Additional sex comb-like 1 (ASXL1) gene encodes the ASXL1 protein, which plays an important role in transcriptional regulation of homeotic gene expression [1]. ASXL1 is frequently mutated in a spectrum of myeloid malignancies, including chronic myelomonocytic leukemia (CMML), myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN), and acute myeloid leukemia (AML) [2, 3]. Importantly, these mutations are associated with poor prognosis, suggesting an important role of ASXL1 mutations in disease progression [2–4]. Most of ASXL1 mutations are nonsense/frameshift, resulting in truncated forms of the protein lacking the C-terminal PHD finger [2, 5], which are detectable in leukemia cell lines [6]. We and others have shown that Asxl1 loss or transgenic expression of a truncated ASXL1 protein in mice (Asxl1Y588XTg) impaired hematopoietic stem/progenitor cell (HSC/HPC) function and led to diverse myeloid malignancies [7–9]. However, the underlying mechanisms remain largely unknown, hindering the development of targeted therapiecs.
ASXL1 exerts its regulatory effects on chromatin through interaction with other protein complexes such as PRC2 [5] and cohesin [10]. ASXL1 is also required for the activity of BAP1 [11–13], a deubiquiting (DUB) enzyme regulating homeobox gene expression by controlling the level of H2AK119Ub in opposition to PRC1-mediated gene repression [11, 12]. Recently, Balasubramani et al. [11] reported that ASXL1 truncation mutations confer an enhanced activity on the ASXL1–BAP1 DUB complex [11], highlighting the significance of the ASXL1–BAP1 complex in normal biological processes and cancer progression. We hypothesize that the ASXL1aa1-587 truncation mutant promotes myeloid malignancies by enhancing BAP1 DUB activity.
To test this hypothesis, we first examined whether deleting one Bap1 allele can delay or even eradicate ASXL1aa1-587-driven myeloid malignancies in vivo. After confirming the successful deletion of one allele of Bap1 (Bap1Δ/+) in hematopoietic cells following polyinosinic: polycytidylic acid (pI:pC) injection (supplemental Figure S1A–C), we analyzed the hematopoietic phenotype of WT, Bap1Δ/+, Asxl1Y588XTg, and Bap1Δ/+;Asxl1Y588XTg mice after 10 months of the injection. Consistent with our previous report [9], Asxl1Y588XTg mice developed diverse myeloid malignancies. Bap1Δ/+ mice did not exhibit detectable abnormalities in hematopoiesis. The peripheral blood (PB) counts of Bap1Δ/+;Asxl1Y588XTg mice were comparable to wild-type (WT) mice, including white blood cells (WBC), neutrophils (NE), hemoglobin (Hb) (Fig. 1a). PB smears from Asxl1Y588XTg mice showed frequent blast cells and/or dysplatic cells, but not in the PB from Bap1Δ/+; Asxl1Y588XTg mice (Fig. 1b). Analysis of bone marrow (BM) cytospin preparations revealed that in contrast to increased blasts in Asxl1Y588XTg BM, the blast cell frequencies in the Bap1Δ/+;Asxl1Y588XTg mice were similar to those in WT mice (Fig. 1c). The spleen sizes of Bap1Δ/+; Asxl1Y588XTg mice were normal, whereas splenomegaly was present in most of the Asxl1Y588XTg mice (supplemental Fig. 1D). Unlike disrupted splenic architecture in Asxl1Y588XTg mice, histological analysis of spleen sections in Bap1Δ/+;Asxl1Y588XTg mice revealed a normal architecture (Fig. 1d). Flow cytometric analyses of the PB, spleen and BM cells showed normalized frequencies of Mac-1+/Gr-1+ myeloid populations in Bap1Δ/+;Asxl1Y588XTg mice compared to that in Asxl1Y588XTg mice (Fig. 1e, f). Furthermore, the frequencies of Lin−Sca1−cKit+CD34+/CD16/32+ granulocyte-macrophage progenitor (GMP) and Lin−Sca1−cKit+CD34+/CD16/32−common myeloid progenitor (CMP) cell populations in the BM of Bap1Δ/+;Asxl1Y588XTg mice were comparable to WT controls (supplemental Figure S1E–F). These data suggest that Bap1 hemizygous deletion in Asxl1Y588XTg mice is sufficient to prevent the ASXL1aa1-587-driven biased myeloid differentiation and myeloid malignancy.
Fig. 1.
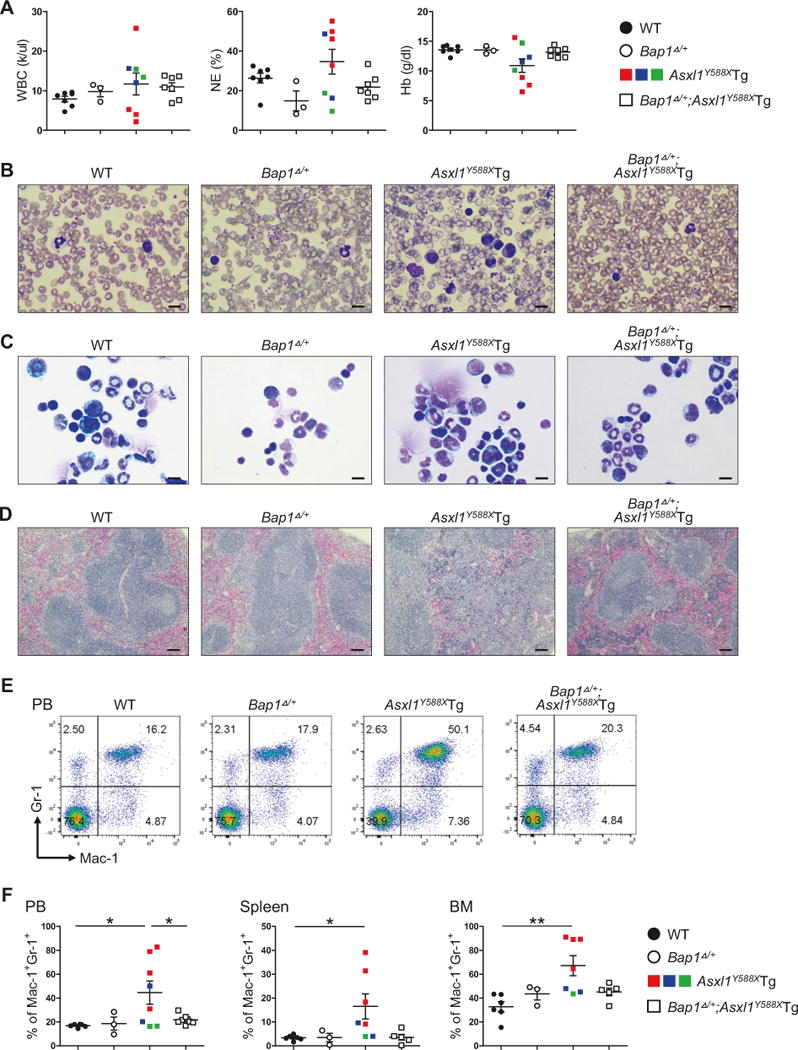
Reduction of BAP1 activity prevents ASXL1aa1-587-driven myeloid malignancies in vivo. a PB counts of WBC, NE and hemoglobin in WT, Bap1Δ/+, Asxl1Y588XTg and Bap1Δ/+;Asxl1Y588XTg mice. b–d Images are May-Giemsa-stained PB smears (b), cytospins prepared from BM cells (c), and H&E stained histologic sections of spleens (d) from representative four groups of mice. Scale bars: (b, c) 10 μm; (d) 100 μm. e Flow cytometric analyses of Mac-1+/Gr-1+ cells in PB of representative four groups of mice. f Quantification of percent Mac-1+/Gr-1+ cells in PB, spleens and BM of four groups of mice. Mice were at 10 months after pI:pC injection. Black circle: WT; open circle: Bap1Δ/+; filled square: Asxl1Y588XTg; open square: Bap1Δ/+; Asxl1Y588XTg. Red square: leukemic mice; blue square: MPN mice; green square: MDS/MPN mice. Error bars represent mean ± standard error of the mean (SEM) from 3 to 8 mice/genotype. (*p < 0.05, **p < 0.01)
Truncated ASXL1 has been reported to enhance the catalytic activity of BAP1 [11]. We found that ASXL1aa1-587 competed with ASXL1 full-length (ASXL1FL) to bind to BAP1 as determined by immunoprecipitation (IP) of BAP1 and western blotting using HEK293T cells expressing BAP1 and ASXL1FL with or without ASXL1aa1-587 (Fig. 2a). Interestingly, western blot analyses showed that there was no dramatic differences in global levels of H2AK119Ub and H3K27me3 in their BM cKit+ cells amongst four genotypes (Fig. 2b, Supplemental Figure S2A). ASXL1aa1-587 expression in cKit+ cells alters the expression of Hoxa and Dcbld1 genes [9]. We next determined the impact of ASXL1aa1-587 expression with a Bap1 hemizygous deletion in cKit+ cells on the Hoxa and Dcbld1 mRNA expression by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Although Asxl1Y588XTg cKit+ cells had a significantly increased expression of Hoxa5, Hoxa7, Hoxa9 and Dcbld1 (Fig. 2c), the expression levels of these genes in Bap1Δ/+;Asxl1Y588XTg cKit+ cells were comparable to WT (Fig. 2c). Furthermore, chromatin immunoprecipitation-qPCR (ChIP-qPCR) using an antibody against H2AK119Ub revealed a significant reduction in H2AK119Ub occupancy at the promoter regions of Hoxa5, Hoxa7, Hoxa9, and Dcbld1 in Asxl1Y588XTg cKit+ cells and in 32D cells expressing ASXL1aa1-587 (Fig. 2d, supplemental Figure S2B). Importantly, the H2AK119Ub occupancy was partially restored at the promoter regions of each of these genes tested in Bap1Δ/+; Asxl1Y588XTg cKit+ cells (Fig. 2d). These data indicate that ASXL1 truncation mutations confer gain-of-function in the pathogenesis of myeloid malignancies by increasing BAP1 DUB activity as reducing BAP1 activity ameliorates the abnormal hematopoietic phenotypes in Asxl1Y588XTg mice.
Fig. 2.
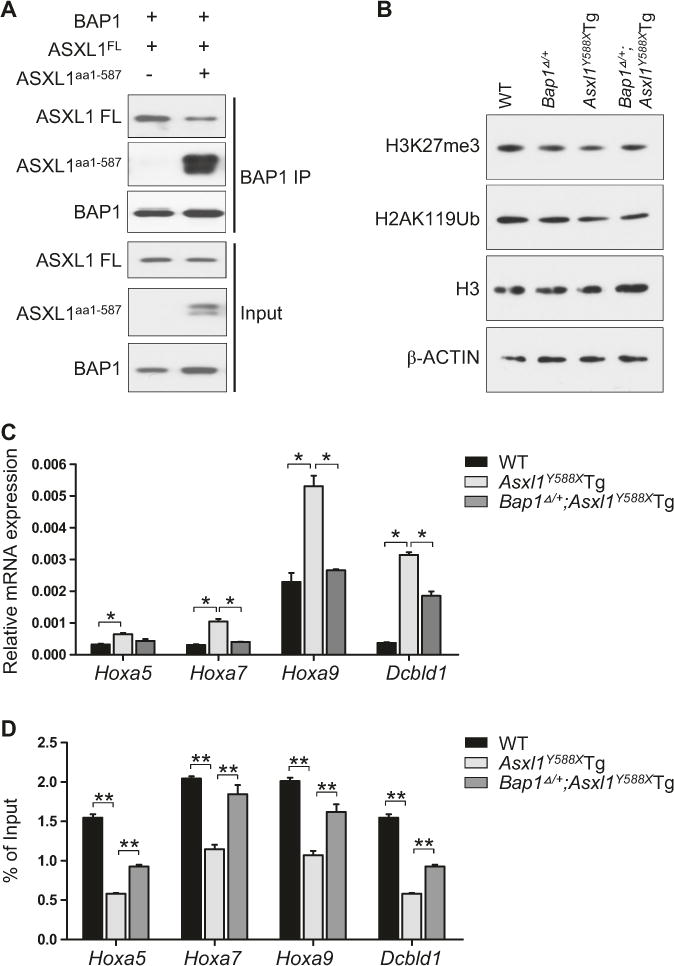
Reduction of BAP1 activity partially restores the expression of dysregulated genes in Asxl1Y588XTg HSC/HPCs. a IP followed by western blotting analysis shows that ASXL1aa1-587 expression dramatically decreased the interaction between ASXL1FL and BAP1. HEK293T cells were transfected with BAP1, FLAG-tagged ASXL1FL with/without FLAG-tagged ASXL1aa1-587. Nuclear extractions were subjected to IP using an antibody against BAP1. Western blots were performed using antibodies against FLAG and BAP1. b Western blot analysis shows the levels of H3K27me3 and H2AK119Ub in cKit+ cells of WT, Bap1Δ/+, Asxl1Y588XTg, and Bap1Δ/+;Asxl1Y588XTg mice. H3 and β-ACTIN were used as loading controls. c Relative mRNA expression of Hoxa5, Hoxa7, Hoxa9, and Dcbld1 was determined in WT, Asxl1Y588XTg, and Bap1Δ/+; Asxl1Y588XTg cKit+ cells by qRT-PCR and normalized with Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. d ChIP for H2AK119Ub followed by qPCR shows the enrichment of H2AK119Ub at the promoter regions of Hoxa5, Hoxa7, Hoxa9, and Dcbld1 genes in BM cKit+ from WT, Asxl1Y588XTg, and Bap1Δ/+;Asxl1Y588XTg mice. Error bars represent mean ± SEM from three mice/genotypes with similar results from two independent experiments. (*p < 0.05, **p < 0.01)
ASXL1 is an obligate regulatory subunit of the BAP1 DUB, and our current findings suggest that a balanced ASXL1–BAP1 axis is critical for normal hematopoiesis. BAP1 is thought to function as a tumor suppressor through its DUB activity. This study uncovers an unexpected oncogenic effect of increased BAP1 activity resulting from truncation of ASXL1 in leukemogenesis. This study discloses, for the first time, that BAP1 may represent a “double-edged sword” in cancer. Future work is warranted to determine whether disruption of the interaction between truncated ASXL1 and BAP1 can circumvent the oncogenic effects of increased BAP1 activity in ASXL1 truncation-driven abnormal hematopoiesis and myeloid malignancy.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA172408 and CA185751 to F-CY and MX; CA125970 to JWH), National Heart, Lung, and Blood Institute (HL112294 to MX), and Leukemia and Lymphoma Society (F-CY, MX, and SDN). JWH was supported by Research to Prevent Blindness, Inc. Senior Scientific Investigator Award, Melanoma Research Alliance—Melanoma Research Foundation Established Investigator Award, Alcon Research Institute, and a generous gift from Dr. Mark J. Daily. The Bascom Palmer Eye Institute received funding from NIH Core Grant P30EY014801, Department of Defense Grant #W81XWH-13-1-0048, and a Research to Prevent Blindness Unrestricted Grant. We thank the services provided by the Satellite Histological Core of Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41375-018-0126-9) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Milne TA, Sinclair DA, Brock HW. The additional sex combs gene of Drosophila is required for activation and repression of homeotic loci, and interacts specifically with Polycomb and super sex combs. Mol Gen Genet. 1999;261:753–61. doi: 10.1007/s004380050018. [DOI] [PubMed] [Google Scholar]
- 2.Gelsi-Boyer V, Trouplin V, Adelaide J, Bonansea J, Cervera N, Carbuccia N, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145:788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- 3.Boultwood J, Perry J, Pellagatti A, Fernandez-Mercado M, Fernandez-Santamaria C, Calasanz MJ, et al. Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia. 2010;24:1062–5. doi: 10.1038/leu.2010.20. [DOI] [PubMed] [Google Scholar]
- 4.Gelsi-Boyer V, Brecqueville M, Devillier R, Murati A, Mozziconacci MJ, Birnbaum D. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases. J Hematol Oncol. 2012;5:12. doi: 10.1186/1756-8722-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–93. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue D, Matsumoto M, Nagase R, Saika M, Fujino T, Nakayama KI, et al. Truncation mutants of ASXL1 observed in myeloid malignancies are expressed at detectable protein levels. Exp Hematol. 2016;44:172–6. e171. doi: 10.1016/j.exphem.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Li Z, He Y, Pan F, Chen S, Rhodes S, et al. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood. 2014;123:541–53. doi: 10.1182/blood-2013-05-500272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Wahab O, Gao J, Adli M, Dey A, Trimarchi T, Chung YR, et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J Exp Med. 2013;210:2641–59. doi: 10.1084/jem.20131141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Kurtenbach S, Guo Y, Lohse I, Durante MA, Li J, et al. Gain of function of ASXL1 truncating protein in the pathogenesis of myeloid malignancies. Blood. 2018;131:328–41. doi: 10.1182/blood-2017-06-789669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Zhang P, Yan A, Guo Z, Ban Y, Li J, et al. ASXL1 interacts with the cohesin complex to maintain chromatid separation and gene expression for normal hematopoiesis. Sci Adv. 2017;3:e1601602. doi: 10.1126/sciadv.1601602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasubramani A, Larjo A, Bassein JA, Chang X, Hastie RB, Togher SM, et al. Cancer-associated ASXL1 mutations may act as gain-of-function mutations of the ASXL1–BAP1 complex. Nat Commun. 2015;6:7307. doi: 10.1038/ncomms8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahtoe DD, van Dijk WJ, Ekkebus R, Ovaa H, Sixma TK. BAP1/ASXL1 recruitment and activation for H2A deubiquitination. Nat Commun. 2016;7:10292. doi: 10.1038/ncomms10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–7. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


