Abstract
Background:
Recent research suggests that posttraumatic stress disorder (PTSD) is associated with altered amygdala and hippocampal resting-state functional connectivity (rsFC). However, less research has examined whether Prolonged Exposure (PE), a first line exposure-based treatment for PTSD, has the potential to alter resting state neural networks.
Methods:
Twenty-four patients with PTSD and 26 matched trauma-exposed healthy controls (TEHCs) underwent resting-state functional magnetic resonance imaging (fMRI) at baseline. PTSD patients were scanned a second time after completing 10-session PE in which patients narrated a detailed trauma account (imaginal exposure) and confronted trauma reminders (in vivo exposure) to extinguish trauma-related fear responses. TEHC were scanned again following a 10-week waiting period. Seed regions of interest (ROIs) included centromedial amygdala (CMA), basolateral amygdala (BLA), and the hippocampus.
Results:
Post- versus pre-treatment comparisons indicated increased rsFC of the BLA and CMA with the orbitofrontal cortex (OFC), and hippocampus-medial prefrontal cortex (mPFC) among patients with PTSD, but not among TEHC participants.
Conclusions:
Enhanced amygdala and hippocampus rsFC with prefrontal cortical regions following PE could underlie improved capacity for inhibition and re-evaluation of threat, and heightened memory encoding and retrieval ability, respectively. These findings encourage further investigation of this circuitry as a therapeutic target in PTSD.
Keywords: Resting-state functional connectivity, fMRI, Amygdala, Hippocampus, PTSD, Prolonged Exposure treatment
INTRODUCTION
Psychotherapy research in posttraumatic stress disorder (PTSD) has recently begun using neuroimaging methods to assess structural (Helpman, Papini, et al., 2016) and functional (Fonzo, Goodkind, Oathes, Zaiko, Harvey, Peng, Weiss, Thompson, Zack, Lindley, et al., 2017; Helpman, Marin, et al., 2016) neural alterations of evidence-based treatments. Prolonged Exposure (PE) therapy is a relatively brief, highly structured, exposure-based treatment, considered to be effective in treating PTSD (Bradley, Greene, Russ, Dutra, & Westen, 2005). PE utilizes repeated imaginal and in vivo exposure procedures to achieve habituation and eventually extinction of fears associated with traumatic memories (Foa, Hembree, & Rothbaum, 2007; Foa & Kozak, 1986; S. A. Rauch, Eftekhari, & Ruzek, 2012). While previous research has established the clinical efficacy of PE (Taylor et al., 2003), the question whether PE has the potential to alter resting state neural networks remains mostly unanswered, with only one well-powered, controlled study addressing this critical question (Fonzo, Goodkind, Oathes, Zaiko, Harvey, Peng, Weiss, Thompson, Zack, Mills-Finnerty, et al., 2017).
Research has implicated several neural pathways warranting further exploration in the context of PE. First, PE has been conceptualized as a “fear-extinction” therapeutic approach (Morrison & Ressler, 2014), during which patients habituate to trauma reminders in a safe environment. Indeed, it was suggested that in PTSD impaired top-down regulation of the amygdala by frontal cortical regions and hippocampus may lead to deficient fear-extinction processes (S. L. Rauch, Shin, & Phelps, 2006). Accordingly, studies have shown aberrant amygdala- and hippocampus-linked circuitry involving the mPFC, insula, and ACC in PTSD. For example, Stevens et al. (2013) found decreased amygdala-ventromedial prefrontal cortex (vmPFC) connectivity in patients with PTSD using an emotional fMRI task (Stevens et al., 2013). Additionally, resting state functional connectivity (rsFC) studies found reduced amygdala-hippocampus and amygdala-rACC connectivity and increased amygdala-insula connectivity in PTSD, which may reflect enhanced attention to threat and biased memory for adverse events (Rabinak et al., 2011; Sripada et al., 2012). Furthermore, recent studies argued that the PFC regulates the basolateral amygdala (BLA), involved in fear acquisition, and the central medial amygdala (CMA), involved in fear expression and execution of fear responses (Duvarci & Pare, 2014), processes implied in PE. Still, no study to date has examined differences in rsFC between these amygdala sub-regions, the hippocampus, and cortical regions, before and after treatment.
Neuroimaging research examining brain-related changes following exposure-based psychotherapies has produced additional relevant, albeit mixed, results. Volumetric studies in civilian patients with PTSD have shown volume reduction in the ACC following PE (Helpman, Papini, et al., 2016), volume increased in the amygdala following eye movement desensitization and reprocessing (EMDR; Laugharne et al., 2016), and volume increased in the hippocampus following cognitive behavioral therapy (CBT; Levy-Gigi, Szabo, Kelemen, & Keri, 2013). In contrast, no changes were noted in these areas in other PE studies (Laugharne et al., 2016; Rubin et al., 2016), brief eclectic psychotherapy (BEP; (Lindauer et al., 2005) or trauma-focused therapy (van Rooij et al., 2015).
Task-based fMRI studies have reported more consistent results for the amygdala and insula in patients with PTSD. Reduced amygdala activation was found following exposure therapy using an affective Stroop task in patients with war-related PTSD (Roy, Costanzo, Blair, & Rizzo, 2014), following CBT using an affective viewing task in battered women (Aupperle et al., 2013) and an emotional face task in a mixed sample (Felmingham et al., 2007), and following exposure-based therapy and cognitive restructuring (ETCR) using a traumatic memory retrieval task among police officers (Peres et al., 2011). Also, reduced insula activity was found following CBT using an affective viewing task in battered women (Aupperle et al., 2013) and following PE using an affective anticipation task in veterans (Simmons, Norman, Spadoni, & Strigo, 2013). Nonetheless, mixed results were reported for the hippocampus, ACC, and PFC in other task-based fMRI studies. For the hippocampus, among heterogeneous PTSD samples, higher activity was found using an emotional facial viewing task following CBT (Felmingham et al., 2007), while reduced activity was reported using a similar emotional face task (Dickie, Brunet, Akerib, & Armony, 2011). Reduced rostral ACC (rACC) has been found following PE using a conditioning-extinction task (Helpman, Marin, et al., 2016) and following CBT using an emotional face task (Felmingham et al., 2007). Per dorsal ACC (dACC), decreased activation was found in physical or sexual assault patients following cognitive behavioral stabilizing group treatment using a Stroop task (Thomaes et al., 2012), while increased activation has been observed in battered women using an affective viewing task (Aupperle et al., 2013) and in military service members following PE using an affective Stroop task (Roy et al., 2014). Finally, reduced vmPFC activation was found following exposure therapy using an affective Stroop task in patients with war-related PTSD (Roy et al., 2014), and decreased dorsal lateral PFC (dlPFC) activity was found following CBT using an affective viewing task in battered women (Aupperle et al., 2013). In contrast, increased activation in the mPFC was found following ETCR among police officers using a traumatic memory retrieval task (Peres et al., 2011), and using an emotional reactivity and regulation task following PE (Fonzo, Goodkind, Oathes, Zaiko, Harvey, Peng, Weiss, Thompson, Zack, Lindley, et al., 2017).
Lastly, to our knowledge, only one study to date has specifically investigated the effects of PE on rsFC patterns in PTSD, reporting increased resting dynamics of the lateral frontopolar cortex among patients with PTSD after treatment (Fonzo, Goodkind, Oathes, Zaiko, Harvey, Peng, Weiss, Thompson, Zack, Mills-Finnerty, et al., 2017). While considered an essential step in understanding the neural substrates of PE, the use of a waiting list as a control group can only provide a partial answer to the effects of PE on rsFC. As suggested by the Fonzo et al. (2017), it remains unclear whether the observed post-treatment functional changes reflected a normalization of neural abnormalities or compensatory neural adaptations.
Here we conducted a resting-state longitudinal fMRI study in patients with PTSD before and after PE to further elucidate its neural markers, utilizing a relatively larger sample than commonly found in treatment studies. Patients with PTSD and trauma-exposed healthy control group (TEHC) were matched on age, sex, and trauma (type and duration). We compared groups before and after treatment to determine whether PE has the potential to alter resting state neural networks. And if so, whether neural changes post-PE reflect a normalization of functional connectivity (i.e., resembling TEHC) or reflect other compensatory mechanisms alleviating PTSD symptoms. In line with the research described above, we hypothesized: 1) PE would be associated with increased connectivity in amygdala-PFC and hippocampal-PFC pathways similar to TEHC, 2) changes in FC post-PE would correlate with PTSD symptom severity reduction, and 3) amygdala sub-regions-PFC connectivity would moderate treatment response. However, given the lack of research investigating amygdala sub-regions we did not have an a priori directional hypothesis.
METHODS
Participants
Progress through the study stages is summarized in CONSORT Figure 1. Forty-three patients with PTSD completed baseline assessment and received a 10-session PE. Thirty-seven TEHCs completed the baseline assessment and allocated to a 10-week waiting period. Fourteen patients with PTSD and 8 TEHCs dropped out of the study, yielding 60 (31 patients with PTSD and 29 TEHCs) participants who completed the study protocol (dropout rate for patients with PTSD: 32.6 %). There was no significant difference between dropouts and completers in the PTSD group for age (p=0.79), sex (p=0.27), years of education (p=0.45), baseline CAPS (CAPS total score: p=0.71; CAPS re-experience: p=0.85; CAPS Avoidance: p=0.73; CAPS Dysphoria: p=0.38; CAPS Hyperarousal: p=0.07; CAPS Dissociative: p=0.71), and HAM-D (p=0.73). There was no significant difference between dropouts and completers in the TEHC group for age (p=0.60), sex (p=0.30), or years of education (p=0.80).
Figure 1:
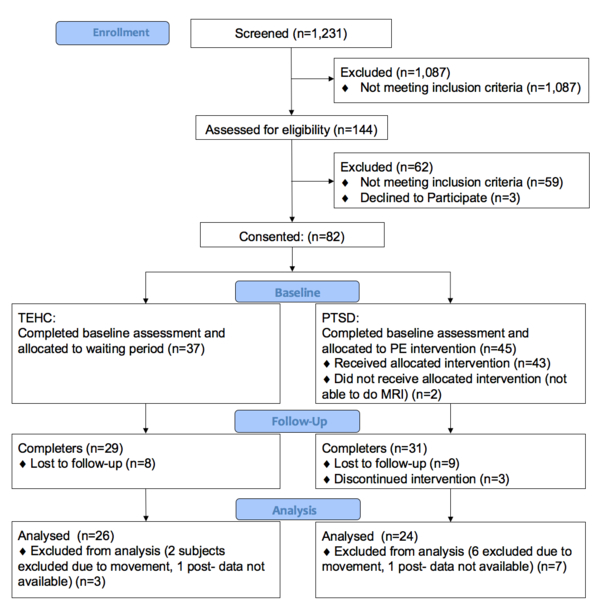
CONSORT figure.
We previously reported functional (Helpman, Marin, et al., 2016) and structural (Helpman, Papini, et al., 2016) PE-related changes in this sample. This report presents their rsFC findings. All participants met DSM-IV-TR PTSD criterion A1 for traumatic events, including vehicular accidents, sexual or physical assaults, and witnessing serious injuries or deaths. Groups were matched on age, sex, ethnicity, and trauma type and duration. An independent Ph.D. clinical evaluator administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) and Clinician-Administered PTSD Scale (CAPS). The New York State Psychiatric Institute Institutional Review Board approved the study.
Patients with PTSD were excluded due to comorbid Axis I psychiatric diagnosis (except comorbid current major depressive disorder and substance or alcohol dependence within the past six months or abuse within past two months). Additional exclusion criteria were any psychotropic medication within four weeks before participation (six weeks for fluoxetine since the half-life of fluoxetine is much longer than other SSRIs; Marken & Munro, 2000), 17-item Hamilton Depression Rating Scale (HAM-D) score >25, or CAPS score <50. TEHC exclusion criteria were current or lifetime Axis I disorders, substance use disorders, and CAPS score >20. Completion of PE treatment protocol was defined as attending the 10 PE session within 12 weeks, followed by a post-treatment assessment. All completers received evaluations and MRI scans at baseline and follow-up.
Treatment
Patients with PTSD underwent a standard PE protocol (Foa et al., 2007), consisting of 10 ninety-minute sessions in which patients narrate an increasingly detailed trauma account (imaginal exposure) and confront trauma reminders for homework (in vivo exposure) to extinguish fear responses. The study therapists were psychologists or clinical social workers. Following established procedures (Markowitz et al., 2015; Schneier et al., 2012), each therapist treated a minimum of two pilot cases to ensure expertise prior to treating patients enrolled in the study. Therapists were audiotaped, monitored for adherence, and supervised by experts to ensure competence. Clinical evaluations and MRI scans were conducted at baseline (week 0) and post-treatment (session 10).
Image procedures
A 1.5T GE Twin Speed MR Scanner operating on the Excite 3 12.0 M4 HD platform equipped with an 8-channel gradient head coil acquired a high-resolution T1-weighted 3D MPRAGE sequence for each participant (repetition time=7.25msec, echo time=3msec, Flip angle=7°, field of view=25.6cm, 256X256 pixel matrix, slice thickness=1mm). Five-minute functional resting-state images were acquired after the structural scan using a gradient echo T2*-weighted sequence (repetition time=3sec, echo time=30msec, flip angle=90°, field of view=22.4cm, 64X64 pixel matrix, slice thickness=2.2mm). Participants were instructed to relax and remain awake while lying still with their eyes open during the 5-minute resting-state scan.
FMRI data were preprocessed using MATLAB version R2016a (The MathWorks, Inc., Natick, MA, USA) and statistical parametric mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, UCL, London, UK). All functional images were slice-time and motion corrected, co-registered to each participant=s T1-weighted structural image, normalized to Montreal Neurological Institute (MNI) canonical template, and smoothed with an 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel (Zhu et al., 2016).
Statistical analysis
Seed-based analysis was carried out using CONN-fMRI FC toolbox v13. Prior to correlation analysis, band-pass filtering with a frequency window of 0.01 to 0.09 Hz was performed. Outliers were detected using Artifact Detection Tools (ART) implemented in CONN. Outlier volumes within each subject identified as having large spiking artifacts (standard deviations>3), or large motion (threshold=0.5mm). Anatomical images were segmented into grey matter, white matter (WM), and cerebrospinal fluid (CSF) regions. The mean WM and CSF time series were calculated by averaging signal over all voxels within WM or CSF mask. Mean WM and CSF time series were then used as covariates of no interest since signal changes in the WM and CSF primarily represent non-neural fluctuations such as physiological artifacts and scanner instabilities. Covariates corresponding to head motion (6 realignment parameters and their derivatives) and outliers detected using ART were also used in the connectivity analysis as covariates of no interest and removed from the BOLD functional time series using linear regression.
Six of the 31 patients with PTSD and 2 of the 29 TEHCs were excluded from further analysis due to movement exceeding 1.5 mm and >20% of each subject’s data points having been detected as outliers at either the baseline or follow-up session. One TEHC and 1 patient with PTSD’s post-treatment scan were not available. The FC analysis thus included 26 TEHC and 24 patients with PTSD. To verify that head motion was equivalent between groups and between pre- and post-scans after excluding these participants, we utilized t-tests to examine motion parameters for group differences at pre- and post-scans or differences between pre- and post-scans. Specifically, two t-tests were used to assess motion parameters group differences, one for baseline scans and one for post-treatment scans. In addition, two t-tests were used to assess pre- and post-motion parameters differences. To acquire a summary measure of motion, the root-mean-square of absolute displacement (RMS_abs: total displacement in any direction relative to a single reference volume) and relative displacement (RMS_rel: total displacement in any direction relative to the preceding volume) were calculated for each participant at pre- and post-scans (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). The motion parameters were not different between groups at baseline (RMS_abs: p=0.45; RMS_rel: p=0.2), or at the post-treatment (RMS_abs: p=0.6; RMS_rel: p=0.5). Additionally, the motion parameters between pre- and post-scans were not different (RMS_abs: p=0.2; RMS_rel: p=0.3).
The mean blood-oxygen-level-dependent (BOLD) time series was computed across all voxels within each seed region of interest (ROI). Bivariate regression analyses were used to determine the linear association of the BOLD time series between seed ROIs to other brain voxels for the whole brain analysis. We examined both positive and negative correlations. The resultant correlation coefficients were transformed into z-scores using Fisher’s transformation to satisfy normality assumptions. In line with previous neuroimaging studies of brain-related changes following exposure-based PTSD treatments, we chose the subcortical regions such as amygdala subregions (BLA and CMA) and hippocampus as seed regions, and we further examined the rsFC to cortical areas such as mPFC, insula, and ACC. Masks were created with the anatomical 70% FSL Harvard-Oxford maximum likelihood subcortical atlases (Desikan et al., 2006), with 6935 voxels in BLA (each side) and 2878 voxels in CMA (each side), after normalization to a standard template (2×2×2). Whole brain connectivity patterns were assessed using the seed ROIs (BLA, CMA, and hippocampus) in PTSD and TEHC at both baseline and follow-up. Consistent with previous studies (Akdeniz et al., 2014; Fernandez-Espejo, Rossit, & Owen, 2015; King et al., 2016; Konova, Moeller, Tomasi, Volkow, & Goldstein, 2013), a threshold of PFWE<0.05 whole brain voxel-wise, family-wise-error-rate (FWE)-corrected with a minimum of 20 voxels was used.
To disentangle treatment effects from habituation to scan procedures or time effects, we carried out a two-by-two group (PTSD, TEHC) by time (pre-, post-) ANOVA. All contrasts were tested within predefined target regions created with the anatomical 70% FSL Harvard-Oxford maximum likelihood cortical and subcortical atlases including the insula, subcallosal cortex, mPFC, OFC, ACC, and thalamus as implicated in previous PTSD neuroimaging research (Garfinkel et al., 2014; Helpman, Marin, et al., 2016; Helpman, Papini, et al., 2016; Levy-Gigi et al., 2013). First, we identified clusters showing significant group-by-time interaction (pFWE<0.05; FWE), and small volume corrected within a priori regions. We then extracted mean cluster values using MarsBaR (http://marsbar.sourceforge.net) and subjected these to post hoc tests. To test the direction of the main effect of group and time, post hoc paired t-tests were used to detect pre- to post- changes in PTSD and TEHC and post hoc independent t-tests were used to detect rsFC difference between patients with PTSD and TEHC at pre- or post-treatment in SPSS 22. Alpha-values were Bonferroni corrected for the number of post hoc t-tests conducted for each pathway and each CAPS sub-scores (e.g., 0.05/24=0.002; Koch et al., 2016). We further assessed the relationships between changes in rsFC and changes in PTSD symptoms on the CAPS and CAPS subscales in the PTSD group using Pearson correlation analysis.
RESULTS
Demographic and Clinical Outcomes
Groups did not differ on age (t=0.03, p=0.98), sex (p=0.86), race (p =0.46), trauma age (p=0.43) and duration (p=1) (see Table 1), but differed in years of education (t=2.70, p=0.009). Following PE, 62.5% of patients with PTSD showed more than 50% reduction in symptoms measured by CAPS, with 33.3% of patients remitted following treatment (i.e., more than 75% reduction in symptoms).
Table 1.
Demographic and Clinical Characteristics of the Sample (N=50)
| TEHC (N=26) |
PTSD (N=24) |
t value | p | |
|---|---|---|---|---|
| Age, M (SD) | 35.3 (10.4) | 35.4 (8.9) | 0.03 | 0.98 |
| Sex, N (%) | 0.86 | |||
| Male | 7 (26.9%) | 7 (29.2%) | ||
| Female | 19 (73.1%) | 17 (70.8%) | ||
| Race, N (%) * | 0.46 | |||
| Caucasian | 11 (42.3%) | 10 (41.7%) | ||
| African-American | 7 (26.9%) | 3 (12.5%) | ||
| Hispanic | 7 (26.9%) | 8 (33.3%) | ||
| Asian | 1 (3.8%) | 1 (4.2%) | ||
| Others | 0 | 2 (8.4%) | ||
| Education, M (SD) | 15.9 (1.9) | 14.4 (1.9) | 2.7 | 0.009 |
| Comorbidities (Current), N (%) |
||||
| MDD | NA | 10 (41.7%) | ||
| SOCPH | NA | 2 (8.4%) | ||
| PANIC | NA | 1 (4.2%) | ||
| SPEPH | NA | 1 (4.2%) | ||
| Primary trauma age (years) |
27 (9) |
29 (9) |
−0.79 |
0.43 |
| Primary trauma duration (years) |
2 (5) |
2 (4) |
0 |
1 |
| HAM-D (baseline), M (SD) |
2.0 (2.3) |
15.7 (5.4) |
10.95 |
<0.001 |
| HAM-D (follow-up), M (SD) |
2.4 (2.8) |
9.0 (6.1) |
4.18 |
<0.001 |
| CAPS-total (baseline), M (SD) |
4.3 (5.1) |
82.0 (15.2) |
22.46 |
<0.001 |
| Re-experiencing | 0.9 (1.9) | 18.7 (4.5) | 17.14 | <0.001 |
| Avoidance | 0.3 (1.2) | 11.0 (3.5) | 13.55 | <0.001 |
| Dysphoria | 0.8 (1.3) | 22.8 (5.5) | 18.02 | <0.001 |
| Hyperarousal | 2.2 (3.1) | 23.8 (6.3) | 14.33 | <0.001 |
| Dissociative | 0.3 (1.1) | 4.8 (3.3) | 6.2 | <0.001 |
| CAPS-total (follow-up), M (SD) |
3.0 (5.7) |
31.2 (22.8) |
5.02 |
<0.001 |
| Re-experiencing | 0.8 (1.7) | 5.3 (5.2) | 3.47 | <0.001 |
| Avoidance | 0.1 (0.4) | 3.8 (4.1) | 3.73 | <0.001 |
| Dysphoria | 0.7 (2.5) | 8.7 (7.4) | 4.29 | <0.001 |
| Hyperarousal | 1.2 (2.5) | 11.3 (8.1) | 4.97 | <0.001 |
| Dissociative | 0.4 (1.1) | 1.8 (2.6) | 2.22 | <0.05 |
1 patient’s race is not available; 2 TEHC CAPS at baseline are not available, 7 TEHC and 1 patient with PTSD CAPS score at follow-up are not available.
Note. SOCPH: social phobia; SPEPH: specific phobia; MDD: major depression disorder; PANIC: panic disorder
Expectedly, patients with PTSD, compared to TEHC, had greater PTSD symptom severity, measured by CAPS, and depression symptom severity, measured by HAM-D, at both baseline (p<0.001 for both measures) and follow-up (p<0.001 for both measures). Patients with PTSD had significantly lower scores on the CAPS and HAM-D at follow-up compared with baseline (mean difference=50.8, t=10.92, p<0.001 for CAPS and mean difference=6.7, t=4.03, p<0.001 for HAM-D), whereas TEHC subjects showed no meaningful symptomatic change (mean difference=1.3, t=1.16, p=0.87 for CAPS, mean difference=0.4, t=−0.56, p=0.29 for HAM-D).
Functional Connectivity
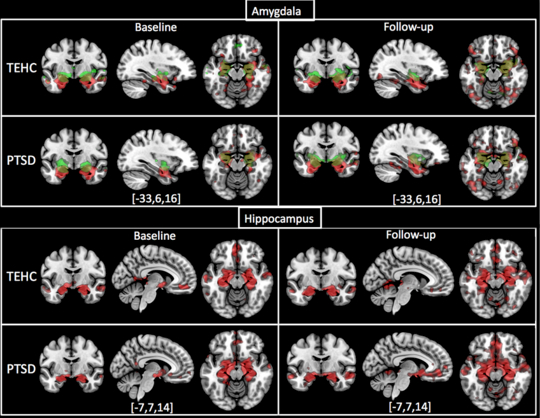
Observed patterns of CMA and BLA FC with the whole brain in PTSD and TEHC at both baseline and follow-up are depicted in Figure 2 (top). Figure 2 (bottom) also shows patterns of hippocampus FC with the whole brain in PTSD and TEHC at baseline and follow-up.
Figure 2:
Whole brain connectivity map with amygdala (top: BLA (red), CMA (green), overlap (yellow)) and hippocampus (bottom: red) as seed ROIs in PTSD and TEHC groups at pre- and post-, p<0.05 whole brain FWE-corrected, 20 voxels
We observed significant group (PTSD vs. TEHC) by time (pre- vs. post-) interactions in rsFC for BLA connectivity with OFC (z=3.71, pFWE=0.01), with vmPFC (z=3.42, pFWE=0.008), and with the thalamus (right: z=3.97, pFWE=0.003, left: z=2.99, pFWE=0.033). Significant interactions also emerged for CMA connectivity with OFC (z=3.76, pFWE=0.009), and for hippocampus connectivity with vmPFC (z=3.84, pFWE=0.003; Figure 3, Table 2). In order to test the direction of the effect of time, we conducted post hoc paired t-tests, and found increased FC in BLA-OFC (p=0.001, df=23, t=3.77), CMA-OFC (p=0.002, df=23, t=3.49), and hippocampus-vmPFC (p=0.004, df=23, t=3.20) for patients with PTSD, whereas TEHC showed no significant pre/post changes in connectivity in any pathways. Additionally, to test the direction of the group effect, we conducted post hoc independent t-tests which revealed reduced rsFC in BLA-OFC (p=0.0054, df=48, t=2.91), BLA-thalamus (p=0.0069, df=48, t=2.82), CMA-OFC (p=0.0003, df=48, t=3.99), and hippocampus-vmPFC (p=0.0045, df=48, t=2.94) among patients with PTSD as compared with TEHC at pre-treatment. Patients with PTSD and TEHC showed no significant differences in connectivity in any of the pathways at post-treatment.
Figure 3.
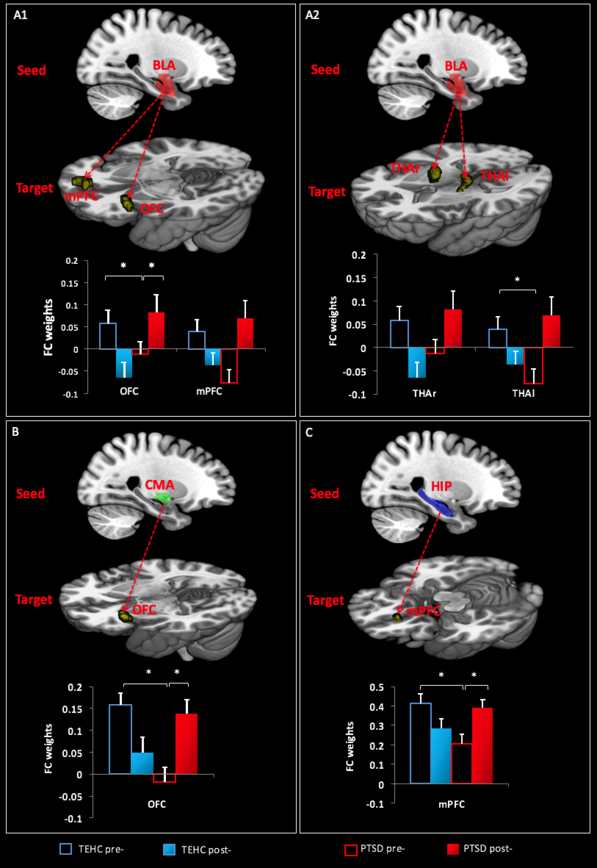
Group-by-time interactions regarding functional connectivity of: (A1) BLA (red cluster)-mPFC and -OFC; (A2) BLA (red cluster)-THA (right and left); (B) CMA (green cluster)-OFC; and (C) Hippocampus (HIP; blue cluster)-medial Prefrontal cortex (mPFC). Arrows represent rsFC between seeds and target regions (yellow clusters). The y-axis represents rsFC strength. Corrected p<0.05.
Table 2.
Group-by-time interaction effects on rsFC using BLA, CMA, and hippocampus (HIP) seeds
| Seed ROI | Cluster size |
z | x (mm) |
y (mm) |
z (mm) |
FWE- corrected p |
|
|---|---|---|---|---|---|---|---|
| BLA | OFC | 46 | 3.71 | −38 | 28 | −2 | 0.01 |
| vmPFC | 37 | 3.42 | −4 | 60 | 6 | 0.008 | |
| THAr | 68 | 3.97 | 10 | −4 | 10 | 0.003 | |
| THAl | 61 | 2.99 | −18 | −16 | 2 | 0.033 | |
| CMA | OFC | 32 | 3.76 | −38 | 26 | −4 | 0.009 |
| Hippocampus | vmPFC | 75 | 3.84 | −4 | 32 | −20 | 0.003 |
Relationship between Functional Connectivity and Clinical Measures
We conducted a post hoc correlation analysis between rsFC changes and reduction in PTSD symptoms from pre- to post-treatment for each of the six pathways mentioned above. Greater increases in CMA-OFC rsFC correlated with greater PTSD symptom reduction in re-experiencing symptoms (R2=0.169, r=0.548, p=0.012, uncorrected) and avoidance symptoms (R2=0.139, r=0.508, p=0.022, uncorrected). Interestingly, we found a negative correlation between increased BLA-vmPFC connectivity and reduction in hyperarousal symptoms (r=−0.496, p=0.026, uncorrected); i.e., increased connectivity post-treatment was associated with less reduction in PTSD hyperarousal severity. No significant relationships were found for any of the other pathways.
DISCUSSION
This study is the first to examine changes in rsFC following PE using amygdala sub-regions (BLA, CMA) and hippocampus seeds in patients with PTSD compared to matched TEHCs. Patients with PTSD exhibited significantly lower BLA- and CMA-OFC and hippocampus-vmPFC rsFC at pre-treatment compared to TEHCs, whereas no group differences were found for these pathways post-treatment. Patients with PTSD exhibited increased rsFC in these same pathways from pre- to post-treatment, while no changes were noted for the TEHC group.
Decreased amygdala-OFC rsFC in patients with PTSD as compared with TEHCs at pre-treatment suggests that lower baseline connectivity levels between these areas may interfere with successful encoding or processing of emotional trauma-related information. The increased amygdala-OFC rsFC following treatment may reflect amygdala-OFC rsFC plastic recovery following PE in patients with PTSD. This post-treatment heightened connectivity may result from the repeated extinction procedures utilized during therapy, namely, imaginal and in vivo exposure, as both amygdala and PFC are considered key components of the corticolimbic circuit involved in emotional processing, where top-down PFC processes regulates amygdala (Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015). These observed changes in amygdala rsFC following PE is in accordance with previous findings. First, amygdala and OFC were found to be anatomically interconnected (Ghashghaei & Barbas, 2002; Wallis, 2007), suggesting that OFC may modulate amygdala reactivity in the regulation of negative emotions (Banks, Eddy, Angstadt, Nathan, & Phan, 2007). In addition, recent research has demonstrated that more efficient crosstalk between amygdala and PFC predicts better emotion regulation and less anxiety in healthy controls (Kim, Gee, Loucks, Davis, & Whalen, 2011). Second, although there have been few rsFC longitudinal studies of psychiatric disorders, and none for PTSD, emerging neuroimaging evidence shows psychotherapies can increased amygdala connectivity with medial OFC (Mansson et al., 2013), dorsolateral PFC (Mason, Peters, Dima, Williams, & Kumari, 2016), dorsomedial PFC (Goldin et al., 2013), and mPFC (Peres et al., 2011). Finally, research suggests baseline amygdala-PFC rsFC predicts symptom change after cognitive behavioral therapy in PTSD (Klumpp, Keutmann, Fitzgerald, Shankman, & Phan, 2014). Still, to our knowledge, no prior study has examined amygdala resting-state connectivity changes following exposure-based psychotherapy for PTSD.
Interestingly, our findings regarding amygdala-OFC pathways involved the left lateral OFC (lOFC), demonstrating robust amygdala connectivity with lOFC among TEHCs, and reduced connectivity in patients with PTSD at baseline, which increased following PE. Different regions of the OFC may have distinct connectivity patterns and specialize in distinct functional roles. The lOFC is characterized by stronger connections with the amygdala, midline thalamus, and insula (Morecraft, Geula, & Mesulam, 1992), with functional connectivity associated in suppressing responses to previously rewarded stimuli (Elliott, Dolan, & Frith, 2000) and responds to aversive outcome (O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001). Previous studies showed evidence that amygdala-OFC connectivity in the left hemisphere reflects a stronger ability to discriminate expression valence, while the right hemisphere connectivity indicates appropriate approach/withdrawal behavioral response (i.e., differentiating happy from angry expressions -left lOFC- and deciding to approach someone -right lOFC; Liang, Zebrowitz, & Aharon, 2009). Other evidence indicated that the left lOFC regulates the influence of emotion on decision-making. Thus, our finding in amygdala-left lOFC connectivity may reflect an enhanced ability of patients with PTSD to discriminate between negative and positive valence following PE.
We also found stronger hippocampal-PFC connectivity in patients with PTSD following PE. Both hippocampus and mPFC are believed to be essential for encoding and retrieving episodic memories with the connectivity between these two regions considered critical for cognitive and emotional regulation of memory processes (Maren, Phan, & Liberzon, 2013) and for regulation of context-dependent fear memory retrieval after extinction (Orsini & Maren, 2012). Neuroimaging studies have shown patients with PTSD consistently display aberrant activity within these brain regions (Milad & Rauch, 2007). Therefore, following PE, stronger hippocampal-PFC connectivity might reflect a better ability in patients to retrieve previously extinguished memories in the appropriate contexts.
Finally, our exploratory analysis revealed CMA-OFC rsFC inversely correlated with re-experiencing and avoidance symptoms in the PTSD group. We also found a positive correlation between BLA-vmPFC pathway rsFC and hyperarousal symptoms. These results may suggest that clinical features of PTSD reflect discrete dysregulated emotional processing systems. The seemingly surprising finding of negative correlation between increases in BLA-vmPFC connectivity and hyperarousal symptom reduction fits well with several studies reporting an association between hyperarousal symptoms and mPFC and amygdala activity. One study found greater mPFC activity associated with threat hyperarousal symptoms during unpredictable threat anticipation (Grupe, Wielgosz, Davidson, & Nitschke, 2016), and another study found increased amygdala activity in response to fearful stimuli to be positively correlated with severity of hyperarousal symptoms in PTSD (Stevens et al., 2013). Consistent with these studies, our finding supports the role of amygdala-PFC connectivity in hyperarousal modulation and raises the possibility that another modulatory area, such as the hippocampus, is required to regulate this hyperarousal output. Our results further suggest FC between hippocampus- and amygdala-cortical areas is needed to process emotionally loaded trauma-related memories. However, as these results were exploratory and found at an uncorrected threshold they should be addressed with caution, and warrant further exploration.
LIMITATIONS
Several study limitations deserve consideration. First, while our overall sample size was substantial for a longitudinal neuroimaging treatment study, it was not large enough to evaluate PTSD subgroups. Thus, differences between responders and non-responders, or between remitters and non-remitters, remain unclear. Future research using larger samples is needed to further explore these potential differences. Second, our sample consisted primarily of female subjects, which limits generalizability and the opportunity to examine sex differences. Importantly, we found no significant sex difference between groups. Still, further studies should aim for a heterogeneous sample. Third, an important unanswered question is whether these neuroanatomical treatment effects endure over time. Also, as scans were only collected pre- and post-treatment, we could not examine intercurrent changes in rsFC during treatment. Future research should assess the long-term effects of PE on rsFC using a follow-up session and might employ an additional mid-treatment assessment to explore intercurrent progress. Additional limitations include using a 1.5T scanner and a relatively short resting-state scan time. In addition, we used FWE correction within hypothesized regions in the group-by-treatment ANOVA test. Further studies may consider using non-parametric permutation test method to produce more stringent results for voxel as well as cluster-wise inference (Eklund et al., 2015). Finally, we did not compare the PE group to a PTSD control group receiving a different type of treatment or in a waiting list, which could provide further information on effects of treatment or time, respectively. Thus, further studies should compare PE to other treatment modalities to elucidate whether the current findings are specific to PE or reflect global treatment response.
CONCLUSION
In summary, our findings elucidate potential neuroanatomical targets and mechanisms of PE by describing post-treatment changes in rsFC of the amygdala and hippocampal with the PFC, three important areas that are engaged during emotional regulation and processing. Exposure-based therapy like PE arguably works by enhancing top-down regulation of cortical areas over the amygdala and hippocampus, as is seen in fear-extinction paradigms. Following PE treatment, patients with PTSD showed increased amygdala and hippocampal rsFC with the PFC, which also correlated with reduced PTSD symptoms. Further work should extend current findings by examining whether rsFC changes are replicable, particularly following other types of treatment and whether PE has a prolonged effect on the processing of emotional trauma-related memories. This study is an initial step towards developing clinically useful brain targets for PE treatment. Future studies with larger samples could evaluate whether these rsFC patterns can improve our ability to predict treatment response and identify those who will benefit most from PE treatment and why thus providing useful predictive features for developing individualized treatment selection.
Acknowledgments
Funding: This research was supported by grants R01MH072833 and R01MH105355 from the National Institute of Mental Health (NIMH) (Dr. Neria, Principal Investigator), and the New York State Psychiatric Institute. Dr. Suarez-Jimenez’s (MH015144) and Lazarov’s (MH020004) work is supported by a T32 grant from the NIMH. Dr. Helpman’s work is supported by T32 grant MH096724 from the NIMH. Funding from the Donald D. Harrington Fellows Program supported Mr. Papini’s work.
Footnotes
Declaration of Interest: Drs. Schneier, Markowitz, and Neria are employees of the New York State Psychiatric Institute. Dr. Markowitz has received research support from the Mack Foundation and book royalties from American Psychiatric Publishing, Basic Books, and Oxford University Press. Dr. Neria has received royalties from Cambridge University Press and Springer, and research support from the Mack Foundation, Stand for the Troops, and the New York Presbyterian Hospital. Dr. Schneier has received royalties from Cambridge University Press, Guilford and Uptodate, research support from Forest labs, and honorarium from Elsevier. The authors report no biomedical financial interests or potential conflicts of interest. The authors thank the individuals who contributed to this study: therapists, evaluators, research assistants, volunteers, the personnel of the New York State Psychiatric Institute Anxiety Disorders clinic, and the patients who participated in this treatment study.
References:
- Akdeniz C, Tost H, Streit F, Haddad L, Wust S, Schafer A, . . . Meyer-Lindenberg A (2014). Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry, 71(6), 672–680. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, . . . Stein MB (2013). Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Res, 214(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci, 2(4), 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, & Westen D (2005). A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry, 162(2), 214–227. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, . . . Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, & Armony JL (2011). Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia, 49(7), 1771–1778. [DOI] [PubMed] [Google Scholar]
- Duvarci S, & Pare D (2014). Amygdala microcircuits controlling learned fear. Neuron, 82(5), 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, & Frith CD (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex, 10(3), 308–317. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, & Bryant R (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci, 18(2), 127–129. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo D, Rossit S, & Owen AM (2015). A Thalamocortical Mechanism for the Absence of Overt Motor Behavior in Covertly Aware Patients. JAMA Neurol, 72(12), 1442–1450. [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, & Rothbaum BO (2007). Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide: Oxford University Press. [Google Scholar]
- Foa EB, & Kozak MJ (1986). Emotional processing of fear: exposure to corrective information. Psychol Bull, 99(1), 20–35. [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, . . . Etkin A (2017). PTSD Psychotherapy Outcome Predicted by Brain Activation During Emotional Reactivity and Regulation. Am J Psychiatry, 174(12), 1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, . . . Etkin A (2017). Selective Effects of Psychotherapy on Frontopolar Cortical Function in PTSD. Am J Psychiatry, 174(12), 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, & Liberzon I (2014). Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci, 34(40), 13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, & Barbas H (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience, 115(4), 1261–1279. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, & Gross JJ (2013). Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry, 70(10), 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Wielgosz J, Davidson RJ, & Nitschke JB (2016). Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol Med, 46(9), 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, . . . Neria Y (2016). Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. Neuroimage Clin, 12, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Papini S, Chhetry BT, Shvil E, Rubin M, Sullivan GM, . . . Neria Y (2016). PTSD Remission after Prolonged Exposure Treatment Is Associated with Anterior Cingulate Cortex Thinning and Volume Reduction. Depress Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex, 21(7), 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, . . . Liberzon I (2016). Altered Default Mode Network (Dmn) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (Ptsd) in Combat Veterans of Afghanistan and Iraq. Depress Anxiety, 33(4), 289–299. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Keutmann MK, Fitzgerald DA, Shankman SA, & Phan KL (2014). Resting state amygdala-prefrontal connectivity predicts symptom change after cognitive behavioral therapy in generalized social anxiety disorder. Biol Mood Anxiety Disord, 4(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2016). Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder. Neuropsychopharmacology, 41(8), 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, & Goldstein RZ (2013). Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry, 70(8), 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugharne J, Kullack C, Lee CW, McGuire T, Brockman S, Drummond PD, & Starkstein S (2016). Amygdala Volumetric Change Following Psychotherapy for Posttraumatic Stress Disorder. J Neuropsychiatry Clin Neurosci, appineuropsych16010006. [DOI] [PubMed] [Google Scholar]
- Levy-Gigi E, Szabo C, Kelemen O, & Keri S (2013). Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol Psychiatry, 74(11), 793–800. [DOI] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, & Aharon I (2009). Effective connectivity between amygdala and orbitofrontal cortex differentiates the perception of facial expressions. Soc Neurosci, 4(2), 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, . . . Gersons BP (2005). Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychol Med, 35(10), 1421–1431. [DOI] [PubMed] [Google Scholar]
- Mansson KN, Carlbring P, Frick A, Engman J, Olsson CJ, Bodlund O, . . . Andersson G (2013). Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res, 214(3), 229–237. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci, 14(6), 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marken PA, & Munro JS (2000). Selecting a Selective Serotonin Reuptake Inhibitor: Clinically Important Distinguishing Features. Prim Care Companion J Clin Psychiatry, 2(6), 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JC, Petkova E, Neria Y, Van Meter PE, Zhao Y, Hembree E, . . . Marshall RD (2015). Is Exposure Necessary? A Randomized Clinical Trial of Interpersonal Psychotherapy for PTSD. Am J Psychiatry, 172(5), 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, Peters ER, Dima D, Williams SC, & Kumari V (2016). Cognitive Behavioral Therapy Normalizes Functional Connectivity for Social Threat in Psychosis. Schizophr Bull, 42(3), 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Rauch SL (2007). The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci, 1121, 546–561. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, & Mesulam MM (1992). Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol, 323(3), 341–358. [DOI] [PubMed] [Google Scholar]
- Morrison FG, & Ressler KJ (2014). From the neurobiology of extinction to improved clinical treatments. Depress Anxiety, 31(4), 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry, 77(3), 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, & Andrews C (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci, 4(1), 95–102. [DOI] [PubMed] [Google Scholar]
- Orsini CA, & Maren S (2012). Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev, 36(7), 1773–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres JF, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, . . . Lederman H (2011). Police officers under attack: resilience implications of an fMRI study. J Psychiatr Res, 45(6), 727–734. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, & Phan KL (2011). Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry, 2, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, Eftekhari A, & Ruzek JI (2012). Review of exposure therapy: a gold standard for PTSD treatment. J Rehabil Res Dev, 49(5), 679–687. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, & Phelps EA (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry, 60(4), 376–382. [DOI] [PubMed] [Google Scholar]
- Roy MJ, Costanzo ME, Blair JR, & Rizzo AA (2014). Compelling Evidence that Exposure Therapy for PTSD Normalizes Brain Function. Stud Health Technol Inform, 199, 61–65. [PubMed] [Google Scholar]
- Rubin M, Shvil E, Papini S, Chhetry BT, Helpman L, Markowitz JC, . . . Neria Y (2016). Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Res, 252, 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Neria Y, Pavlicova M, Hembree E, Suh EJ, Amsel L, & Marshall RD (2012). Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: a randomized controlled trial. Am J Psychiatry, 169(1), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Norman SB, Spadoni AD, & Strigo IA (2013). Neurosubstrates of remission following prolonged exposure therapy in veterans with posttraumatic stress disorder. Psychother Psychosom, 82(6), 382–389. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, & Liberzon I (2012). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci, 37(4), 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, & Ressler KJ (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res, 47(10), 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Thordarson DS, Maxfield L, Fedoroff IC, Lovell K, & Ogrodniczuk J (2003). Comparative efficacy, speed, and adverse effects of three PTSD treatments: exposure therapy, EMDR, and relaxation training. J Consult Clin Psychol, 71(2), 330–338. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, van Balkom AJ, . . . Veltman DJ (2012). Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychol Med, 42(11), 2337–2349. [DOI] [PubMed] [Google Scholar]
- van Rooij SJ, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, & Geuze E (2015). Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med, 45(13), 2737–2746. [DOI] [PubMed] [Google Scholar]
- Wallis JD (2007). Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci, 30, 31–56. doi: 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, . . . Neria Y (2016). Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]