Abstract
The brain functions through chemical interactions between many different cell types, including neurons and glia. Acquiring comprehensive information on complex, heterogeneous systems requires multiple analytical tools, each of which have unique chemical specificity and spatial resolution. Multimodal imaging generates complementary chemical information via spatially localized molecular maps, ideally from the same sample, but requires method enhancements that span from data acquisition to interpretation. We devised a protocol for performing matrix-assisted laser desorption/ionization (MALDI)-Fourier transform ion cyclotron resonance-mass spectrometry imaging (MSI), followed by infrared (IR) spectroscopic imaging on the same specimen. Multimodal measurements from the same tissue provide precise spatial alignment between modalities, enabling more advanced image processing such as image fusion and sharpening. Performing MSI first produces higher quality data from each technique compared to performing IR imaging before MSI. The difference is likely due to fixing the tissue section during MALDI matrix removal, thereby preventing analyte degradation occurring during IR imaging from unfixed specimen. Leveraging the unique capabilities of each modality, we utilized pan sharpening of MS (mass spectrometry) ion images with selected bands from IR spectroscopy and midlevel data fusion. In comparison to sharpening with histological images, pan sharpening can employ a plethora of IR bands, producing sharpened MS images while retaining the fidelity of the initial ion images. Using Laplacian pyramid sharpening, we determine the localization of several lipids present within the hippocampus with high mass accuracy at 5 μm pixel widths. Further, through midlevel data fusion of the imaging datasets combined with k-means clustering, the combined dataset discriminates between additional anatomical structures unrecognized by the individual imaging approaches. Significant differences between molecular ion abundances are detected between relevant structures within the hippocampus, such as the CA1 and CA3 regions. Our methodology provides high quality multiplex and multimodal chemical imaging of the same tissue sample, enabling more advanced data processing and analysis routines.
Keywords: Mass Spectrometry Imaging, Quantum Cascade Laser, Infrared Spectroscopic Imaging, Pan Sharpening, Multimodal Imaging
For TOC only
INTRODUCTION
The brain is a complex organ that is incompletely understood, partly due to its vast chemical and cellular heterogeneity. Communication between different cell types via the exchange of chemical signals contributes to emergent functions, including episodic and spatial memory, in which the hippocampus plays a critical role.1, 2 While many morphological regions of the hippocampus are involved in the formation and maintenance of different memory types, the dentate gyrus (DG) is implicated in the formation of spatial and long-term memory.3, 4 A wide range of distinct cell types are present in the DG, including granule cells, astrocytes, ependyma, radial glia, neuroblasts, and inhibitory neurons.5 Several analytical approaches have effectively been used to advance our understanding of brain function, e.g., electrophysiology,6 immunohistochemistry,7 behavioral analysis,8 magnetic resonance imaging,9 among others.10–13 Despite this progress, there remains a need for techniques capable of untargeted, multiplex chemical analysis at spatial resolutions relevant to cellular length scales.
Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging (MSI) is capable of multiplex detection and structural characterization of hundreds to thousands of analytes within a sample at femtomole detection limits.14 However, MALDI MSI typically obtains spatial resolutions greater than 20 μm,15 precluding subcellular imaging of tissue sections using most commercial instruments. Several labs have built prototype MALDI mass spectrometers capable of subcellular resolution,16, 17 or used oversampling techniques on commercial instruments18 to achieve smaller pixel sizes. However, these approaches can number of analytes present in a single pixel of the chemical image.
Although instrument modifications are one approach to achieving higher spatial resolutions, an alternative is to enhance the resolution of mass spectrometry (MS) ion images through sharpening using another imaging modality19–21 When an ion distribution image is correlated to an underlying, high spatial resolution image, the results of sharpening are encouraging. However, generally only a single, high spatial resolution image is available for sharpening. Pan sharpening ion images with contrasting high spatial resolution images can induce artifacts or degrade the quality of the original MS image. Therefore, a singular, high spatial resolution image limits the number and diversity of ion images suitable for sharpening, possibly excluding important analytes. Here, we overcome this limitation by sharpening MS images with those obtained using another chemical imaging approach.
Infrared (IR) spectroscopic imaging is a non-destructive, optical imaging method that provides rich spectral information at micron spatial resolution.22, 23 Numerical algorithms can relate the data to histologic identity or transformation of constituent cells,24–28 and provide images that mimic those obtained from conventional staining protocols.29, 30 The collected data can be used to identify classes of chemicals in tissue31 but not most individual metabolites in complex biological samples. Thus, coupling IR and MSI presents a unique opportunity to integrate complementary spectral data and spatial resolutions. We applied this combined approach to measure chemical distributions present within the hippocampus, including the DG.
Because of its promise, the integration of MSI and vibrational spectroscopy has been a long-term goal.32–34 Practitioners have recorded IR imaging data prior to MSI by combining synchrotron Fourier transform (FT)-IR microspectroscopy with time-of-flight (TOF)-secondary ion mass spectrometry to study histopathological changes in hepatic steatosis.35, 36 Recently, hyperspectral imaging was performed with FT-IR spectroscopy, confocal Raman spectroscopy, and MALDI MSI on hamster brains.37 In that work, the FT-IR and Raman spectroscopic analyses were conducted on the same tissue section, and MALDI MSI was used to study an adjacent section.
Our goal was to demonstrate enhanced registration and image correlations using both modalities to interrogate the same sample. The choice of recording IR data first seems intuitive and straightforward. However, as IR spatial resolution increases, the image acquisition time also rises to at least several hours, over which analytes in the fresh tissue (as required for MSI) may degrade. While MALDI MS is generally considered destructive, prior reports have shown that only a fraction of the sample is consumed during the measurement process.38 After single cell MALDI MS, sufficient material remains for follow-up analyses.39, 40 In imaging applications, tissue sections are frequently stained following MSI.41–45 The MALDI matrix 2,5-dihydroxybenzoic acid (DHB) is utilized industrially as an antioxidant46, 47 and the vacuum created inside the ion source reduces oxidation during analysis.48 This may be one of the first explorations of using these two imaging modalities, at their native optimal configurations, in which MS is performed first. Given the persistence of diagnostic information in IR imaging, despite the influence of various preparation conditions,25, 49–51 it is likely that IR chemical signatures arise from robust structural features, and different regions of the brain are likely conserved post-MALDI analysis as well.31
We developed a combined chemical imaging approach that allows broadened spatio-chemical characterization of a variety of biological samples via sequential analysis of specimens using MSI followed by IR imaging. While counterintuitive in terms of analysis order, the methodology preserves the information acquired by both analytical modalities. The resulting multimodal imaging dataset can be easily spatially registered through affine transformations and is suitable for data fusion, including pan sharpening. Compared to prior approaches for sharpening MS ion images utilizing optical monochrome morphological information, we used “chemical pan sharpening” as a means to leverage both the higher spatial resolution IR image and spectral content to optimally fit the ion distribution images. Finally, we determined that data fusion of the image sets enables discrimination between anatomically relevant brain regions.
EXPERIMENTAL
Chemicals.
All chemicals were purchased from Millipore Sigma (St. Louis, MO) and used without further purification unless otherwise specified.
Animals.
Ten- to twelve-week old LE/BluGill rats (University of Illinois at Urbana-Champaign) were used for all studies. Use of this inbred strain greatly reduces inter-experimental variation common to outbred animals and allows achievement of high statistical significance with small sample sizes. A dense genome scan was performed at a 10 cM interval between markers on LE/BluGill progenitors. The results of this scan, performed by the Medical College of Wisconsin Human and Molecular Genetics Center as part of the National Heart, Lung, and Blood Institute (NHLBI) Programs for Genomic Applications (PGA) U01 HL66579, demonstrated that the colony is inbred, yielding one allele at each locus tested (http://pga.mcw.edu/pga-bin/straindesc.cgi).
All animal-related experimental procedures were conducted at the University of Illinois at Urbana-Champaign under protocols approved by the Institutional Animal Care and Use Committee under Animal Welfare Assurance number A3118–01. All animal care and experiments were conducted in full compliance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue Sectioning.
Coronal brain sections containing the hippocampus were prepared from adult LE/BluGill rats. Rats were sacrificed by decapitation 2 h after lights were turned on in the donor colony Brains were quickly removed and placed on dry ice crushed to a fine powder. Coronal brains were sectioned (20 μm) by cryostat at −17°C (Microm HM550, Thermo Fisher Scientific, Waltham, MA). Sections containing a similar hippocampal rostralcaudal plane from three separate animals were placed on the same low emission (low-E) glass slide (Kevley Technologies, Chesterland, OH) and stored with Drierite desiccant at −80°C until further analysis. Placing the three biological replicates on a single slide reduces batch variations for IR analysis and correlated MS analysis.
Sample Preparation and MS Analysis.
Tissue sections for MS analysis were warmed to room temperature in a dry nitrogen box for an hour before MALDI matrix application. DHB was sublimed onto tissue sections, forming a fine crystal layer with a thickness of ~0.3 mg/cm2 enabling MS-based lipid analysis52, 53 using a custom sublimation chamber described previously.54 Following sublimation, slides were returned to 22°C within a dry nitrogen box for at least 15 min. Prior to quantum cascade laser (QCL)-IR analysis (either before, after, or without MALDI MS analysis), sections were placed in a vacuum desiccator for 2 h.
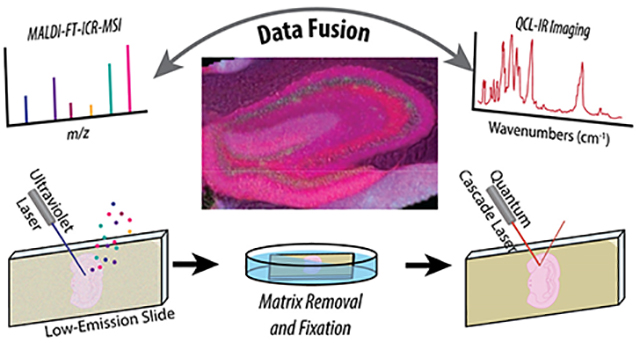
Coated with DHB, hippocampal tissue sections were imaged at 1200 dpi with a flatbed scanner (Canon U.S.A. Inc., Melville, NY) to guide MSI. Optical images were loaded into flexImaging ver. 4.1 (Bruker Corp., Billerica, MA) and registered with a solariX XR 7T FT-ion cyclotron resonance (ICR) mass spectrometer (Bruker Corp.). Figure 1 displays the optimized protocol for correlated MALDI MSI and QCL-IR spectroscopy. Spectra were acquired in positive mode covering a mass range of m/z 150 to 3000, requiring a 0.7340 s transient. All MS data were acquired using the “minimum” laser probe setting (~25 μm diameter). The tissue was imaged with a 25 μm pixel size and two additional biological replicates were collected with 50 μm pixel sizes, with corresponding data presented in the supporting information. Data at each pixel was generated by accumulating ions produced during ten laser shots at 25% power and 1000 Hz, unless otherwise specified. Reduced profile spectra were saved via FTMS control and a reduced file was generated within flexImaging for later import into SCiLS 2016b ver. 4.01.8720 (Bruker Corp). Within SCiLS, spectra were root mean square (RMS) normalized and exported in imzml format for further analysis.
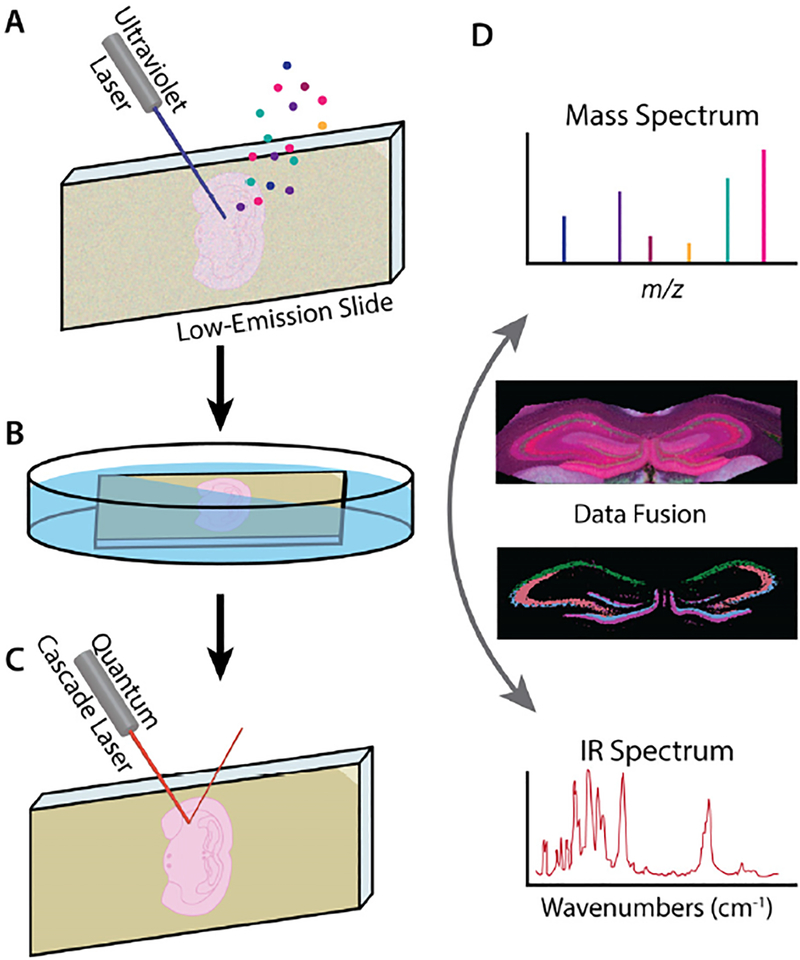
Figure 1.
Schematic of the combined MALDI-FT-ICR-MSI with IR imaging approach. (A) MALDI MSI is performed first, followed by (B) matrix removal and tissue fixation prior to (C) IR imaging. Analyzing the same tissue sample simplifies (D) data fusion compared to the more common use of adjacent sections.
Matrix Removal and Sample Fixation.
After MSI analysis, hippocampal tissue sections were placed within a solution of 4%o paraformaldehyde (PFA) in 1× phosphate buffered saline (PBS; ThermoFisher Scientific) for 15 min to remove the MALDI matrix and concurrently fix the tissue sections. Tissues were washed in PBS for 3 min following fixation and briefly rinsed with Milli-Q water (Millipore, Billerica, MA) to remove any remaining salts. Tissues were dried under a stream of nitrogen gas and placed within a desiccator for 2 h before QCL-IR imaging.
Infrared Analysis.
IR spectroscopic images were acquired using a prototype QCL-IR system (Agilent Technologies, Santa Clara, CA).55 The discrete frequency QCL-IR system uses a room temperature single-point bolometer detector, with a 0.72 NA objective lens. Spectra were acquired in the mid-IR range, 1900–800 cm−1, at 8 cm−1 spectral resolution and 5 μm image pixel size in reflection mode. Spectral processing steps were implemented in MATLAB 2015b (MathWorks, Inc., Natick, MA) and the ENVI-IDL 4.8 environment, and saved as .mat files for registration with MALDI images.
Data Registration and Analysis.
MSI datasets were imported into MATLAB as imzml files using a modified version of MSIreader.56 The series of mass spectra corresponding to each pixel with the MS image consisted of m/z intensity pairs of centroid data. Mass spectra were aligned with non-uniform bin widths because mass resolution changes as a function of m/z value for FT-ICR MS. For example, the bin width at m/z 150 was 0.0004 Da and 0.05 Da at m/z 2500, with 10 additional divisions in between. Bin widths were estimated as the average peak width at m/z values over the range of the mass spectra. Bins were constructed as piecewise linear divisions over the entire spectral range. Next, the centroided m/z values of each peak in the spectrum were counted and placed into finer bin divisions (one-eighth the values provided above) for outlier noise removal. The distribution of peak frequency was peak-picked with a minimum peak distance of the original bin width and a height threshold of 0.1% of the number of pixels. These operations removed peaks found in a small subset of pixels, producing a tentative m/z list for the aligned data set.
Next, the rough m/z list was refined by determining the center of mass for all peaks falling within the bin range. Thus, while binning was performed for alignment, the recorded m/z value retained high mass accuracy from the FT-ICR MS data. Finally, the signal intensity matrix was populated from the exact m/z list by summing the intensities within the recorded m/z value ± the bin width. The resulting data matrix was suitable for performing multivariate data analysis.
Image registration between MS and IR images was performed on the score image of principal component 1 (PC1) determined during principal component analysis (PCA) of the data matrix, which captures the majority of variance in each imaging mode. To speed computation, a subset of 1000 pixels was randomly chosen from each set of images to estimate the coefficient matrix by PCA. The entire score image was estimated by multiplying the signal intensity matrix by the coefficients determined with a subset of pixels. The score image resulting from PCA on a subset of pixels was qualitatively identical to the entire dataset and produced adequate alignment through image registration.
Next, the score images were roughly overlain by manually selecting control points from anatomical features present in each image. The initial, affine transformation was utilized to seed intensity-based registration, which produces better overlap. Manually determining the initial transformation was necessary to ensure consistent convergence of the intensity-based registration. The resulting affine transformation was then utilized to map the MS image onto the IR image space.
Pan Sharpening.
Pan sharpening was performed with a Laplacian pyramid method, which utilizes high spatial frequency components from the higher spatial resolution IR image to sharpen chemical images obtained by MSI.21 Briefly, this implementation incorporates the affine transformation from registration to estimate a scaling factor, and can match arbitrary differences in scale. First, the scale of the transformation is utilized to determine the number of iterations to down-sample the IR image. Each downsample increases the pixel size by a factor of two. To match the image modalities for fusion, the MS image is upsampled slightly to the next matching size, e.g., fusing the 5 μm IR pixels with the 25 μm MSI pixels required interpolating the MS image to 20 μm for two iterations of downsampling.
At each iteration, the IR image is reduced with the kernel specified by Burt and Adelson, as implemented in the MATLAB function impyramid.57 The difference between the effectively blurred reconstruction and the current image retains the high frequency features of the image, which is stored in the Laplacian pyramid. Once the IR image is resized to match the MS image, the process is repeated in reverse. At each iteration, the MS image is expanded by a factor of two and the Laplacian pyramid imparts the high frequency information from the IR image.
Midlevel Data Fusion.
Data fusion of each image set was performed by up-sampling the MS images to match IR image spatial resolution. Briefly, both MS and IR images were subjected to PCA for dimensionality reduction while retaining 99% of variance for the IR data and PC1-PC10 for the MSI (~64–81% of variance). The choice of principal components attempted to balance the contributions of each modality while rejecting any unnecessary noise. To up-sample the MSI data, each score image was spatially transformed to the IR image with bilinear interpolation. Data fusion was conducted pixel by pixel between the IR and MS images by concatenating the PC scores and standardizing each principal component to be mean centered with unit variance. The resulting, fused image was again subjected to PCA to identify the highest-variance contributions.
For unsupervised, multivariate classification, similar pixels were grouped together by k-means clustering for each IR spectral feature image, interpolated MS image, and fused score image. Within the hippocampus, clustering highlights anatomical regions that are chemically similar to each other. The optimum number of clusters was determined by Davies-Bouldin cluster evaluation. Statistically significant differences in ion intensity between anatomical regions were tested by one-way ANOVA with multiple comparison false discovery correction. A post-hoc Tukey-Karmer method was used for multiple comparison tests between significantly different ion intensities.
RESULTS AND DISCUSSION
Optimized Acquisition of Multimodal Imaging Datasets.
Combining disparate analytical techniques frequently requires compromising established protocols to prepare a sample suitable for analysis by each method. The suboptimal performance of a given method is an acceptable compromise if the orthogonal information acquired from the multimodal approach offsets the degraded data quality and/or analyte coverage compared to using a single method. To optimize multimodal data acquisition, a systematic assessment of QCL-IR and MALDI MS analyses and their interactions was performed.
To analyze the same tissue section, some compromises between MALDI MS and IR imaging were required. IR imaging of tissue sections frequently employs salt plates with low IR absorbance, or low-E glass slides that reflect IR light effectively. Due to their lower cost, we utilized low-E glass slides for the IR acquisition. Initial attempts to increase low-E slide conductivity for MALDI-TOF analysis included gold sputtering following MALDI matrix application. This treatment introduced additional variance during sample preparation and left an insoluble residue after MALDI matrix removal, complicating subsequent IR analysis. Thus, we used a MALDI FT-ICR mass spectrometer, which functions appropriately with non-conductive substrates, likely because of the decoupling of analyte desorption/ionization and analysis, as well as the medium level of vacuum in the ion source.58 We also found that spray-based MALDI matrix application led to unacceptable levels of analyte delocalization, as was evident in the resulting IR images. Thus, we applied MALDI matrix by sublimation, thereby improving IR imaging.
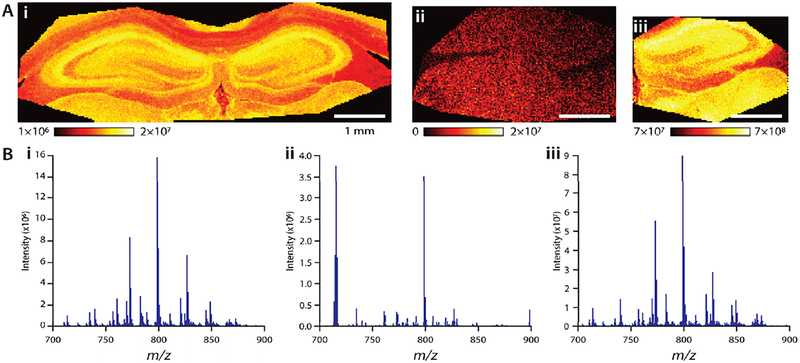
Since IR microspectroscopy is generally assumed to be non-destructive, in our initial experiments, IR imaging of tissue sections was conducted first. The post-IR imaging tissues were degraded in quality for further MSI measurements; many chemical compounds became undetectable and the overall signal intensity was lower compared to control tissue analyzed directly with MALDI MSI. To validate this finding, adjacent tissue sections were imaged by MALDI MS with and without IR analysis (Figure 2). Optimum signal intensity was obtained using MALDI MS with 25% laser power and 10 laser shots for the control sample (Figure 2A(i)). Using the same settings we were unable to acquire high quality mass spectra from tissue after IR imaging (Figure 2A(ii)). For example, the RMS-normalized intensity of the base peak in the average mass spectrum decreased from 1.6×107 to 4×106 counts following IR imaging (Figure 2B(i) and (ii)). As shown in Figure 2A(iii), doubling the laser power and number of laser shots were necessary to regain similar signal intensity (9×107 counts) but not the morphological detail of the ion images. While doubling the laser power increased the raw intensity above values obtained in the control measurement, the observed noise level also became disproportionally higher (~100 fold), demonstrating that the signal-to-noise ratio was still worse overall. Moreover, tissue exposed to higher MALDI MS laser power was physically damaged, preventing follow-up staining with hematoxylin and eosin, or immuno-histochemistry. Our finding that the tissue had degraded MS signals after IR imaging is somewhat counterintuitive as studies have shown that nano-IR causes small temperature changes with QCL illumination.59 However, lipid degradation in unfixed tissue at ambient conditions has been shown to reduce MSI signal quality.60 Exploration of the mechanism and magnitude of tissue degradation is beyond the scope of this manuscript. Regardless of the cause(s), acquiring IR data first not only leads to poorer mass spectral data but may also increase variability in the IR measurement itself as the sample changes during acquisition. One option to mitigate damage could be to use a tightly controlled protocol that may also involve nitrogen purging, but this option was not explored here.
Figure 2.
Influence of conducting IR spectroscopic imaging on the quality of MALDI MSI data. (A) MALDI MS ion image of the hippocampus (m/z 798.5407; [PC 34:1 + K]+) for (i) MaLDI MS performed prior to IR spectroscopic imaging, (ii) after IR analysis with identical conditions - left side of brain section was analyzed, and (iii) right side of the same tissue section as in (ii) with twice the laser power and number of laser shots. (B) Average, RMS normalized spectra for each image.
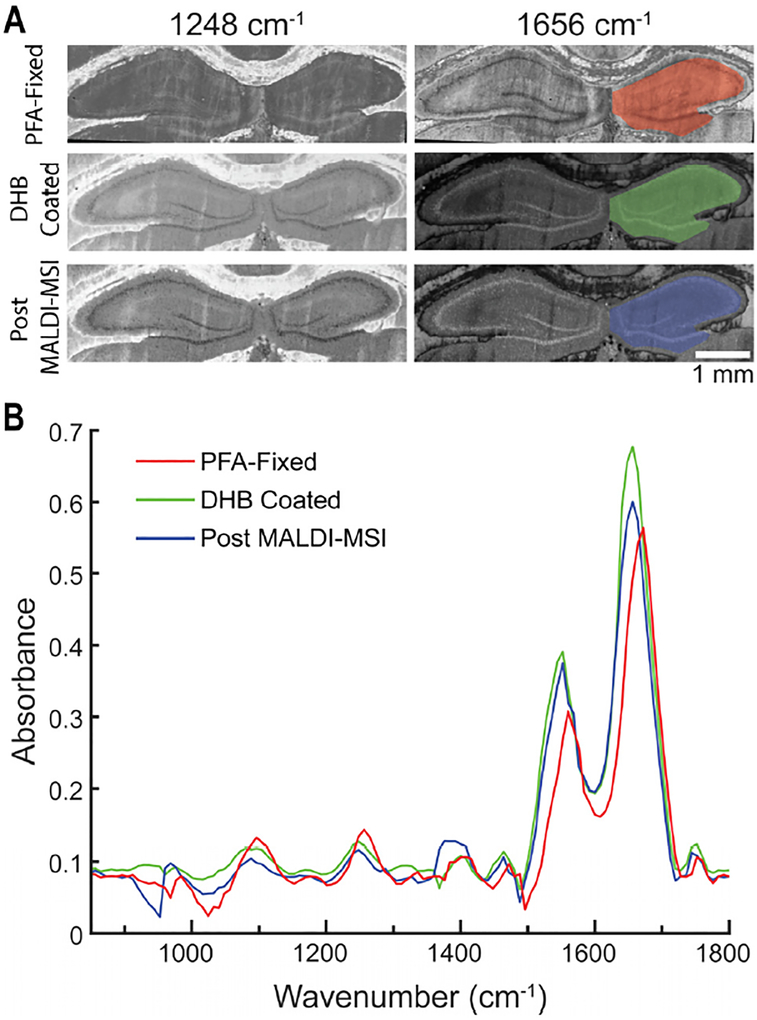
Next, we explored reversing the order of the multimodal measurements by first examining how MALDI MS alters the results of IR measurements. Adjacent hippocampus tissue sections were analyzed with IR imaging with various perturbations from the MALDI MSI process. Tissues weree ither 1) directly fixed using PFA, 2) coated with DHB and then fixed, or 3) coated with DHB, analyzed by MALDI MS, and fixed. Figure 3A displays absorbance images acquired at wavenumbers for the asymmetric PO2− stretch (1248 cm-1) and amide II stretch (1656 cm−1). Qualitatively, the images indicate no damage from MALDI desorption and no apparent distortion or blurring of expected morphological image features. The average absorption spectrum was calculated from the hippocampal region, shown as shaded regions of interest, and plotted for direct comparison (Figure 3B). The spectra indicate a slight red shift in IR spectra collected from tissues exposed to DHB, with no change upon laser ablation. Similar shifts have been reported for different fixation treatments in other studies and could arise from a change in the optical refractive index mismatch within tissue61–65 as well as chemical changes to the protein backbone. Moreover, the shift between the amide I and amide II bands may be the result of an altered microenvironment, loss of some chemical species, or exposure to atmosphere. The tissue that was sublimed with DHB but not subjected to MALDI MS analysis had the highest absorbance values, whereas the tissue analyzed by MALDI MS more closely resembled the fixed tissue section. The spectral differences may be from residual DHB absorbing in the region of 1500–1750 cm−1. Overall, most IR spectral features are similar between the adjacent tissue sections, demonstrating that performing MALDI MS before IR spectroscopy results only in minor changes to the IR imaging data. Compared to the drastic differences seen in MALDI MS performed after IR spectroscopy, the order of multimodal analysis described here provides an acceptable compromise.
Figure 3.
Influence of MALDI MSI on IR imaging data quality for rat hippocampus images. (A) Discrete frequency IR images of the 1248 cm−1 and 1656 cm−1 bands of adjacent tissue sections subjected to either (top) PFA treatment, (middle) sublimation of DHB with subsequent PFA treatment, or (bottom) sublimation of DHB and MALDI MSI analysis, with subsequent PFA treatment. (B) The average absorbance spectra from the color-coded regions of the hippocampus are overlaid. While there are several morphological differences between the three tissue sections, the IR spectra match.
Chemical Pan Sharpening of Ion Images.
A benefit of combining different imaging approaches is the increased flexibility in data analysis and visualization. Various types of microscopy data sets (e.g., haemotoxylin staining, eosin staining, and electron microscopy) are utilized for pan sharpening of chemical imaging outputs. However, not all chemically unique morphological features can be observed in singleplex images. Also, using contrasting distributions can introduce artifacts or fail to improve sharpness, which is especially problematic when only a single image is available for pan sharpening. With additional choices for sharpening, pan sharpening can be optimized to enhance a given ion image without introducing artifacts. Multiplex IR datasets display a “mesoscale” level of detail in tissue composition, such as overall lipid, protein, and nucleotide distributions, but at higher spatial resolution than MSI. Thus, these images present unique opportunities for pan sharpening of MSI data sets. Through chemical pan sharpening, more ion images are suitable for sharpening as more corresponding contrast distributions can be found. Figures S1–6 show the effects of sharpening of single ion images with different IR bands. Two different lipid ion mages (Figures S1–6C, F) are pan sharpened with absorbance images at two QCL-IR bands (Figure S1–6A, B) associated with lipids and proteins. Overall, Figures S1, S3, S5D, E show similar distributions of signal intensities because Laplacian pyramid sharpening utilizes the low frequency components from the MS ion image. Within the DG (Figures S2, S4, S6), many small, cellular structures arise in sharpened images from high frequency information contained in the 1656 cm−1 band. However, because the celllike structures are not seen in the ion image, the features must arise from IR absorbance contrast and cannot be used for making assumptions of analyte localization within the cell bodies. While more apparent in Figure S5E, H, the data also demonstrate the ability of IR microscopy to detect fine tissue features that are invisible to MSI that may be native or arise during sample handling.
Careful handling is paramount during tissue sampling and sectioning as sample preparation artifacts can be propagated to the pan-sharpened image. In Figure S6, the sharpened image in panel G appears blurrier than the image in panel H, which is sharpened by an IR band exhibiting higher contrast around the DG. The example images demonstrate that the quality of pan sharpening depends on the contrast and distributions present in the underlying, higher spatial resolution images. Pan sharpening with IR distributions provides additional options for selecting appropriate bands for sharpening, but care is required when extrapolating beyond distributions present in the low spatial resolution, MS images.
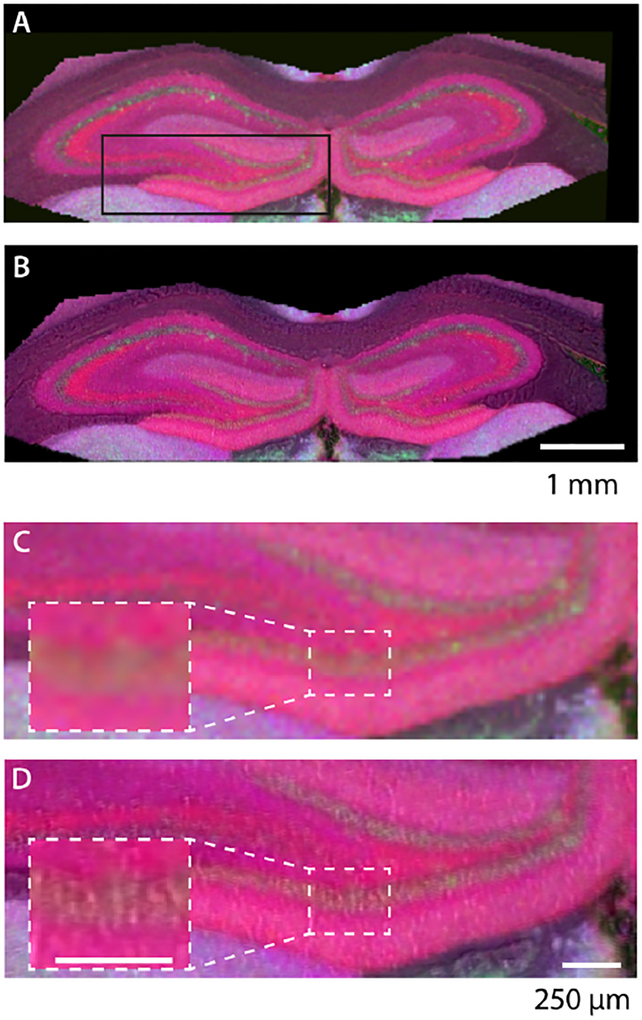
Since Laplacian pyramid fusion functions on individual ion distributions, sharpened ion images can be combined and presented as an RGB composite (Figures 4, S7, S8). Figure 4A shows the RGB MS image overlaying the distribution of three compounds in the hippocampus. While different layers within the hippocampus are visible, the spatial resolution at which MSI was acquired is insufficient for distinguishing clear regional boundaries and, in some cases cellular localizations, within the brain tissue. However, sharpening each ion image with an absorbance image arising from the amide II band (1656 cm−1, Figures 4B, D and S7, S8, panels B, D) accentuates boundaries between different layers within the hippocampus. Focusing on the DG (Figures 4 and S7, S8, panels C, D) highlights the improvement in image quality, especially for replicates acquired at 50 μm×50 μm pixels (Figures S7, S8). After fusion, tissue morphology associated with known cellular structures becomes more apparent; e.g., the round structures within the bottom portion of the DG that appear as blurry, indistinct hot spots (Figure 4C). The resulting sharpened image is a qualitative fusion of the multimodal datasets, with color and brightness determined by ion abundances within a pixel and high frequency spatial information from the amide II stretching band.
Figure 4.
RGB composite ion image: R = [PC 32:0 + K]+ (m/z 772.5253), G = [PC 40:6 + K]+ (m/z 872.5566), and B = [PC 34:0 + k]+ (m/z 800.5567). (A) Unsharpened MS image of the hippocampus taken with MSI at 25 μm spatial resolution. (B) MS image in A, sharpened with QCL-IR band at 1656 cm−1. (C) Magnification of the area marked in A containing an unsharpened MS image of the DG (magnified inset) and one end of the CA3 region. (D) Magnification of sharpened MS image shown in B.
Midlevel Data Fusion of IR and MALDI MS Images.
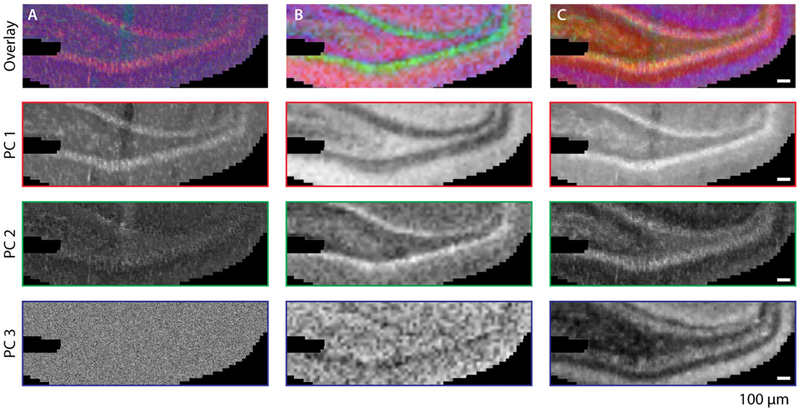
Hyperspectral PCA score images provide measures of the tissue variance, where pixels of the same color represent a closer distance in the score space and therefore similar chemical composition. Figure 5 shows hyperspectral images derived from spectral PCA of IR imaging (Figure 5A) and FT-ICR-MSI (Figure 5B) data. The overlaid IR image produces a higher spatial resolution view of the DG with some cellular features visible (Figure 5A); however, only the first two principal components display meaningful morphological information as the third component appears to be noise. The hyperspectral PCA score images of FT-ICR-MSI (Figure 5B) have noticeably poorer spatial resolution but display more chemically distinct regions. Distributions of individual principal components higher than PC2 demonstrate more noise but depict some morphological detail (Figure 5B). Combining the PCA score images and performing PCA on the fused data produces the midlevel fusion image (Figure 5C). The fused dataset shows additional layers within the hippocampus at an improved spatial resolution. The improvement from midlevel fusion is especially striking for replicates shown in Figures S9, S10, where some artifacts visible in the IR images are removed from the fused images.
Figure 5.
Hyperspectral PCA score images and individual principal components showing the expected structures within the DG region of the rat hippocampus. Images are from (A) IR spectra, (B) MALDI FT-ICR-MSI, and (C) midlevel data fusion of data presented in panels A and B.
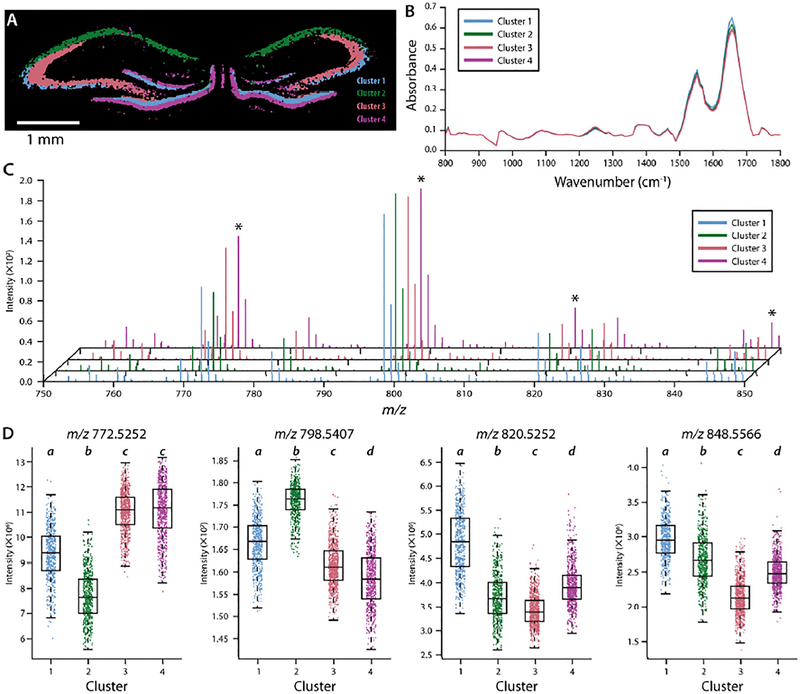
For biological replicate 1, acquired at 25 μm pixel widths for MSI, Davies-Bouldin optimization indicates the data is well modeled with 11 unique clusters (Figure S11), which associate well with histologically defined morphological regions within the hippocampus. Notably, we can parse out the lucidum and alveus layers separately in segments 3 and 6, respectively. The unsupervised clustering effectively annotates the tissue morphology according to previous histological classifications. With a segment map, average IR and MS spectra for each region can reveal their unique chemical profiles. A representative example in shown in Figure 6 (with additional replicates shown in Figures S12–S14); segments 1–4 are overlaid to show a clear chemical separation of CA1 (blue), the lucidum layer of the CA3 (pink), the rest of CA3 (green), and the DG (purple). A comparison of the average absorption spectra reveals differences around 1250cm−1, 1550 cm−1, and 1680 cm−1 (Figure 6B), which are largely related to differences in protein. Additionally, the average mass spectra show several, significant differences between phosphatidylcholine (PC) lipid signal intensities, as summarized in Figure 6C, D. The box plots (Figure 6D) show four example lipid species ([PC 32:0+K]+, [PC 34:1+K]+, [PC 36:4+K]+, and [PC 38:4+K]+) with differing abundance between segments corresponding to CA1, the lucidum layer of CA3, the rest of CA3, and the DG. Through data fusion and unsupervised clustering, significant chemical differences are detectable between physiologically similar regions. Neither method alone provides the chemical specificity and spatial resolution to differentiate between each layer. Multimodal imaging, optimized for high data quality, is vital for exploring chemical heterogeneity present in complex biological systems, including the brain.
Figure 6.
Results of k-means clustering of fused data sets. (A) Segments associated with the CA1 (blue), lucidum layer of CA3 (pink), rest of CA3 (green), and the DG (purple) layers within the hippocampus. (B) Average absorbance spectra for the four clusters. (C) Average mass spectra for the specified clusters. Asterisks denote the m/z values used for the 4 boxplots presented in D. (D) Boxplots of selected signal intensities within each highlighted cluster. Boxplots for corresponding salt adducts can be found in Figure S15. Labels above each box denote significantly different groups by post-hoc Tukey’s honestly significant difference multiple comparison test.
CONCLUSIONS
We developed an analytical workflow that allows MALDI MS and IR spectroscopy to be performed on the same tissue. The workflow allows for chemical pan sharpening of MS ion images with a variety of IR absorption bands, providing optimized pan sharpened ion images with minimal artifacts. The inherent spatial agreement between modalities allowed us to determine lipid distributions within the DG, a morphologically and chemically complex, and functionally important, brain structure. Further, we developed a midlevel data fusion approach that allows chemical information from each technique to be combined, enhancing discrimination of structurally important regions by k-means clustering. Because MALDI MSI is a gentle ionization approach, laser damage was not observed in the IR images, making the incorporation of traditional staining methods feasible. Immunohistochemistry would further augment the multimodal datasets with information on localization of targeted antigens within the hippocampus. The multimodal imaging approach presented here can be easily extended to other biologically important structures in the nervous system, such as the supraoptic and suprachiasmatic nuclei.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge support from the National Institutes of Health, Award Number 1U01 MH109062 from the National Institute of Mental Health and P30 DA018310 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. E.K.N. acknowledges support from the National Science Foundation Graduate Research Fellowship Program and the Springborn Fellowship. This work is also partially supported by the Agilent Thought Leader Award to R.B.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information includes Figures S1–15, providing additional details on biological replicates and validation experiments, as noted in the text. The Supporting Information is available free of charge on the ACS Publications website.
REFERENCES
- 1.Scoville WB; Milner B, Loss of recent memory after bilateral hippocampal lesions. J. Neurol., Neurosurg. Psychiatry 1957, 20 (1), 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Strien NM; Cappaert NLM; Witter MP, The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci 2009, 10, 272. [DOI] [PubMed] [Google Scholar]
- 3.Kee N; Teixeira CM; Wang AH; Frankland PW, Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci 2007, 10, 355. [DOI] [PubMed] [Google Scholar]
- 4.Bruel-Jungerman E; Laroche S; Rampon C, New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci 2005, 21 (2), 513521. [DOI] [PubMed] [Google Scholar]
- 5.Seri B; García-Verdugo JM; Collado-Morente L; McEwen BS; Alvarez-Buylla A, Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol 2004, 478 (4), 359–378. [DOI] [PubMed] [Google Scholar]
- 6.James Surmeier D; Wilson CJ; Eberwine J, Patch-Clamp Techniques for Studying Potassium Currents in Mammalian Brain Neurons. 1994; Vol. 19, p 39–67. [Google Scholar]
- 7.Jennes L; Stumpf WE; Kalivas PW, Neurotensin: Topographical distribution in rat brain by immunohistochemistry. J. Comp. Neurol 1982, 210 (3), 211–224. [DOI] [PubMed] [Google Scholar]
- 8.Glover LR, S. T, Karlsson R-M, Bannerman DM, Cameron HA Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues. PLoS Biol 2017, 15 (4), e2001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones DK, Studying connections in the living human brain with diffusion MRI. Cortex 2008, 44 (8), 936952. [DOI] [PubMed] [Google Scholar]
- 10.Fenno L; Yizhar O; Deisseroth K, The Development and Application of Optogenetics. Annu. Rev. Neurosci 2011, 34 (1), 389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livet J; Weissman TA; Kang H; Draft RW; Lu J; Bennis RA; Sanes JR; Lichtman JW, Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007, 450, 56. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich M; Zhang F, Applications of CRISPR-Cas systems in neuroscience. Nat. Rev. Neurosci 2015, 17, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okaty BW; Sugino K; Nelson SB, Cell Type-Specific Transcriptomics in the Brain. J. Neurosci 2011, 31 (19), 6939–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Aneed A; Cohen A; Banoub J, Mass Spectrometry, Review of the Basics: Electrospray, MALDI, and Commonly Used Mass Analyzers. Appl. Spectrosc. Rev 2009, 44 (3), 210–230. [Google Scholar]
- 15.Gessel MM; Norris JL; Caprioli RM, MALDI imaging mass spectrometry: Spatial molecular analysis to enable a new age of discovery. J. Proteomics 2014, 107 (Supplement C), 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zavalin A; Todd EM; Rawhouser PD; Yang J; Norris JL; Caprioli RM, Direct imaging of single cells and tissue at sub-cellular spatial resolution using transmission geometry MALDI MS. J. Mass Spectrom 2012, 47 (11), 1473–1481. [DOI] [PubMed] [Google Scholar]
- 17.Kompauer M; Heiles S; Spengler B, Atmospheric pressure MALDI mass spectrometry imaging of tissues and cells at 1.4-μm lateral resolution. Nat. Methods 2016, 14, 90. [DOI] [PubMed] [Google Scholar]
- 18.Jurchen JC; Rubakhin SS; Sweedler JV, MALDI-MS imaging of features smaller than the size of the laser beam. J. Am. Soc. Mass Spectrom 2005, 16 (10), 16541659. [DOI] [PubMed] [Google Scholar]
- 19.Van de Plas R; Yang J; Spraggins J; Caprioli RM, Image fusion of mass spectrometry and microscopy: a multimodality paradigm for molecular tissue mapping. Nat. Methods 2015, 12, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarolli JG; Jackson LM; Winograd N, Improving Secondary Ion Mass Spectrometry Image Quality with Image Fusion. J. Am. Soc. Mass Spectrom 2014, 25 (12), 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollnhals F; Audinot J-N; Wirtz T; Mercier-Bonin M; Fourquaux I; Schroeppel B; Kraushaar U; Lev-Ram V; Ellisman MH; Eswara S, Correlative Microscopy Combining Secondary Ion Mass Spectrometry and Electron Microscopy: Comparison of Intensity-Hue-Saturation and Laplacian Pyramid Methods for Image Fusion. Anal. Chem 2017, 89 (20), 10702–10710. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava R, Infrared Spectroscopic Imaging: The Next Generation. Appl. Spectrosc 2012, 66 (10), 10911120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasse MJ; Walsh MJ; Mattson EC; Reininger R; Kajdacsy-Balla A; Macias V; Bhargava R; Hirschmugl CJ, High-resolution Fourier-transform infrared chemical imaging with multiple synchrotron beams. Nat. Methods 2011, 8, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez DC; Bhargava R; Hewitt SM; Levin IW, Infrared spectroscopic imaging for histopathologic recognition. Nat. Biotechnol 2005, 23, 469. [DOI] [PubMed] [Google Scholar]
- 25.Hackett MJ; McQuillan JA; El-Assaad F; Aitken JB; Levina A; Cohen DD; Siegele R; Carter EA; Grau GE; Hunt NH, Chemical alterations to murine brain tissue induced by formalin fixation: implications for biospectroscopic imaging and mapping studies of disease pathogenesis. Analyst 2011, 136 (14), 2941–2952. [DOI] [PubMed] [Google Scholar]
- 26.Bergner N; Romeike BF; Reichart R; Kalff R; Krafft C; Popp J, Tumor margin identification and prediction of the primary tumor from brain metastases using FTIR imaging and support vector machines. Analyst 2013, 138 (14), 3983–3990. [DOI] [PubMed] [Google Scholar]
- 27.Kastyak-Ibrahim M; Nasse M; Rak M; Hirschmugl C; Del Bigio M; Albensi B; Gough KM, Biochemical label-free tissue imaging with subcellular-resolution synchrotron FTIR with focal plane array detector. Neuroimage 2012, 60 (1), 376–383. [DOI] [PubMed] [Google Scholar]
- 28.Leslie LS; Wrobel TP; Mayerich D; Bindra S; Emmadi R; Bhargava R, High definition infrared spectroscopic imaging for lymph node histopathology. PLoS ONE 2015, 10 (6), e0127238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayerich D; Walsh MJ; Kadjacsy-Balla A; Ray PS; Hewitt SM; Bhargava R, Stain-less staining for computed histopathology. Technology 2015, 3 (01), 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhargava R; Madabhushi A, Emerging themes in image informatics and molecular analysis for digital pathology. Annu. Rev. Biomed. Eng 2016, 18, 387–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker MJ; Trevisan J; Bassan P; Bhargava R; Butler HJ; Dorling KM; Fielden PR; Fogarty SW; Fullwood NJ; Heys KA Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc 2014, 9 (8), 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masyuko R; Lanni EJ; Sweedler JV; Bohn PW, Correlated imaging - a grand challenge in chemical analysis. Analyst 2013, 138 (7), 1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanni EJ; Masyuko RN; Driscoll CM; Dunham SJB; Shrout JD; Bohn PW; Sweedler JV, Correlated Imaging with C60-SIMS and Confocal Raman Microscopy: Visualization of Cell-Scale Molecular Distributions in Bacterial Biofilms. Anal. Chem 2014, 86 (21), 10885–10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z; Chu L-Q; Sweedler JV; Bohn PW, Spatial Correlation of Confocal Raman Scattering and Secondary Ion Mass Spectrometric Molecular Images of Lignocel-lulosic Materials. Anal. Chem 2010, 82 (7), 2608–2611. [DOI] [PubMed] [Google Scholar]
- 35.Petit VW; Réfrégiers M; Guettier C; Jamme F; Sebanayakam K; Brunelle A; Laprévote O; Dumas P; Le Naour F, Multimodal Spectroscopy Combining Time-of-Flight-Secondary Ion Mass Spectrometry, Syn-chrotron-FT-IR, and Synchrotron-UV Microspectroscopies on the Same Tissue Section. Anal. Chem 2010, 82 (9), 3963–3968. [DOI] [PubMed] [Google Scholar]
- 36.Le Naour F; Bralet M-P; Debois D; Sandt C; Guettier C; Dumas P; Brunelle A; Laprévote O, Chemical Imaging on Liver Steatosis Using Synchrotron Infrared and ToF-SIMS Microspectroscopies. PLoS ONE 2009, 4 (10), e7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasch P; Noda I, Two-Dimensional Correlation Spectroscopy for Multimodal Analysis of FT-IR, Raman, and MALDI-TOF MS Hyperspectral Images with Hamster Brain Tissue. Anal. Chem 2017, 89 (9), 5008–5016. [DOI] [PubMed] [Google Scholar]
- 38.Page JS; Sweedler JV, Sample Depletion of the Matrix-Assisted Laser Desorption Process Monitored Using Radionuclide Detection. Anal. Chem 2002, 74 (24), 62006204. [DOI] [PubMed] [Google Scholar]
- 39.Comi TJ; Neumann EK; Do TD; Sweedler JV microMS: A Python Platform for Image-Guided Mass Spectrometry Profiling. J. Am. Soc. Mass Spectrom 2017, 28 (9), 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comi TJ; Makurath MA; Philip MC; Rubakhin SS; Sweedler JV, MALDI MS Guided Liquid Microjunction Extraction for Capillary Electrophoresis-Electrospray Ionization MS Analysis of Single Pancreatic Islet Cells. Anal. Chem 2017, 89 (14), 7765–7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dufresne M; Guneysu D; Patterson NH; Marcinkiewicz MM; Regina A; Demeule M; Chaurand P, Multimodal detection of GM2 and GM3 lipid species in the brain of mucopolysaccharidosis type II mouse by serial imaging mass spectrometry and immunohistochemistry. Anal. Bioanal. Chem 2017, 409 (5), 1425–1433. [DOI] [PubMed] [Google Scholar]
- 42.Kaya I; Michno W; Brinet D; Iacone Y; Zanni G,; Blennow K; Zetterberg H; Hanrieder J, Histology-Compatible MALDI Mass Spectrometry Based Imaging of Neuronal Lipids for Subsequent Immunofluorescent Staining. Anal. Chem 2017, 89 (8), 4685–4694. [DOI] [PubMed] [Google Scholar]
- 43.Chaurand P; Schwartz SA; Billheimer D; Xu BJ; Crecelius A; Caprioli RM, Integrating Histology and Imaging Mass Spectrometry. Anal. Chem 2004, 76 (4), 1145–1155. [DOI] [PubMed] [Google Scholar]
- 44.Bich C; Vianello S; Guérineau V; Touboul D; De La Porte S; Brunelle A, Compatibility between TOF-SIMS lipid imaging and histological staining on a rat brain section. Surf. Interface Anal 2013, 45 (1), 260–263. [Google Scholar]
- 45.Deutskens F; Yang J; Caprioli RM, High spatial resolution imaging mass spectrometry and classical histology on a single tissue section. J. Mass Spectrom 2011, 46 (6), 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brand-Williams W; Cuvelier ME; Berset C, Use of a free radical method to evaluate antioxidant activity. LWT--Food Sci. Technol 1995, 28 (1), 25–30. [Google Scholar]
- 47.Ashidate K; Kawamura M; Mimura D; Tohda,;Miyazaki S; Teramoto T; Yamamoto Y; Hirata Y, Gentisic acid, an aspirin metabolite, inhibits oxidation of low-density lipoprotein and the formation of cholesterol ester hydroperoxides in human plasma. Eur. J. Pharmacol 2005, 513 (3), 173–179. [DOI] [PubMed] [Google Scholar]
- 48.Sproß J, Analysis of Volatile and Oxidation Sensitive Compounds Using a Cold Inlet System and Electron Impact Mass Spectrometry. J.Vis. Exp 2014, (91), 51858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aparicio S; Doty S; Camacho N; Paschalis E; Spevak L; Mendelsohn R; Boskey A, Optimal methods for processing mineralized tissues for Fourier transform infrared microspectroscopy. Calcif. Tissue Int 2002, 70 (5), 422–429. [DOI] [PubMed] [Google Scholar]
- 50.Lyng F; Gazi E; Gardner P, Preparation of tissues and cells for infrared and Raman spectroscopy and imaging In Biomedical Applications of Synchrotron Infrared Microspectroscopy: A Practical Approach Moss D, Ed. Royal Society of Chemistry: Cambridge, 2011; pp 147–185. [Google Scholar]
- 51.Kwak JT; Reddy R; Sinha S; Bhargava R, Analysis of variance in spectroscopic imaging data from human tissues. Anal. Chem 2011, 84 (2), 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaurand P; Cornett DS; Angel PM; Caprioli RM, From whole-body sections down to cellular level, multiscale imaging of phospholipids by MALDI mass spectrometry. Mol. Cell. Proteomics 2011, 10 (2), 0110.004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hankin JA; Farias SE; Barkley RM; Heidenreich K; Frey LC; Hamazaki K; Kim HY; Murphy RC, MALDI mass spectrometric imaging of lipids in rat brain injury models. J. Am. Soc. Mass Spectrom 2011, 22 (6), 1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanni EJ; Dunham SJB; Nemes P; Rubakhin SS; Sweedler JV, Biomolecular Imaging with a C60-SIMS/MALDI Dual Ion Source Hybrid Mass Spec-trometer: Instrumentation, Matrix Enhancement, and Single Cell Analysis. J. Am. Soc. Mass Spectrom 2014, 25 (11), 1897–1907. [DOI] [PubMed] [Google Scholar]
- 55.Tiwari S; Raman J; Reddy V; Ghetler A; Tella RP; Han Y; Moon CR; Hoke CD; Bhargava R, Towards Translation of Discrete Frequency Infrared Spectroscopic Imaging for Digital Histopathology of Clinical Biopsy Samples. Anal. Chem 2016, 88 (20), 10183–10190. [DOI] [PubMed] [Google Scholar]
- 56.Robichaud G; Garrard KP; Barry JA; Muddiman DC, MSiReader: An Open-Source Interface to View and Analyze High Resolving Power MS Imaging Files on Matlab Platform. J. Am. Soc. Mass Spectrom 2013, 24 (5), 718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burt P; Adelson E, The Laplacian Pyramid as a Compact Image Code. iEEE Transactions on Communications 1983, 31 (4), 532–540. [Google Scholar]
- 58.Garrett TJ; Prieto-Conaway MC; Kovtoun V; Bui H; Izgarian N; Stafford G; Yost RA Imaging of small molecules in tissue sections with a new intermedi-ate-pressure MALDI linear ion trap mass spectrometer. Int. J. Mass Spectrom 2007, 260 (2), 166–176. [Google Scholar]
- 59.Donaldson PM; Kelley CS; Frogley MD; Filik J; Wehbe K; Cinque G, Broadband near-field infrared spectromicroscopy using photothermal probes and synchrotron radiation. Opt. Express 2016, 24 (3), 1852–64. [DOI] [PubMed] [Google Scholar]
- 60.Patterson NH; Thomas A; Chaurand P, Monitoring time-dependent degradation of phospholipids insectioned tissues by MALDI imaging mass spectrometry. J. Mass Spectrom 2014, 49 (7), 622–7. [DOI] [PubMed] [Google Scholar]
- 61.Mohlenhoff B; Romeo M; Diem M; Wood BR, Mie-type scattering and non-Beer-Lambert absorption behavior of human cells in infrared microspectroscopy. Biophys. J 2005, 88 (5), 3635–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassan P; Byrne HJ; Bonnier F; Lee J; Dumas P; Gardner P, Resonant Mie scattering in infrared spectroscopy of biological materials-understanding the ‘dispersion artefact. Analyst 2009, 134 (8), 1586–1593. [DOI] [PubMed] [Google Scholar]
- 63.Perez-Guaita D; Heraud P; Marzec KM; De La Guardia M; Kiupel M; Wood BR, Comparison of transflection and transmission FTIR imaging measurements performed on differentially fixed tissue sections. Analyst 2015, 140 (7), 2376–2382. [DOI] [PubMed] [Google Scholar]
- 64.Davis BJ; Carney PS; Bhargava R, Theory of Mid-infrared Absorption Microspectroscopy: II. Heterogeneous Samples. Anal. Chem 2010, 82 (9), 3487–3499. [DOI] [PubMed] [Google Scholar]
- 65.Davis BJ; Carney PS; Bhargava R, Theory of Midinfrared Absorption Microspectroscopy: I. Homogeneous Samples. Anal. Chem 2010, 82 (9), 3474–3486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.