Abstract
Introduction
Chronic rhinosinusitis(CRS) is a diverse clinical syndrome with a heterogeneous pathophysiology. Early attempts to identify CRS endotypes and biomarkers have largely relied on analysis of surgically obtained tissue, thus limiting their practical utility. This study examined the ability of mucus Th2 biomarkers to predict CRS disease severity and clinical characteristics.
Methods
CRS(n=90) and healthy control subjects(n=17) were prospectively enrolled prior to surgical intervention and mucus levels of IL-4,IL-5,and IL-13 were determined using a multiplex cytometric bead assay. Data for relevant cytokines was then scaled, normalized, and later combined to develop standardized metrics indicative of Th2-associated inflammation. Th2-high and Th2-low subgroups were consequently identified and validated against factors associated with disease severity and clinical outcomes.
Results
Mucus levels of IL-5, and IL-13 were elevated in CRS subjects compared to controls, while no significant difference was noted for IL-4. IL-5 and IL-13 high CRS were associated with worse objective measures of disease severity and greater rates of revision surgery. Similar relationships were noted for both cytokines when CRSwNP patients were analyzed separately. Th2-high CRS and Th2-low CRS were then categorized using a scaled IL-5/IL-13 metric. Th2-high CRS was characterized by an increased number of subjects with nasal polyps and comorbid asthma, and worse symptom and CT scores.
Conclusions
The Th2-associated cytokines, IL-5 and IL-13, are detectable in sinonasal mucus and their levels can be used to define Th2-high and Th2-low CRS. Identification of Th2-high and Th2-low endotypes using mucus-based biomarkers could facilitate stratification of CRS subgroups and guide personalized therapies.
Keywords: rhinosinusitis, Th2 cell, eosinophils, mucus, biomarker, endotype
INTRODUCTION
Chronic rhinosinusitis is a debilitating inflammatory airway disease that affects up to 5% of the U.S. population1. Despite its substantial economic and quality of life burden, the pathophysiology of CRS is still poorly defined. CRS subgroups have classically been phenotypically classified based on the presence (CRSwNP) or absence (CRSsNP) of nasal polyps, with each being associated with unique etiologies and inflammatory mediators. While CRSwNP and CRSsNP have historically been linked with Th2- and Th1-associated inflammation, respectively, these distinctions have more recently come into question, with a mixed Th1/Th2 picture being identified in many cases, regardless of polyp status2-4. The CRSwNP phenotype is frequently linked with tissue eosinophilia, a characteristic strongly associated with greater disease severity, poorer outcomes, and increased rates of polyp recurrence5-8. Unfortunately, many of these patients are identified post hoc, with a reliance on operative histopathology specimens for diagnosis. The failure of phenotypic characteristics to effectively predict response to medical or surgical therapy suggests a need for biomarkers that can identify clinically relevant disease endotypes.
Recent introduction of monoclonal antibody-based therapeutics that target IL-4/IL-13 and IL-5 have shown great promise for managing recalcitrant asthma9-13. CRS appears to share both pathophysiology and overlapping inflammatory signatures with asthma, and consequently previous and ongoing clinical trials are now evaluating these therapeutics as potential treatments for CRS and nasal polyposis, with promising results14-16. Nonetheless, methodology for identifying patients who may benefit from these therapies has not been clearly elucidated, with Th2-associated CRS generally being defined loosely by the presence of nasal polyps on physical exam or by eosinophilic inflammation identified within surgical specimens. Efforts to endotype asthmatic patients have progressed rapidly over previous years, with development of tissue-, sputum-, and epithelial-based approaches that can categorize asthma endotypes through structured and unstructured approaches17-21. Similar efforts are just now being introduced for CRS. Thomassen et al. recently characterized 10 potential CRS endotypes based on cluster analysis of different inflammatory mediators within surgical tissue specimens2, while our group recently identified 6 potential CRS endotypes based on analysis of cytokine signatures in CRS mucus22. A subsequent large multinational study found that molecular and immunologic signatures can vary widely based on geographic location, suggesting that patient response to biologic and other immune-directed therapeutics may depend in part on endotypic differences that are inherent to the local environment3.
Improved understanding of CRS pathophysiology and early attempts to identify CRS endotypes and phenotypes have largely depended on analysis of sinonasal tissue, typically in the form of surgically excised biopsy specimens. However, this approach is inherently invasive and is limited by differences in localized marker expression throughout the nasal and sinus cavity23. The ideal CRS biomarker should be easily assayed, allow for accurate identification of disease subtypes with clinical relevance, and identify patients who may benefit from individualized therapies. Such biomarkers should also be measurable via non- or minimally-invasive approaches. Recent studies characterizing CRS endotypes have confirmed that severe CRS is associated with a Th2 signature, however reliance on a large number of inflammatory markers and/or variables, and use of complex mathematical modeling and cluster analysis makes these approaches unsuitable for clinical practice. We hypothesized that simplified identification of a Th2-associated cytokine signature in sinonasal mucus using a small number of biological variables could help categorize patients based on immunologic factors rather than phenotypic variables such as nasal polyps. The ultimate goal of the current study is to identify Th2-high CRS endotypes via minimally-invasive approaches, with the potential to characterize disease severity and predict outcomes.
METHODS
Study Design and Population
This study was approved by the Vanderbilt University Institutional Review Board. Patients presented to the Vanderbilt Asthma, Sinus, and Allergy Program (ASAP) and Otolaryngology clinic at the Vanderbilt Bill Wilkerson Center. CRS was diagnosed according to the European Position Paper on Rhinosinusitis and Nasal Polyps and the International Consensus Statement on Allergy and Rhinology and therefore were initially managed medically24,25. CRS patients offered surgery had previously failed adequate medical therapy that included two or more weeks of oral prednisone, two or more weeks of broad-spectrum or culture-directed antibiotics, and a combination of other additional therapies including oral/topical antihistamines, topical nasal steroid sprays, oral decongestants, mucolytics, or saline rinses. Patients with continued symptoms who elected to undergo endoscopic sinus surgery were prospectively enrolled and signed informed consent. Control cases included patients undergoing pituitary or skull base surgery without a history or radiographic evidence of CRS. Patients were excluded if they had received systemic steroids within 4 weeks of surgery. Patients with cystic fibrosis, autoimmune, or granulomatous diseases or who were receiving immune-directed monoclonal antibodies were excluded. The presence of concomitant allergic rhinitis and asthma was recorded. Allergic rhinitis was diagnosed based on positive skin prick testing and/or prior physician diagnosis and clinical history suggestive of seasonal variation of atopic symptoms with improvement following use of topical nasal steroid or oral antihistamine. Asthma was diagnosed based on a positive methacholine challenge or consistent pulmonary function studies, or by prior diagnosis by a pulmonologist. Allergic Fungal Rhinosinusitis (AFRS) was diagnosed according to published criteria26, including presence of fungal elements and allergic mucin on pathology, and concomitant positive allergy testing to fungal allergen(s). Aspirin exacerbated respiratory disease (AERD) was diagnosed based on presence of asthma and nasal polyposis, as well as a prior history of positive aspirin challenge or at least two episodes of respiratory reaction to aspirin or non-steroidal anti-inflammatory drugs. Patient reported symptom severity was measured utilizing the Sinonasal Outcome Test-22 (SNOT-22)27. All patients underwent a high resolution CT scan of the paranasal sinuses. Each scan was evaluated by two physicians who were blinded to subject identifiers and diagnosis. A standard Lund Mackay scoring system was used to assess overall extent of CRS. Subjects enrolled in the study also completed the 40-item Smell Identification Test (SIT) immediately prior to surgery. The SIT has excellent sensitivity, correlates closely with scores attained via formal threshold testing, and has the advantage of being easily and quickly administered to subjects on the day of surgical intervention28. Raw scores were adjusted for patient age and gender by subtracting the mean normative age- and sex-appropriate SIT score from the total SIT score for each subject6. Thus a negative adjusted SIT score represents reduced sense of smell compared to the mean for that subject’s age and gender. Normative SIT scores were extracted from the Smell Identification Test Administration Manual (Sensonics International; Haddon Heights, NJ).
Mucus Collection and Histopathologic Evaluation of Sinonasal Tissue
At the beginning of surgery, 9 × 24mm polyurethane sponges (Summit Medical; St. Paul, MN) were placed into the middle meatus of each subject under endoscopic guidance. Each sponge was removed after 5 minutes, placed in a sterile microcentrifuge tube and immediately processed. Sponges were placed into a microporous centrifugal filter device (MilliporeSigma; Billerica, MA) and centrifuged at 14,000 × g for 10 minutes to elute mucus. Samples were then gently vortexed and again centrifuged for 5 minutes to remove any cellular debris. Supernatants were removed, placed into a new microcentrifuge tube, and frozen at −80°C for later analysis.
Cytokine assays were performed using a standard sensitivity multiplex cytokine bead assay (BD Biosciences; Franklin Lakes, NJ) according to the manufacturer’s protocol. Briefly, 50 μL of mucus was incubated with 50 μl of mixed capture beads for each measured inflammatory mediator and incubated for 1 hour. 50 μL of mixed detection reagent was then added to each sample and standard, and incubated for an additional 2 hours. After addition of 1 mL wash buffer, samples were centrifuged at 200 × g for 5 minutes and the supernatant was discarded. The beads were then resuspended in 300 μL wash buffer and analyzed on an LSR Fortessa flow cytometer (BD Biosciences). Data was analyzed using BD FCAP Array Software version 3.0.
Sinonasal tissue was collected from the ethmoid bulla or ethmoid sinus in all patients undergoing endoscopic sinus surgery for CRS. Tissue from healthy controls was collected from either the ethmoid sinus or sphenoid face. Histopathological evaluation of excised tissue was performed by a pathologist in a blinded fashion and the mean number of eosinophils counted over 5 randomly selected high powered fields (HPF) was recorded.
Identification and Characterization of Th2-high and Th2-low CRS
Raw mucus IL-5 and IL-13 levels were log normalized. An IL-5 high and IL-13 high cut-off was then identified by adding 2 standard deviations to the mean value of each cytokine for the control population. A standardized metric representing combined IL-5 and IL-13 high disease was defined by scaling and centering data so that each cytokine had the same mean and standard deviation. The Th2-high cut-off was again defined by adding 2 standard deviations to the Th2 mean for the control population, similar to a previous approach17. Low and high cytokine groups were then compared against clinical and demographic factors.
Statistics
Normality of data in each group was assessed using the D’Agostino-Pearson omnibus test. Variables with a normal distribution were compared using a student’s t-test while nonparametric data was analyzed using the Mann-Whitney test. Comparative data was presented as means +/− standard deviation or medians with interquartile range, respectively. The Spearman correlation coefficient was used to calculate correlations between individual cytokines. A p value of 0.05 was considered statistically significant for all comparisons. Statistical analyses were performed with Prism 6 software (Graphpad; La Jolla, CA).
RESULTS
Patient Population and Demographics
The patient population is part of an ongoing prospective translational study evaluating biological markers and clinical outcomes in CRS, and has been partially characterized elsewhere22. Ninety CRS patients and 17 healthy controls were enrolled in the study (Table 1). A majority of CRS patients had nasal polyps, while comorbid asthma and allergic rhinitis were reported in 49% and 62%, respectively. 12 patients had AERD, while 10 were diagnosed with AFRS. Almost half of enrolled subjects had undergone prior endoscopic sinus surgery. Disease burden was significant, with a mean SNOT-22 score of 47.1 and median CT score of 16.0. Consistent with the overall disease burden, a majority of patients had SIT testing consistent with moderate to severe hyposmia, with a mean age- and sex-adjusted SIT score of −7.0. Among healthy controls, only 1 of 17 patients had a history of allergic rhinitis and none were asthmatic.
Table 1. Study Population and Demographics for Healthy Control and CRS Patients.
Data is presented as frequencies (percentages), means +/− standard deviation or medians with interquartile range.
| Healthy Control | All CRS | CRSsNP | CRSwNP | |
|---|---|---|---|---|
| No. | 17 | 90 | 37 | 53 |
| Age (years) | 50.5 +/− 13.1 | 48.5 +/− 13.1 | 47.7 +/− 13.3 | 49.0 +/− 13.1 |
| Sex, no. (% female) | 13 (76) | 42 (47) | 21 (57) | 21 (40) |
| Asthma, no. (%) | 0 (0) | 44 (49) | 12 (32) | 32 (60) |
| Allergic Rhinitis, no. (%) | 1 (6) | 56 (62) | 20 (54) | 36 (68) |
| AERD, no. (%) | - | 11 (12) | 0 (0) | 11 (21) |
| AFS, no. (%) | - | 10 (11) | 0 (0) | 11 (21) |
| SNOT-22 score | - | 47.1 +/− 18.7 | 48.5 +/− 16.3 | 46.1 +/− 20.4 |
| CT score | 1.0 (0.0-3.6) | 16.0 (11.0-20.0) | 12.0 (9.0-15.3) | 17.5 (14.8-22.0) |
| SIT score | −4.0(−7.0–1.0) | −7.0(−24.5–3.0) | −3.0 (−6.8–1.0) | −20.0 (−27.0–7.0) |
| Prior surgery, no. (%) | - | 43/90 (48) | 13 (35) | 30 (57) |
AERD, aspirin-exacerbated respiratory disease; AFRS, allergic fungal rhinosinusitis; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; CT, computed tomography; SIT, smell identification test; SNOT-22, sinonasal outcome test-22.
IL-4, IL-5, and IL-13 are Detectable in Sinonasal Mucus and are Closely Correlated
IL-4, IL-5, and IL-13 are pro-inflammatory cytokines that are hallmarks of the Th2 signature29,30. We assessed levels of these cytokines in sinonasal mucus as potential minimally invasive biomarkers of Th2-high CRS. Mucus derived from CRS patients had significantly elevated levels of IL-5 (mean 93.1 +/− 286.5 vs. 0.5 +/− 0.8 pg/mL, p < 0.001) and IL-13 (mean 67.5 +/− 145.6 vs. 6.1 +/− 11.6 pg/mL, p < 0.02) compared to healthy controls (Figure 1A). IL-4 levels were not significantly different between CRS and healthy control patients (mean 1.5 +/− 3.0 vs. 0.9 +/− 1.3 pg/mL, p = 0.53). Levels of IL-4, IL-5, and IL-13 were all moderately to strongly correlated (p< 0.001 for all comparisons) (Figure 1B). Due to a lack of significant differences between healthy control and CRS patients, and weaker correlation with IL-5 and IL-13 levels, IL-4 was excluded from subsequent analysis.
Figure 1.
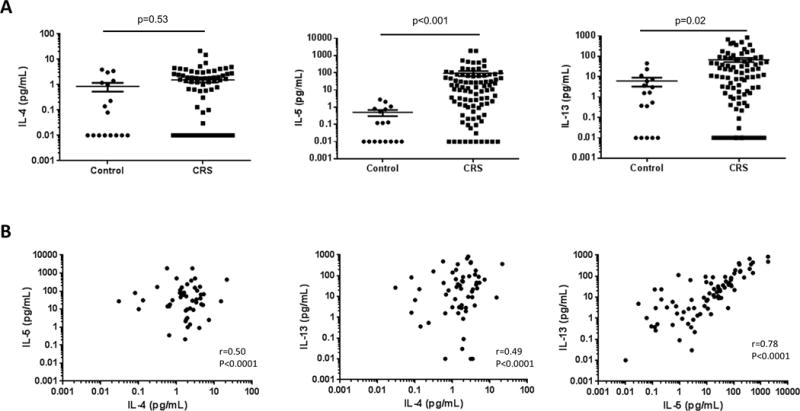
(A) Comparison of mucus IL-4, IL-5, and IL-13 levels between healthy control and CRS patients. IL-5 and IL-13 levels were significantly elevated in CRS patients compared to controls, while no significant difference was found for IL-4. Solid lines indicate the mean +/− SEM. (B) Correlation between mucus levels of IL-4, IL-5, and IL-13 in CRS. All correlations were moderate or strong (p<0.0001 for all comparisons).
Establishing IL-5-high and IL-13-high Metrics to Differentiate CRS Patients
In order to further evaluate mucus IL-5 and IL-13 levels as clinically relevant biomarkers, values were log normalized and compared between healthy controls and both CRSsNP and CRSwNP patients. An IL-5/IL-13 high cut-off was then defined by adding two standard deviations to the mean value of each cytokine for healthy control patients. As shown in Figure 2A, an overwhelming majority of CRSwNP patients fell into the IL-5 high group. Conversely, most CRSsNP patients were IL-5 low. Due to wider variability of IL-13 levels in healthy control patients, a small number of patients fell into the IL-13 high group (CRSsNP, 4.3%; CRSwNP, 26.4%) (Figure 2B).
Figure 2. Calculation of a combined Th2 low/high metric.
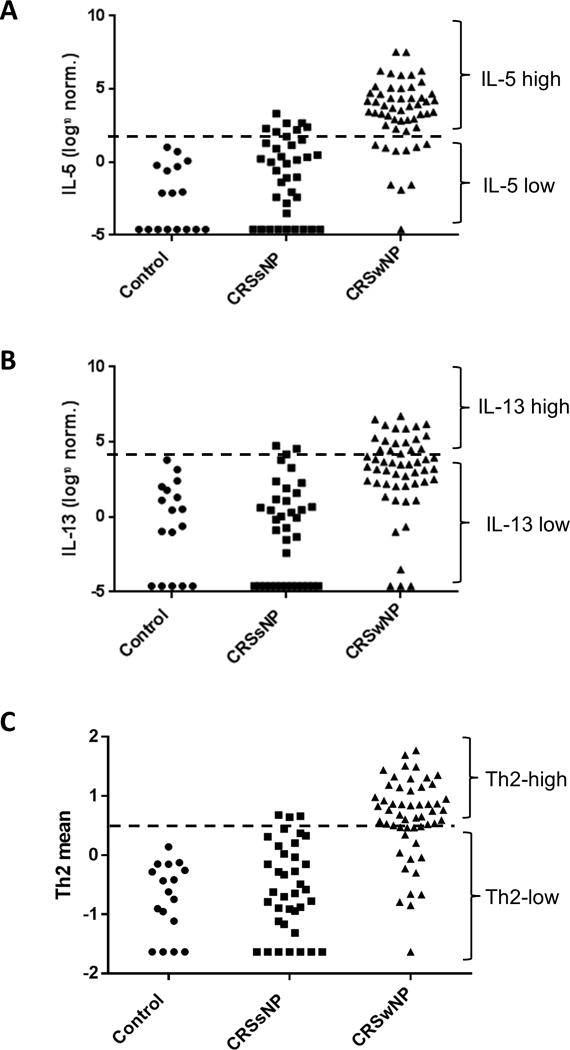
Mucus cytokine levels were log normalized and an IL-5- (A) and IL-13-high (B) cut-off was identified by adding 2 SDs to the mean data for healthy controls (dashed line). Patients above the line were defined as IL-5/IL-13 high, while those below the line were defined as IL-5/IL-13 low. (C) Log normalized mucus cytokine levels for IL-5 and IL-13 were scaled, combined, and centered. A Th2-high cut-off was then identified by adding 2 SDs to the mean data for healthy controls (dashed line). Patients above the line were defined as Th2-high, while those below the line were defined as Th2-low.
Clinical Characteristics of IL-5 high and IL-13 high CRS
The clinical relevance of each Th2 biomarker was next evaluated (Table 2). Patients with IL-5 high CRS were more likely to have nasal polyps (p<0.0001), asthma (p=0.006), AERD (p=0.001), and AFRS (p=0.04) than those with IL-5 low CRS. IL-5 high patients also had more severe disease as evidenced by higher preoperative CT scores (p=0.01), worse objective olfactory function (p<0.0001), and elevated tissue eosinophilia (p=0.001). As further evidence of their recalcitrant disease, IL-5 high patients were more likely to have had prior sinus surgery (p=0.01) and had a higher number of average prior procedures (p=0.02), compared to IL-5 low patients. Similar observations were noted among patients with IL-13 high CRS, though these patients additionally had worse preoperative SNOT-22 scores (p=0.03).
Table 2. Clinical and Demographic Data for IL-5 low/high and IL-13 low/high CRS.
Data is presented as frequencies (percentages) means +/− standard deviation or medians with intraquartile range.
| IL-5-low CRS | IL-5-high CRS | p-value | IL-13-low CRS | IL-13-high CRS | p-value | |
|---|---|---|---|---|---|---|
| No. | 40 | 50 | 74 | 16 | ||
| Age (years) | 50.2 +/− 12.0 | 47.1 +/− 13.9 | 0.27 | 48.1 +/− 13.0 | 49.9 +/− 14.2 | 0.64 |
| Sex, no. (% female) | 21 (53) | 21 (42) | 0.40 | 38 (51) | 4 (25) | 0.10 |
| Nasal polyps, no. (%) | 10 (25) | 43 (86) | <0.0001 | 39 (53) | 14 (88) | 0.01 |
| Asthma, no. (%) | 12(30) | 30 (60) | 0.006 | 29 (38) | 13 (81) | 0.005 |
| Allergic Rhinitis, no. (%) | 21 (50) | 35 (70) | 0.13 | 46 (61) | 10 (63) | 1.00 |
| AERD, no. (%) | 0 (0) | 12 (24) | 0.001 | 6 (8) | 6 (38) | 0.006 |
| AFRS, no. (%) | 1 (3) | 9 (18) | 0.04 | 3 (4) | 7 (44) | 0.0001 |
| SNOT-22 score | 48.9 +/− 16.7 | 45.7 +/− 20.2 | 0.55 | 44.4 +/− 17.3 | 58.9 +/− 20.6 | 0.03 |
| CT score | 13.5 (10.0-17.0) | 17.3 (13.0-22.0) | 0.0003 | 14.5 (11.0-17.6) | 20.8 (10.0-22.2) | <0.0001 |
| SIT score | −4 (−8 - -1) | −20.5 (−27.8 - -7.0) | <0.0001 | −5.0(−20.9–2.0) | −21.5(−29.5–16.0) | 0.001 |
| Prior surgery, no. (%) | 12 (30) | 29 (58) | 0.01 | 31 (42) | 11 (69) | 0.06 |
| # of prior surgeries | 0.48 +/− 0.96 | 1.08 +/− 1.32 | 0.02 | 0.68 +/− 1.05 | 1.38 +/− 1.59 | 0.03 |
| Tissue eosinophils/HPF | 3.5 (0-29.5) | 56.0 (24.5-100.0) | <0.0001 | 25.0 (1.0-85.0) | 61.5 (33.5-156.3) | 0.01 |
AERD, aspirin-exacerbated respiratory disease; AFRS, allergic fungal rhinosinusitis; CT, computed tomography; SIT, smell identification test; SNOT-22, sinonasal outcome test-22; HPF, high power field. Bold, p<0.05.
Since differences in clinical characteristics could potentially be attributable to higher numbers of CRSwNP subjects in the IL-5/13 high groups, we removed this possible confounder by evaluating CRSwNP separately (Table 3). Among these phenotypically similar patients, those with IL-5 high CRS had worse SIT scores (p=0.03) and greater tissue eosinophilia (p=0.002). Likewise, CRSwNP patients in the IL-13 high group had worse SIT (p<0.0001) and CT scores (p=0.005), compared to those with IL-13 low disease. Though statistically limited by the small number of patients in the IL-13 high group, there was a clear trend toward worse SNOT-22 scores (p=0.07) and higher tissue eosinophil counts (p=0.10) compared to IL-13 low patients.
Table 3. Clinical and Demographic Data for IL-5 low/high and IL-13 low/high CRSwNP.
Data is presented as frequencies (percentages) means +/− standard deviation or medians with intraquartile range.
| IL-5-low CRSwNP | IL-5-high CRSwNP | p-value | IL-13-low CRSwNP | IL-13-high CRSwNP | p-value | |
|---|---|---|---|---|---|---|
| No. | 10 | 43 | 39 | 14 | ||
| Age (years) | 52.6 +/− 11.3 | 48.1 +/− 13.5 | 0.34 | 48.2 +/− 12.8 | 51.4 +/− 14.3 | 0.44 |
| Sex, no. (% female) | 4 (40) | 17 (40) | 1.00 | 18 (46) | 3 (21) | 0.13 |
| Asthma, no. (%) | 4 (40) | 27 (63) | 0.29 | 19 (49) | 12 (86) | 0.03 |
| Allergic Rhinitis, no. (%) | 6 (60) | 30 (70) | 0.71 | 27 (69) | 9 (64) | 0.75 |
| AERD, no. (%) | 0 (0) | 12 (28) | 0.09 | 6 (15) | 6 (43) | 0.06 |
| AFRS, no. (%) | 1 (20) | 9 (21) | 0.67 | 3 (10) | 7 (50) | 0.002 |
| SNOT-22 score | 52.5 +/− 19.5 | 44.5 +/− 20.7 | 0.40 | 42.2 +/− 18.8 | 57.1 +/− 22.1 | 0.07 |
| CT score | 17.0 (13.5-18.8) | 15.5 (9.0-18.0) | 0.28 | 16.5 (13.0-20.0) | 22.8 (20.8-24.0) | <0.0001 |
| SIT score | −6.5(−17.3–4.3) | −24.0(−28.0–8.0) | 0.03 | −11.5(−25.9–4.3) | −28.0(−30–20) | 0.005 |
| Prior surgery, no. (%) | 4 (40) | 27 (63) | 0.54 | 22 (56) | 9 (64) | 0.76 |
| # of prior surgeries | 0.50 +/− 0.71 | 1.16 +/− 1.34 | 0.14 | 0.90 +/− 1.07 | 1.43 +/− 1.70 | 0.18 |
| Tissue eosinophils/HPF | 14.5 (0.8-35.0) | 73.0 (35.0-100.0) | 0.002 | 50.0 (15.0-100.0) | 74.0(50.0-177.5) | 0.10 |
AERD, aspirin-exacerbated respiratory disease; AFRS, allergic fungal rhinosinusitis; CT, computed tomography; SIT, smell identification test; SNOT-22, sinonasal outcome test-22; HPF, high power field. Bold, p<0.05.
Clinical characteristics of Th2-high and Th2-low CRS
In order to establish a metric of Th2 inflammation, each cytokine value was scaled and combined into a mean representing Th2 inflammation. A Th2 cutoff was defined by adding two standard deviations to the mean Th2 value for the healthy control population. Distribution of subjects is shown in Figure 2C, with a majority of CRSsNP subjects in the Th2 low group and a majority of CRSwNP subjects in the Th2 high group. Consistent with results for both IL-5 and IL-13, the combined IL-5/13 high group was associated with higher rates of nasal polyposis (p<0.0001), AERD (p=0.002), worse preoperative CT (p<0.0001) and SIT (p=0.0002) scores, eosinophilia on histopathology (p<0.0001), and higher rates of revision surgery (p=0.02) (Table 4). When CRSwNP patients were evaluated separately, those who were Th2-high had elevated tissue eosinophil counts (p=0.002) and a trend toward worse CT (p=0.07) and SIT (p=0.21) scores.
Table 4. Clinical and Demographic Data for Th2 low/high CRS (A) and Th2 low/hogh CRSwNP (B).
Data is presented as frequencies (percentages) means +/− standard deviation or medians with intraquartile range.
| Th2-low CRS | Th2-high CRS | p-value | Th2-low CRSwNP | Th2-high CRSwNP | p-value | |
|---|---|---|---|---|---|---|
| No. | 49 | 41 | 15 | 38 | ||
| Age (years) | 48.8 +/− 12.3 | 48.1 +/− 12.1 | 0.79 | 48.3 +/− 11.2 | 49.3 +/− 13.9 | 0.82 |
| Sex, no. (% female) | 27 (55) | 15 (37) | 0.09 | 7 | 14 | 0.55 |
| Nasal polyps, no. (%) | 15 (31) | 38 (93) | <0.0001 | - | - | - |
| Asthma, no. (%) | 16 (33) | 25 (61) | 0.06 | 7 (47) | 24 (63) | 0.36 |
| Allergic Rhinitis, no. (%) | 26 (53) | 29 (71) | 0.20 | 9 (60) | 27 (71) | 0.52 |
| AERD, no. (%) | 1 (2) | 11 (27) | 0.002 | 1 (7) | 11 (29) | 0.14 |
| AFRS, no. (%) | 1 (4) | 9 (22) | 0.07 | 1 (13) | 9 (24) | 0.25 |
| SNOT-22 score | 47.8 +/−15.9 | 44.5 +/− 20.2 | 0.50 | 50.0 +/− 17.1 | 44.7 +/− 21.6 | 0.54 |
| CT score | 14.0 (10.0-17.0) | 18.0 (14.3-22.3) | <0.0001 | 16.0 (13.0-18.0) | 20.0 (15.9-22.6) | 0.07 |
| SIT score | −4.0(−8.8–1.3) | −21.0(−28.0–7.0) | 0.0002 | −8.0(−25.0–5.0) | −23.8(−28.0–9.0) | 0.21 |
| Prior surgery, no. (%) | 17 | 25 | 0.02 | 7 (47) | 24 (63) | 0.36 |
| # of prior surgeries | 0.55 +/− 0.96 | 1.10 +/− 1.36 | 0.01 | 0.73 +/− 0.88 | 1.16 +/− 1.39 | 0.37 |
| Tissue eosinophils/HPF | 4.5 (1.0-35.0) | 67.0 (29.0-120.0) | <0.0001 | 25.0 (1.0-50.0) | 74.0 (46.9-142.5) | 0.002 |
AERD, aspirin-exacerbated respiratory disease; AFRS, allergic fungal rhinosinusitis; CT, computed tomography; SIT, smell identification test; SNOT-22, sinonasal outcome test-22; HPF, high power field. Bold, p<0.05.
DISCUSSION
Classification of CRS has historically depended on the absence or presence of nasal polyps, which has often driven treatment algorithms and outcomes. However, this is largely a phenotypic classification that is not based on disease pathophysiology or endotype. As evidenced by this and other studies, CRSsNP and CRSwNP can both present with Th2-associated inflammation. The current report details a minimally-invasive approach for identifying patients with Th2-high CRS that utilizes biomarkers in sinonasal mucus. While prior studies have shown variability of individual cytokines and inflammatory mediators in surgically-derived tissue and even used this information to cluster patients into endotypes2,3,31, the strength of the current study is its ability to categorize patients without invasive acquisition of tissue and with a limited number of biomarkers.
We evaluated sinonasal mucus for the Th2-associated cytokines, IL-5 and IL-13, and used this data to derive a scaled and combined Th2 cutoff value. This approach has been used successfully for asthma, which has similar pathophysiology to CRS, resulting in the differentiation of clinically relevant patient groups18. We were able to successfully separate CRS patients into Th2-low and Th2-high endotypes, with the Th2-high group composed primarily of CRSwNP patients and enriched in asthmatics. A subset of CRSsNP patients also fell into the Th2-high group, highlighting the heterogeneity of CRS and the failure of phenotype-based classification to account for underlying inflammatory etiology. Despite apparent pathophysiological relevance32-35, our study found that IL-4 was a poor biomarker for Th2-high CRS, largely because mucus levels were indistinguishable from healthy controls. This is in line with recent studies that have also shown small or no differences in IL-4 levels between individual CRS phenotypes or compared to healthy controls36-39, but is in contrast to some prior studies that did confirm elevated levels of IL-4 in CRS compared to controls40-42. We suspect that much of these conflicting results may be attributable to differences between assays and variability in approaches for mucus collection and processing. It is also possible that the inability of IL-4 to function as a reliable biomarker in our study could be secondary to the limited sensitivity of antibody-based approaches used here and in prior studies, rather than to the biological function and/or quantity of IL-4 itself.
The goal of the current study was not only to classify patients based on Th2 signature, but also to determine whether these classifications were clinically relevant. The Th2 endotype was defined by more severe clinical disease, with worse preoperative CT scores and worse objective olfactory function. These patients also were more likely to have had prior endoscopic sinus surgery, and had a greater degree of eosinophilia in surgically-derived tissue, a marker of more severe and recalcitrant sinus disease. The mucus-based diagnostics used in this study create the potential for point-of-care endotyping that can predict both disease severity and outcomes. For example, high surgical failure rates in the Th2-high group as evidenced by a greater number of prior surgeries, would suggest that these patients may be more effectively treated with more aggressive upfront surgery, or non-surgical treatments that target underlying inflammation.
In subgroup analysis, we sought to determine whether CRSwNP patients could be further subgrouped based on their Th2 signature. Though a majority of CRSwNP patients fell into the Th2-high group, approximately 40% did not reach this threshold, with many showing levels comparable to healthy control or CRSsNP patients. The heterogeneity of CRSwNP is well documented with recent studies suggesting variable pathophysiology based on inflammatory subtype/endotype and geographic location2,3,22. Presumably, many of these patients may be expected to respond poorly to anti-IL-5 or anti-IL-4/-13R biologic medications. In support of this, a small study evaluating reslizumab for treatment of nasal polyposis found that responders had increased nasal IL-5 levels43. However, it is still unclear whether treatment response was secondary to increased local IL-5 levels or due to greater disease burden that could be associated with higher levels of IL-5 or other cytokines. The current study showed that CRSwNP patients with an IL-5 high or IL-13 high signature had worse preoperative disease severity than their IL-5 and IL-13 low counterparts. The combined Th2 high metric was likewise associated with tissue eosinophilia and trended toward worse objective measures of disease severity. Though our original study was designed to distinguish Th2-low and Th2-high CRS patients, irrespective of polyp status, larger follow-up studies may help to confirm the ability of a combined Th2 signature to differentiate CRSwNP patients into clinically relevant subgroups. Nonetheless, data in the current study suggests that Th2 status can be used as a prognostic factor in patients who are otherwise phenotypically uniform.
Strengths of the current study include its prospective design and use of minimally-invasive measures to characterize CRS inflammation. While we included strict inclusion and exclusion criteria in our study, it is possible that other comorbid factors could have an impact on the levels of cytokines and other pro-inflammatory mediators in sinonasal mucus. It is also possible that mucus levels of certain cytokines may not correlate to those in sinonasal tissue. However, close correlation between cytokine levels in sinonasal tissue and mucus is supported by a previous study44. Finally, it is likely that temporal variations in cytokine levels may occur among individual patients, an issue that may affect diagnostic accuracy. For example, we have purposefully excluded patients from our study who had received systemic steroids within 4 weeks of mucus collection, as this factor may substantially impact cytokine levels. Likewise, variations based on CRS exacerbations, or exacerbations within other comborbid diseases such as asthma or allergic rhinitis, could potentially alter results. Future prospective studies that assess seasonal and diurnal variations in mucus cytokine levels are needed to confirm this approach as an accurate and consistent biomarker.
CONCLUSIONS
CRS patients can be differentiated based on the absence or presence of a Th2 signature using minimally invasive collection of sinonasal mucus. Patients with Th2-high CRS were more likely to have nasal polyps, tissue eosinophilia, and asthma, and had worse preoperative disease severity.
Acknowledgments
This project was supported by NIH RO3 DC014809 (J.H.T.), L30 AI113795 (J.H.T.), and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
ABBREVIATIONS
- AERD
aspirin-exacerbated respiratory disease
- AFRS
allergic fungal rhinosinusitis
- CRSsNP
chronic rhinosinusitis without nasal polyps
- CRSwNP
chronic rhinosinusitis with nasal polyps
- SIT
smell identification test
- SNOT-22
22 item sinonasal outcome test
Footnotes
Financial disclosures: No relevant disclosures
Conflicts of interest: None
Presented at the Spring Meeting of the American Rhinologic Society at COSM; April 20, 2018; National Harbor, MD.
References
- 1.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: national health interview survey, 2012. 2014. pp. 1–161. (Vital and health statistics Series 10, Data from the National Health Survey). [PubMed] [Google Scholar]
- 2.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. The Journal of allergy and clinical immunology. 2016;137:1449–1456 e1444. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. The Journal of allergy and clinical immunology. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Tan BK, Klingler AI, Poposki JA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. The Journal of allergy and clinical immunology. 2017;139:699–703 e697. doi: 10.1016/j.jaci.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. American journal of rhinology & allergy. 2014;28:192–198. doi: 10.2500/ajra.2014.28.4033. [DOI] [PubMed] [Google Scholar]
- 6.Hauser LJ, Chandra RK, Li P, Turner JH. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. International forum of allergy & rhinology. 2017;7:957–962. doi: 10.1002/alr.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2009;141:454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;142:64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. The Lancet Respiratory medicine. 2017;5:390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 10.Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2017;47:129–138. doi: 10.1111/cea.12853. [DOI] [PubMed] [Google Scholar]
- 11.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. The New England journal of medicine. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. The New England journal of medicine. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 14.Bachert C, Mannent L, Naclerio RM, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. Jama. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 15.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. The Journal of allergy and clinical immunology. 2011;128:989–995. e981–988. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 16.Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. The Journal of allergy and clinical immunology. 2017;140:1024–1031 e1014. doi: 10.1016/j.jaci.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Silkoff PE, Laviolette M, Singh D, et al. Identification of airway mucosal type 2 inflammation by using clinical biomarkers in asthmatic patients. The Journal of allergy and clinical immunology. 2017;140:710–719. doi: 10.1016/j.jaci.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. The Journal of allergy and clinical immunology. 2014;133:388–394. doi: 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinks TS, Brown T, Lau LC, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. The Journal of allergy and clinical immunology. 2016;138:61–75. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo CS, Pavlidis S, Loza M, et al. A Transcriptome-driven Analysis of Epithelial Brushings and Bronchial Biopsies to Define Asthma Phenotypes in U-BIOPRED. American journal of respiratory and critical care medicine. 2017;195:443–455. doi: 10.1164/rccm.201512-2452OC. [DOI] [PubMed] [Google Scholar]
- 21.Cheng D, Xue Z, Yi L, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. American journal of respiratory and critical care medicine. 2014;190:639–648. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner JH, Chandra RK, Li P, Bonnet K, Schlundt DG. Identification of Clinically Relevant Chronic Rhinosinusitis Endotypes using Cluster Analysis of Mucus Cytokines. The Journal of allergy and clinical immunology. 2018 doi: 10.1016/j.jaci.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weibman AR, Huang JH, Stevens WW, et al. A prospective analysis evaluating tissue biopsy location and its clinical relevance in chronic rhinosinusitis with nasal polyps. International forum of allergy & rhinology. 2017 doi: 10.1002/alr.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology Supplement. 2012;23:3. preceding table of contents, 1-298. [PubMed] [Google Scholar]
- 25.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. International forum of allergy & rhinology. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 26.Bent JP, 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1994;111:580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical otolaryngology: official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 28.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. The Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nature reviews Immunology. 2017 doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 30.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nature reviews Immunology. 2015;15:271–282. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 31.Divekar R, Rank M, Squillace D, Kita H, Lal D. Unsupervised network mapping of commercially available immunoassay yields three distinct chronic rhinosinusitis endotypes. International forum of allergy & rhinology. 2017;7:373–379. doi: 10.1002/alr.21904. [DOI] [PubMed] [Google Scholar]
- 32.Ramanathan M, Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. American journal of rhinology. 2008;22:115–121. doi: 10.2500/ajr.2008.22.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. The Journal of allergy and clinical immunology. 2012;130:1087–1096 e1010. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 34.Hong HY, Chen FH, Sun YQ, et al. Local IL-25 contributes to Th2-biased inflammatory profiles in nasal polyps. Allergy. 2018;73:459–469. doi: 10.1111/all.13267. [DOI] [PubMed] [Google Scholar]
- 35.Bal SM, Bernink JH, Nagasawa M, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nature immunology. 2016;17:636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 36.Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. American journal of respiratory and critical care medicine. 2015;192:682–694. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Chandra RK, Li P, Hull BP, Turner JH. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. The Laryngoscope. 2018 doi: 10.1002/lary.27112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nabavi M, Arshi S, Bahrami A, et al. Increased level of interleukin-13, but not interleukin-4 and interferon-gamma in chronic rhinosinusitis with nasal polyps. Allergologia et immunopathologia. 2014;42:465–471. doi: 10.1016/j.aller.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Schlosser RJ, Mulligan JK, Hyer JM, Karnezis TT, Gudis DA, Soler ZM. Mucous Cytokine Levels in Chronic Rhinosinusitis-Associated Olfactory Loss. JAMA otolaryngology– head & neck surgery. 2016;142:731–737. doi: 10.1001/jamaoto.2016.0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Burner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2005;35:1186–1191. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 41.Scavuzzo MC, Fattori B, Ruffoli R, et al. Inflammatory mediators and eosinophilia in atopic and non-atopic patients with nasal polyposis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2005;59:323–329. doi: 10.1016/j.biopha.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Riccio AM, Tosca MA, Cosentino C, et al. Cytokine pattern in allergic and non-allergic chronic rhinosinusitis in asthmatic children. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2002;32:422–426. doi: 10.1046/j.1365-2222.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 43.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. The Journal of allergy and clinical immunology. 2006;118:1133–1141. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. The Laryngoscope. 2013;123:E72–78. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]


