Abstract
Visceral leishmaniasis (VL) is a life-threatening outcome of Leishmania infantum or L. donovani infection. Dogs are the primary domestic reservoir of L. infantum parasites and ownership of infected dogs increases the risk of human VL. Controlling infection within dog populations is regarded as critical to VL management in endemic countries, both preventing progression of canine disease and limiting parasite transmission to humans and dogs. Here we discuss various strategies that are used to diagnose canine visceral leishmaniasis (CVL) and the possibilities of adapting these for use within population screening and control programs. In addition, given the variable transmissibility of L. infantum to the sand fly vector, we outline some possibilities for the preferential identification of “super-spreader” dogs among the overall infected population.
Keywords: Leishmaniasis, canine, asymptomatic, diagnosis, serology
Leishmania infection and visceral leishmaniasis
Visceral leishmaniasis
(VL, see Glossary) is a vector–borne disease characterized by prolonged fever, wasting, splenomegaly, and hepatomegaly, resulting in >90% case–fatality within two years in the absence of treatment [1]. Estimates of the number of new human VL (HVL) cases per year range from 25,000 to 200,000 accounting for underreporting [2, 3], and although therapeutic drug options are available, the control portfolio to prevent transmission are limited. VL due to Leishmania donovani is considered anthroponotic, with likely zoonotic transmission in East Africa, whereas VL due to L. infantum is considered a zoonosis reliant on domestic dogs as the sole proven reservoir [4, 5] (Figure 1). L. infantum infection of dogs can lead to canine VL (CVL), a multisystemic disease with a range of clinical signs typically including dermatitis, lymphadenomegaly, general muscular atrophy, and renal disease [6].
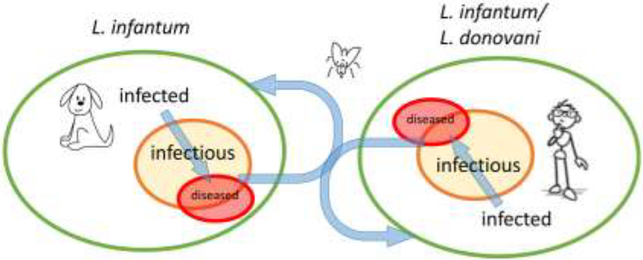
Figure 1. Transmission of VL-causing Leishmania among dogs and humans.
A Venn diagram depicting the progression of Leishmania-infected dogs and/or humans to an infectious state culminating in presentation of VL. While L. infantum can circulate in, and be transferred between, dog and human populations via sand fly vectors, L. donovani is limited to human populations.
Anthroponotic VL has been targeted for elimination as a public health problem (defined as an incidence of <1 VL case/10,000 people per year in specified geographical scales) in the Indian subcontinent by 2020 [7]. Substantial investments and political advocacy have been used to implement novel case detection strategies, rapid diagnostic testing and vector control activities [1, 7]. In contrast, despite the large burdens typically in young children, efforts to reduce zoonotic VL (ZVL) in endemic regions of Latin America, East Africa, and central Asia are less advanced [3]. The zoonotic nature of L. infantum transmission suggests that controlling infection within dog populations, is a reasonable strategy to reduce infection rates in humans [8, 9]. The majority of ZVL cases in the Americas occur in Brazil [3], where intervening measures of indoor residual spraying (IRS) and dog test-and-slaughter are national policies [10]. The test-and-slaughter policy has, however, been legally challenged in some Brazilian states with the outcome that treatment is now permitted. There is, however, a lack of evidence that these strategies have significantly reduced canine or human ZVL incidence [11, 12], and alternative or complimentary control methods are presently under evaluation [13–15]. Preventative measures against canine infection or disease include the use of topical or oral insecticides, and canine anti-Leishmania vaccination, that are shown to provide varying levels of protection to individual dogs [16]. That any canine control option corresponds to a reduction in human infection still requires well designed randomized field trials [17].
Given that many more asymptomatic than symptomatic canine (and human) Leishmania infections are identified in cross-sectional surveys, surveillance for both infection and disease are essential for the future success of control programs [6]. In this article, we discuss various diagnostic strategies, with emphasis on current and new developments in antigen-based serological diagnostic tests, their applications, and performance requirements for different settings. In doing so, we identify current issues and research priorities.
Diagnosing infection and CVL
The clinical signs of CVL are quite general and therefore not particularly useful for detecting symptomatic infection or differentiating Leishmania from other etiologies [16]. Numerous tools are, however, available to aid the diagnosis of both canine and human infection, covering an array of direct and indirect detection methods (Figure 2). Direct methods include xenodiagnosis and parasite observance in ex vivo cultures, or recognition of amastigotes following biopsy or microscopy. Parasite genome equivalents can be detected by molecular DNA-based techniques. In clinical settings, confirmation of cure requires demonstrating absence of tissue parasites, which depends on the sensitivity of methods and sample type to detect low-level parasite burdens. Leishmania burdens generally decline during treatment [18–20], although residual parasites were detected in up to 50% of human patients that were considered cured based on the resolution of symptoms [19, 21]. Similarly, alleviation of symptoms and decreased parasite loads are considered indicators of treatment success in CVL patients, though complete parasitological clearance is often not achieved (reviewed by [22]). Some diagnostic techniques used routinely in research laboratories are not suited for widespread use in field settings. For example, PCR/qPCR requires nucleic acid extraction, amplification in a thermocycler and gel-based analyses to demonstrate specific gene expression, each step requiring specialist technical conditions; it may require invasive sample collection and is generally costly. Point-of-care assays such as loop-mediated isothermal amplification (LAMP) and nanoparticle-based lateral flow biosensors for DNA are being developed to address some of these issues [23–28]. The commercially available Leishmania OligoC-TesT kit that incorporates standardized PCR reagents with rapid oligochromatographic dipstick detection of the PCR products provides greater sensitivity for polysymptomatic and oligosymptomatic dogs than for asymptomatic dogs, a trend observed for various other qualitative PCR methods [29]. Real-time qPCR typically provides the highest sensitivity. Among the recent advances towards development of a field applicable PCR type test for CVL, is a combined, duplex qPCR protocol that detects L. infantum kinetoplast (k)DNA simultaneously with host (canine 18S) rRNA in a single tube, in a ready-to-use (gelified and freezer-free) format [30]. Duplexing of these qPCR protocols permits generation of quality-controlled results with reduced overall cost relative to running each assay separately. Higher test sensitivities (e.g. 92.9%) were achieved using splenic aspirates than skin or blood samples (50% and 35.7%, respectively) but relies on invasive sampling [30]. Similarly, an in-clinic point-of-care PCR for the diagnosis of canine Leishmania infection (PCRun; Biogal Galed Labs ACS) provided reliable confirmation of CVL infection, but was constrained by an inverse correlation between assay sensitivity and simplicity of sample collection (ranging from 58.8% in lymph node aspirates to 10.0% in blood) [31].
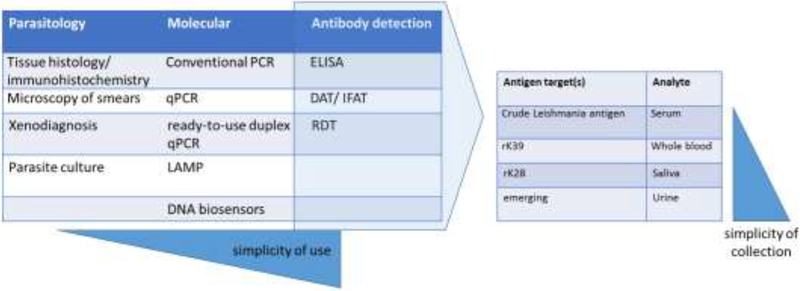
Figure 2. Diagnostic and detection strategies.
The progression of diagnostic strategies from direct detection methods to indirect, antibody-detection techniques is depicted. As antibodydetection methods are refined, the possibility of using “simpler-to-collect” (i.e., less invasive) samples that are more permissive for large-scale surveillance programs is presenting themselves.
Despite molecular advances, indirect approaches centered on immune responses to Leishmania antigens still provide the most tangible possibilities to support CVL and HVL diagnosis in low resource settings. While dogs resistant to developing CVL typically have undetectable or low, often transient, anti-Leishmania antibody responses, non-resistant dogs that develop signs of CVL typically have high serum antibody titers that positively correlate with tissue parasite density and clinical severity [32–34]. Therefore, antibody-based test sensitivities are unlikely to be similar for asymptomatic and symptomatic infections [35]. High initial antibody titers against crude Leishmania antigen (CLA) are associated with increased risk of developing clinical signs [36], and positive rk39 rapid diagnostic test (RDT) results are strongly associated with high infection levels and disease progression [35]. In humans, high anti-Leishmania antibody levels during the asymptomatic infection phases are similarly indicative of likely progression to VL: in India, 12% of individuals with direct agglutination test (DAT) titers >25,600 subsequently developed clinical disease, compared to only 1% of those with low but positive DAT results [37]. Likewise, in Bangladesh 29% of strongly rK39-seropositive individuals at study intake progressed to VL compared to less than 4% of low seropositive or seronegative individuals [38]. Comparable estimates using alternative antibody threshold definitions were reported by others [37].
Diagnostic tests require different attributes dependent upon the particular application. High sensitivity is required to prevent inadvertent introduction of infected human or canine blood into blood banks; to monitor success of treatment/intervention of either HVL or CVL; to detect spread of Leishmania-infected dogs across international borders, and for epidemiological studies or trials to differentiate infected and non-infected individuals in the population. Refinement from the use of crude Leishmania extracts to purified recombinant antigens includes several recombinant antigen-based immunoassays, foremost among these, are platforms based on the chimeric polyprotein rK39 used to confirm HVL and CVL infection [39–41]. The incorporation of rK39 into lateral flow-based rapid tests (Kalazar Detect™, InBios International Inc., Seattle, WA) provided a simple field-friendly format capable of providing diagnosis of HVL at the point of care [42, 43]. Modifications of the rK39 antigen yielded a next generation rK28 RDT-based rapid diagnostic test for enhanced diagnosis of HVL in Africa (Onsite Leishmania Ab Combo Rapid Test, CTK Biotech, San Diego, CA) [44–46]. A dog-specific variant of this, DPP® CVL (Chembio Diagnostics, Medford, NJ), retains high performance [47–49]. and is now established within the diagnostic recommendations of the Brazilian Ministry of Health. Removal of the laboratory requirements associated with ELISA render these lateral flow-based tests applicable in a wider array of settings.
New antigen targets and refinements to provide further incremental improvements in the diagnostic performance of antibody-detection tests are being proposed. For example, the use of recombinant protein rLc36 in ELISA enabled differentiation of positive and negative sera and showed a sensitivity of 85% and specificity of 71% [50]. Combinatorial approaches can enhance sensitivity, while continued screening of genomic expression libraries or adapted bioinformatics searches are providing new hits. Indeed, combining rKLO8 and rK26 increased sensitivity and specificity (85% and 93%, respectively) over the levels achieved by either antigen alone (sensitivities and specificities of 68% and 92% for KLO8, and of 77% and 91% for rK26, respectively) [51]. Following selection from a L. infantum amastigote cDNA library, rLci1A and rLci2B were combined and incorporated into a prototype rLci1A/rLci2B dual path platform rapid test. Evaluation of 154 sera from CVL dogs across endemic regions in Brazil yielded performance specifications comparable to those attained with DPP® CVL (sensitivity of 87% and specificity of 100% for rLci1A/rLci2B DPP versus 88% sensitivity and 97% specificity for DPP® CVL, respectively) [52]. Immunoproteomics identified LiHyD, a hypothetical protein, that yielded perfect (100%) sensitivity and specificity when tested by ELISA against sera from 44 CVL and 9 asymptomatic L. infantum-infected dogs [53]. In terms of detecting disease, novel protein-coding gene fragments that had not previously been studied were used to generate polyproteins that in serum ELISA demonstrated better performance to detect CVL than HVL: no effective detection of HVL (26–52% sensitivity) was demonstrated whereas 48–91% of CVL dogs were detected. These three antigens performed better than rK39 in this particular evaluation panel of 46 CVL sera [54].
In addition to novel targets, practical advancements are being sought. As mentioned, lateral flow-based tests can be used in a wide array of settings and prototype rLci1A/rLci2B DPP have been generated [52]. Removal of the laboratory requirements expands applicability and other examples fulfill this criterion. Covalent coupling of a k28-like protein onto polystyrene latex to provide a substrate for antibody binding yielded an agglutination test capable of producing visual results within 5 minutes, with sensitivity, specificity and diagnostic accuracy of 78%; 100% and over 80%, respectively [55]. Besides formatting, adjustments (or simply alternative use) to generate results from other body fluids are also being examined. Anti-rK28 antibodies are readily detectable in the urine of HVL patients, with performance only marginally lower than that obtained with serum [56]. It is likely that similar results would be achieved from infected canine urine. Saliva presents another easy way to collect alternative analyte, and a time-resolved immunofluorometric assay (TR-IFMA) quantifying anti-rK39 IgG2 antibodies indicated that greater discrimination between seropositive and seronegative dogs was achieved using saliva than serum [57].
Detecting Leishmania infection in surveillance and control programs
VL surveillance programs and epidemiological studies that aim to detect Leishmania infection pertinent to either HVL or CVL control require tests with high sensitivity. Under specific conditions, such as reservoir control programs that deploy a test-and-slaughter policy, low compliance by dog owners fearing the potential removal of their apparently healthy but seropositive dogs has a major impact on program success. Thus, high specificity to differentiate clinically suspect cases from other etiological agents is of paramount importance, as is the need for high sensitivity to detect asymptomatic infections. In the advent of treatment of CVL, prompt and specific differential diagnosis is required by veterinary clinics to indicate Leishmania chemotherapy. On the other hand, for prevention of canine infection, high test sensitivity and specificity to confirm lack of Leishmania infection are required prior to application of preventative topical or oral insecticides, or canine vaccination.
For both humans and dogs, tissue parasite numbers are generally low in asymptomatic L. donovani or L. infantum infections [58]. An Indian study recorded an average 500-fold lower blood parasite loads in asymptomatic L. donovani-infected individuals than in HVL patients [59], but indicated that asymptomatic individuals with higher parasite loads were at increased risk of developing VL [60]. The most sensitive technique for diagnosis of infection in dogs is qPCR, with qPCR results often, but not always, becoming positive in early infection before seroconversion occurs [34, 61]. Comparative evaluations indicate that seroprevalence may be below 30% in dog populations where prevalence of DNA detection by PCR can be as high as 80% [6, 62, 63]. Comparison between foci and tests is difficult but annual incidence estimates in Europe appear to vary greatly (between 40–92%) [61, 64, 65]. Test standardization for surveillance would help in data interpretation, but detection of blood or tissue parasites by PCR/qPCR is currently not operational within population-based surveillance programs for reasons discussed above.
Tests detecting immune responses appear more tenable for larger surveillance programs than many other current methods. Dogs resistant to developing CVL exhibit protective immunity mediated by CD4+ T helper 1 cell responses with prominent expression of IFN-y and IL-4 cytokines in association with Th2 regulatory responses [32]. Among immune-based assays for HVL, Leishmania-specific T cell recall tests have been develop that range from the leishmanin skin test, which induces an in vivo delayed type hypersensitivity (DTH) response [66, 67], to ex vivo assays measuring secretion of cytokines into stimulated blood [68, 69]. Compared to cell-based assays, serological antibody-detection tests, including indirect fluorescent antibody test (IFAT), direct agglutination test (DAT) and ELISA, are relatively cheap and, although generally more sensitive at detecting symptomatic than asymptomatic infections [70], their durability and practicality is considered an advantage particularly for low resource settings. Simple strategies can also render DPP CVL semi-quantitative, providing a more detailed diagnostic assessment and potentially a broader use in monitoring [71]. Truly asymptomatic infections usually revert to seronegative and/or low to moderate titers within months of infection and accurate quantification of responses is important in capturing this [72].
The Brazilian Ministry of Health changed the diagnostic protocol for CVL in December 2011, with the current protocol reducing false-positive results relative to the previous protocol. A key action within the program has traditionally included euthanasia of all Leishmania seropositive or confirmed infected dogs, and not only those that displayed clinical signs. This has, however, been legally challenged in several states with the courts generally preferring the option of treatment similar to the widely accepted practice in Europe. The current criteria of canine infection requires firstly a positive result in DPP CVL, followed by another positive result in a confirmatory ELISA using soluble antigens of L. infantum promastigotes. Recent studies have demonstrated that adjusting the sequence of testing, from that used in the current protocol (DDP followed by ELISA) to the reverse order (ELISA followed by DDP), did not cause a significant alteration in the final number of infected dogs detected [73, 74]. Sensitivity and specificity were, respectively, 82.3% and 92.8% for DDP, and 85% and 92.3% for ELISA [74]. However, conflicting test results lead to interpretation issues, which is perhaps inevitable as the tests target different antigens and have variable levels of sensitivity and specificity. Based on the surveillance protocol, of 1130 dogs first examined by DPP CVL under field conditions in Minais Gerais, then confirmed by lab-based ELISA evaluation, seroprevalence was assessed at 7.8% (8/101). This rate was slightly lower than the 8.9% (101/1130) that could be attributed by a positive result in either the DPP CVL test or ELISA (in addition to a lab-based DPP CVL) [75]. It was noteworthy, but perhaps not too surprising, that in animals initially positive by DDP CVL, seroconversion was more frequent in dogs that in follow-up testing were ELISA indeterminate, compared to those that were clearly ELISA negative. In another study surveying 975 dogs with DPP, ELISA and qPCR, almost 1 in 5 (174/887; 19.6%) of the dogs that were negative in DPP CVL tested positive by qPCR of either blood or lymph node aspirates [76]. Further evaluation in a second cohort of the DPP negative dogs revealed that almost half (36/79; 45.6%) were positively detected in qPCR of at least one of their blood, lymph node or conjunctival swab samples that were collected.
Identifying infectious individuals as a means toward controlling transmission
A major question for HVL control efforts on the Indian subcontinent is whether people with asymptomatic infection are sufficiently infectious to sand flies to represent a significant reservoir for L. donovani [77]. While it has been demonstrated that HVL and post kala-azar dermal leishmaniasis (PKDL) patients can be highly infectious [4, 78], the epidemiological significance of asymptomatic L. donovani infection in transmission remains unclear. Given that there appear to be between 4- to 17-fold more asymptomatic individuals than HVL patients, some believe that even if a subset of asymptomatic individuals are infectious at a very low level, the asymptomatic population can make a critical contribution to ongoing transmission [72, 79–83]. By cross-sectional study, parasites have been detected in human blood in up to 58% of asymptomatic infections with L. infantum [19, 58, 84] and L. donovani [59, 85]. Large variance also occurs in tissue parasite loads. Fourteen percent of 4695 asymptomatic Ethiopians were qPCR-positive in blood, of which 3.2% had high genome equivalent counts [85], suggesting that a small proportion of individuals may have a disproportionate role in onward transmission. In longitudinal studies, 83% (80/97) of asymptomatic L. infantum-infected individuals living in an active transmission region of Brazil tested PCR-positive during 6 years follow-up [86].
In the case of zoonotic transmission, whilst it is clear that both asymptomatic and symptomatic dogs can infect vectors such as Lu. longipalpis, their relative roles in maintaining endemic transmission is best explored by mathematical modelling [5, 87–91]. Meta-analysis of the relatively few canine xenodiagnosis studies [35] indicate that the proportion of symptomatic dogs that are infectious is generally high (80%), and that this proportion may be higher in Europe than in Latin America (86% versus 45%). The meta-analysis also indicated that 29% of dogs reported as asymptomatic were also infectious, although the definition of true asymptomatic infection requires careful clinical, biochemical and hematological classification during infection development. While some longitudinally followed dogs contribute little or nothing to transmission, there is usually a small fraction of infected dogs that contribute disproportionately: current studies indicate that 15% to 44% of dogs are responsible for more than 80% of all sand fly infections [5, 92, 93]. A high transmission potential corresponds to skin and bone marrow Leishmania parasite loads being higher relative to loads in less infectious and non-infectious dogs [94]. Such animals are considered “super-spreaders”, as noted similarly in other infection systems [95–97].
An alternative to blanket intervention programs against dogs may be to target those dogs that are most infectious to the sand fly vector, as opposed to targeting L. infantum infection or CVL per se. To date, attempts to use serological tests to specifically detect infectious individuals have been limited [92, 96], and as mentioned above, high test specificity is required to better inform dog owners and ensure compliance with control program recommendations. One longitudinal study showed high sensitivities for identifying highly infectious dogs with rK39 and CLA (79% and 97%, respectively), but low specificity for the detection of non-infectious dogs (50% and 13%, respectively). Alternative thresholds for defining positive results derived from receiver operating characteristic curve analysis improved the specificity to 68% with respect to non-infectious dogs, and provided an 82% sensitivity for predicting highly infectious dogs; differentiation of mildly infectious dogs from non-infectious dogs (both contributing relatively little to transmission in xenodiagnosis) was not achieved [92]. Similarly in another study, evaluating multiple current serological tests (ELISA, IFAT80, DPP CVL, rK39 tests, fast agglutination screening tests (FAST), and DAT) failed to discriminate those dogs that transmitted L. infantum from those that did not [98]. Further longitudinal evaluations of diagnostic tests to detect the epidemiologically important infectious portions of the infected CVL and HVL populations are urgently needed.
Concluding remarks
CVL is an important veterinary complication that not only deserves attention in its own right but could potentially be highly informative for HVL control programs (see Outstanding Questions). Control of infection in dogs in L. infantum-endemic regions would likely create the additional benefit of limiting a critical reservoir that maintains the threat of human infection and disease. As discussed, many tools currently used to diagnose CVL are now being evolved to allow identification and quantification of infection irrespective of symptoms, and both serological and molecular assays are being adapted to suit field conditions. Test attributes have to be tailored to be fit-for-purpose, conversely, test standardization is desirable to facilitate comparative interpretation and for meta-analyses. As alternative preventative measures are being evaluated to combat transmission, there are opportunities to develop immunological and molecular assays to identify infectious, in addition to infected and diseased individuals.
Highlights
Visceral leishmaniasis (VL) is an important disease of dogs and humans, with dogs being the primary domestic reservoir of L. infantum parasites.
Detection of diseased, infected and parasite-transmitting “super-spreader” dogs is likely critical for control
Dependent upon scale, direct and indirect detection methods can be used for surveillance
Novel molecular assays with point-of-care potential are emerging
New antigen targets are being characterized to enhance performance of serological assays
Adaptation to provide point-of-care serological tests to permit Leishmania detection and facilitate large-scale screening programs
Outstanding questions
Does control of the parasite reservoir in dogs impact on the incidence of human infection and HVL?
Are any tests capable of differentiating between diseased, infected and parasite-transmitting “super-spreader” dogs?
Can novel molecular assays be implemented in large-scale screening programs?
Does asymptomatic infection elicit sufficiently potent antibody responses to permit detection?
Can new antigens enhance the performance of serological assays?
Are saliva and urine capable of providing performance levels in antibody-detection tests similar to those achieved with serum?
Can tests developed for canine VL be informative for human VL?
How are canine test-and-control options best deployed to provide a protective community effect?
Acknowledgements
Diagnostic work at IDRI has been funded by National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI025038. Additional program support came from the Bill and Melinda Gates Foundation under grants #631 and #39129). Aurore Lison is funded by the European Union’s horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 642609. Orin Courtenay acknowledges the continued support of the Wellcome Trust, UK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Glossary:
- visceral leishmaniasis
the vector–borne disease caused by infection with either Leishmania donovani or L. infantum
- asymptomatic
infection in the absence of clinical signs or symptoms
- super-spreader
infected dogs, or humans, that make a disproportionally high contribution to ongoing parasite transmission to the intermediate sand fly host
- rK39
the gold standard recombinant, chimeric polyprotein rK39 used to confirm VL
- DPP CVL
Dual-Path Platform technology (DPP®CVL rapid test) for the serodiagnosis of canine visceral. A lateral flow-based test that detects canine IgG antibodies against the rK28 antigen
Footnotes
Conflict of Interest Statement
Malcolm Duthie is a co-inventor on a patent for leishmaniasis vaccine development and IDRI supplies antigens to commercial partners for manufacture of diagnostic tests for VL. Orin Courtenay and Aurore Lison have stated no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO: Kala-azar elimination programme: Report of a WHO consultation of partners. In. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Global leishmaniasis update, 2006–2015: a turning point in leishmaniasis surveillance. Wkly Epidemiol Rec 2017, 92(38):557–565. [PubMed] [Google Scholar]
- 3.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Team WHOLC: Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012, 7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinnell RJ, Courtenay O: Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136(14):1915–1934. [DOI] [PubMed] [Google Scholar]
- 5.Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C: Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis 2002, 186(9):1314–1320. [DOI] [PubMed] [Google Scholar]
- 6.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L: Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends in Parasitology 2008, 24(7):324–330. [DOI] [PubMed] [Google Scholar]
- 7.Singh OP, Epco Hasker E, Boelaert M, Sundar S: Elimination of visceral leishmaniasis on the Indian subcontinent. The Lancet Infectious Diseases 2016, 16(12):e304–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palatnik-de-Sousa CB, Silva-Antunes I, Morgado Ade A, Menz I, Palatnik M, Lavor C: Decrease of the incidence of human and canine visceral leishmaniasis after dog vaccination with Leishmune in Brazilian endemic areas. Vaccine 2009, 27(27):3505–3512. [DOI] [PubMed] [Google Scholar]
- 9.Seva AP, Ovallos FG, Amaku M, Carrillo E, Moreno J, Galati EA, Lopes EG, Soares RM, Ferreira F: Canine-Based Strategies for Prevention and Control of Visceral Leishmaniasis in Brazil. PLoS One 2016, 11(7):e0160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brazil MoH: Manual of monitoring and control of visceral leishmaniasis. . In. Edited by Surveillance DoH, Surveillance DoE, vol. 1; 2014: 1–120. [Google Scholar]
- 11.Romero GAS, Boelaert M: Control of Visceral Leishmaniasis in Latin America-A Systematic Review. Plos Neglected Tropical Diseases 2010, 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werneck GL: The control of visceral leishmaniasis in Brazil: end of a cycle? Cadernos De Saude Publica 2016, 32(6). [DOI] [PubMed] [Google Scholar]
- 13.Bray DP, Bandi KK, Brazil RP, Oliveira AG, Hamilton JGC: Synthetic Sex Pheromone Attracts the Leishmaniasis Vector Lutzomyia longipalpis (Diptera: Psychodidae) to Traps in the Field. Journal of Medical Entomology 2009, 46(3):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.e Silva RA, de Andrade AJ, Quint BB, Raffoul GES, Werneck GL, Rangel EF, Romero GAS: Effectiveness of dog collars impregnated with 4% deltamethrin in controlling visceral leishmaniasis in Lutzomyia longipalpis (Diptera: Psychodidade: Phlebotominae) populations. Memorias Do Instituto Oswaldo Cruz 2018, 113(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miro G, Petersen C, Cardoso L, Bourdeau P, Baneth G, Solano-Gallego L, Pennisi MG, Ferrer L, Oliva G: Novel Areas for Prevention and Control of Canine Leishmaniosis. Trends in Parasitology 2017, 33(9):718–730. [DOI] [PubMed] [Google Scholar]
- 16.Solano-Gallego L, Koutinas A, Miro G, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G: Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Veterinary Parasitology 2009, 165(1–2):1–18. [DOI] [PubMed] [Google Scholar]
- 17.Gavgani AS, Hodjati MH, Mohite H, Davies CR: Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet 2002, 360(9330):374–379. [DOI] [PubMed] [Google Scholar]
- 18.Kip AE, Balasegaram M, Beijnen JH, Schellens JH, de Vries PJ, Dorlo TP: Systematic review of biomarkers to monitor therapeutic response in leishmaniasis. Antimicrob Agents Chemother 2015, 59(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourabbas B, Moghadam AG, Pouladfar G, Rezaee Z, Alborzi A: Quantification of Leishmania infantum Kinetoplast DNA for Monitoring the Response to Meglumine Antimoniate Therapy in Visceral Leishmaniasis. American Journal of Tropical Medicine and Hygiene 2013, 88(5): 868–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudarshan M, Weirather JL, Wilson ME, Sundar S: Study of parasite kinetics with antileishmanial drugs using real-time quantitative PCR in Indian visceral leishmaniasis. Journal of Antimicrobial Chemotherapy 2011, 66(8):1751–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma S, Kumar R, Katara GK, Singh LC, Negi NS, Ramesh V, Salotra P: Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One 2010, 5(4):e10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travi BL, Cordeiro-da-Silva A, Dantas-Torres F, Miro G: Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis 2018, 12(1):e0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agallou M, Margaroni M, Athanasiou E, Toubanaki DK, Kontonikola K, Karidi K, Kammona O, Kiparissides C, Karagouni E: Identification of BALB/c Immune Markers Correlated with a Partial Protection to Leishmania infantum after Vaccination with a Rationally Designed Multi-epitope Cysteine Protease A Peptide-Based Nanovaccine. PLoS Negl Trop Dis 2017, 11(1):e0005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriworarat C, Phumee A, Mungthin M, Leelayoova S, Siriyasatien P: Development of loopmediated isothermal amplification (LAMP) for simple detection of Leishmania infection. Parasit Vectors 2015, 8:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao CH, Ding D, Wang JY, Steverding D, Wang X, Yang YT, Shi F: Development of a LAMP assay for detection of Leishmania infantum infection in dogs using conjunctival swab samples. Parasit Vectors 2015, 8:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaouch M, Mhadhbi M, Adams ER, Schoone GJ, Limam S, Gharbi Z, Darghouth MA, Guizani I, BenAbderrazak S: Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of Leishmania infantum in canine leishmaniasis based on cysteine protease B genes. Vet Parasitol 2013, 198(1–2):78–84. [DOI] [PubMed] [Google Scholar]
- 27.Adams ER, Schoone GJ, Ageed AF, Safi SE, Schallig HD: Development of a reverse transcriptase loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Leishmania parasites in clinical samples. Am J Trop Med Hyg 2010, 82(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toubanaki DK, Athanasiou E, Karagouni E: Gold nanoparticle-based lateral flow biosensor for rapid visual detection of Leishmania-specific DNA amplification products. J Microbiol Methods 2016, 127:51–58. [DOI] [PubMed] [Google Scholar]
- 29.Carson C, Quinnell RJ, Holden J, Garcez LM, Deborggraeve S, Courtenay O: Comparison of Leishmania OligoC-TesT PCR with conventional and real-time PCR for Diagnosis of canine Leishmania infection. J Clin Microbiol 2010, 48(9):3325–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rampazzo RCP, Solca MDS, Santos LCS, Pereira LN, Guedes JCO, Jr., Veras PST, Fraga DBM, Krieger MA, Costa ADT: A ready-to-use duplex qPCR to detect Leishmania infantum DNA in naturally infected dogs. Vet Parasitol 2017, 246:100–107. [DOI] [PubMed] [Google Scholar]
- 31.Selder R, Weber K, Bergmann M, Geisweid K, Hartmann K: Sensitivity and specificity of an inclinic point-of-care PCR test for the diagnosis of canine leishmaniasis. Vet J 2018, 232:46–51. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri CL: Immunology of canine leishmaniasis. Parasite Immunology 2006, 28(7):329–337. [DOI] [PubMed] [Google Scholar]
- 33.Reis LES, Coura-Vital W, Roatt BM, Bouillet LEM, Ker HG, de Brito RCF, Resende DD, Carneiro M, Giunchetti RC, Marques MJ et al. : Molecular diagnosis of canine visceral leishmaniasis: A.comparative study of three methods using skin and spleen from dogs with natural Leishmania infantum infection. Veterinary Parasitology 2013, 197(3–4):498–503. [DOI] [PubMed] [Google Scholar]
- 34.Quinnell RJ, Courtenay O, Davidson S, Garcez L, Lambson B, Ramos P, Shaw JJ, Shaw MA, Dye C: Detection of Leishmania infantum by PCR, serology and cellular immune response in a cohort study of Brazilian dogs. Parasitology 2001, 122(Pt 3):253–261. [DOI] [PubMed] [Google Scholar]
- 35.Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O: Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis 2013, 7(1):e1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinnell RJ, Courtenay O, Shaw MA, Day MJ, Garcez LM, Dye C, Kaye PM: Tissue cytokine responses in canine visceral leishmaniasis. Journal of Infectious Diseases 2001, 183(9):1421–1424. [DOI] [PubMed] [Google Scholar]
- 37.Hasker E, Chourasia A, Malviya P, Gidwani K, Picado A, Ostyn B, Kansal S, Singh RP, Singh OP, Singh AK et al. : Strong association between serological status and probability of progression to clinical visceral leishmaniasis in prospective cohort studies in India and Nepal. International Journal of Infectious Diseases 2014, 21:250–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman LAC, Dyson L, Courtenay O, Chowdhury R, Bern C, Medley GF, Hollingsworth TD:Quantification of the natural history of visceral leishmaniasis and consequences for control. Parasites & Vectors 2015, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns JM Jr., Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG: Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci U S A 1993, 90(2):775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL et al. : rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis 1996, 173(3):758–761. [DOI] [PubMed] [Google Scholar]
- 41.Boarino A, Scalone A, Gradoni L, Ferroglio E, Vitale F, Zanatta R, Giuffrida MG, Rosati S: Development of recombinant chimeric antigen expressing immunodominant B epitopes of Leishmania infantum for serodiagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 2005, 12(5):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch RJ, Anderson BL, Litwin CM: Rapid immunochromatographic strip test for detection of anti-K39 immunoglobulin G antibodies for diagnosis of visceral leishmaniasis. Clin Vaccine Immunol 2008, 15(9):1483–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalho SF, Lemos EM, Corey R, Dietze R: Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. Am J Trop Med Hyg 2003, 68(3):321–324. [PubMed] [Google Scholar]
- 44.Pattabhi S, Whittle J, Mohamath R, El-Safi S, Moulton GG, Guderian JA, Colombara D, Abdoon AO, Mukhtar MM, Mondal D et al. : Design, development and evaluation of rK28-based point-ofcare tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis 2010, 4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bezuneh A, Mukhtar M, Abdoun A, Teferi T, Takele Y, Diro E, Jemaneh A, Shiferaw W, Wondimu H, Bhatia A et al. : Comparison of point-of-care tests for the rapid diagnosis of visceral leishmaniasis in East. Am J Trop Med Hyg 2014:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhtar M, Abdoun A, Ahmed AE, Ghalib H, Reed SG, Boelaert M, Menten J, Khair MM, Howard RF: Diagnostic accuracy of rK28-based immunochromatographic rapid diagnostic tests for visceral leishmaniasis: a prospective clinical cohort study in Sudan. Trans R Soc Trop Med Hyg 2015, 109(9):594–600. [DOI] [PubMed] [Google Scholar]
- 47.Grimaldi G Jr., Teva A, Ferreira AL, dos Santos CB, Pinto I, de-Azevedo CT, Falqueto A: Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP(R) CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg 2012, 106(1):54–59. [DOI] [PubMed] [Google Scholar]
- 48.Laurenti MD Jr., de Santana Leandro MV, Tomokane TY, De Lucca HR, Aschar M, Souza CS, Silva RM, Marcondes M, da Matta VL: Comparative evaluation of the DPP((R)) CVL rapid test for canine serodiagnosis in area of visceral leishmaniasis. Vet Parasitol 2014, 205(3–4):444–450. [DOI] [PubMed] [Google Scholar]
- 49.Fraga DB, Pacheco LV, Borja LS, Tuy PG, Bastos LA, Solca Mda S, Amorim LD, Veras PS: The Rapid Test Based on Leishmania infantum Chimeric rK28 Protein Improves the Diagnosis of Canine Visceral Leishmaniasis by Reducing the Detection of False-Positive Dogs. PLoS Negl Trop Dis 2016, 10(1):e0004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogueira CT, Cistia MLD, Urbaczek AC, Jusi MM, Velasquez AMA, Machado RZ, Ferreira H, Henrique-Silva F, Langoni H, Costa PID et al. : Potential application of rLc36 protein for diagnosis of canine visceral leishmaniasis. Mem Inst Oswaldo Cruz 2018, 113(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez Abad LP, Almeida CS, Mattos AMM, Mendonca ACP, Alves MJM, Pinheiro AC, Porrozzi R, Abass E, Steinhoff U, Teixeira HC: Diagnostic accuracy of rKLO8 versus rK26 ELISAs for screening of canine visceral leishmaniasis. Acta Trop 2017, 166:133–138. [DOI] [PubMed] [Google Scholar]
- 52.Fraga DB, da Silva ED, Pacheco LV, Borja LS, de Oliveira IQ, Coura-Vital W, Monteiro GR, Oliveira GG, Jeronimo SM, Reis AB et al. : A multicentric evaluation of the recombinant Leishmania infantum antigen-based immunochromatographic assay for the serodiagnosis of canine visceral leishmaniasis. Parasit Vectors 2014, 7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lage DP, Martins VT, Duarte MC, Costa LE, Garde E, Dimer LM, Kursancew AC, Chavez-Fumagalli MA, de Magalhaes-Soares DF, Menezes-Souza D et al. : A new Leishmania-specific hypothetical protein and its non-described specific B cell conformational epitope applied in the serodiagnosis of canine visceral leishmaniasis. Parasitol Res 2016, 115(4):1649–1658. [DOI] [PubMed] [Google Scholar]
- 54.Magalhaes FB, Castro Neto AL, Nascimento MB, Santos WJT, Medeiros ZM, Lima Neto AS, Costa DL, Costa CHN, Dos Santos WLC, Pontes de Carvalho LC et al. : Evaluation of a new set of recombinant antigens for the serological diagnosis of human and canine visceral leishmaniasis. PLoS One 2017, 12(9):e0184867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia VS, Gonzalez VDG, Gugliotta L, Burna A, Demonte A, Arias DG, Cabeza MS, Guerrero SA: Development of a simple and economical diagnostic test for canine leishmaniasis. Exp Parasitol 2017, 182:9–15. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh P, Bhaskar KR, Hossain F, Khan MA, Vallur AC, Duthie MS, Hamano S, Salam MA, Huda MM, Khan MG et al. : Evaluation of diagnostic performance of rK28 ELISA using urine for diagnosis of visceral leishmaniasis. Parasit Vectors 2016, 9(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cantos-Barreda A, Escribano D, Bernal LJ, Ceron JJ, Martinez-Subiela S: Quantification of antiLeishmania antibodies in saliva of dogs. Vet Parasitol 2017, 242:54–58. [DOI] [PubMed] [Google Scholar]
- 58.Michel G, Pomares C, Ferrua B, Marty P: Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Tropica 2011, 119(2–3):69–75. [DOI] [PubMed] [Google Scholar]
- 59.Sudarshan M, Sundar S: Parasite load estimation by qPCR differentiates between asymptomatic and symptomatic infection in Indian visceral leishmaniasis. Diagn Microbiol Infect Dis 2014, 80(1):40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudarshan M, Singh T, Singh AK, Chourasia A, Singh B, Wilson ME, Chakravarty J, Sundar S: Quantitative PCR in epidemiology for early detection of visceral leishmaniasis cases in India. PLoS Negl Trop Dis 2014, 8(12):e3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliva G, Scalone A, Manzillo VF, Gramiccia M, Pagano A, Di Muccio T, Gradoni L: Incidence and time course of Leishmania infantum infections examined by parasitological, serologic, and nested-PCR techniques in a cohort of naive dogs exposed to three consecutive transmission seasons. Journal of Clinical Microbiology 2006, 44(4):1318–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L: Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. Journal of Clinical Microbiology 2001, 39(2):560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lachaud L, Chabbert E, Dubessay P, Dereure J, Lamothe J, Dedet JP, Bastien P: Value of two PCR methods for the diagnosis of canine visceral leishmaniasis and the detection of asymptomatic carriers. Parasitology 2002, 125:197–207. [DOI] [PubMed] [Google Scholar]
- 64.Dye C, Vidor E, Dereure J: SEROLOGICAL DIAGNOSIS OF LEISHMANIASIS - ON DETECTING INFECTION AS WELL AS DISEASE. Epidemiology and Infection 1993, 110(3):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antoniou M, Messaritakis I, Christodoulou V, Ascoksilaki I, Kanavakis N, Sutton AJ, Carson C, Courtenay O: Increasing Incidence of Zoonotic Visceral Leishmaniasis on Crete, Greece. Emerging Infectious Diseases 2009, 15(6):932–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cardoso L, Neto F, Sousa JC, Rodrigues M, Cabral M: Use of a leishmanin skin test in the detection of canine Leishmania-specific cellular immunity. Vet Parasitol 1998, 79(3):213–220. [DOI] [PubMed] [Google Scholar]
- 67.Cardoso L, Schallig HD, Cordeiro-da-Silva A, Cabral M, Alunda JM, Rodrigues M: Anti-Leishmania humoral and cellular immune responses in naturally infected symptomatic and asymptomatic dogs. Vet Immunol Immunopathol 2007, 117(1–2):35–41. [DOI] [PubMed] [Google Scholar]
- 68.Carrillo E, Moreno J: Cytokine profiles in canine visceral leishmaniasis. Vet Immunol Immunopathol 2009, 128(1–3):67–70. [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Orellana P, Mari-Martorell D, Montserrat-Sangra S, Ordeix L, Baneth G, Solano-Gallego L: Leishmania infantum-specific IFN-gamma production in stimulated blood from dogs with clinical leishmaniosis at diagnosis and during treatment. Vet Parasitol 2017, 248:39–47. [DOI] [PubMed] [Google Scholar]
- 70.Miro G, Cardoso L, Pennisi MG, Oliva G, Baneth G: Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part two. Trends in Parasitology 2008, 24(8):371–377. [DOI] [PubMed] [Google Scholar]
- 71.Larson M, Toepp A, Scott B, Epid, Kurtz M, Fowler H, Esfandiari J, Howard RF, Vallur AC, Duthie MS et al. : Semi-quantitative measurement of asymptomatic L. infantum infection and symptomatic visceral leishmaniasis in dogs using Dual-Path Platform(R) CVL. Appl Microbiol Biotechnol 2017, 101(1):381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bern C, Haque R, Chowdhury R, Ali M, Kurkjian KM, Vaz L, Amann J, Wahed MA, Wagatsuma Y, Breiman RF et al. : The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am J Trop Med Hyg 2007, 76(5):909–914. [PubMed] [Google Scholar]
- 73.Coura-Vital W, Ker HG, Roatt BM, Aguiar-Soares RD, Leal GG, Moreira N, Oliveira LA, de Menezes Machado EM, Morais MH, Correa-Oliveira R et al. : Evaluation of change in canine diagnosis protocol adopted by the visceral leishmaniasis control program in Brazil and a new proposal for diagnosis. PLoS One 2014, 9(3):e91009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almeida SS, Gomes CL, Silva ES, Brandao STR, Aviz WP, Pinheiro L, Paciello MO, Sander A, Cangussu R, de Souza Aguiar RW et al. : Dual-Path Platform (DPP) and Enzyme-Linked Immunosorbent Assay (ELISA): Change the Sequence of the Tests Does Not Change the Number of Positive Dogs for Canine Visceral Leishmaniasis. African Journal of Microbiology Research 2017, 11(3):106–109. [Google Scholar]
- 75.Belo VS, Gregorio EA, Teixeira-Neto RG, da Rocha Lima A, Pereira AAS, Marcelino AP, Paz GF, da Silva ES: Reliability of techniques used in the diagnosis of canine visceral leishmaniasis by the national control program in Brazil: A survey in an area of recent transmission. Prev Vet Med 2017, 146:10–15. [DOI] [PubMed] [Google Scholar]
- 76.Lopes EG, Seva AP, Ferreira F, Nunes CM, Keid LB, Hiramoto RM, Ferreira HL, Oliveira T, Bigotto MFD, Galvis-Ovallos F et al. : Serological and molecular diagnostic tests for canine visceral leishmaniasis in Brazilian endemic area: one out of five seronegative dogs are infected. Epidemiol Infect 2017, 145(12):2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stauch A, Sarkar RR, Picado A, Ostyn B, Sundar S, Rijal S, Boelaert M, Dujardin JC, Duerr HP: Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis 2011, 5(11):e1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molina R, Ghosh D, Carrillo E, Monnerat S, Bern C, Mondal D, Alvar J: Infectivity of Post-Kalaazar Dermal Leishmaniasis Patients to Sand Flies: Revisiting a Proof of Concept in the Context of the Kala-azar Elimination Program in the Indian Subcontinent. Clin Infect Dis 2017, 65(1):150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Das S, Matlashewski G, Bhunia GS, Kesari S, Das P: Asymptomatic Leishmania infections in northern India: a threat for the elimination programme? Transactions of the Royal Society of Tropical Medicine and Hygiene 2014, 108(11):679–684. [DOI] [PubMed] [Google Scholar]
- 80.Das VNR, Siddiqui NA, Verma RB, Topno RK, Singh D, Das S, Ranjan A, Pandey K, Kumar N, Das P:Asymptomatic infection of visceral leishmaniasis in hyperendemic areas of Vaishali district, Bihar, India: a challenge to kala-azar elimination programmes. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011, 105(11):661–666. [DOI] [PubMed] [Google Scholar]
- 81.Hirve S, Boelaert M, Matlashewski G, Mondal D, Arana B, Kroeger A, Olliaro P: Transmission Dynamics of Visceral Leishmaniasis in the Indian Subcontinent - A Systematic Literature Review. PLoS Negl Trop Dis 2016, 10(8):e0004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zijlstra EE, el-Hassan AM, Ismael A, Ghalib HW: Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg 1994, 51(6):826–836. [DOI] [PubMed] [Google Scholar]
- 83.Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S: Asymptomatic Leishmania infection: a new challenge for Leishmania control. Clin Infect Dis 2014, 58(10):1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mary C, Faraut F, Drogoul MP, Xeridat B, Schleinitz N, Cuisenier B, Dumon H: Reference values for Leishmania infantum parasitemia in different clinical presentations: Quantitative polymerase chain reaction for therapeutic monitoring and patient follow-up. American Journal of Tropical Medicine and Hygiene 2006, 75(5):858–863. [PubMed] [Google Scholar]
- 85.Miller E, Warburg A, Novikov I, Hailu A, Volf P, Seblova V, Huppert A: Quantifying the contribution of hosts with different parasite concentrations to the transmission of visceral leishmaniasis in Ethiopia. PLoS Negl Trop Dis 2014, 8(10):e3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carneiro M, Moreno EC, Gonçalves AV, Lambertucci JR, Antunes CMF: Visceral Leishmaniasis: Challenges in identifying subclinical Leishmania infection. Drug Development Research 2011, 72(6):442–450. [Google Scholar]
- 87.Rock KS, Quinnell RJ, Medley GF, Courtenay O: Progress in the Mathematical Modelling of Visceral Leishmaniasis. Adv Parasitol 2016, 94:49–131. [DOI] [PubMed] [Google Scholar]
- 88.Laurenti MD, Rossi CN, da Matta VL, Tomokane TY, Corbett CE, Secundino NF, Pimenta PF, Marcondes M: Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet Parasitol 2013, 196(3–4):296–300. [DOI] [PubMed] [Google Scholar]
- 89.Travi BL, Ferro C, Cadena H, Montoya-Lerma J, Adler GH: Canine visceral leishmaniasis: dog infectivity to sand flies from non-endemic areas. Res Vet Sci 2002, 72(1):83–86. [DOI] [PubMed] [Google Scholar]
- 90.Michalsky EM, Rocha MF, da Rocha Lima AC, Franca-Silva JC, Pires MQ, Oliveira FS, Pacheco RS, dos Santos SL, Barata RA, Romanha AJ et al. : Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet Parasitol 2007, 147(1–2):67–76. [DOI] [PubMed] [Google Scholar]
- 91.Vercosa BL, Lemos CM, Mendonca IL, Silva SM, de Carvalho SM, Goto H, Costa FA: Transmission potential, skin inflammatory response, and parasitism of symptomatic and asymptomatic dogs with visceral leishmaniasis. BMC Vet Res 2008, 4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Courtenay O, Carson C, Calvo-Bado L, Garcez LM, Quinnell RJ: Heterogeneities in Leishmania infantum infection: using skin parasite burdens to identify highly infectious dogs. PLoS Negl Trop Dis 2014, 8(1):e2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molina R, Amela C, Nieto J, Sanandres M, Gonzalez F, Castillo JA, Lucientes J, Alvar J:INFECTIVITY OF DOGS NATURALLY INFECTED WITH LEISHMANIA-INFANTUM TO COLONIZED PHLEBOTOMUS-PERNICIOSUS. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994, 88(4):491–493. [DOI] [PubMed] [Google Scholar]
- 94.Courtenay O, Peters NC, Rogers ME, Bern C: Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform leishmaniasis transmission dynamics and control. PLoS pathogens 2017, 13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Enriquez GF, Bua J, Orozco MM, Wirth S, Schijman AG, Gurtler RE, Cardinal MV: High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites for domestic transmission. Infection Genetics and Evolution 2014, 25:36–43. [DOI] [PubMed] [Google Scholar]
- 96.Chase-Topping M, Gally D, Low C, Matthews L, Woolhouse M: Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat Rev Microbiol 2008, 6(12):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM: Superspreading and the effect of individual variation on disease emergence. Nature 2005, 438(7066):355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mendonca IL, Batista JF, Werneck GL, Soares MRA, Costa DL, Costa CHN: Serological tests fail to discriminate dogs with visceral leishmaniasis that transmit Leishmania infantum to the vector Lutzomyia longipalpis. Rev Soc Bras Med Trop 2017, 50(4):483–488. [DOI] [PubMed] [Google Scholar]