Abstract
Peripheral inflammation often causes changes in mood and emergence of depressive behavior, and is characterized by a group of physical manifestations including lethargy, malaise, listlessness, decreased appetite, anhedonia, and fever. These behavioral changes are induced at the molecular level by pro-inflammatory cytokines like interleukin (IL)-1β, IL-6 and TNF-α. The basolateral amygdala (BLA) is a key brain region involved in mood and may mediate some of the behavioral effects of inflammation. However, it is unknown whether peripheral inflammatory state affects the activity of BLA neurons. To test this, adult male Sprague Dawley rats were treated with IL-1β (1 μg, intraperitoneal (i.p.)), and behavioral and electrophysiological measures were obtained. IL-1β reduced locomotion in the open-field test and also reduced home-cage mobility, consistent with features of sickness-like behavior. Using in vivo single-unit extracellular electrophysiological recordings from anesthetized rats, we found that spontaneous BLA neuronal firing was acutely (<30 min) increased after IL-1β, followed by a return to baseline level, particularly in the basal nucleus of the BLA complex. To verify and expand on effects of peripheral inflammation, we tested whether another, longer lasting inflammagen also changes BLA neuronal firing. Lipopolysaccharide (250 μg/kg, i.p.) increased BLA firing rate acutely (<30 min) and persistently. The findings demonstrate a rapid effect of peripheral inflammation on BLA activity and suggest a link between BLA neuronal firing and triggering of behavioral consequences of peripheral inflammation. These findings are a first step towards understanding the neuronal basis of depressive behavior caused by acute peripheral inflammation.
Keywords: Basolateral amygdala, In vivo extracellular electrophysiology, Inflammation, Interleukin-1β, Lipopolysaccharide, Neuronal firing
INTRODUCTION
Peripheral inflammation and infection are associated with changes in mood and are characterized by a group of physical manifestations including lethargy, malaise, listlessness, decreased appetite, anhedonia, and fever (Dunn and Swiergiel, 1998; Konsman et al., 2002; Anisman and Merali, 2003; Dantzer, 2009). Evidence suggests the coexistence of immune dysfunction with psychological changes in patients suffering from a wide range of neuropsychiatric and non-neurological disorders (Segerstrom and Miller, 2004; Schneiderman et al., 2005; Hunter, 2012). Immunotherapies are increasingly used clinically, and often produce effects on mood (Raison et al., 2005; Hodes et al., 2015; Kovacs et al., 2016).
Psychological stress and depression have been intimately linked to peripheral inflammation (Dunn and Swiergiel, 1998; Kiecolt-Glaser and Glaser, 2002; Kiecolt-Glaser et al., 2002; Anisman and Merali, 2003; Dantzer and Kelley, 2007; Dantzer et al., 2008; Howren et al., 2009; Felger and Lotrich, 2013). Patients with depression have increased levels of circulating pro-inflammatory cytokines, including interleukin (IL)-6 and tumor necrosis factor (TNF)-α (Dantzer, 2009; Howren et al., 2009; Felger and Lotrich, 2013; Lindqvist et al., 2009; Levine et al, 1999). Antagonism of TNF by infliximab improves depressive symptoms in a subset of treatment-resistant patients with high baseline inflammatory biomarkers (Raison et al., 2013). In addition, interferon (IFN)-α used in treatment of some viral infections and cancers increases peripheral IL-6 and (TNF)-α and induces symptoms of depression (Capuron et al., 2002; Maddock et al., 2005; Raison et al., 2005). While it has received less attention, IFN-α also increases IL-1 (Sissolak et al., 1992), and there is evidence for a link between IL-1 and mood. Increased IL-1β levels have been found in peripheral samples from several different populations of patients with depression (Mota et al., 2013; Owen et al., 2001; Thomas et al., 2005), including middle-aged patients with unipolar depression (Mota et al., 2013) and it correlated with unipolar depression severity in geriatric patients (Thomas et al., 2005). IL-1β levels in cerebrospinal fluid are elevated in patients with unipolar depression (Levine et al, 1999) or correlated with severity of unipolar depression (Martinez et al, 2012). This is further validated by meta-analysis that confirms correlation between IL-1β levels and unipolar depression (Howren et al., 2009). There are also hints toward a link between increased IL-1β and mood in rodent studies. Elevation in brain IL- 1 levels is necessary and sufficient to cause depression in mice (Goshen et al., 2008). Furthermore, in a rodent model of peripheral nerve injury, upregulation of IL-1β, both in the periphery and within the brain was sufficient to produce depression-like behavior, and it can be blocked by IL-1 receptor antagonist (Gui et al., 2016; Norman et al., 2010). Together, these studies suggest that inflammatory cytokines may trigger the development of depression (Felger and Lotrich, 2013).
While there is substantial and increasing clinical evidence for effects of immune modulators on mood, the mechanism for this is not clear. Systemic inflammation induced by peripheral injection of the pro-inflammatory cytokine IL-1β in rats is known to cause a time- and dose-dependent increase in the expression of c-Fos in the corticotrophin releasing factor (CRF) and oxytocin producing cells of the paraventricular nucleus of the hypothalamus (PVH), along with similar activation of neurons in the bed nucleus of stria terminalis, central amygdala, lateral parabrachial nucleus, dorsomedial and ventrolateral medulla (Ericsson et al., 1994). The systemic inflammation with IL-1 can also induce the release of prostaglandin (PG) E2 from the intramedullary perivascular cells, which in turn activates the PVH neurons and thereby stimulating the hypothalamo-pituitary-adrenal axis (Ericsson et al., 1997). Several neuroimaging studies in humans have shown that specific brain regions respond to peripheral inflammation. For example, increased peripheral inflammatory cytokines was associated with fMRI activation of ventral prefrontal cortex, temporal cortex, cuneus and fusiform gyrus in women undergoing a grief elicitation task (O’Connor et al., 2009). Escherichia coli endotoxin, which induces acute inflammation, increased fMRI activity in insular and posterior cingulate cortex, precuneus, temporal sulcus and pole, medial prefrontal cortex, and dorsomedial prefrontal cortex, with activation of the latter three structures correlated with depressed mood (Eisenberger et al., 2009). A similar endotoxin challenge enhanced amygdala activity in response to socially threatening images and was associated with greater feelings of social disconnection (Inagaki et al. 2012). This hints toward cortical and amygdala targets for the immune system in its effects on mood. In rodents, intracerebroventricular treatment with IL-1β triggers Fos in the amygdala in addition to many other brain regions (Konsman et al., 2000), further supporting the amygdala as a potential target for the effects of inflammation on mood.
Although peripheral immune activation has effects on mood and emotion and can induce expression of amygdala immediate early genes within hours, it is unknown whether inflammation can alter the firing of amygdala neurons and whether this can occur on a rapid time scale, or is associated with prolonged changes in amygdala neuronal firing. In the present study we used electrophysiological approaches to test if acute peripheral inflammation, induced by an IL-1β dose high enough to induce classical behavioral features of sickness, rapidly changes the in vivo activity of neurons in an amygdala region that plays a prominent role in mood and emotion, the basolateral amygdala (BLA).
EXPERIMENTAL PROCEDURES
Ethical approval
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Rosalind Franklin University of Medicine and Science, and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Measures were taken to reduce any distress to the animals and the total number of animals used for the study.
Animal subjects
Adult male Sprague Dawley rats (Envigo, Indianapolis, IN; post-natal day 59–63) were housed 2–3 per cage in the climate controlled Biological Resource Facility at Rosalind Franklin University of Medicine and Science, with ad libitum access to food and water. They were allowed to acclimate for at least 7 days before experiments. Lights in the housing room were on a reversed 12h light / dark schedule (light off: 07:00–19:00). All efforts were taken to avoid single housing of the rats; in very rare instance, single housing was restricted for a very short period (1 – 2 days). Peripheral inflammation can be acutely induced by peripheral injection of IL-1β, or more persistently by LPS (Lacosta et al., 1998; Copeland et al., 2005; Lukens et al., 2012; Biesmans et al., 2013). Rats were randomly assigned to receive a single injection (i.p.) of either 0.9% saline (control) or IL-1β in the first experiments; saline or LPS in the second experiments. A total of 127 rats were used in the study and their distribution for individual experiment is mentioned under the specific experimental procedures below. Briefly, 40 rats for behavioral experiments, 39 rats for electrophysiological experiments and 48 rats for ELISA experiments were used in the study.
Materials
Recombinant rat IL-1β (GenScript, Piscataway, NJ) at 1μg / 250μL (i.p.) was chosen based on demonstrated effectiveness in sickness behavior induction (e.g. Lacosta et al., 1998) and our pilot experiments that replicated these results in rats. Lipopolysaccharide (LPS; from Escherichia coli O127:B8, Sigma-Aldrich, St. Louis, MO) at 250 μg / kg body weight in a volume of 1 mL / kg is effective at causing sickness behavior in rats; additionally, this dose and route of LPS administration has been shown previously to be effective in inducing inflammatory changes in different brain regions via a fast conducting neural pathway as well as a slow conducting humoral pathway producing a longer lasting inflammatory effect in the brain (Bluthe et al., 1992; Konsman et al., 2002; Konsman et al., 2008). Control rats were treated with same volume of 0.9% sterile saline i.p.
Behavioral Experiments
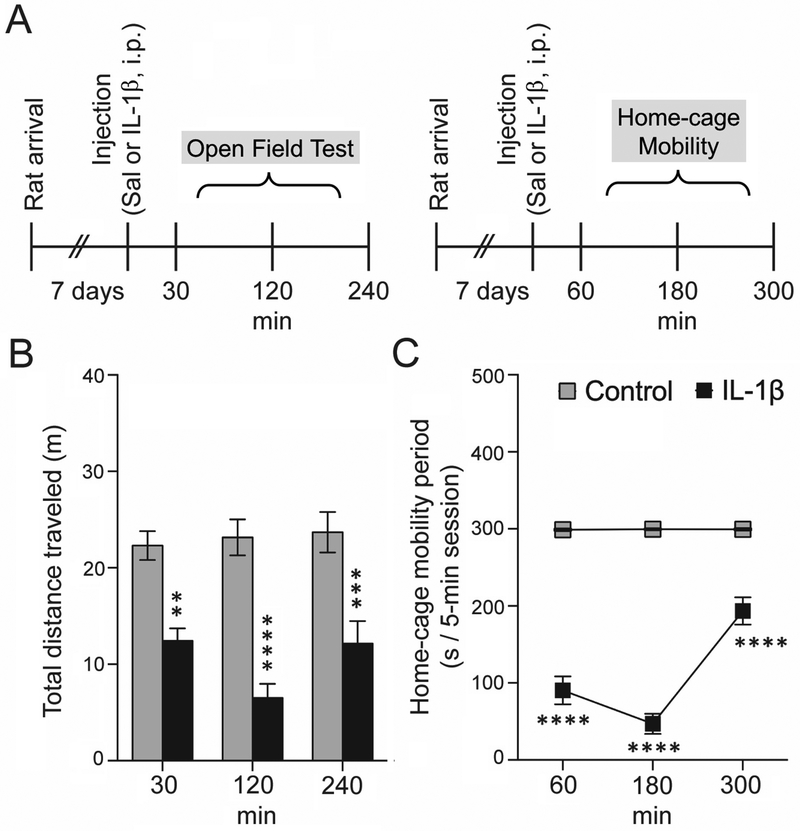
Substantially reduced activity level is a key feature of sickness behavior upon peripheral inflammation (Bluthe et al., 1992; Biesmans et al., 2013). This reduced activity is expected to persist across a range of environments. To verify use of a behaviorally meaningful dose, IL-1β (1 μg / 250μL i.p.) or saline was administered to rats at predetermined times before measuring their activity in the open-field and home-cage.
a. Open field test.
The open field test (black opaque, 24 in.x35 in. or 60.96 cmx88.90 cm) was performed in a dimly lit room (20–25 lx) with computer-generated white noise (65–70 dB) for 5min at 30, 120 and 240min post-treatment. Behavioral recordings were obtained using IR-sensitive cameras (Fire-i, Unibrain, San Ramon, CA) connected to a computer (Dell E6500, Round Rock, TX) and were saved for off-line analysis using ANY-Maze version 4.99 z (Wood Dale, IL). The field was thoroughly cleaned with 70% ethanol between rats. The total distance traveled in the field was quantified and compared. Different cohorts of rats were used at different time points and hence analysis of the data has been done using two-way ANOVA. A total of 25 rats were used in the open field test (14 rats for control group, 11 rats for IL-1β treated group).
b. Home-cage mobility period.
Treated rats were monitored in their home cages at 60, 180 and 300 min post-treatment. The immobility time was recorded by digital stopwatch. The mobility period per session was calculated by subtracting the immobility period from 5min. The same rats were tested at the different time points and hence twoway repeated measures (RM)-ANOVA has been used for analysis of the data. A total of 15 rats were used in the home cage mobility test (8 rats for control group, 7 rats for IL- 1β treated groups).
In vivo Extracellular Electrophysiology
BLA neuronal firing rate was determined using in vivo extracellular electrophysiological recordings following previously published methods (Zhang and Rosenkranz, 2012). A total of 39 rats were used in the electrophysiology study. For the IL-1β treated group, 72 neurons from 16 rats (1 – 18 neurons / rat; median: 4 neurons / rat; mean: 4.5 neurons / rat) and for its corresponding control 73 neurons from 9 rats (1 – 16 neurons / rat; median: 9 neurons / rat; mean: 8.1 neurons / rat) were included. For the LPS treated group, 29 neurons from 7 rats (1 – 8 neurons / rat; median: 2 neurons / rat; mean: 2.8 neurons / rat) and for its corresponding control 15 neurons from 4 rats (2 – 6 neurons / rat; median: 3.5 neurons / rat; mean: 3.7 neurons / rat) were included. This is because only these neurons fulfilled all the criteria mentioned in the data analysis section. For simplicity, the specific number of neurons from the number of rats at different time periods are mentioned below in the result section under the section describing the firing rates of the neurons.
Briefly, rats were anesthetized by urethane (1.5 g/kg i.p.; Sigma-Aldrich) and positioned in a stereotaxic apparatus (Stoelting, Wood Dale, IL). Body temperature measured rectally was maintained at 36–37 C (Model TC-1000, CWE Inc, Ardmore, PA). Burr holes were drilled in the skull and a concentric bipolar electrode (0.25 mm outer diameter, Rhodes Medical Instrument, Summerland, CA) was lowered to the medial prefrontal cortex (+2.7 to +3.2 mm anterior, −0.5 to −0.7 mm lateral, −4.2 to −5.4 mm ventral from bregma) and used only to measure EEG-like local field potential activity for the studies described here. Adequate depth of anesthesia was confirmed by slow rhythmic EEG-like activity (~1 Hz). Dura overlying the BLA (−2.5 to −3.6 mm caudal and - 4.4 to −5.1 mm lateral from bregma) was removed. Single-barrel glass electrodes filled with 2% Pontamine Sky Blue (Alfa Aesar, Ward Hill, MA) in 2 M sodium chloride (Fisher Scientific, Pittsburgh, PA) were slowly lowered into the amygdala using a hydraulic microdrive (Model MO-10, Narishige, East Meadow, NY). A minimum of 45 min was allowed from the time of electrode implantation before any recording was attempted, in order to let the animals achieve hemodynamic stability. This was followed by a period of baseline (pre-treatment) recordings (30–60 min).Rats were then injected i.p. with either IL-1β (1 μg / 250μL), saline (250μL), LPS (250 μg / kg body weight in a volume of 1 mL / kg) or saline (1 mL / kg). Post-treatment recordings were started as soon as any neuronal firing was obtained after the injection.
Electrophysiological signals collected by the recording electrode were amplified and filtered (0.3 Hz – 3 kHz; Dagan Corp., Minneapolis, MN). Signal were audially monitored (Model AM8 Grass Instruments, West Warwick, RI) and digitized (5–10 kHz; Model ITC-18, HEKA, Bellmore, NY) for display and analysis (AxoGraph X version 1.6.4, Sydney, Australia), and storage (Mac Pro/2.8 Apple, Cupertino, CA).
At the end of electrophysiological recording, Pontamine Sky Blue was ejected (−29 μA, 30–45 min) to mark the recording site. Brains were removed and stored in 4% paraformaldehyde (Fisher Scientific) in 0.1 M phosphate buffer overnight at 4C followed by cryoprotection in 25% sucrose (Sigma-Aldrich) in 0.1 M phosphate buffer. Brains were then sliced into 60 mm thick sections using a freezing microtome (Leica Microsystems Inc., Buffalo Grove, IL) and stained with Cresyl Violet (Sigma-Aldrich). All recording sites were reconstructed in atlas representations based on the histology.
Quantification of Serum IL-1β after Peripheral Immune Challenge
Serum levels of cytokine IL-1β were assessed by ELISA at different time points (<30min, 60–120min and >240min) using the Quantikine ELISA Rat IL-1β kit (RLB00; R&D Systems, Minneapolis, MN). Single i.p. injection of saline, IL-1β or LPS (as above) was followed by urethane (≤1.5 g/kg body weight), decapitation within 15–30 min of the anesthesia injection, and trunk blood collection. Serum was separated by centrifugation and stored (<7days; −20C freezer) until quantitative ELISA was performed in duplicate or triplicate following kit literature (R&D Systems, Minneapolis, MN). Actual time windows of blood collection from the time of treatment with saline (control), IL-1β or LPS have been listed in Figure 7E.
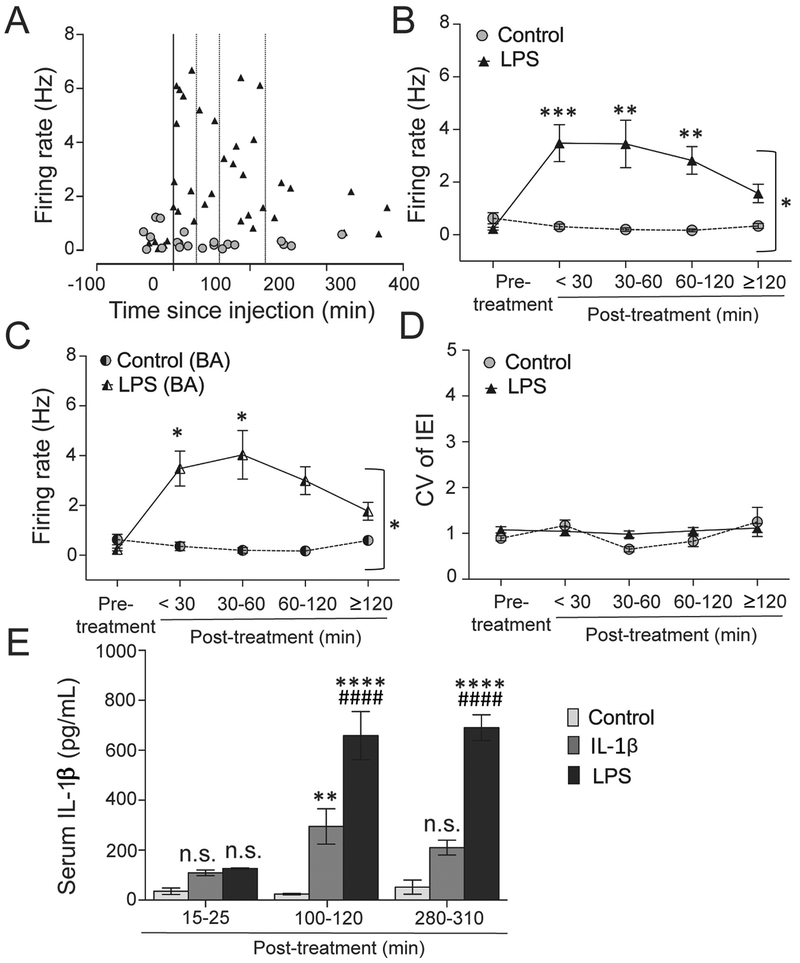
Figure 7: LPS treatment causes prolonged increase in BLA firing rate.
(A) The firing of BLA neurons was measured and plotted against time after saline (control) or LPS treatment. The line through 0 time-point represents time of treatment. Dotted lines represent 30 min, 60 min and 120 min post-treatment. (B) The BLA firing rate between the two groups was compared among five different time-period windows (pre-treatment (baseline), <30 min, 30–60 min, 60–120 min, and ≥120 min post-treatment). There was a significant interaction between treatment over time (p=0.010, two-way ANOVA). The LPS group showed a significant increase in the firing rate at <30 min 30 – 60 min and 60–120 min (i.e. <120 min) after treatment compared to the control. **p<0.01, ***p<0.001 by Holm-Sidak’s post hoc test after significance in twoway ANOVA. (C) Firing rate was significantly increased in the basal (BA) nuclei after LPS treatment at < 30 min. *p<0.05 by Holm-Sidak’s post hoc test after significance in two-way ANOVA. (D) CV of IEI is not changed in the LPS-treated group compared to control (Treatment × time interaction: p=0.224, two-way ANOVA). (E) Pro-inflammatory state induced by IL-1β and LPS treatment in the periphery can be estimated by the eventual infiltration of IL-1β into circulation. Serum levels of IL-1β were assessed by ELISA at different time points (<30 min (15 – 25 min window), 60–120 min (100 – 115 min window) and >240 min (280 – 310 min window)) after single i.p. injection in saline (control group; 250 μL), IL-1β (1 μg in 250 μL) or LPS (250 μg/kg in a volume of 1 mL/kg) treated rats. Serum IL-1β levels were unchanged at <30 min (15 – 25 min window) post-treatment in all the three groups, but was significantly increased in the IL-1β and LPS treated groups at the 60–120 min post-treatment time-point compared to the control. At 60–120 min time-point (100 – 120 min window), level of IL-1β in the serum was also significantly higher in the LPS-treated rats compared to the IL-1β treated group. After 240 min (280 – 310 min window) of treatment, serum IL-1β remained elevated only in the LPS-treated group compared to the other two groups. **p<0.01, ****p<0.0001 vs. control; ####p<0.0001 vs. IL-1β group at respective time points; n.s. indicates non-significance vs. control at that time-point; Holm-Sidak’s post hoc test after significance in two-way ANOVA. Serum samples from N = 5 rats (control), 7 rats (IL-1β) and 4 rats (LPS) / group at each time point were tested from a total of 48 rats in duplicate or triplicate. Plots show mean ± S.E.M.
Data Analysis
BLA neurons were included if: (i) Their locations were within the confines of the BLA complex, as determined by histological reconstruction. The BLA was delineated in Cresyl Violet stained sections based on the borders defined in stereotaxic atlas representations (Paxinos and Watson, 2009). (ii) Their recorded action potentials (APs) had a clear signal-to-noise ratio (>3:1). (iii) At least 3 continuous minutes of data were obtained. The spontaneous firing rate of BLA neurons was measured as the number of APs per second (Hz). (iv) AP half-width > 200 μs is used as a conservative criterion to exclude potential interneurons (Zhang and Rosenkranz, 2012; Zhang and Rosenkranz, 2016). BLA activity was also assessed by quantifying the number of spontaneously active neurons recorded per electrode track, a gross estimation of the relative number of spontaneously active neurons. This was quantified only for electrode tracks that penetrated through the entire BLA.
Statistical analysis was performed using Microsoft Excel and GraphPad Prism version 6.0h (La Jolla, CA). Data distribution was tested using the D’Agostino and Pearson omnibus normality test (α=0.05). For testing variance homogeneity between two groups, F-test was performed, where p < 0.05 indicated non-homogeneity in variance. For more than two groups, variance homogeneity was tested using the Brown-Forsythe test, where p < 0.05 indicated that the variance was significantly different and hence non-homogeneity in variance. When conditions of both normality and variance homogeneity were satisfied, parametric tests were performed (unpaired t-test for comparison between two groups, and analysis of variance (ANOVA) for comparison among more than two groups followed by Holm-Sidak’s post hoc test when p-value became significant). When either of the two conditions were not satisfied either non-parametric test (Mann-Whitney U test for comparison between two groups) or parametric test with Holm-Sidak’s correction method (α=0.05) without assuming consistent standard deviation (S.D.) test (for comparison among more than two groups) was performed. Statistical significance was set at p<0.05. Values are expressed either as mean ± S.E.M. (for parametric result) or median with distribution-based measures (box and whisker plot for non-parametric result).
When data of multiple neurons were obtained from single rat for an overall measure, within-subject averaging was performed. However, when analysis was performed across time, this approach could not be used. Because of the nature of in vivo recordings, the time at which a firing neuron is recorded cannot be predicted, leading to data in which different neurons from the same rat are spread across different time epochs, and not every rat has a neuron recorded in every time epoch. This second factor prohibits the use of two-way repeated measures ANOVA. To diminish concerns related to this approach, the number of neurons per rat recorded for any time epoch was minimized (<3), and results were scrutinized in order to ensure that no single rat disproportionately influenced results.
RESULTS
Effect of IL-1β on locomotor behavior
IL-1β decreased the total distance traveled in the OFT (Figure 1; main-effect of treatment: F(1,62)=74.05, p<0.0001, two-way ANOVA). This was observed at all measured time points [30min (Ncontrol=14 rats, NIL-1β=10 rats), 120min (Ncontrol=13 rats, NIL-1β=11 rats), and 240min (Ncontrol=12 rats, NIL-1β=8 rats), p<0.001, Holm-Sidak’s post hoc test]. Sickness behavior induced by IL-1β reduced the overall activity of the rats in open field significantly (as evidenced by decreased total distance traveled). This substantial locomotor decrease made it difficult to accurately interpret a decrease in central area time and central area exploration of the open field (data not shown) was due to anxiety. Similarly, IL-1β decreased home-cage mobility time (Figure 1; main effect of treatment: F(1,13)=1565, p<0.0001, two-way RM-ANOVA) at each time point assessed [60min, 180min and 300min (Ncontrol=8 rats, NIL-1β=7 rats at each time point), p<0.0001, Holm-Sidak’s post hoc test without assuming consistent S.D., t-ratio = 12.384 (60 min), 20.802 (180 min), 6.466 (300 min) vs. control at respective time-point].
Figure 1: IL-1β treatment produces a reduction in locomotor activity.
To confirm effectiveness of IL-1β in inducing sickness-like behavior, locomotion was measured in an open field and in their home cages at different time points. (A) Experimental timeline at which locomotion in the open field test (30 min, 120 min and 240 min) and home-cage mobility (60 min, 180 min and 300 min) was measured. (B) IL-1β caused a significant decrease in the total distance traveled in the open field (C) IL-1β caused a significant decrease in home-cage mobility at all the time points tested. **p<0.01, ***p<0.001, ****p<0.0001 vs. control at respective time points; Holm-Sidak’s post hoc test after significance in (B) two-way ANOVA, and (C) two-way RM-ANOVA. N=7–14 rats at each time point per group. Plots show mean ± S.E.M.
BLA Neuronal Recordings
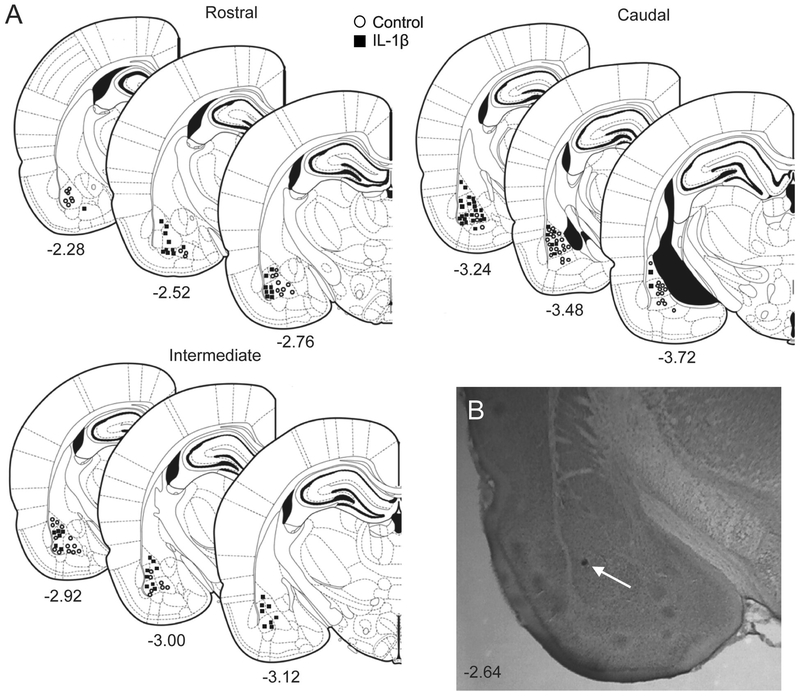
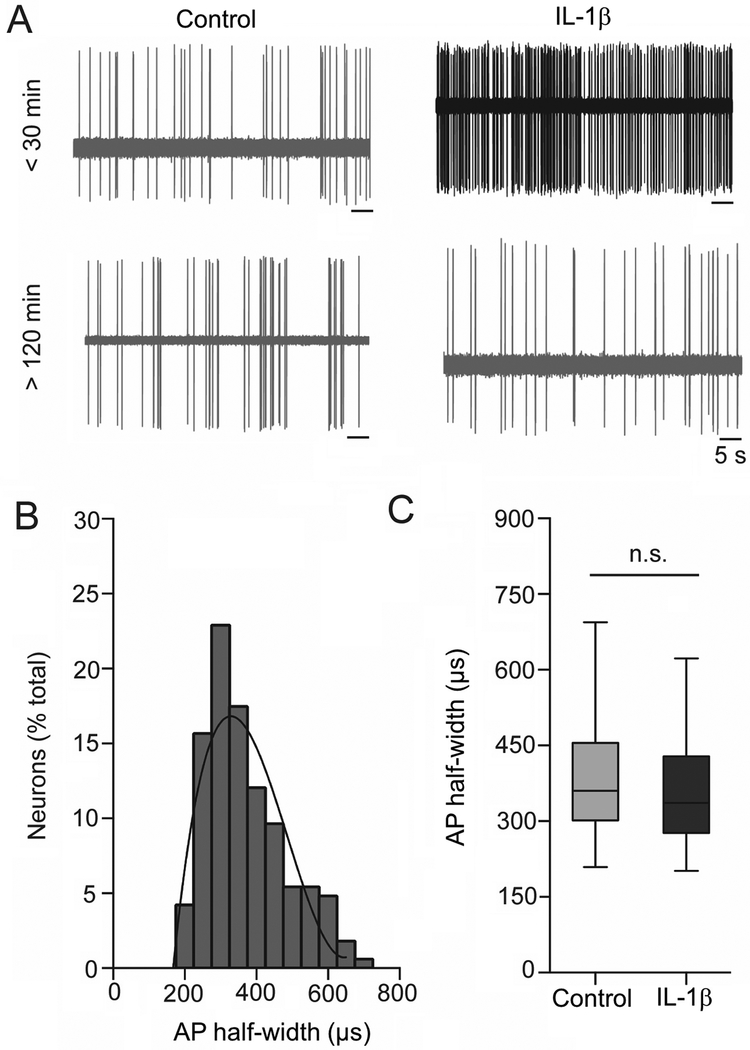
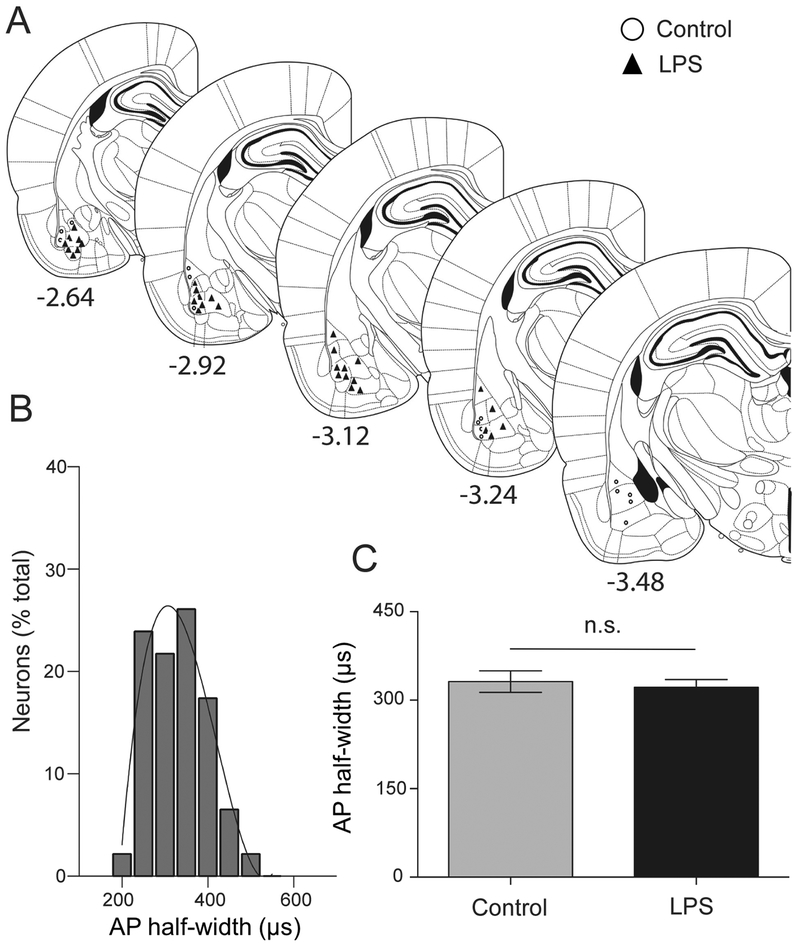
The recording sites for IL-1β and control groups were spread throughout the BLA (Figure 2). The BLA is comprised of projection neurons (approximately 80%) and interneurons (approximately 15–20%), which can be tentatively parsed based on AP half-width (Washburn and Moises, 1992; Rainnie et al., 1993; Rosenkranz and Grace, 1999). Neurons with AP half-width above 200 μs were only considered in the present study. AP half-width from all recorded BLA neurons was plotted as a frequency distribution and fit tested to a single polynomial or two polynomials. A total of 133 neurons from 22 rats were included post-treatment. The frequency distribution data fit best to a single polynomial (3rd order) with a normal distribution (Figure 3B), consistent with a single population. The longer duration half-width of recorded neurons is consistent with BLA projection neurons. The AP half-width of neurons from the two treatment groups were also similar (Figure 3C; U=2328, mediancontrol=360, median IL-1β=338.2, Ncontrol=73 neurons / 9 rats, NIL-1β= 72 neurons / 16 rats, p=0.236, Mann- Whitney U test). These data suggest that the analyzed neuronal data were sampled from a similar population of projection neurons across groups.
Figure 2: Location of recording sites in BLA.
Recording sites in the BLA were reconstructed from brain sections (Paxinos and Watson, 2009) after localization of the Pontamine Sky Blue dye ejected at the conclusion of in vivo recording. (A) Brain sections containing BLA recording sites are shown schematically, listed by bregma level. Recording sites from control group are marked by open circles while those from IL-1β group are shown by black boxes. The numbers below each section indicates distance (in mm) from bregma. (B) An example histological section of a rat brain counterstained with Cresyl Violet showing the location of ejected Pontamine Sky Blue dye in the BLA from an electrophysiological recording (indicated by white arrow).
Figure 3: Extracellular electrophysiological recordings from BLA neurons.
(A) Neuronal firing traces in the control and IL-1β groups at <30 min (upper panel) and later time points (>120 min, lower panel) post-treatment. (B) BLA neurons can be tentatively separated into projection neurons and interneurons based on AP half-width. Frequency distribution of the AP half-widths (μs) from all recorded neurons is plotted here. The data distribution is best fit with one third-order polynomial curve, instead of two curves, indicating that all recordings were obtained from a single population of neurons, most likely BLA projection neurons. (C) There is no significant difference between AP half-widths of neurons from post-treatment control and IL-1β groups (p=0.355, Mann-Whitney U test), indicating that similar population of BLA neurons were sampled in both groups. n.s. indicates non-significance. Data represented with box and whisker plot showing the minimum, 25th percentile, median, 75th percentile and maximum values.
Effects of IL-1β on Spontaneous Firing Rate of BLA neurons
a. Spontaneous firing rate.
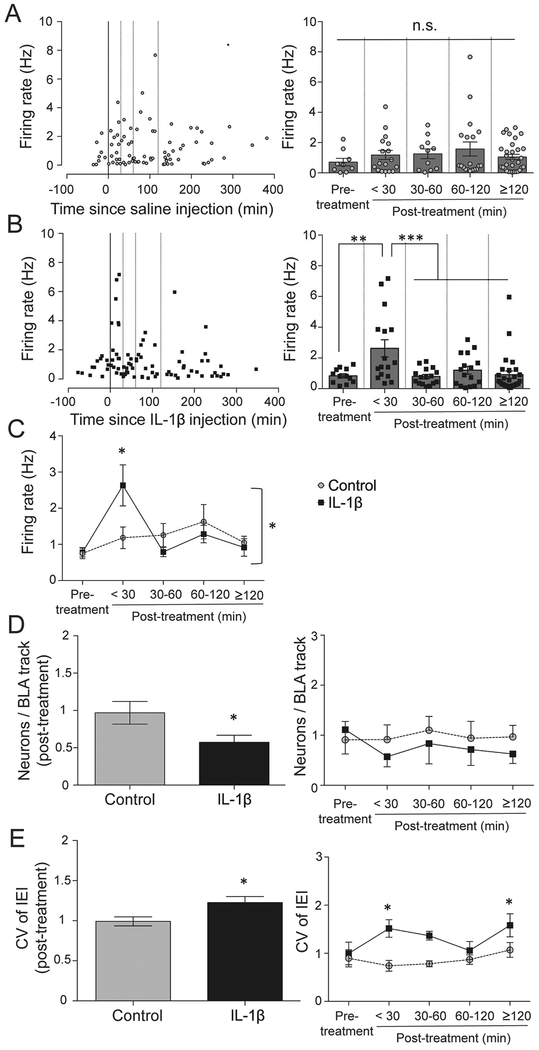
The firing of BLA neurons was recorded pre-treatment and at variable times after saline control (Figure 4A; pre-treatment: 8 neurons from 4 rats; post-treatment: 73 neurons from 9 rats) or IL-1β injection (Figure 4B; pre-treatment: 12 neurons from 7 rats; post-treatment: 72 neurons from 16 rats). The firing rate of neurons recorded from the BLA complex was compared among five different time-period windows [Figure 4; pre-treatment (Ncontrol=8 neurons / 4 rats, NIL-1β=12 neurons / 7 rats), <30min (Ncontrol=14 neurons / 6 rats, NIL-1β=15 neurons / 9 rats), 30–60 min (Ncontrol=11 neurons / 5 rats, NIL-1β=14 neurons / 7 rats), 60–120min (Ncontrol=19 neurons / 7 rats, NIL-1β=16 neurons / 6 rats) and ≥120min (Ncontrol=29 neurons / 7 rats, NIL-1β=27 neurons / 7 rats) after treatment]. There was no difference in the pre-treatment firing rate between control and IL-1β groups, therefore the pre-treatment values were combined together (n=20 neurons / 11 rats) during analysis. There was a significant main-effect of time (F(4,185)=4.703, p=0.001; Figure 4C) and a significant interaction when analyzing the effects of treatment (control / IL-1β) over time (Treatment × time interaction: F(4,185)=3.133, p=0.016, two-way ANOVA). Further analysis revealed a significant increase in the firing rate of the IL-1β group at <30min from treatment compared to the control (Figure 4C; DF=185, t=3.319, p<0.05; Holm-Sidak’s post hoc test without assuming consistent S.D.; t-ratio = 2.300 (< 30 min), 1.511 (30 – 60 min), 0.611 (60 – 120 min), 0.467 (≥120 min)) as well as compared to all other time-period windows (Figure 3B, right; vs. baseline: p<0.05; vs. 30–60min: p<0.05; vs. 60–120min: p<0.05; vs. ≥120min: p<0.01; Holm-Sidak’s post hoc test without assuming consistent S.D. after significance in one-way ANOVA). The firing rate was unaffected in the control group across all the time points (Figure 4A, right; and Figure 4C).
Figure 4: IL-1β treatment acutely increases firing rate in the BLA.
(A) Control group. Firing rate of individual BLA neuron plotted against time from treatment (left). Neurons were grouped into time bins based on the time at which they were recorded. There was no significant effect of control treatment on firing across the time points tested, namely: pre-treatment (baseline), <30 min, 30–60 min, 60–120 min, and ≥120 min post-treatment (right; p>0.05; one-way ANOVA). (B) IL-1β treated group. Firing rate of individual BLA neuron is plotted against time from treatment (left). When analyzed over time, there was a significant effect of IL-1β on firing (right; p=0.001; oneway ANOVA). (C) IL-1β group shows a significant increase in the firing rate at <30 min compared to the control, but returns to the baseline value at later time points. There is a significant interaction between treatment and time [(p=0.016, two-way ANOVA); *p<0.05 by Holm-Sidak’s post hoc test without assuming consistent S.D. after significance in two-way ANOVA]. (D) IL-1β treatment significantly reduced the number of neurons per track in BLA (left; *p<0.05 vs. control, unpaired t-test) and showed a trend towards a reduction in the number of neurons per track over time compared to the control group (right; F(1,193)=1.293, p=0.256, two-way ANOVA). (E) There is a significant post-treatment increase in the CV of IEI of neuronal firing in the IL-1β group compared to the control group (left; *p<0.05, unpaired t-test). Further analysis shows that the CV of IEI is significantly increased at <30 min and ≥120 min (right; *p<0.05 vs. control, Holm-Sidak’s post hoc test after significance in two-way ANOVA). The line through 0 time-point represents time of treatment. Dotted lines represent 30 min, 60 min and 120 min post-treatment. Plots show mean ± S.E.M.
The firing pattern can reflect functional neuronal changes, even in the absence of a change in firing and can be quantified as the coefficient of variation (CV) of the inter-event interval (IEI). Increased CV is consistent with a shift towards irregular or bursting firing patterns. IL-1β caused an increase of CV compared to control (Figure 4E; t = 2.130, df = 23, p = 0.044, CV of IEIcontrol = 0.991±0.056, CV of IEIIL-1β=1.225±0.075, within-subject averaging of CV has been performed; Ncontrol=73 neurons / 9 rats, NIL-1β=72 neurons / 16 rats, p=0.0001, unpaired t-test). CV of firing was increased (main effect of treatment: F(1,163)=12.01, p=0.0007, two-way ANOVA) at the <30min window (Figure 4E, right; DF=163, t=2.935, p<0.05, Holm-Sidak’s post hoc test) and ≥120 min (Figure 4E, right; DF=163, t=2.567, p<0.05, Holm-Sidak’s post hoc test) compared to the control. These data indicate that the firing pattern became more irregular after IL-1β-challenge.
b. Neurons per track.
Number of spontaneously firing neurons encountered per track in the BLA is a gross estimate of global relative BLA activity (Zhang and Rosenkranz, 2012). Our data show that the number of neurons per track was significantly reduced in the IL-1β treated group compared to the control (Figure 4D, left; t = 2.340, df = 20, p=0.029, within-subject averaging of neurons per track was performed; number of tracks: 81 tracks / 9 rats (control) and 102 tracks / 13 rats (IL-1β); Control: 0.967± 0.151, IL-1β: 0.573 ±0.094; unpaired t-test). Analysis across time showed only a trend towards reduction in the number of neurons per track after IL-1β (Figure 4D, right; treatment effect: F(1,193)=1.293, p=0.256, two-way ANOVA).
Different Effects of IL-1β Treatment on Basal and Lateral Nuclei
The lateral and basal nuclei have complementary roles in emotion, but may be differentially impacted by peripheral inflammation. Therefore, the data were separated and analyzed by nucleus.
a. Basal nucleus (BA):
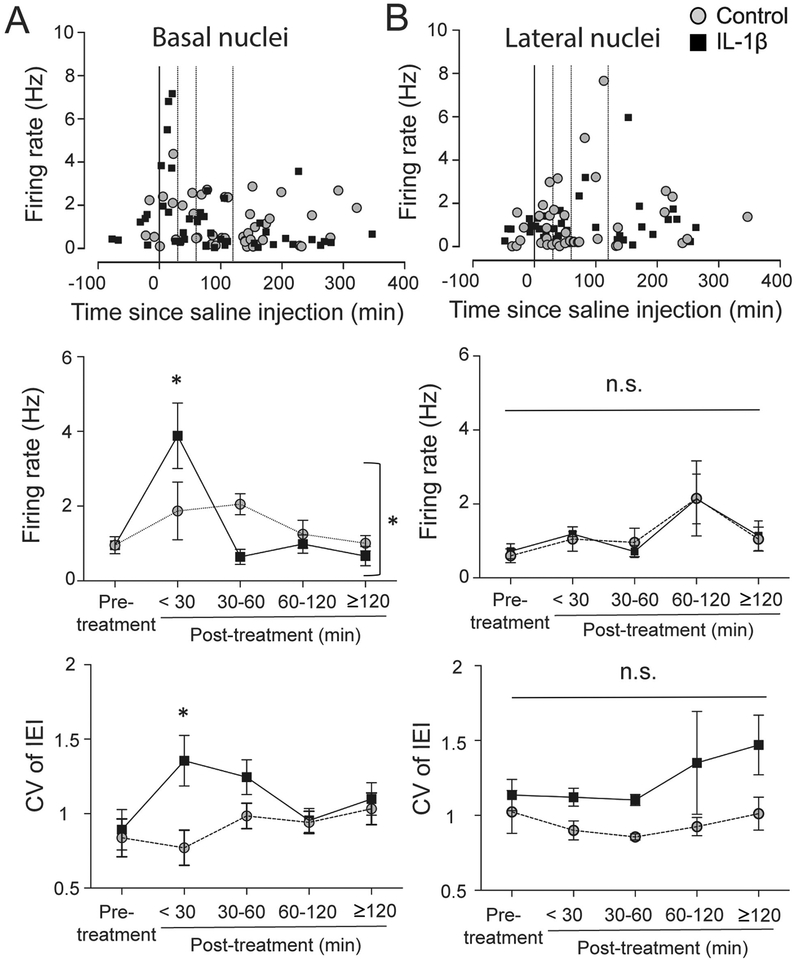
IL-1β significantly impacted the firing of BA neurons (Figure 5A; main effect of treatment, F(4,85)=7.461, p<0.0001), and a significant interaction when analyzing treatment effects over time (Figure 5A; Treatment × time interaction: F(4,86)=3.283, p=0.014, two-way ANOVA). IL-1β increased BA neuronal firing rate at <30min compared to control (Figure 5A, middle; Holm-Sidak’s post hoc test, DF=86, t=3.057, p<0.05). The firing rate was unaffected in the control group across all the time points (Figure 5A, middle). In addition, IL-1β increased the firing CV at <30min compared to control (Figure 5A, lower; treatment effect: F(1,77)=4.302, p=0.041, twoway ANOVA; DF=77, t=2.771, p<0.05, Holm-Sidak’s post hoc test). In the control group, a total of 39 neurons from BA nuclei fulfilled the criteria for analysis which was spread out as follows: 5 neurons / 3 rats (< 30 min), 3 neurons / 3 rats (30 – 60 min), 11 neurons / 5 rats (60 – 120 min) and 20 neurons / 7 rats (≥ 120 min). In the IL-1β treated group, a total of 39 neurons from BA nuclei fulfilled the criteria for analysis and were spread out as follows: 8 neurons / 4 rats (< 30 min), 5 neurons / 4 rats (30 – 60 min), 13 neurons / 5 rats (60 – 120 min) and 13 neurons / 5 rats (≥ 120 min).
Figure 5: IL-1β preferentially impacted firing of neurons in the basal nucleus.
(A) Basal nucleus (BA) of the BLA complex. Firing rate of individual neurons from the BA is plotted against time (upper). The firing rate between the two groups was compared among five different time windows (middle; pre-treatment (baseline), <30 min, 30–60 min, 60–120 min, and ≥120 min post-treatment). There was a significant interaction between treatment and time (middle; p=0.014, two-way ANOVA). CV of IEI was significantly increased at <30 min window in IL-1β group (lower). *p<0.05 by Holm-Sidak’s post hoc test after significance in two-way ANOVA. (B) Lateral nucleus (LAT) of the BLA complex. Firing rate of individual neurons from the lateral nucleus is plotted against time (upper). The firing rate between the two groups was compared among the same five different time windows. There was no significant interaction (p=0.992, twoway ANOVA) and no significant main effects. There was a main effect of treatment on CV of IEI (p=0.003, two-way ANOVA), though no single IL-1β treatment time point emerged as significant compared to control in post hoc tests (p>0.05, Holm-Sidak’s post hoc test). The line through 0 time-point represents time of treatment. Dotted lines represent 30 min, 60 min and 120 min post-treatment. Plots show mean ± S.E.M.
b. Lateral nucleus (LAT):
There were no significant effects of IL-1β on the firing of LAT neurons (Figure 5B; Treatment × time interaction: F(4,72)=0.063, p=0.992, two-way ANOVA). However, IL-1β increased the CV of LAT neuron firing (main effect of treatment F(1,68)=9.443, p=0.003, two-way ANOVA), though no single time point emerged with a significant increase in CV compared to control (Figure 5B, lower; p>0.05, Holm-Sidak’s post hoc test). In the control group, a total of 34 neurons from LAT nuclei fulfilled the criteria for analysis which was spread out as follows: 9 neurons / 4 rats (< 30 min), 8 neurons / 3 rats (30 – 60 min), 8 neurons / 3 rats (60 – 120 min) and 9 neurons / 5 rats (≥ 120 min). In the IL-1β treated group, a total of 33 neurons from LAT nuclei fulfilled the criteria for analysis and were spread out as follows: 7 neurons / 4 rats (< 30 min), 9 neurons / 4 rats (30 – 60 min), 3 neurons / 2 rats (60 – 120 min) and 14 neurons / 7 rats (≥ 120 min).
LPS effects on firing of BLA neurons
The transient effects of IL-1β on BLA firing were unexpected, given the potential for IL-1β to ramp up immune activity. IL-1β can induce c-Fos in the BLA (Brady et al.,1994; Konsman et al.,2000). However, c-Fos induction could be related to brief or prolonged increases of BLA neuron activity. Therefore, we also tested the effects of an inflammagen with a known long duration of action, LPS.
a. Effect of peripheral LPS treatment on BLA neuronal firing.
The AP half-width distribution of BLA neurons showed a unimodal normal distribution curve (Figure 6B), and the AP half-width was similar in both groups (Figure 6C; t=0.429, df=42, Control: 331.5±18.33, LPS: 321.8±13.15, Ncontrol=15 neurons / 4 rats, NLPS=29 neurons / 7 rats, p=0.669, unpaired t-test). This is consistent with recordings from one population of BLA neurons, likely pyramidal neurons.
Figure 6: BLA neuronal recording after peripheral LPS treatment.
(A) Recording sites of BLA neurons from LPS and control groups were reconstructed and plotted onto a stereotaxic rat brain atlas (Paxinos and Watson, 2009). Recording sites from control group are marked by open circles and those from LPS-treated group are shown by black triangles. The numbers (mm) indicate bregma level of the sections (B) Frequency distribution of AP half-widths is consistent with a single population of neurons recorded from the BLA (best fit to one curve instead of two). (C) The AP half-width of neurons in the LPS treated group was similar to the control (p=0.669, unpaired t-test). n.s. indicates non-significance. Plot shows mean ± S.E.M.
LPS significantly increased the firing rate of BLA neurons compared to the control group (U=2, mediancontrol=0.223, medianLPS=2.300, Ncontrol=15 neurons, NLPS=29 neurons, p<0.0001, Mann-Whitney U test; data not shown in figure). To determine if LPS causes long lasting effects on BLA firing, the firing rate was compared among four different time-period windows (pre-treatment (Ncontrol=6 neurons, NLPS=5 neurons), < 30min (Ncontrol=5 neurons, NLPS=9 neurons), 30–60 min (Ncontrol=3 neurons, NLPS=4 neurons), 60–120min (Ncontrol=3 neurons, NLPS=10 neurons), and ≥120min (Ncontrol=4 neurons, NLPS=6 neurons) after treatment). There was a significant main effect of LPS on firing (Figure 7B; F(1,45)=27.75, p<0.0001) and a significant interaction (Treatment × time interaction: F(4,45)=3.734, p=0.010, two-way ANOVA). LPS increased the firing of BLA neurons at <30min (DF=45, t=0.504, p<0.001, Holm-Sidak’s post hoc test), between 30–60 min (DF=45, t=3.286, p<0.01) and also between 60–120min (DF=45, t=3.113, p<0.01) compared to the control (Figure 7B). Results above indicated an effect of IL-1β on firing of BA neurons. Therefore, BA neurons were the focus of examination for the effects of LPS. On further scrutiny, a significant effect of LPS on the firing rate of neurons of the BA nuclei was found (Figure 7C; Treatment × time interaction: F(4,34)=2.932, p=0.034; main effect of treatment: F(1,34)=14.16, p=0.0006). While there was no significant effect on the firing of LAT neurons (Treatment × time interaction: F(3,12)=2.272, p=0.132). There was no significant effect of LPS on BLA neuron firing pattern overall (CV of IEI, p=0.857, t=0.185, df=9, within-subject averaging of CV of IEI was performed; Ncontrol=15 neurons / 4 rats, NLPS=29 neurons / 7 rats; unpaired t-test; data not shown in figure), nor when assessed over time (Figure 7D; Treatment × time interaction effect: F(4,45)=1.478, p=0.224, two-way ANOVA). Thus, immune activation can produce longer lasting effects on BLA neuron activity.
b. Time-dependent effect of IL-1β and LPS on serum pro-inflammatory state.
LPS induces an increase of peripheral IL-1β, and IL-1β level can reflect the time course of LPS immune-activating effects. IL-1β administration itself acts physiologically at many targets, and may also induce further production of IL-1β levels. Therefore, measurement of IL-1β levels in the periphery can reflect the time course of the capacity of LPS or IL-1β treatment to exert immune-mediated effects. To confirm that differences between IL-1β and LPS could be due to differences in the time course of their systemic effects, IL-1β levels in serum were measured after IL-1β and LPS treatment. Both IL-1β and LPS increased serum IL-1β levels (Figure 7D; main effect of time, F(2,39)=25.40, p<0.0001; main effect of treatment: F(2,39)=65.09, p<0.0001; Treatment × time interaction: F(4,39)=11.56, p<0.0001, two-way ANOVA). The serum level of IL-1β was similar in all the groups at <30min (15 – 25 min window) post-treatment. However, it was significantly higher in IL-1β treated group (t=4.472, DF=39, p<0.01; Holm-Sidak’s post hoc test) and LPS treated group (t=9.145, DF=39, p<0.0001; Holm-Sidak’s post hoc test) 60–120min (100 – 120 min window) post-treatment compared to control. LPS had a greater impact than IL-1β treatment (t=5.610, DF=39, p<0.0001; Holm-Sidak’s post hoc test). Beyond 240 min (280 – 310 min window) post-treatment, serum level of IL-1β remained elevated only in the LPS treated group compared to both the IL-1β treated and the control groups (t=9.209, DF=39, p<0.0001 vs. IL-1β treated group; t=7.415, DF=39, p<0.0001 vs. control; Holm-Sidak’s post hoc test). There was no significant difference between the IL-1β -treated and control groups at this time period (t=2.613, DF=39, p>0.05; Holm-Sidak’s post hoc test).
DISCUSSION
This study aimed to understand the effects of peripheral administration of an inflammagen, in a dose that causes sickness-like behavior, on the firing of BLA neurons. Earlier studies in rats have shown that peripheral immune activation using LPS or streptococcal enterotoxin B alters EEG-like activity in central amygdala 125–200min post-treatment (Engler et al., 2011; Prager et al., 2013) and caudal amygdala at 110min post-treatment (Doenlen et al., 2011). Those studies did not observe rapid effects in the amygdala, perhaps because EEG-like activity lacks the resolution of single neurons or because it might reflect a change in synaptic inputs or volume conduction, and might not reflect a change in the firing of amygdala neurons. We found that peripheral inflammagens rapidly increase the firing of BLA neurons, thereby providing a clue as to how it might trigger changes in behavior. This finding provides a functional interpretation for previous observations of BLA immediate early gene transcription after inflammagen exposure, and greatly refines the time course for BLA activation. Furthermore, the rats in these experiments were anesthetized, so it is unlikely that effects on BLA activity were an indirect consequence of malaise instead of immune activation. This effect of peripheral immune challenge on BLA neurons may be the first step in the enduring effects of inflammation on mood and emotion.
Previous studies have reported different effects on rodent open field behavior depending on the dose and time post-treatment of IL-1β. Rat IL-1β in a dose of 1 μg i.p. increased locomotor activity while a lower dose (10 ng) increased grooming and rearing activities (Song et al., 2006). On the other hand, Swiergiel and Dunn found that decreased line crossings in the center of the open field was associated with decreased line crossings in the periphery as well as total number of line crossings at doses of 100 ng or more, but not with 30 ng dose, of IL-1β i.p. in mice 30 or 60 min post-treatment (Swiergiel and Dunn, 2007). However, the effects of peripherally injected IL-1β on BLA firing are not necessarily related to the effects on locomotor activity observed in the present study. The behavioral tests were performed only to confirm the effectiveness of IL-1β in causing sickness-like behavior.
Peripheral inflammation and infection lead to the production and release of pro-inflammatory cytokines like IL-1β, IL-6 and TNF-α (Zhang and An, 2007; Dantzer, 2009). The acute elevation of peripheral pro-inflammatory cytokines produces classical features of sickness behavior and depressed mood. Some of these symptoms may be triggered through the BLA. It is now known that peripheral immune stimuli utilize multiple routes to relay their signals to the brain (Dantzer et al., 2000; Konsman et al., 2002; Felger and Lotrich, 2013). One of the fastest routes is via stimulation of the vagus nerve; other rapid routes include humoral pathways to circumventricular organs, (Katsuura et al., 1990; Dantzer et al., 2000; Konsman et al., 2002), specific saturable transporters and activation of blood-brain barrier endothelium and subsequent activation of perivascular macrophages (Banks et al., 1995; Dantzer, 2009). The rapid effects of IL-1β on BLA neuronal firing in the current study are most parsimoniously explained by effects mediated by the vagus nerve upon i.p. administration. The relatively short duration of the effects on BLA firing may be explained by decreased effects on the vagus as IL-1β moves from the peritoneal cavity to distribute in other tissue and blood. In fact, effects of IL-1β i.p. injection on firing already diminished by the time peak IL-1β is observed in the serum (Figures 4C and7E). Further consistent with this, IL-1 injection rapidly increases NE release within the brain (Dunn, 2006), as would be expected by activation of the vagus nerve. NE causes a predominantly excitatory effect on BLA neuron firing (Buffalari and Grace, 2009), which may underlie BLA activation after peripheral immunoactivation.
However, it is likely that IL-1β will exert a range of slower actions via other described cascades, and may induce effects on BLA function that are missed by focusing on the firing rate. IL-1 receptors are found in the BLA and there are hints of a prolonged effect of IL-1β on firing pattern of BLA neurons (Figure 4E, right), and perhaps even a later suppression of BA neuron firing at longer latencies after IL-1β injection. BLA neurons are relatively quiescent under anesthetized recording conditions, and usually fire only in response to a barrage of synaptic input (Rosenkranz and Grace, 1999; Rosenkranz, 2011). Therefore, the alteration of BLA neuron firing pattern after IL-1β might be due to modulation of excitatory and/or inhibitory inputs to BLA neurons. Indeed, in vitro BLA recordings indicate that IL-1β may shift the balance of excitatory and inhibitory synaptic inputs to BLA neurons (Yu and Shinnick-Gallagher, 1994). In contrast, LPS did not modify the firing pattern of BLA neurons, but did cause a prolonged increase of firing. We speculate that this may reflect differences in the effects of IL-1β and LPS, at these doses, on synaptic input to the BLA.
Several recent studies have demonstrated that pro-inflammatory cytokines may increase glutamatergic release in the amygdala. In a mouse model of complete Freund’s adjuvant (CFA)-induced chronic inflammatory pain, using in vitro whole-cell patch-clamp recording it has been recently shown that peripheral inflammation increased glutamatergic transmission in the BLA, as evidenced by increase in the frequency and amplitude of the miniature excitatory post-synaptic currents (mEPSCs) and inhibition of GABAergic transmission as evidenced by decrease in the frequency and amplitude of the miniature inhibitory post-synaptic currents (mIPSCs) (Chen et al., 2013). In addition, exogenous pro-inflammatory cytokine TNF-α significantly increased mEPSC frequency and amplitude while significantly decreasing mIPSC frequency and amplitude in the BLA, without changing the resting membrane potential, thereby suggesting that TNF-α contributes to the changes in the BLA synaptic transmission (Chen et al., 2013). Another recent study using similar in vitro whole-cell patch-clamp recordings has shown that i.p. challenge with LPS in adult male mice increases glutamatergic release in the amygdala through the chemokine CXCL12 and its receptor CXCR4 thereby causing anxiety-like behavior in the mice (Yang et al., 2016). Similar recruitment of chemokines might play a role in the alteration of the BLA neuronal firing observed in our study. One study has shown that the pro-inflammatory cytokine TNF-α increases the firing rate and GABA release in neurons in rat brain slices from a different amygdala region, the central amygdala (CeA; Knapp et al., 2011). Another whole-cell recording study has found that application of IL-1β (50 ng/mL) for 12 – 15 min in brain slices caused dual effects on mIPSC frequency and amplitude recorded from CeA neurons, as evidenced by increased mIPSC frequency in 62% neurons with decreased mIPSC frequency in 28% neurons, as well as decreased mIPSC amplitude in 38% of neurons, no effect on mIPSC amplitude in 48% of neurons and both increase in frequency and amplitude of mIPSC in 14% of neurons (Bajo et al., 2015), suggesting the existence of the effect of IL-1β on the spontaneous action potential-independent transmission of GABA via both pre- and post-synaptic mechanisms, with predominance of post-synaptic mechanism, in the CeA neurons. These studies suggest that modulation of synaptic activity may be an important mechanism for the effects of inflammation on amygdala neuronal activity. In addition, the ligand-gated non-selective cation channels transient receptor potential vanilloid 1 (TRPV1) are expressed in very high density within the BLA where they increase neuronal excitability (Xiao et al., 2016) and can mediate the effects of IL-1β and other inflammatory cytokines on excitability in the brain (Musumeci et al., 2011; Rossi et al., 2011).
LAT and BA nuclei subserve different, but complementary role in associative memory and emotion (Calandreau et al., 2005; Blume et al., 2017). We found more robust effects of IL-1β challenge on BA neurons. Because the LAT is more heavily implicated in short-duration responses to cues (Erlich et al., 2012), while the BA is involved in longer duration responses (Orsini et al., 2011), one might expect that inflammagen would preferentially modulate longer lasting emotion states and mood, such as depressive-like behavior and generalized anxiety. This effect of inflammagen on the neural substrates of long-lasting emotion states may provide an initial neurobiological hint to the association between depressive disorders and inflammation in humans.
We acknowledge a few limitations in this study. First, we have used urethane anesthesia before blood collection for serum IL-1β measurement, which itself may impact cytokine levels. This approach was preferred in this instance to more approximate recording conditions. Although we had similar urethane-anesthetized rats in the control group, we acknowledge that this can interfere with accurate assessment of absolute levels of cytokines. Second, since we injected the rats with recombinant rat IL-1β, serum ELISA detected both endogenous and exogenous IL-1β, which reflects the overall IL-1β load, not only the effects of exogenous IL-1β on endogenous levels. Third, we have used only one dose of IL-1β and LPS to see their effect on BLA neuronal physiology. This may contribute to differences in the duration of their effects.
Sickness behavior induced by pro-inflammatory cytokines resembles some of the clinical signs and symptoms of depression. Depression is associated with significantly elevated pro-inflammatory cytokines. Inflammatory states that are sufficient to cause sickness behaviors may also result in the emergence of depressive disorders in vulnerable subjects (Dantzer, 2009). BLA hyperactivation is often observed in patients with depression (Frodl et al., 2002; Rubinow et al., 2016). The current results provide a potential link between hyperactivation of the BLA upon inflammation, and amygdala hyperactivation in depression. The goal of our study was to examine the effects of peripheral inflammation on BLA neuronal firing in vivo. Although we have not tested the effects of peripheral inflammation in behavioral mood assays in this study, it would be important to examine how the effects of peripheral inflammation on mood might relate to effects on BLA neuronal firing.
HIGHLIGHTS:
IL-1β (1 μg, i.p.) induced sickness-like behavior in adult male Sprague Dawley rats.
It increased the firing rate in the BLA complex acutely with a change in firing pattern.
The effect was more prominent in the basal nucleus of the BLA complex.
LPS (250 μg/kg, i.p.) increased BLA firing rate acutely and persistently.
Immunogen challenge increased BLA firing rate before serum IL-1β reached its peak.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Drs. Anthony R. West, Gloria E. Meredith, Grace E. Stutzmann, Janice H. Urban and Robert A. Marr for valuable discussion and assistance. The authors thank Dr. Robert J. Bridges for helpful discussion and guidance on ELISA experiments. The authors also thank Mallika Padival and Matthew Anagnostopoulos for technical support.
FUNDING AND DISCLOSURE
This study was supported by the National Institutes of Health grants MH084970 and MH109484 (to J.A.R.). A portion of the study was also supported by the Grant-in-Aid of Research Award from the National Academy of Sciences, administered by Sigma Xi, The Scientific Research Society (to S.M.). The funding bodies had no role in the design of the study, collection and analysis of data and decision to publish. The authors declare no conflicts of interest.
ABBREVIATIONS:
- AP
Action potential
- BA
Basal nucleus
- BLA
Basolateral amygdala
- EEG
Electroencephalogram
- ELISA
Enzyme-linked immunosorbent assay
- IEI
Inter-event interval
- IL
Interleukin
- LAT
Lateral nucleus
- LPS
Lipopolysaccharide
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anisman H and Merali Z (2003) Cytokines, stress and depressive illness: brain-immune interactions. Ann Med 35: 2–11. [DOI] [PubMed] [Google Scholar]
- Bajo M, Varodayan FP, Madamba SG, Robert AJ, Casal LM, Oleata CS, Siggins GR, Roberto M (2015) IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front Pharmacol 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD (1995) Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 2: 241–248. [DOI] [PubMed] [Google Scholar]
- Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P et al. (2013) Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm 2013: 271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ et al. (2017) Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala. J Neurosci 37: 10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW (1992) Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res 573: 318–320. [DOI] [PubMed] [Google Scholar]
- Brady LS, Lynn AB, Herkenham M, Gottesfeld Z (1994) Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci 14: 4951–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM and Grace AA (2009) Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol 12: 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandreau L, Desmedt A, Decorte L, Jaffard R (2005) A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learn Mem 12: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R (2002) Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry (England) 7:468–473. [DOI] [PubMed] [Google Scholar]
- Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu XJ et al. (2013) The contribution of TNF-alpha in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett 541: 275–280. [DOI] [PubMed] [Google Scholar]
- Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D (2005) Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 12: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R (2009). Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 29: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthe RM, Kelley KW (2000) Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci 85: 60–65. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenlen R, Krugel U, Wirth T, Riether C, Engler A, Prager G et al. (2011) Electrical activity in rat cortico-limbic structures after single or repeated administration of lipopolysaccharide or staphylococcal enterotoxin B. Proc Biol Sci 278: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ (2006) Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res 6: 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ and Swiergiel AH (1998) The role of cytokines in infection-related behavior. Ann N Y Acad Sci 840: 577–585. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2009) An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage 47: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi MB et al. (2011) Acute amygdaloid response to systemic inflammation. Brain Behav Immun 25: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Arias C, Sawchenko PE (1997) Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci 17:7166–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE (1994) A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 14:897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Bush DE, Ledoux JE (2012) The role of the lateral amygdala in the retrieval and maintenance of fear-memories formed by repeated probabilistic reinforcement. Front Behav Neurosci 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC and Lotrich FE (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246: 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C et al. (2002) Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 51: 708–714. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R (2008) Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13:717–728. [DOI] [PubMed] [Google Scholar]
- Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG (2016) Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 12:10.1177/1744806916646784. Print 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, Russo SJ (2015) Neuroimmune mechanisms of depression. Nat Neurosci 18: 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Hunter P (2012) The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep 13: 968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI (2012) Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 59: 3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuura G, Arimura A, Koves K, Gottschall PE (1990) Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am J Physiol 258: E163–71. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK and Glaser R (2002) Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res 53: 873–876. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R (2002) Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol 70: 537–547. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Whitman BA, Wills TA, Angel RA, Overstreet DH, Criswell HE, Ming Z, Breese GR (2011) Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain Behav Immun 25 Suppl 1:S146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R (2002) Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 25: 154–159. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Tridon V, Dantzer R (2000) Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience 101: 957–967. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R (2008) Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci 28: 2499–2510. [DOI] [PubMed] [Google Scholar]
- Kovacs D, Kovacs P, Eszlari N, Gonda X, Juhasz G (2016) Psychological side effects of immune therapies: symptoms and pathomechanism. Curr Opin Pharmacol 29: 97–103. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H (1998) Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology 137: 351–361. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V (1999) Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 40:171–176. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L (2009) Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 66:287–292. [DOI] [PubMed] [Google Scholar]
- Lukens JR, Gross JM, Kanneganti TD (2012) IL-1 family cytokines trigger sterile inflammatory disease. Front Immunol 3: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock C, Landau S, Barry K, Maulayah P, Hotopf M, Cleare AJ, Norris S, Pariante CM (2005) Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry 10:332–333. [DOI] [PubMed] [Google Scholar]
- Martinez JM, Garakani A, Yehuda R, Gorman JM (2012) Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety 29:32–38. [DOI] [PubMed] [Google Scholar]
- Mota R, Gazal M, Acosta BA, de Leon PB, Jansen K, Pinheiro RT, Souza LD, Silva RA, Oses JP, Quevedo L, Lara DR, Ghisleni G, Kaster MP (2013) Interleukin-1beta is associated with depressive episode in major depression but not in bipolar disorder. J Psychiatr Res 47:2011–2014. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Grasselli G, Rossi S, De Chiara V, Musella A, Motta C et al. (2011) Transient receptor potential vanilloid 1 channels modulate the synaptic effects of TNF-alpha and of IL-1beta in experimental autoimmune encephalomyelitis. Neurobiol Dis 43: 669–677. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals. National Academies Press. [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC (2010) Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry 15:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Irwin MR, Wellisch DK (2009) When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage 47: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S (2011) Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci 31: 17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Eccleston D, Ferrier IN, Young AH (2001) Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand 103:226–228. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2009) The Rat Brain in Stereotaxic Coordinates: Compact, 6th edn Academic Press: New York, NY. [Google Scholar]
- Prager G, Hadamitzky M, Engler A, Doenlen R, Wirth T, Pacheco-Lopez G et al. (2013) Amygdaloid signature of peripheral immune activation by bacterial lipopolysaccharide or staphylococcal enterotoxin B. J Neuroimmune Pharmacol 8: 42–50. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P (1993) Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol 69: 1350–1362. [DOI] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH (2005) Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 19: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF et al. (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA (2011) Neuronal activity causes rapid changes of lateral amygdala neuronal membrane properties and reduction of synaptic integration and synaptic plasticity in vivo. J Neurosci 31: 6108–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA and Grace AA (1999) Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci 19: 11027–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Muzio L, De Chiara V, Grasselli G, Musella A, Musumeci G et al. (2011) Impaired striatal GABA transmission in experimental autoimmune encephalomyelitis. Brain Behav Immun 25: 947–956. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Mahajan G, May W, Overholser JC, Jurjus GJ, Dieter L et al. (2016) Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct 221: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD (2005) Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol 1: 607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC and Miller GE (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130: 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissolak G, Hoffbrand AV, Mehta AB, Ganeshaguru K (1992) Effects of interferon-alpha (IFN) on the expression of interleukin 1-beta (IL-1), interleukin 6 (IL-6), granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor-alpha (TNF) in acute myeloid leukemia (AML) blasts. Leukemia 6:1155–1160. [PubMed] [Google Scholar]
- Song C, Horrobin DF, Leonard BE (2006) The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry 39: 88–99. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH and Dunn AJ (2007) Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav 86: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT (2005) Increase in interleukin-1beta in late-life depression. Am J Psychiatry 162:175–177. [DOI] [PubMed] [Google Scholar]
- Washburn MS and Moises HC (1992) Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 12: 4066–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Chen X, Zhang PA, Xu Q, Zheng H, Xu GY (2016) TRPV1-mediated presynaptic transmission in basolateral amygdala contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Sci Rep 6: 29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang Q et al. (2016) Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun 56: 352–362. [DOI] [PubMed] [Google Scholar]
- Yu B and Shinnick-Gallagher P (1994) Interleukin-1 beta inhibits synaptic transmission and induces membrane hyperpolarization in amygdala neurons. J Pharmacol Exp Ther 271: 590–600. [PubMed] [Google Scholar]
- Zhang JM and An J (2007) Cytokines, inflammation, and pain. Int Anesthesiol Clin 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W and Rosenkranz JA (2012) Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience 226: 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA (2016) Effects of repeated stress on age-dependent GABAergic regulation of the lateral nucleus of the amygdala. Neuropsychopharm 41:2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]