guallar E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis. 2010;210:643–648. doi: 10.1016/j.atherosclerosis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obeid O, Elfakhani M, Iskandar SM, Batal M, Mouneimne Y, Adra N, Hwalla N. Plasma copper, zinc, and selenium levels and correlates with metabolic syndrome components of lebanese adults. Biol Trace Elem Res. 2008;123:58–65. doi: 10.1007/s12011-008-8112-0. [DOI] [PubMed] [Google Scholar]
- 53.Yang KC, Lee LT, Lee YS, Huang HY, Chen CY, Huang KC. Serum selenium concentration is associated with metabolic factors in the elderly: a cross-sectional study. Nutr Metab. 2010;7:38–38. doi: 10.1186/1743-7075-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stranges S, Laclaustra M, Chen J, Cappuccio FP, Navasacien A, Ordovas JM, Rayman M, Guallar E. Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr. 2010;140:81–87. doi: 10.3945/jn.109.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stranges S, Tabák AG, Guallar E, Rayman MP, Akbaraly TN, Laclaustra M, Alfthan G, Mussalo-Rauhamaa H, Viikari JSA, Raitakari OT. Selenium status and blood lipids: the cardiovascular risk in Young Finns study. J Intern Med. 2011;270:469–477. doi: 10.1111/j.1365-2796.2011.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galanchilet I, Guallar E, Martinescudero JC, De MG, Dominguezlucas A, Gonzalezmanzano I, Lopezizquierdo R, Redon J, Chaves FJ, Tellezplaza M. Do genes modify the association of selenium and lipid levels? Antioxid Redox Signal. 2015;22:1352–1362. doi: 10.1089/ars.2015.6248. [DOI] [PubMed] [Google Scholar]
- 57.Gonzálezestecha M, Palazónbru I, Bodaspinedo A, Trasobares E, Palazónbru A, Fuentes M, Cuadradocenzual MÁ, Calvomanuel E. Relationship between serum selenium, sociodemographic variables, other trace elements and lipid profile in an adult Spanish population. J Trace Elem Med Biol. 2017;43:93–105. doi: 10.1016/j.jtemb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Boskabadi H, Maamouri G, Rezagholizade OF, Mafinejad S, Tara F, Rayman MP, Ghayourmobarhan M, Sahebkar A, Tavallaie S, Shakeri MT. Effect of prenatal selenium supplementation on cord blood selenium and lipid profile. Pediatr Neonatol. 2012;53:334–339. doi: 10.1016/j.pedneo.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Hercberg S, Bertrais S, Czernichow S, Noisette N, Galan P, Jaouen A, Tichet J, Briancon S, Favier A, Mennen L. Alterations of the lipid profile after 7.5 years of low-dose antioxidant supplementation in the SU.VI.MAX study. Lipids. 2005;40:335–342. doi: 10.1007/s11745-006-1391-3. [DOI] [PubMed] [Google Scholar]
- 60.Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E. Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med. 2011;154:656–665. doi: 10.7326/0003-4819-154-10-201105170-00005. [DOI] [PubMed] [Google Scholar]
- 61.Chen C, Jin Y, Unverzagt FW, Cheng Y, Hake AM, Liang C, Feng M, Su L, Liu J, Bian J. The association between selenium and lipid levels: A longitudinal study in rural elderly Chinese. Arch Gerontol Geriatr. 2015;60:147–152. doi: 10.1016/j.archger.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alissa EM, Ahmed WH, Alama N, Ferns GAA. Selenium status and cardiovascular risk profile in healthy adult Saudi males. Molecules. 2009;14:141–59. doi: 10.3390/molecules14010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr. 2009;139:199–200. doi: 10.3945/jn.108.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li K, Zhao H, Zhou JC, Tang JY, Lei XG, Ning K. Wang, Differentially expressed genes in subcutaneous fat tissue in an obese pig model induced by a high-fat diet. J Anim Vet Adv. 2011;10:1804–1810. [Google Scholar]
- 65.Zhao H, Li K, Tang JY, Zhou JC, Wang KN, Xia XJ, Lei XG. Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J Nutr. 2015;145:1394–1401. doi: 10.3945/jn.115.211318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin X, Hidiroglou N, Lok E, Taylor M, Kapal K, Ross N, Sarafin K, Lau A, De SA, Chan HM. Dietary selenium (Se) and vitamin E (V(E)) supplementation modulated methylmercury-mediated changes in markers of cardiovascular diseases in rats. Cardiovasc Toxicol. 2012;12:10–24. doi: 10.1007/s12012-011-9134-y. [DOI] [PubMed] [Google Scholar]
- 67.Lippman SMK, EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baburao JA, Anand JV. Vitamin E, its beneficial role in diabetes mellitus (DM) and its complications. J Clin Diagn Res. 2012;6:1624–1628. doi: 10.7860/JCDR/2012/4791.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghaffari T, Nouri M, Irannejad E, Rashidi MR. Effect of vitamin E and selenium supplement on paraoxonase-1 activity, oxidized low density lipoprotein and antioxidant defense in diabetic rats. Bioimpacts Bi. 2011;1:121–128. doi: 10.5681/bi.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng WH, Valentine BA, Lei XG. High levels of dietary vitamin E do not replace cellular glutathione peroxidase in protecting mice from acute oxidative stress. J Nutr. 1999;129:1951–1957. doi: 10.1093/jn/129.11.1951. [DOI] [PubMed] [Google Scholar]
- 71.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibit T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm G, Geissler E, Thomas S, Stockwell B, O'Donnell V, Kagan V, Schick J, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu W, Chen Z, Zhang H, Wang Y, Luo Y, Huang P. ZNF143 transcription factor mediates cell survival through upregulation of the GPX1 activity in the mitochondrial respiratory dysfunction. Cell Death Dis. 2012;3:e422. doi: 10.1038/cddis.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yun JW, Lum K, Xin GL. A novel upregulation of glutathione peroxidase 1 by knockout of liver-regenerating protein Reg3β aggravates acetaminophen-induced hepatic protein nitration. Free Radical Biol Med. 2013;65:291–300. doi: 10.1016/j.freeradbiomed.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albert N, Natacha D, Maya M, Eric T. Epigenetic regulatory effect of exercise on glutathione peroxidase 1 expression in the skeletal muscle of severely dyslipidemic mice. Plos One. 2016;11:e0151526. doi: 10.1371/journal.pone.0151526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merry TL, Tran M, Stathopoulos M, Wiede F, Fam BC, Dodd GT, Clarke I, Watt MJ, Andrikopoulos S, Tiganis T. High-fat-fed obese glutathione peroxidase 1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxid Redox Signal. 2014;20:2114–2129. doi: 10.1089/ars.2013.5428. [DOI] [PubMed] [Google Scholar]
- 77.Merry TL, Tran M, Dodd GT, Mangiafico SP, Wiede F, Kaur S, Mclean CL, Andrikopoulos S, Tiganis T. Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice. Diabetologia. 2016;59:2632–2644. doi: 10.1007/s00125-016-4084-3. [DOI] [PubMed] [Google Scholar]
- 78.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 79.McClung JP, Roneker CA, Mu WP, Lisk DJ, Langlais P, Liu F, Lei XG, Cousins RJ. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Nat Acad Sci USA. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali MA, Rmhm E, Hanafi MY. Vitamin C and E chronic supplementation differentially affect hepatic insulin signaling in rats. Life Sci. 2017;3205:30689–30696. doi: 10.1016/j.lfs.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 81.Mauvais-Jarvis F, Kahn CR. Understanding the pathogenesis and treatment of insulin resistance and type 2 diabetes mellitus: what can we learn from transgenic and knockout mice? Diabetes Metab. 2000;26:433–448. [PubMed] [Google Scholar]
- 82.Whiteman EL, Han C, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 83.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 84.Espinola-Klein C, Rupprecht HJ, Bickel C, Schnabel R, Genth-Zotz S, Torzewski M, Lackner K, Munzel T, Blankenberg S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am J Cardiol. 2007;99:808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 85.Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 2004;53:2455–2460. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- 86.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallasbonke V, Jandeleitdahm KA, Allen TJ, Kola I, Cooper ME. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E–deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 87.de Haan JB, Witting PK, Stefanovic N, Pete J, Daskalakis M, Kola I, Stocker R, Smolich JJ. Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet. J Lipid Res. 2006;47:1157–1167. doi: 10.1194/jlr.M500377-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice, Arterioscler. Thromb Vasc Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 89.Chew P, Yuen DY, Koh P, Stefanovic N, Febbraio MA, Kola I, Cooper ME, de Haan JB. Site-specific antiatherogenic effect of the antioxidant ebselen in the diabetic apolipoprotein E-deficient mouse. Arterioscler Thromb Vasc Biol. 2009;29:823–830. doi: 10.1161/ATVBAHA.109.186619. [DOI] [PubMed] [Google Scholar]
- 90.Chew P, Yuen DYC, Stefanovic N, Pete J, Coughlan MT, Jandeleitdahm KA, Thomas MC, Rosenfeldt F, Cooper ME, Haan JBD. Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPX1-double knockout mouse. Diabetes. 2010;59:3198–3207. doi: 10.2337/db10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasmiena AA, Stefanovic N, Huynh K, Tan R, Barlow CK, Tull D, Dehaan JB, Meikle PJ. Plasmalogen modulation attenuates atherosclerosis in ApoE- and ApoE/GPx1-deficient mice. Atherosclerosis. 2015;243:598–608. doi: 10.1016/j.atherosclerosis.2015.10.096. [DOI] [PubMed] [Google Scholar]
- 92.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 93.Esworthy RS, Ho YS, Chu FF. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch Biochem Biophys. 1997;340:59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- 94.Soboll S, Grundel S, Harris J, Kolb-Bachofen V, Ketterer B, Sies H. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of cell fractionation. Biochem J. 1995;311:889–894. doi: 10.1042/bj3110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esposito L, Kokoszka J, Kg, Cottrell B, Macgregor G, Wallace D. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radical Bio Med. 2000;28:754–766. doi: 10.1016/s0891-5849(00)00161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng WH, Ho YS, Ross DA, Han Y, CG, Lei XG. Overexpression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–680. doi: 10.1093/jn/127.5.675. [DOI] [PubMed] [Google Scholar]
- 97.Cheng WH, Ho YS, Ross DA, Valentine BA, Combs GF, Lei XG. Cellular glutathione peroxidase knockout mice express normal levels of selenium-dependent plasma and phospholipid hydroperoxide glutathione peroxidases in various tissues. J Nutr. 1997;127:1445–1450. doi: 10.1093/jn/127.8.1445. [DOI] [PubMed] [Google Scholar]
- 98.Gouaze V, Andrieu-Abadie N, Cuvillier O, Malagarie-Cazenave S, Frisach M, Mirault M, Levade T. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem. 2002;277:42867–42874. doi: 10.1074/jbc.M203067200. [DOI] [PubMed] [Google Scholar]
- 99.Mirault ME, Tremblay A, Beaudoin N, Tremblay M. Overexpression of seleno-glutathione peroxidase by gene transfer enhances the resistance of T47D human breast cells to clastogenic oxidants. J Biol Chem. 1991;266:20752–20760. [PubMed] [Google Scholar]
- 100.Kipp AP, Muller MF. Diversity of selenium functions in health and disease Abingdon. USA: CRC Press, Taylor & Francis Group; 2015. Dual functions of selenoproteins in cancer-Glutathione peroxidase 2; pp. 189–203. [Google Scholar]
- 101.Florian S, Krehl S, Loewinger M, Kipp A, Banning A, Esworthy S, Chu FF, Brigeliusflohé R. Loss of Gpx2 increases apoptosis, mitosis, and Gpx1 expression in the intestine of mice. Free Radical Bio Med. 2010;49:1694–1702. doi: 10.1016/j.freeradbiomed.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dittrich AM, Meyer HA, Krokowski M, Quarcoo D, Ahrens B, Kube SM, Witzenrath M, Esworthy RS, Chu FF, Hamelmann E. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur Respir J. 2010;35:1148–1154. doi: 10.1183/09031936.00026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Esworthy RS, Aranda R, Martín MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 104.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 105.Esworthy RS, Yang L, Frankel PH, Chu FF. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr. 2005;135:740–745. doi: 10.1093/jn/135.4.740. [DOI] [PubMed] [Google Scholar]
- 106.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochimica et Biophysica Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 107.Winfrey VP, Motley AK, Austin LM. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am J Physiol Renal Physiology. 2010;298:F1244–F1253. doi: 10.1152/ajprenal.00662.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cardoso BR, Hare DJ, Bush AI, Roberts BR. Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry. 2016;22:328–335. doi: 10.1038/mp.2016.196. [DOI] [PubMed] [Google Scholar]
- 109.Arai M, Imai H, Sumi D, Imanaka T, Takano T, Chiba N, Nakagawa Y. Import into mitochondria of phospholipid hydroperoxide glutathione peroxidase requires a leader sequence. Biochem Biophys Res Commun. 1996;227:433–439. doi: 10.1006/bbrc.1996.1525. [DOI] [PubMed] [Google Scholar]
- 110.Conrad M, Moreno SG, Sinowatz F, Ursini F, Kölle S, Roveri A, Brielmeier M, Wurst W, Maiorino M, Bornkamm GW. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol. 2005;25:7637–7644. doi: 10.1128/MCB.25.17.7637-7644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carlson BA, Ryuta T, Elena Y, Tsuji PA, Hoffmann VJ, Ulrich S, Gladyshev VN, Hatfield DL, Marcus C. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Bio. 2016;9:22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dear TN, Campbell K, Rabbitts TH. Molecular cloning of putative odorant-binding and odorant-metabolizing proteins. Biochemistry. 1991;30:10376–10382. doi: 10.1021/bi00107a003. [DOI] [PubMed] [Google Scholar]
- 113.Ravnharen G, Olsen A, Tjønneland A, Dragsted LO, Nexø BA, Wallin H, Overvad K, Raaschounielsen O, Vogel U. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27:820–825. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- 114.Cominetti C, Bortoli MD, Purgatto E, Ong TP, Moreno FS, AG Associations between glutathione peroxidase-1 Pro198Leu polymorphism, selenium status, and DNA damage levels in obese women after consumption of Brazil nuts. Nutrition. 2011;27:891–896. doi: 10.1016/j.nut.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Kuzuya M, Ando F, Iguchi A, Shimokata H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am J Clin Nutr. 2008;87:1939–1944. doi: 10.1093/ajcn/87.6.1939. [DOI] [PubMed] [Google Scholar]
- 116.Buraczynska M, Buraczynska K, Dragan M, Ksiazek A. Pro198Leu polymorphism in the glutathione peroxidase 1 gene contributes to diabetic peripheral neuropathy in type 2 diabetes patients. Neuromolecular Med. 2017;19:147–153. doi: 10.1007/s12017-016-8438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hernández GC, Hernández CP, Martínez CN, Parra CA, García DS, Pérez LA. Glutathione peroxidase-1 pro200leu polymorphism (rs1050450) is associated with morbid obesity independently of the presence of prediabetes or diabetes in women from central mexico. Nutr Hosp. 2015;32:1516–1525. doi: 10.3305/nh.2015.32.4.9500. [DOI] [PubMed] [Google Scholar]
- 118.Shuvalova YA, Kaminnyi AI, Meshkov AN, Kukharchuk VV. Pro198Leu polymorphism of GPx-1 gene and activity of erythrocytic glutathione peroxidase and lipid peroxidation products. Bull Exp Biol Med. 2010;149:743–745. doi: 10.1007/s10517-010-1041-x. [DOI] [PubMed] [Google Scholar]
- 119.Forsberg L, De FU, Marklund SL, Andersson PM, Stegmayr B, Morgenstern R. Phenotype determination of a common Pro-Leu polymorphism in human glutathione peroxidase 1. Blood Cells Mol Dis. 2000;26:423–426. doi: 10.1006/bcmd.2000.0325. [DOI] [PubMed] [Google Scholar]
- 120.Liu LM, Zheng TS, Li M, Xu J, Lu HJ, Jia WP, Xiang KS. Pro1981eu of Gpx-1 gene in the development of nephropathy and coronary heart desease in chinese type 2 diabetes mellitus. Diabetes. 2007;27:770–774. [Google Scholar]
- 121.Ewens KG, George RK, Ziyadeh FN, Spielman RS. Assessment of 115 candidate genes for diabetic nephropathy by transmission/disequilibrium test. Diabetes. 2005;54:3305–3318. doi: 10.2337/diabetes.54.11.3305. [DOI] [PubMed] [Google Scholar]
- 122.Cho CS, Lee S, Lee GT, Woo HA, Choi EJ, Rhee SG. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid Redox Signal. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liddell JR, Hoepken HH, Crack PJ, Robinson SR, Dringen R. Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity. J Neurosci Res. 2006;84:578–586. doi: 10.1002/jnr.20957. [DOI] [PubMed] [Google Scholar]
- 124.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14:2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng WH, Ho YS, Valentine BA, Ross DA, CG, Lei XG. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998;128:1070–1076. doi: 10.1093/jn/128.7.1070. [DOI] [PubMed] [Google Scholar]
- 126.Cheng WH, GFC, Lei XG. Knockout of cellular glutathione peroxidase affects selenium-dependent parameters similarly in mice fed adequate and excessive dietary selenium. Biofactors. 1998;7:311–321. doi: 10.1002/biof.5520070403. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Yun JW, Lei XG. Glutathione peroxidase mimic ebselen improves glucose-stimulated insulin secretion in murine islets. Antioxid Redox Signal. 2014;20:191–203. doi: 10.1089/ars.2013.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sies H, Arteel GE. Interaction of peroxynitrite with selenoproteins and glutathione peroxidase mimics. Free Radic Biol Med. 2000;28:1451–1455. doi: 10.1016/s0891-5849(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 129.Mai HN, Chung YH, Shin EJ, Kim DJ, Ji HJ, Nguyen TTL, Nam Y, Yu JL, Nah SY, Yu DY. Genetic depletion of glutathione peroxidase-1 potentiates nephrotoxicity induced by multiple doses of cocaine via activation of angiotensin II AT1 receptor. Free Radical Res. 2016;50:467–483. doi: 10.3109/10715762.2016.1143097. [DOI] [PubMed] [Google Scholar]
- 130.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pi J, Bai Y, Zhang Q, Wong V, Floering L, Daniel K, Reece J, Deeney J, Andersen M, Corkey B. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 132.Donadio JS, Guerra-Shinohara EM, Rogero MM, Cozzolino MF. Influence of gender and SNPs inGPX1 gene on biomarkers of selenium status in healthy brazilians. Nutrients. 2016;8:81–93. doi: 10.3390/nu8050081. [DOI] [PMC free article] [PubMed] [Google Scholar]