Abstract
Purpose
Uterine-serous-carcinoma (USC) is a rare and aggressive variant of endometrial cancer. Whole-exome-sequencing (WES) studies have recently reported c-Myc gene amplification in large number of USC suggesting c-Myc as a potential therapeutic target. We investigated the activity of novel BET bromodomain inhibitors (GS-5829 and GS-626510, Gilead-Science-Inc.) and JQ1 against primary USC-cultures and USC-xenografts.
Experimental Design
We evaluated c-Myc expression by qRT-PCR in a total of 45 USC including fresh-frozen-tumor-tissues and primary USC-cell-lines. We also performed immunohistochemistry (IHC) and Western-Blot experiments in 8 USC tumors. USC cultures were evaluated for sensitivity to GS-5829, GS-626510 and JQ1 in-vitro using proliferation, viability and apoptosis-assays. Finally, the in-vivo activity of GS-5829, GS-626510 and JQ1 was studied in USC-ARK1 and USC-ARK2 mouse-xenografts.
Results
Fresh-frozen USC and primary USC cell-lines overexpressed c-Myc when compared to normal tissues (p =0.0009 and =0.0083, respectively). High c-Myc expression was found in 7 of 8 of primary USC cell lines tested by qRT-PCR and 5 of 8 tested by IHC. In-vitro experiments demonstrated high sensitivity of USC cell-lines to the exposure to GS-5829, GS-626510 and JQ1 with BET-inhibitors causing a dose-dependent decrease in the phosphorylated levels of c-Myc and a dose-dependent increase in caspase activation (apoptosis). In comparative in-vivo experiments GS-5829 and/or GS-626510 were found more effective than JQ1 at the concentrations/doses used in decreasing tumor-growth in both USC-ARK1 and USC-ARK2 mouse-xenograft-models.
Conclusions
GS-5829 and GS-626510 may represent novel, highly effective therapeutics agents against recurrent/chemotherapy resistant USC overexpressing c-Myc. Clinical studies with GS-5829 in USC-patients harboring chemotherapy-resistant disease are warranted.
Keywords: Uterine serous papillary cancer, bromodomain proteins, endometrial carcinoma, whole exome sequencing, GS-5829 and GS-626510
INTRODUCTION
Endometrial cancer is the most common gynecologic malignancy with approximately 61,380 new cases and 10,920 estimated disease-related deaths in the United States in 2017 [1]. Uterine serous carcinoma (USC) is a highly aggressive variant of endometrial cancer. While this histologic subtype represents only 3% to 10% of all uterine tumors, it accounts for 40% of all deaths due to its rapid myometrial invasion and intra-abdominal as well as distant spread [2–5]. Consistent with this view, at the time of staging surgery, up to two-third of comprehensively staged USC patients are found to have disease outside the uterus (i.e., stage III and IV) [2–5]. While after surgery USC patients, including women harboring stage I disease, are typically treated with platinum/taxane combination chemotherapy, responses are often non-durable with a median progression-free survival of only 13 months [2–5]. The poor prognosis of USC patients underscores the need to identify novel effective treatment options for patients with advanced or recurrent USC resistant to conventional chemotherapy.
Whole exome sequencing (WES) studies from our laboratory [6] and the Cancer Genome Atlas Network (TCGA) [7] have recently demonstrated the mutational profile of USC is characterized by high genomic instability (ie, copy-number high) and consists of alterations in multiple genes including TP53, PIK3CA, PPP2R1A, CHD4/Mi2b and TAF1, as well as gain of function mutations in multiple oncogenes including but not limited to HER2/neu, CCNE and c-Myc. On the basis of this knowledge, multiple preclinical studies and prospective Phase II investigator-initiated clinical trials targeting HER2/neu gene amplifications with Trastuzumab (ie, a humanized monoclonal antibody targeting HER2/neu, Genentech/Roche) or Afatinib (ie, an irreversible pan-c-erb inhibitor, Boehrineger Ingelheim) or targeting PIK3CA gene mutations with Copanlisib (ie, a novel PIK3CA inhibitor, Bayer) have been recently reported or are currently enrolling USC patients in the United States (clinicaltrials.gov/ct2/show/NCT02491099 and NCT02728258, respectively) [8–11].
The oncogenic role of the c-Myc gene in lymphoma/leukemia and in multiple solid tumors have been extensively documented [12, 13]. Since c-Myc is critically implicated in cell self-renewal, survival and tumorigenesis, targeting c-Myc-addicted tumors with c-Myc specific inhibitors may represent a novel, potentially highly effective therapeutic strategy against multiple human tumors including USC patients harboring recurrent disease resistant to chemotherapy. In agreement with this view, several groups have recently reported the possibility of indirectly abrogating c-Myc function using JQ1, a selective small-molecule inhibitor able to target one bromodomain-containing family important in gene transcription (ie, bromodomain and extra-terminal domain such as BRD2, BRD3, BRD4 and BRDT), which is followed by genome downregulation of MYC-dependent target genes [14–16]. In this study we preclinically characterized the activity of two novel BET inhibitors (ie, GS-626510 and GS-5829, Gilead Science Inc, Foster City, CA), that similarly to JQ1 may reversibly bind the BET bromodomains proteins BRD2, BRD3, BRD4 and BRDT, and prevent protein-protein interaction between BET proteins and acetylated histones and transcription factors [16, 17], against multiple primary USC cell lines overexpressing c-Myc. We provide the first evidence that these BET inhibitors are endowed with remarkable in vitro and in vivo activity against primary USC cell lines as well as USC xenografts. Clinical studies with GS-5829 (ie, clinical compound) in patients harboring chemotherapy-resistant USC are warranted.
MATERIALS AND METHODS
Cell line establishment
Study approval was obtained from the Institutional Review Board at Yale University, and all patients signed consent prior to tissue collection according to the institutional guidelines and in accordance with the Declaration of Helsinki. A total of 8 primary USC cell lines (cell lines characteristics and tissue source are described in Supplementary Table S1) were established from patients at the time of primary staging surgery or tumor recurrence after sterile processing of fresh tumor biopsy samples, as described previously and evaluated in our study [6]. Briefly, tumors were processed by mechanical disruption in an enzymatic solution of 0.14% collagenase type I (Sigma) and 0.01% DNase (Sigma) in RPMI 1640 (Gibco, Life Technologies, Grand Island, NY). The resulting solution was incubated while stirring for 45 minutes at room temperature. The samples were then washed twice with RPMI 1640 10% FBS and plated in Petri dishes as a monolayer in RPMI 1640 containing 10% FBS (Gemini, Woodland, CA), 1% Fungizone (Gibco, Life Technologies, Grand Island, NY) and 1% penicillin/streptomycin (Gibco, Life Technologies, Grand Island, NY). Cells were incubated at 37° C in a humidified atmosphere of 95% air/5% CO2. Primary cell lines were authenticated by whole exome sequencing (WES) at the Yale Center for Genome Analysis [6] and cryopreserved. Tumors were staged according to the International Federation of Gynecology and Obstetrics staging system. All revived cell lines were used within 20 passages, and cultured for less than 6 months. Primary USC cell lines with limited passages were used in the experiments listed below and corresponding cell blocks were analyzed for c-Myc surface expression by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH).
GS-5829 and GS-626510
GS-5829 (clinical compound), GS-626510 (tool compound) and JQ1, were obtained from Gilead Sciences Inc, Foster City, CA. Briefly, compounds were dissolved in DMSO as a 10 mM stock solution and diluted in culture medium immediately before use.
Quantitative real-time PCR
RNA was extracted from a total of 58 samples including 37 fresh frozen USC, 8 primary USC cell lines and 13 normal control tissue samples (from gynecologic organs including normal cervix, uterus and ovaries). RT-PCR was performed using AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. Total RNA (5 μg) was reverse-transcribed using Superscript III (Invitrogen, Carlsbad, CA). Quantitative PCR was carried out to evaluate the expression level of c-Myc (c-Myc, Assay ID: Hs00905030_m1, Applied Biosystems) in all samples with a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s protocol. Each reaction was run in duplicate. The internal control, glyceraldehyde-3-phosphate dehydrogenase, (GAPDH, Assay ID: Hs99999905_ml, Applied Biosystems), was used to normalize variations in cDNA quantities from different samples. The comparative threshold cycle (Ct) method was used for the calculation of amplification fold as specified by the manufacturer. Analyses were performed using SDS software 2.2.2 (Applied Biosystems/Life Technologies).
Immunohistochemistry
Cell blocks were created for the 8 USC cell lines, and immunohistochemistry was performed to evaluate the expression of c-Myc at the protein level. 3x107 USC viable cells were pelleted in a 50 mL conical tube, washed once in PBS, then gently overlaid with formalin (10% NBF) and fixed overnight. The pellet was dehydrated with 70% EtOH, 80% EtOH, 90% EtOH, 100% EtOH (5 minutes each), then HistoGel was applied (Richard Allan Scientific, Cat# HG-4000-012) and allowed to solidify by cooling to near room temperature (<20° C). On the sections deparaffinization and antigen retrieval processes were performed on the Ventana Discovery Ultra autostainer in 3 cycles at 68°C for 8 minutes. The antigen retrieval uses heat-induced enzyme retrieval with Cell Conditioning 1 (CC1) which is a proprietary solution by Ventana, but is congruent to EDTA pH=8. Antigen retrieval conditions are 95°C for 64 minutes. All staining procedures were done on the Ventana Discovery Ultra. One drop of Inhibitor CM, which comes packaged with the Chromomap DAB kit, was automatically dispensed onto each slide and incubated for 8 minutes. With slide heaters disabled, one drop of Anti-c-Myc (#ab32072, RbmAb Clone Y69, Abcam, Burlingame, CA) 1μg/mL in da Vinci green diluent (Biocare Medical, Concord, CA) was manually dispensed and incubated for 40 minutes. The Ventana HQ detection method uses Anti-Rabbit HQ secondary antibody incubated for 32 minutes at 37°C and the Anti-HQ HRP conjugate incubated for 32 minutes. DAB CM was added and incubated for 8 minutes. Lastly, Hematoxylin II was added as the counterstain and incubated for 12 minutes. The slides were rinsed, dehydrated, covered with mounting medium (Dako), and coverslipped. Immunostaining results from tumor tissues excised from ARK2 xenografted animals (ie, PK/PD study and the in vivo efficacy study) were interpreted using an automated computational image analysis system (Definiens Tissue Studio software, Munich, Germany). For each stain, we used the Tissue Studio software to define an intensity and size threshold for nucleus identification and to define an intensity threshold for nuclear stain positivity essentially as previously described [18, 19]. The automated analysis software was trained for scoring only the appropriate epithelial regions of the tissue. Based on these criteria, each cell was classified as positive or negative and depending on intensity of the nuclear stain, positive cells were further categorized into low-positive, medium-positive, or high-positive. For each sample, we estimated the mean percentage of stain-positive cells (at any intensity) across the cores, by weighting each core by its total cell count. Results are aggregated per animal and expressed as mean per group ± SD. Statistical differences between experimental and control groups were measured by the two-tailed Student t test.
Cell viability assay
To determine dose response, cells were aliquoted into 6-well microtiter plates at 40,000 cells per well. After 24 hours the cells were treated with scalar amounts of each drug ranging from 0.001 μM to 5 μM. Three days after treatment the contents of each well were harvested in their entirety and stained with propidium iodide (Sigma Life Sciences, St. Louis, MO) (2 μL of 500 μg/mL stock solution in PBS), to be counted by flow cytometry. The number of viable cells in each well was normalized to the number of viable cells in the control well. The IC50 of each cell line was then determined by comparing the log base 10 of drug concentration in each well to the percentage of viable cells using a non-parametric 3 parameter regression. All IC50 data were calculated using Prism 6 software (GraphPad Prism Software Inc., San Diego, CA). All experiments were completed in at least triplicate.
Immunoblotting
Cells were seeded at 1,000,000 cells/well/2 mL into 6-well plates. After an overnight incubation, cells were treated with GS-626510 for indicated time points for Western blot analysis. Cells were washed with ice-cold PBS post treatment and resuspended in 200 μL with RIPA buffer containing Protease and Phosphatase Inhibitor (ThermoFisher Scientific, cat#78430). Proteins (10 μg) were resolved using SDS-polyacrylamide gels and blotted onto nitrocellulose membranes. Membranes were washed with TBS (140 mmol/L NaCl, 50 mmol/L Tris-HCl; pH 7.2) containing 0.1%Tween20 (TBST) and 5% skimmed milk to block nonspecific protein binding. Membranes were incubated with antibody against c-Myc (1:1000, 5605S, Cell Signaling Technology), BRD4 (1:500, ab128874, Abcam), c-Myc-phsphoT58 (1:500, ab28842, Abcam), c-Myc-phospho S62 (clone 33A12E10) (1:500, ab78318, Abcam), or GAPDH (1:10000) in TBST for overnight at 4°C, washed three times with TBST, and then incubated with the fluorescently-labeled secondary antibody (1:10000) for 1 hour at room temperature. Immunoreactive proteins were scanned and quantified using Odyssey fluorescence imaging system.
Antibody information
Mouse anti c-Myc (Cell Signaling, #5605); mouse anti c-Myc (phospho S62) (Abcam, #Ab78318); rabbit anti-Myc (phospho T58) (Abcam, #Ab28842); rabbit anti-BRD4 (Abcam, #Ab128874); rabbit anti-GAPDH (Santa Cruz Biotechnology, #SC-25778); mouse anti-GAPDH (Santa Cruz Biotechnology, #SC-32233); IRDye® 800CW Coat anti-Rabbit IgG (LI-COR Biosciences, #926-68171); IRDye® 680RD Goat anti-Mouse IgG (LI-COR Biosciences, #926-68070).
IncuCyte Caspase 3/7 activity
Cells were cultured in MEM with 10% FBS in 96-well plate at 3000 cell/well and treated with 3-fold serial dilution (1.5 nM to 10 μM) of GS-626510. DMSO was used as negative control and 6 replicates were done for each treatment. After adding the inhibitor, the 96-well plate was placed in an IncuCyte FLR with 10X objective in a standard cell culture incubator. The kinetic activation of Caspase 3/7 was monitored using CellPlayer™ 96-Well Kinetic Caspase-3/7 Reagent (Essen BioScience, cat#4440). Three images per well were collected every 3 hours in both phase-contrast and fluorescence for 72 hours.
Caspase 3/7 Glo activity
Cells were treated with indicated concentrations of GS-626510. Caspase-Glo® 3/7 Assay was performed after 24 hours treatment using the protocol recommend by the manufacturer (Promega, G8093).
Pharmacokinetic and Pharmacodynamic studies in mice
Female CB17/lcrHsd-Prkd/scid mice (15–19 g) bearing USC-ARK2 tumors were treated with a single dose of GS-5829 at 40, 20, 10 mg/kg, GS-626510 at 10 mg/kg and vehicle for oral gavage studies. Approximately 0.5 mL of blood were taken from animals via cardiac puncture under anesthesia with Isoflurane into K2EDTA tubes at 1 and 6 hours post treatment. Three animals were analyzed per time point. Blood samples were centrifuged (2000xg, 5 minutes) to obtain plasma supernatant and red blood cell pellets. Plasma samples were flash frozen and stored at −80°C until analysis was performed. To the remaining blood cell pellets were added 0.5 mL of TRIzol® Reagent and flash frozen. Following blood collection the tumors were excised and halved. One half was flash frozen and stored at −80°C for pharmacodynamics studies. The second half was fixed in formalin for 24 hours and then transferred in Ethanol 70%. The formalin-fixed tissues were sent to IDEXX BioResearch for paraffin embedment and preparation of slides, for biomarker and histopathological evaluation.
Determination of GS-5829 and GS-626510 concentration in mouse plasma
An aliquot of 10–25 μL of each plasma sample was added to a 96 well plate and cold acetonitrile/internal standard solution was added. After the protein precipitation, an aliquot of the supernatant was transferred to a 96-well plate and diluted with water so as not to exceed the calibration range of the instrument. An aliquot of the above solution was injected to an AB Sciex API-5000 triple quadrupole LC/MS/MS system. A Zorbax Extend Rx-C18 HPLC column (50 x 2.1 mm, 3.5μm), from Agilent (Part # 735700-902) was used for separation. Mobile phase A contained 1% acetonitrile and 5% methanol in 10 mM ammonium formate adjusted to pH 3.0 with formic acid. Mobile phase B contained 5% methanol and 10% 10 mM ammonium formate in acetonitrile adjusted to pH 5.0 with formic acid. A Thermo Aria multiplexer with two identical Agilent 1200 series binary pumps (P/N G1312A Bin Pump) was used for elution and separation. Sampling was performed by a HTS Pal autosampler (LEAP Technologies, Carrboro, NC). Mass spectrometry was performed on an API-5000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA), using positive ion electrospray ionization and multiple reaction monitoring.
Xenograft implantation and in vivo drug study
The cell line ARK1 and ARK2 were expanded in T-150 tissue culture flasks. The cells were collected and washed in PBS. Four groups of 10 female CB17/lcrHsd-Prkd/scid mice each were injected with ARK2 tumor cells and two groups of 6 female CB17/lcrHsd-Prkd/scid mice each were injected with ARK1 tumor cells (7 x106 cells in 0.3ml of PBS with 50% Matrigel® (BD Biosciences)) subcutaneously into the lower abdomen area. Mice were triaged into treatment groups when mean tumor burden was 150–300mg (target 200 mg) or 0.119–0.238 cm3 (target 0.159 cm3). Dosing begun upon reaching target size and was delivered to the ARK2 xenografts according to Supplementary Table S2 for 28 days (every day), and to the ARK1 xenografts according to Supplementary Table S3 for 3 weeks (5 days/week). Tumor measurements and mouse weights were recorded at least two times weekly and reported for each mouse (individually identified). Tumor volumes were calculated using the formula 0.5 × (width2 × height). On day 28 at the specified time point post last dose (Supplementary Table S2), ARK2 animals were humanely euthanized. Blood and tumors were collected and tumor tissue weights were recorded upon collection. On day 19 at the specified time point post last dose (Supplementary Table S3), ARK1 animals were humanely euthanized. Blood and tumors were collected and tumor tissue weights were recorded upon collection. All mice were housed and treated in accordance with the policies set forth by the Institutional Animal Care and Use Committee (IACUC) at Yale University.
Statistical analysis
GraphPad Prism version 6 (GraphPad Software, Inc. San Diego, CA) was used to determine statistical significance by the student’s t-test. Differences in all comparisons were considered statistically significant at p-values < 0.05.
RESULTS
c-Myc overexpression in fresh frozen USC and primary USC cell lines by RT-PCR and IHC
A recent comprehensive NGS study by our group found c-Myc amplification in over 40% of USC samples when analyzed by WES [6]. To assess/validate c-Myc transcript expression in USC in this study we performed real-time PCR in a total of 45 tumor samples including 37 fresh frozen USC and 8 primary USC cell lines as well as 13 normal tissue controls obtained from cervix/uterus and ovaries collected from patients undergoing hysterectomies for benign conditions (ie, endometriosis and uterine leiomyoma). We found c-Myc expression in fresh frozen tumors (mean ± SEM relative mRNA values = 5.36 ± 1.16) and primary USC cell lines (mean ± SEM = 14.56 ± 3.68) to be significantly higher when compared to normal tissues (mean ± SEM = 1.16 ± 0.14) (p = 0.0009 and = 0.0083, respectively) (Figure 1A). Next, we performed immunohistochemistry (IHC) on cell blocks obtained from the primary USC cell lines to evaluate the expression of c-Myc at the protein level. Moderate to high levels (ie, 2+ and 3+ staining) of c-Myc protein expression were detected in 62.5% (5 out of 8) of the USC cell lines, with the remaining 3 USC cell lines demonstrating 1+ c-Myc expression (Figure 1B).
Figure 1.
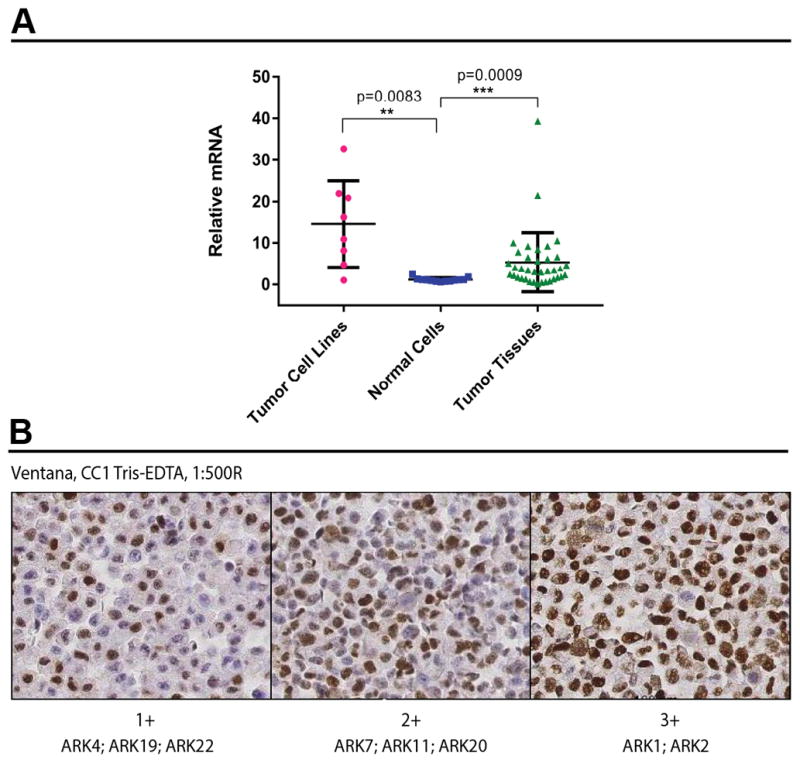
A) c-Myc over expression by RT-PCR in primary USC cell lines, normal cells and fresh frozen USC. B) Expression of c-Myc at the protein level, assessed by immunohistochemistry on 8 primary USC cell lines.
GS-5829 and GS-626510 inhibited cell proliferation in primary USC cell lines
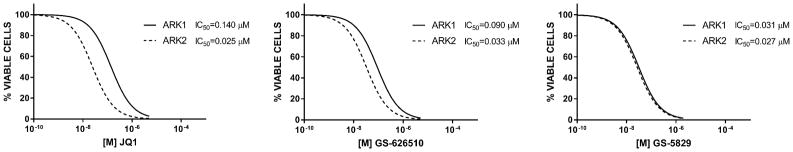
We investigated the in vitro effects of GS-5829 and GS-626510 on the growth of the 8 primary USC cell lines using flow cytometric-based assays as described in the Methods. As representatively shown for ARK1 and ARK2 cell lines (Figure 2), after incubation with varying concentrations of GS-5829 or GS-626510 or JQ1 we found a progressive, dose-response decrease in cell proliferation in all USC tested. No significant differences were noted in the IC50 of GS-5829, GS-626510 or JQ1 in ARK1 and ARK2 (Figure 2) or in any of the 6 additional USC cell line evaluated in vitro (data not shown).
Figure 2.
In vitro cell proliferation of ARK1 and ARK2 following 72 hours incubation with JQ1, GS-626510 or GS-5829 at increasing concentrations.
Treatment with GS-626510 induces apoptosis in ARK1 and ARK2 USC cells
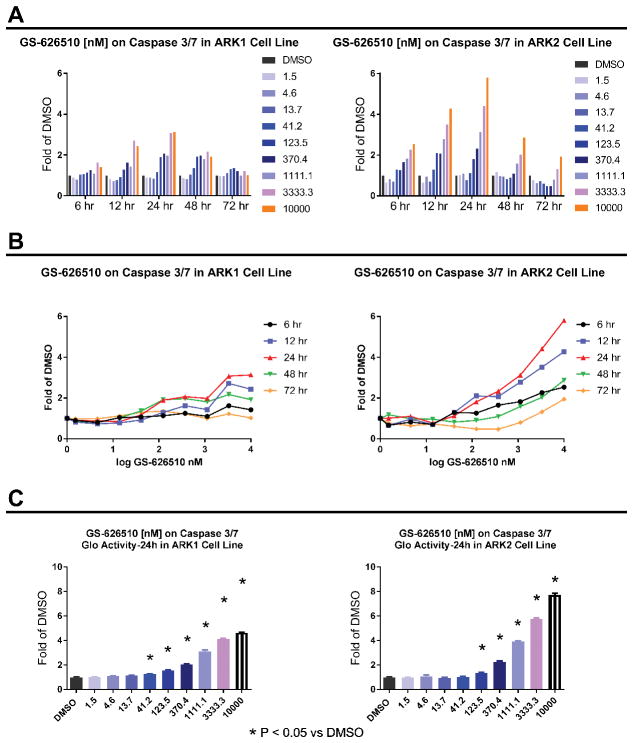
To examine whether BET-inhibitors inhibit the survival of ARK1 and ARK2 cells by triggering the apoptotic pathway, we measured the increase in caspase 3/7 activity following treatment with GS-626510. Real time increase in caspase 3/7 activity was monitored by using the IncuCyte FLR imaging system. Caspase 3/7 activity was found consistently increased in the treatment groups (0–10 μM) with time. Figure 3A and Figure 3B show the apoptotic activity peaks at 24 hours. Consistent with the IncuCyte Casapse3/7 assay results, GS-626510 induced the Casapse3/7 Glo activity in both cell lines in a concentration dependent manner. As shown in Figure 3C, we found ARK1 and ARK2 cell growth to be significantly inhibited by activation of apoptosis after a 24 hours treatment with GS-626510 starting at 41.2 nM and 123.5 nM, respectively.
Figure 3.
A) and B) Increase in caspase 3/7 activity in ARK1 and ARK2 cells, following treatment with GS-626510. C) Caspase 3/7 Glo activity in ARK1 and ARK2 cells, following a 24 hours treatment with increasing concentrations of GS-626510.
c-Myc protein levels by Western Blot after exposure to BET-inhibitors
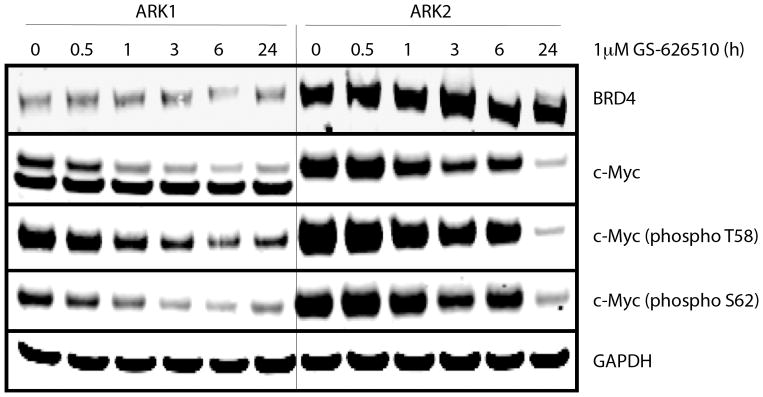
To determine whether the compounds effectively target c-Myc, western blotting was performed on ARK1 and ARK2 cells after exposure to GS-626510 for 0–24 hours. Both cell lines expressed high levels of the c-Myc protein, which was markedly suppressed by GS-626510 in a dose dependent manner (Figure 4). Some cell lines may have up to 3 different c-Myc isoforms with slightly different molecular weight [20] and accordingly, ARK-1 cell lines was found to express two different c-Myc isoforms by Western Blot. The BET inhibitor GS-626510 reduced both total and phospho c-Myc proteins at 0.5 hour and 1 hour in ARK1 and ARK2 cells, respectively, with the decrease reaching its peak at 24 hours after treatment. The BRD4 protein expression level was higher in ARK2 cells when compared to ARK1 cells. However, the inhibitor had not detectable effect on BRD4 protein expression in both cell lines (Figure 4).
Figure 4.
c-Myc and BRD4 protein levels expression in ARK1 and ARK2 cells, after exposure to GS-626510 (1 μM) for 0–24 hours.
BET-inhibitors GS-5829 and GS-626510 exhibit excellent oral bioavailability after a single administration in animals bearing USC-ARK2 tumors
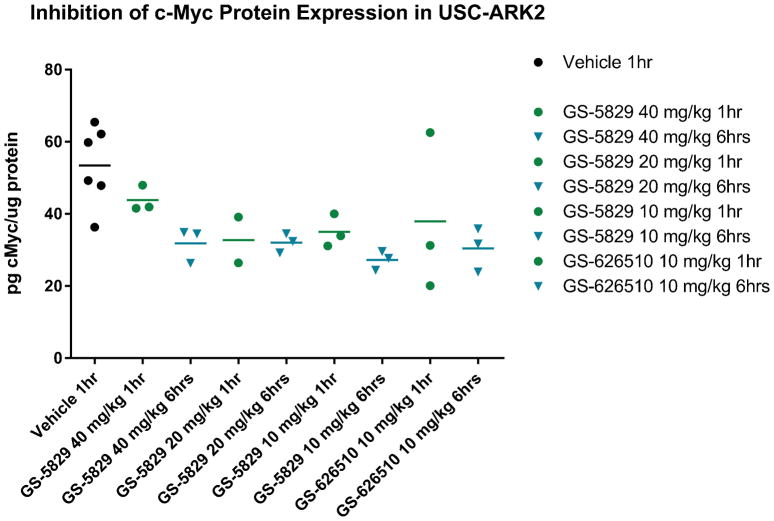
Xenografts bearing USC-ARK2 tumors were treated with a single dose of GS-5829 at 40, 20, 10 mg/kg, GS-626510 at 10 mg/kg or vehicle through oral gavage and blood samples were collected at different time points for PK and PD studies. As shown in Figure 5, no significant difference in inhibition of c-Myc protein expression were detected at the dose of 10 mg/kg to 40 mg/kg for both drugs, and such inhibition was significantly greater when compared to the control group (ie. vehicle group). Pharmacokinetic analysis of collected plasma showed excellent oral bioavailability for both GS-5829 and GS-626510 in the mouse model (data not shown).
Figure 5.
Inhibition of c-Myc protein expression after a single treatment with GS-5829 or GS-626510 in animals bearing USC-ARK2 tumors.
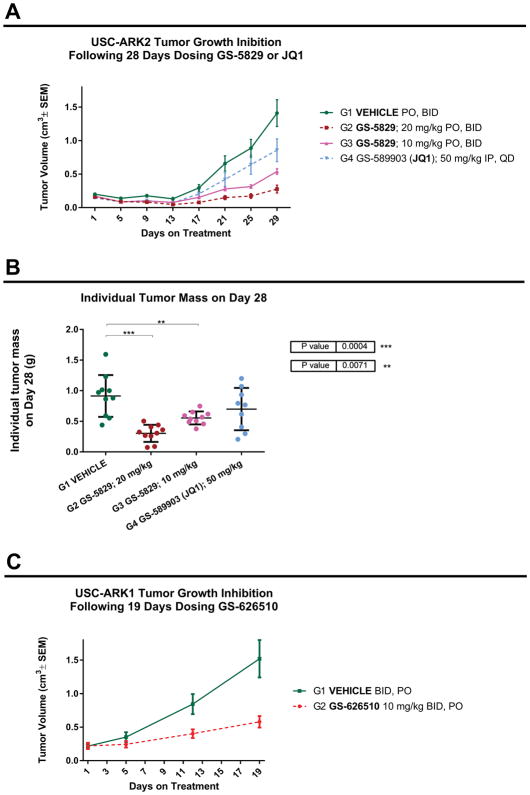
GS-5829 impaired USC-ARK2 xenograft tumor growth in vivo
We next evaluated the impact of GS-5829 in vivo in xenograft models generated by injecting subcutaneously ARK2 cells into female CB17/lcrHsd-Prkd/scid mice. For comparison we also tested the BET inhibitor JQ1, dosed by intraperitoneal injection (50 mg/kg/day). Twice daily oral doses of GS-5829 10 mg/kg and 20 mg/kg were well tolerated with no clear impact on body weight compared with vehicle control. As shown in Figure 6A, mice undergoing twice daily oral treatment with GS-5829 (20 mg/kg and 10 mg/kg) exhibited a significantly slower rate of tumor growth, compared to vehicle control and to mice undergoing daily treatment with JQ1 (50 mg/kg/day IP). This difference was statistically significant starting on dosing day 21 (p value <0.05). The tumor tissue weights recorded on day 28 (last day of treatment) confirmed a statistically significant difference between the control group and the animals treated with both 20 and 10 mg/kg (Figure 6B).
Figure 6.
A) USC-ARK2 tumor growth inhibition in vivo, following 28 days dosing GS-5829 or JQ1. B) Individual tumor mass of USC-ARK2 xenograft model, recorded on day 28, after dosing GS-5829 or JQ1. C) USC-ARK1 tumor growth inhibition in vivo, following 19 days dosing GS-626510.
GS-626510 impaired USC-ARK1 xenograft tumor growth in vivo
To generalize our in vivo findings with GS-626510 we developed a second xenograft model by injecting subcutaneously ARK1 cells into female CB17/lcrHsd-Prkd/scid mice. As shown in Figure 6C, similarly to the results obtained with the ARK2 xenograft model, after 19 days of treatment, mice undergoing twice daily oral treatment with GS-626510 (10 mg/kg) exhibited a significantly slower rate of tumor growth (P<0.05), with no clear impact on body weight, compared with vehicle control.
BET-inhibitors GS-5829 and GS-626510 significantly reduced c-Myc protein expression in ARK2 xenograft tumor samples
IHC was initially performed on the tumor tissues collected after a single treatment with GS-5829 or GS-626510 in animals bearing USC ARK2 xenografts (ie. PK/PD study) and an average quantitative nuclear H-Score was given to each sample through morphometric analysis as described in the methods. A significant in vivo modulation of c-Myc expression was observed in all treated groups when compared to the vehicle-treated control group (p < 0.05) (Supplementary figure S1 and Table S4). Similarly, IHC was performed on the tumor tissues excised from ARK2 xenografted animals after 28 days of twice daily oral treatment with GS-5829 (20 mg/kg and 10 mg/kg) or daily intraperitoneal treatment with JQ1 (50 mg/kg) (Supplementary figure S2 and Table S5). These experiments revealed a significant modulation of c-Myc expression in all groups when compared to vehicle treated group (P< 0.05).
DISCUSSION
Our laboratory has recently used next-generation sequencing (ie, WES) to analyze the genetic landscape of a large number of fresh frozen USC as well as multiple primary USC cell lines [6]. In this comprehensive genetic analysis we found somatic copy number variations (CNV) to play a major role in the pathogenesis of USC [6]. Of interest, CNV analyses identified amplification of HER2 gene in 44% of the whole exome-sequenced USC with an additional 16% and 52% of the USC samples, respectively, demonstrating SNV mutations or CNV gain in the phosphoinositide 3-kinase (PI3K) gene [6]. Importantly, gain of function mutations in c-Myc were detected in 40% of the sequenced USC [6]. These results strongly suggested that MYC gain of function mutation may represent a novel target for USC patients with advanced/recurrent disease unresponsive to chemotherapy.
c-Myc has long been considered an “undruggable target”. In the last few years however multiple preclinical studies have validated the inhibition of BET bromodomain (using JQ1 as prototype BET inhibitor), as an alternative, highly effective strategy to indirectly downregulate c-Myc activity in c-Myc-driven cancers [12, 14–16]. Accordingly, in this study we initially evaluated c-Myc expression in fresh frozen USC and multiple primary USC cell lines available to our laboratory and subsequently tested the activity of two novel orally active BET inhibitors indirectly targeting c-Myc by reversibly binding the BET proteins BRD2, BRD3, BRD4 and BRDT in in vitro and in vivo experiments. GS-5829 is currently being evaluated in multiple phase 1–2 clinical trials in solid tumors and lymphomas, including but not limited to metastatic castrate-resistant prostate cancer and in advanced estrogen receptor positive HER2-negative breast cancer (ClinicalTrials.gov identifier: NCT02392611, NCT02607228, NCT02983604) while GS-626510, due to its shorter half-life, has so far been assessed only in pre-clinical studies. The results demonstrate high RNA expression of c-Myc in over 70% of the fresh frozen USC tested and in 7 out of 8 of the primary USC cell lines available to this study confirming that a large number of USC may be addicted to overactivation of the c-Myc pathway. IHC and western blot experiments confirmed protein expression of different isoforms of c-Myc in primary USC cell lines indicating the potential existence of different biological mechanisms driving proliferation and/or survival in these endometrial tumors. Taken together, these data are in agreement with previous NGS studies from our groups [6] as well the TCGA Network demonstrating common gain of function mutation in c-Myc in copy-number high (ie, serous-like) endometrial cancer patients [7].
Next, we preclinically investigated the therapeutic potential of GS-5829 or GS-626510 against multiple well characterized (ie, WES sequenced) primary USC cell lines. We found both the tool and clinical BET inhibitor compounds to significantly inhibit tumor cell proliferation in USC cell lines by causing apoptosis. This biologic effect was associated with a dose dependent decrease in the phosphorylated levels of c-Myc in the USC cell lines exposed to scalar concentration of GS-5829 or GS-626510 and a dose dependent increase in caspase activation (ie, apoptosis). Importantly, we found that activity of the BET inhibitors was not affected by the presence or absence of gain of function mutations in the PIK3CA gene, a genetic feature we previously correlated with resistance to trastuzumab treatment in USC [21], as demonstrated by the high sensitivity to GS-5829 and GS-626510 of both USC-ARK1 (PIK3CA-mutated) and USC-ARK2 (PIK3CA-wild type) cell lines [6]. Importantly, the novel BET inhibitors were both active in vivo in decreasing tumor growth in xenografts and their activity was more effective at the concentrations/doses used when compared to JQ1. It is worth noting that no evidence of acute or chronic toxicity was detected in the treated animals as demonstrated by the lack of significant variation in behavior or body weights when compared to the mice in the control groups. Taken together, these results demonstrate promising activity of GS-5829 or GS-626510 against c-Myc-amplified USC cell lines both in vitro as well as in vivo.
In conclusion, using a highly annotated collection of USC cell lines we have demonstrated that GS-5829 and GS-626510, two novel BET-inhibitors, have impressive activity against USC primary tumors as well as USC xenografts. Assessment of c-Myc expression in tumors exposed to the BET-inhibitors demonstrated down-regulation of both total and phospho c-Myc proteins. The clinical compound GS-5829 demonstrated excellent bioavailability after oral administration and was significantly more effective than JQ1 at the doses used in comparative experiments in vivo against USC xenografts. Clinical studies with GS-5829 in USC patients harboring disease resistant to standard salvage chemotherapy are warranted.
Supplementary Material
Cell lines characteristics and tissue source.
Groups and dosing description of the in vivo drug study on USC-ARK2 xenograft.
Groups and dosing description of the in vivo drug study on USC-ARK1 xenograft.
Average quantitative nuclear histological score associated with the ex vivo c-Myc expression assessed by IHC in the ARK2 xenograft tumor samples, excised 1 hour and 6 hours after a single dose of GS-5829 at 40, 20, 10 mg/kg, GS-626510 at 10 mg/kg and vehicle for oral gavage studies.
Average quantitative nuclear histological score associated with the ex vivo c-Myc expression assessed by IHC in the ARK2 xenograft tumor samples, excised 1 hour, 6 hours and 12 hours after the last dose of GS-5829 at 20, 10 mg/kg, JQ1 at 50 mg/kg and vehicle for oral gavage studies, following 28 days of treatment.
Ex vivo c-Myc expression assessed by IHC on the ARK2 xenograft tumor samples, excised 1 hour and 6 hours after a single dose of GS-5829 at 40, 20, 10 mg/kg, GS-626510 at 10 mg/kg and vehicle for oral gavage studies.
Ex vivo c-Myc expression assessed by IHC on the ARK2 xenograft tumor samples, excised 1 hour, 6 hours and 12 hours after the last dose of GS-5829 at 20, 10 mg/kg, JQ1 at 50 mg/kg and vehicle for oral gavage studies, following 28 days of treatment.
TRANSLATIONAL RELEVANCE.
Uterine serous carcinoma (USC) is a rare but highly aggressive variant of endometrial cancer accounting for a disproportionately large number of deaths. Whole exome sequencing (WES) results have recently demonstrated gain of function mutations in the c-Myc oncogene in a large number of USC. In an effort to develop innovative treatments against this deadly gynecologic malignancy, we preclinically evaluated the activity of two novel BET bromodomain inhibitors (ie, GS-5829 and GS-626510, Gilead Science, Foster City, CA), against multiple primary USC cell lines and USC xenografts. Our results demonstrated remarkable activity of GS-5829 and GS-626510 both in vitro as well as in vivo. Clinical studies with GS-5829 in patients harboring chemotherapy-resistant USC are warranted.
Acknowledgments
Financial support: This work was supported in part by grants from NIH U01 CA176067-01A1, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to A.D.S., and Gilead Sciences Inc., Foster City, CA. This investigation was also supported by NIH Research Grant CA-16359 from NCI and Stand-up-to-cancer (SU2C) convergence 2.0 grant to Alessandro Santin.
Abbreviations
- USC
uterine serous carcinoma
- RT-PCR
reverse transcriptase-polymerase chain reaction
- LOH
loss of heterozygosity
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson M, et al. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Fader AN, Santin AD, Gehrig PA. Early stage uterine serous carcinoma: management updates and genomic advances. Gynecol Oncol. 2013;129(1):244–50. doi: 10.1016/j.ygyno.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PE. The management of serous papillary uterine cancer. Curr Opin Oncol. 2006;18(5):494–9. doi: 10.1097/01.cco.0000239890.36408.75. [DOI] [PubMed] [Google Scholar]
- 5.Goff BA, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994;54(3):264–8. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 6.Zhao S, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110(8):2916–21. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research, N., et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menderes G, et al. Superior in vitro and in vivo activity of trastuzumab-emtansine (T-DM1) in comparison to trastuzumab, pertuzumab and their combination in epithelial ovarian carcinoma with high HER2/neu expression. Gynecol Oncol. 2017;147(1):145–152. doi: 10.1016/j.ygyno.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab CL, et al. Afatinib demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro and in vivo. Br J Cancer. 2014;111(9):1750–6. doi: 10.1038/bjc.2014.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez S, et al. Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol. 2014;135(2):312–7. doi: 10.1016/j.ygyno.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fader AN, Roque DM, Siegel E. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress Her2/neu. J Clin Oncol. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 12.Zuber J, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–8. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller DM, et al. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18(20):5546–53. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertz JA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011;108(40):16669–74. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–17. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi X, et al. Loss of TRIM33 causes resistance to BET bromodomain inhibitors through MYC- and TGF-beta-dependent mechanisms. Proc Natl Acad Sci U S A. 2016;113(31):E4558–66. doi: 10.1073/pnas.1608319113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell RK, et al. VEGF-A/VEGFR Inhibition Restores Hematopoietic Homeostasis in the Bone Marrow and Attenuates Tumor Growth. Cancer Res. 2016;76(3):517–24. doi: 10.1158/0008-5472.CAN-14-3023. [DOI] [PubMed] [Google Scholar]
- 19.Oh H, et al. Expression of estrogen receptor, progesterone receptor, and Ki67 in normal breast tissue in relation to subsequent risk of breast cancer. NPJ Breast Cancer. 2016:2. doi: 10.1038/npjbcancer.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benassayag C, et al. Human c-Myc isoforms differentially regulate cell growth and apoptosis in Drosophila melanogaster. Mol Cell Biol. 2005;25(22):9897–909. doi: 10.1128/MCB.25.22.9897-9909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black JD, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015;113(7):1020–6. doi: 10.1038/bjc.2015.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell lines characteristics and tissue source.
Groups and dosing description of the in vivo drug study on USC-ARK2 xenograft.
Groups and dosing description of the in vivo drug study on USC-ARK1 xenograft.
Average quantitative nuclear histological score associated with the ex vivo c-Myc expression assessed by IHC in the ARK2 xenograft tumor samples, excised 1 hour and 6 hours after a single dose of GS-5829 at 40, 20, 10 mg/kg, GS-626510 at 10 mg/kg and vehicle for oral gavage studies.
Average quantitative nuclear histological score associated with the ex vivo c-Myc expression assessed by IHC in the ARK2 xenograft tumor samples, excised 1 hour, 6 hours and 12 hours after the last dose of GS-5829 at 20, 10 mg/kg, JQ1 at 50 mg/kg and vehicle for oral gavage studies, following 28 days of treatment.
Ex vivo c-Myc expression assessed by IHC on the ARK2 xenograft tumor samples, excised 1 hour and 6 hours after a single dose of GS-5829 at 40, 20, 10 mg/kg, GS-626510 at 10 mg/kg and vehicle for oral gavage studies.
Ex vivo c-Myc expression assessed by IHC on the ARK2 xenograft tumor samples, excised 1 hour, 6 hours and 12 hours after the last dose of GS-5829 at 20, 10 mg/kg, JQ1 at 50 mg/kg and vehicle for oral gavage studies, following 28 days of treatment.