Abstract
Many people restrict their palatable food intake. In animal models, time-limiting access to palatable foods increases their intake while decreasing intake of less preferred alternatives; negative emotional withdrawal-like behavior is sometimes reported. In drug addiction models, intermittent extended access drives greater changes in use than brief access. When it comes to palatable food, the impact of briefer vs. longer access durations within intermittent access conditions remains unclear. Here, we provided male rats with chow or with weekday access to a preferred, sucrose-rich diet (PREF) (2, 4, or 8h daily) with chow otherwise available. Despite normal energy intake, all restricted access conditions increased weight gain by 6 weeks and shifted diet acceptance within 1 week. They increased daily and 2-h intake of PREF with individual vulnerability and decreased chow intake. Rats with the briefest access had the greatest binge-like (2-h) intake, did not lose weight on weekends despite undereating chow, and were fattier by 12 weeks. Extended access rats (8h) showed the greatest daily intake of preferred food and corresponding undereating of chow, slower weight gain when PREF was unavailable, and more variable daily energy intake from week to week. Increased fasting glucose was seen in 2-h and 8-h access rats. During acute withdrawal from PREF to chow diet, restricted access rats showed increased locomotor activity. Thus, intermittent access broadly promoted weight gain, fasting hyperglycemia and psychomotor arousal during early withdrawal. More restricted access promoted greater binge-like intake and fat accumulation, whereas longer access promoted evidence of greater food reward tolerance.
Keywords: binge eating, intermittent availability, palatable food intake, anxiety, withdrawal, duration
1. Introduction
Health or body image concerns lead some individuals to restrict intake of high-calorie “forbidden” foods(Anseloni & Brandão, 1997; Brink & Ferguson, 1998; Calder & Mussap, 2015; Lowes & Tiggemann, 2003; Mase, Miyawaki, Ohara, & Nakamura, 2015; Vandervoort, Aimé, & Green-Demers, 2015), which tend to contain sugar or fat and thereby are palatable. Animal models of limited access to palatable foods have been used to study resulting changes in the appetitive qualities of certain foods (Cottone P, Sabino V, Steardo L, 2008; Cottone, Sabino, Steardo, & Zorrilla, 2008b; Johnson & Kenny, 2010; Klump, Racine, Hildebrandt, & Sisk, 2013; Kreisler, Garcia, Spierling, Hui, & Zorrilla, 2017; Parylak, Cottone, Sabino, Rice, & Zorrilla, 2012; Parylak, Koob, & Zorrilla, 2011; Rogers, 1985) and emotional function towards understanding dieting-related pathologic eating behavior in humans (Avena, Gold, Kroll, & Gold, 2012; Avena, Rada, & Hoebel, 2008; R. L. Corwin, Avena, & Boggiano, 2011; R. L. W. Corwin & Babbs, 2012). Limiting the duration of access to preferred food (and other reinforcers(S. H. Ahmed, 2011; S. Ahmed, Walker, & Koob, 2000; Cohen, Koob, & George, 2012; Kitamura, Wee, Specio, Koob, & Pulvirenti, 2006)) promotes increased (“binge-like”) intake. Limiting access also often leads to reduced intake of the more available, but less preferred, food(Cottone P, Sabino V, Steardo L, 2008). The degrees of undereating of nonpreferred food vs. overeating of preferred food are dissociable, however, and undereating can persist despite normal bodyweight and adiposity(Johnson & Kenny, 2010; Kreisler et al., 2017). Undereating has been interpreted not to be corrective homeostasis/caloric conditioning alone, but rather food rejection due to an adjustment in reward function that leads to development of compulsive eating(Spierling et al., 2018) via negative reinforcement mechanisms(Cottone P, Sabino V, Steardo L, 2008; Cottone et al., 2008b; Johnson & Kenny, 2010; Kreisler et al., 2017; Parylak et al., 2011). Consistent with this hypothesis, abstinence from preferred foods is associated with hostile mood in humans(Wells, Read, Laugharne, & Ahluwalia, 1998) and, in animal models, elicits activation of amygdala stress circuitry(Cottone, Sabino, Roberto, et al., 2009; Teegarden & Bale, 2007), anti-reward adaptations(Johnson & Kenny, 2010), and a negative emotional state with psychomotor arousal and anxiety-, depressive-, and irritability-like behavior (Avena et al., 2008; Cottone P, Sabino V, Steardo L, 2008; Cottone, Sabino, Roberto, et al., 2009; Cottone et al., 2008b; Cottone, Sabino, Steardo, & Zorrilla, 2009; Iemolo et al., 2012; Spierling et al., 2018; Teegarden & Bale, 2007). Resumption of intake relieves the state, thereby promoting renewed overconsumption of the forbidden foods, which is often followed by further dietary restriction. Such intake “cycling” is a putative causal factor in bulimia nervosa, some forms of binge-eating disorder (Goldschmidt, Wall, Loth, Le Grange, & Neumark-Sztainer, 2012; Mathes, Brownley, Mo, & Bulik, 2009; Polivy & Herman, 1985) and obesity(Cannon, 2005; M. R. Lowe, 2015; Michael R Lowe, Doshi, Katterman, & Feig, 2013) and may have adverse long-term metabolic consequences (M. R. Lowe, 2015; Montani, Schutz, & Dulloo, 2015).
For example, despite less total caloric intake, rats with a history of limited access to preferred food often gain body weight (BW) more efficiently and showed increased adiposity and pro-inflammatory adipokine levels(Cottone, Sabino, Steardo, et al., 2009; Kreisler et al., 2017; Parylak et al., 2012). Furthermore, limited access rats showed decreased thermogenic brown adipose tissue mass in direct relation to their degree of voluntary caloric restriction on non-access days(Kreisler et al., 2017); despite abstinence, they regained more weight following renewed access to preferred food(Kreisler et al., 2017; Montani et al., 2015). Both findings suggest thrifty metabolic adjustments. Furthermore, weight cycling is associated with indicators of insulin resistance, even in normal weight subjects (Montani et al., 2015), and type II diabetes is more prevalent in patients with binge-eating disorder than those without (Raevuori et al., 2015; Thornton et al., 2017). Distributing caloric load throughout the day rather than consuming them in one large meal improves fasting glucose levels(Carlson et al., 2007; Jenkins et al., 1992; Taylor, Hubbard, & Anderson, 1999). Thus, diet cycling/binge-eating may contribute to maladaptive adaptations, independent of weight gain, that promote long-term metabolic disease.
Food intake cycling may be homologous to cycles of drug use and abstinence that promote the transition to addiction(Avena et al., 2008; Volkow, Wang, Tomasi, & Baler, 2013). In animal models of addiction, the duration of intermittent phases of access to drugs of abuse is a key determinant of escalated drug-taking and negative emotional withdrawal behavior. Longer intermittent access periods (e.g., 6–23h) promote escalated intake of several psychostimulants and opiates, whereas briefer intermittent access periods (e.g., 1–3h) do not produce this phenotype(S. H. Ahmed, 2011; J. C. M. Vendruscolo et al., 2017; L. F. Vendruscolo et al., 2011). Longer access periods are also associated with increasing reinforcing value of drugs, compulsive drug-seeking despite punishment or risk, resistance to extinction(S. H. Ahmed, 2011; S. Ahmed et al., 2000; Chen et al., 2006; Cohen et al., 2012; Hwa et al., 2011; Kenny, Boutrel, Gasparini, Koob, & Markou, 2005; Kitamura et al., 2006; Koob et al., 2014; Lenoir & Ahmed, 2008; O’Dell et al., 2007; J. C. M. Vendruscolo et al., 2017) , negative emotional(S. H. Ahmed, 2011; Koob & Le Moal, 2005) withdrawal symptoms (S. Ahmed et al., 2000; O’Dell et al., 2007; Park et al., 2013; L. F. Vendruscolo et al., 2011), and recruitment of brain stress circuitry(Greenwell et al., 2009; Greenwell, Walker, Cottone, Zorrilla, & Koob, n.d.; Specio et al., 2008; Zorrilla et al., 2012).
The impact of duration of access to palatable food is less clear. We recently showed that rats given intermittent (Monday-Wednesday-Friday), extended (24 hr/day) access to a highly preferred food uniquely develop weight cycling with cyclic day-to-day energy intake due to overconsumption on access days and marked rejection of otherwise acceptable chow on non-access days(Kreisler et al., 2017; Spierling et al., 2018). Daily cycling was not seen in rats receiving intermittent brief access (30 min/day). Rats with extended access (23h/day) to a cafeteria diet developed impaired brain reward function and chow hypophagia, whereas rats with briefer access (1h/day) did not(Johnson & Kenny, 2010). On the other hand, rats with intermittent brief vs. extended access showed similarly increased operant self-administration, timeout responding, and punishment-resistant intake(Spierling et al., 2018). Also, when free-feeding, short access rats binged more early in renewed access (30 min) than did extended access rats(Kreisler et al., 2017), contrary to what is seen for many substances of abuse(S. H. Ahmed, 2011).
Here, we further test the hypothesis that the duration of access influences the development of binge-like intake, excess daily energy intake and chow hypophagia. Duration of access also has not been studied with regard to the development of reviewed emotional and metabolic phenotypes associated with intermittent access to preferred food. Thus, the present study exposed rats to daily 2, 4, or 8h weekday access to a preferred food while quantifying palatable food and chow intake; psychomotor arousal and anxiety-related behavior during withdrawal; and weight gain, adiposity and fasting glucose.
2. Method
2.1. Animals
Adult, male Wistar rats (Charles River, Hollister, CA; n= 48, in 2 cohorts of 24) were singlehoused in wire-topped plastic cages in a temperature- (22 °C) and humidity- (60%) controlled vivarium with a 12:12 h reverse light cycle (lights off at 0900h). Prior to experimental procedures, all rats had ad libitum access to standard chow (Harlan 7012 Teklad LM-485, Indianapolis, IN) and water. Body weights and chow and water intake were recorded daily for 2–4 days before the feeding schedule. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Care and Use Committee of The Scripps Research Institute.
2.2. Feeding schedule
The preferred diet (PREF, 91% preference ratio over chow in a 24-hr preference test(Cottone, Sabino, Steardo, & Zorrilla, 2008a) used was a sucrose-rich pelleted formula (AIN-76A, Test Diets, St. Louis, MO, 3.4 kcal/g) with similar macronutrient composition (~66% carbohydrates, 21% protein, and 13% fat by kcal) to chow (3.1 kcal/g). Upon arrival, rats were assigned to one of the following four feeding schedule groups (n = 12/group), matching for body weight (BW) and both 8-h and 24-h chow intake.
2h daily access to PREF/22h chow access (2h group)
4h daily access to PREF/20h chow access (4h group)
8h daily access to PREF/16h chow access (8h group)
0h daily access to PREF/24h chow access (Chow group)
We chose these durations of access to resemble those that have been used for short vs. extended access drug self-administration paradigms (S. H. Ahmed, 2011; J. C. M. Vendruscolo et al., 2017; L. F. Vendruscolo et al., 2011) but wherein all access durations would be briefer than the 12-hr dark cycle. Thus, access during the prominent feeding phase of the dark cycle would be restricted for all groups. All food was presented in the home cage in excess and was consumed ad libitum within a given access period. PREF access occurred on weekdays (Monday-Friday, M-F) and began at dark onset (0900h) each day. All rats received only chow over the weekends (Sat-Sun). The feeding schedule occurred for 12 weeks.
2.3. Food intake and BW
Food intake was determined to 0.1 g precision as the difference between the amount of food presented and the amount of food remaining at the end of a given period of time. In all groups, food was weighed 2, 4, 8 and 24h after dark onset. Saturday measurements of food intake were unavailable for Cohort 2 due to technical error, therefore, weekend food intake analyses are for Cohort 1 only. Cumulative food intake was defined as the sum of daily (Fig 1) or weekly (Fig 2) averages. BWs were recorded at dark onset. BW change was calculated for access days (Fig 7a, averaged across weeks for the 4-day period from M-Th, excluding Fridays on which behavior tests were performed), across the entire first 6 weeks of the experiment (Fig. 7b), and for nonaccess weekends (Fig. 7c). To assess the stability of individual differences in diet intake across time, single measure, two-way, random effect intraclass correlations of consistency (Shrout & Fleiss, 1979) were calculated for average daily intake over weeks 2–6 of the feeding schedules. Intraclass correlation coefficients (ICCs) are reported accordingly, with higher values indicating greater stability of individual differences over time.
Figure 1: Daily food intake during week 1.
Effect of daily (M-F only) access to a highly preferred sucrose-rich, chocolate-flavored diet (PREF) on daily energy intake during week 1 of exposure. PREF was presented for either 0h (“Chow”), 2h, 4h, or 8h each day. Chow was freely available outside of PREF access. Daily intakes are presented on days 1–4 (M-Th only) and yaxes in a-c represent group means + SEM (error bars may be too small to be visible). *Group x Day interaction; &Chow different than all other groups; #8h different than 2h
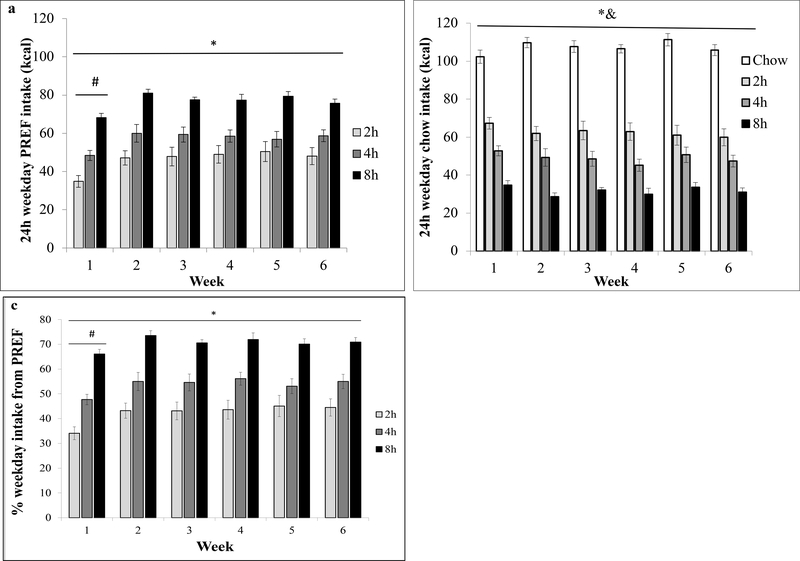
Figure 2: Average daily food intake of various diets during weeks 1–6.
(a) Daily PREF intake on weekdays; (b) Daily chow intake on weekdays; (c) % of daily intake from PREF. Intakes are presented as weekly averages ± SE *main effect of Group; #main effect of Week; &Group x Week interaction
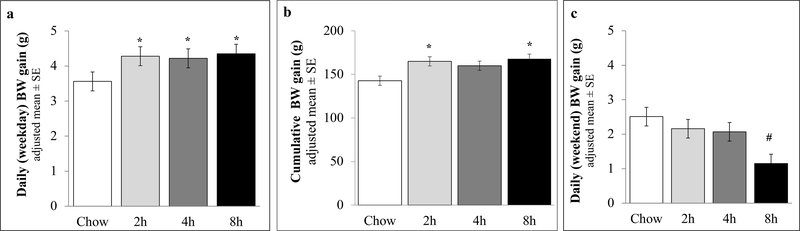
Figure 7: BW gain.
BW gain was analyzed as (a) weekly (average of all M-Th periods) expressed as daily average; (b) Cumulative (weeks 1–6); and (c) weekends (average of all Sat-Sun periods, except weeks 3–4), expressed as daily average; BW means and SEs were adjusted for BW at diet schedule onset. *different than Chow (p < .05); # different than Chow and 2h groups.
2.4. Carcass composition
Carcasses from cohort 1 were reserved for a different study, but chemical analysis of carcass composition was performed on cohort 2 (n = 24, 6 per group). Sacrifices occurred between 1400h and 1700h after rats had been on their respective feeding schedules for 12 weeks. All rats received 48h of chow access prior to sacrifice by guillotine under brief isoflurane anesthesia. Carcasses were dried (60 °C) to constant mass to determine water content and extracted with petroleum ether in a Soxhlet apparatus to determine fat mass and fat-free dry mass (protein and ash)(Harris & Martin, 1984).
2.5. Elevated plus maze (EPM)
To test the hypothesis that rats receiving intermittent access to PREF rapidly showed increased anxiety-like behavior when withdrawn from PREF, rats were tested in the elevated plus-maze (EPM) after 3 weeks of the feeding schedule. On the day prior to EPM testing, rats were habituated to the EPM anteroom for 2h, but were not exposed to the apparatus.
In order to standardize the time interval between last PREF access and the EPM test, all rats in the 2h, 4h, and 8h access groups received 2h of PREF access on the day of EPM testing (from 0900–1100h in their home cages). At 1100h, all rats were moved to the anteroom and switched to chow until plus-maze testing that began after a 2-hr habituation period. White noise (70 dB) was present throughout habituation and testing. Beginning at 1300h, rats were placed individually onto the center of the EPM (to which they were naive) facing a closed arm. The EPM was made of dark Plexiglas and consisted of four arms (50 cm long×10 cm wide): two closed arms (enclosed by 40 cm high dark walls) and two open arms (0.5 cm high ledges). The EPM was elevated to a height of 50 cm. The anteroom and test room were both dark, to match the lighting conditions of the vivarium. Lighting on the open arms was 1.5–2.0 lux. Sessions were 5-min long and were scored from videotape by a rater unaware of the rat’s treatment condition. The number of entries into as well as the amount of time spent in the center, open and closed arms, were recorded for each rat. Due to video recording failure, a few measures were not available for cohort 1, but all were available for cohort 2 (n = 24) (Table S1, Fig 5a). All rats received chow after the EPM test until the next scheduled preferred access period. The primary dependent measures were the time spent in the open arms, a measure inversely related to anxiety-like behavior, and the number of entries into the closed arms, reflecting locomotor activity (Anseloni & Brandão, 1997; Boguszewski & Zagrodzka, 2002; Doremus, Varlinskaya, & Spear, 2006; Zhao, Weiss, & Zorrilla, 2007).
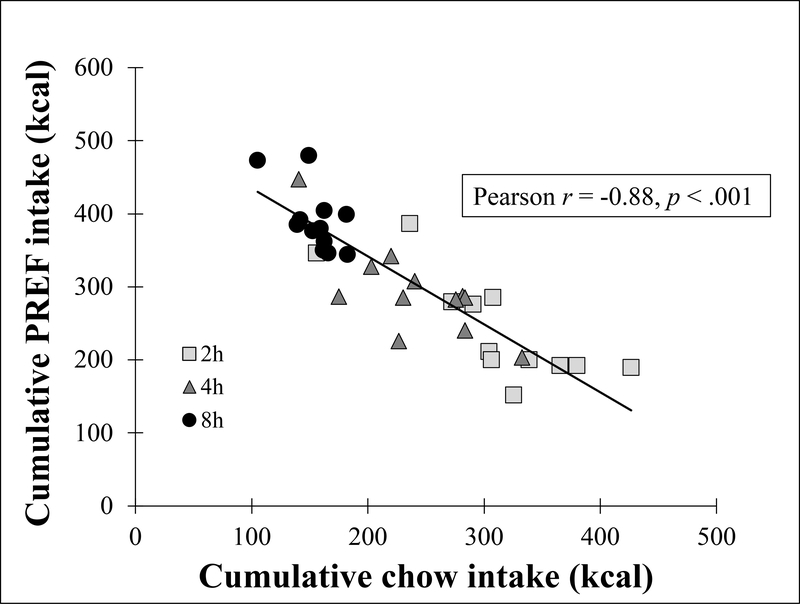
Figure 5: Chow vs. PREF intake.
Cumulative (sum of week 2–6 averages) chow vs. cumulative PREF intake.
2.6. Fasting blood glucose
To test the hypothesis that rats receiving intermittent access to PREF showed elevated fasting glucose levels, rats were fasted for 16h prior to onset of the dark cycle (1700–0900). One hour prior to blood collection, rats were placed in the testing room to acclimate to the testing environment. At 0900h, blood was collected via tail nick, and whole blood glucose levels were quantified via glucometer (Ascensia ELITE, Bayer Healthcare, Morristown, NJ). Blood glucose testing occurred on two separate occasions: once after 4–5 weeks and then again after 6–8 weeks of the feeding schedule. Following blood collection, rats subsequently received their daily PREF access periods beginning at 1100h.
2.7. Motor activation
Locomotor assessment was used in order to test the hypothesis that rats receiving intermittent access to PREF showed psychomotor activation following removal of PREF. On two consecutive days, rats were placed in individual wire-mesh cage (20×25×36 cm) equipped with two horizontal infrared photobeams placed along the long axis of the cage, 2 cm above the floor and 16 cm from one another. The first day was an acclimation session, and the second day was used for data analysis. Testing took place after 5–7 weeks on the feeding schedule. On both days, rats were individually placed in the test cages for 6 h, beginning at dark onset. Testing was performed in darkness, to match the lighting conditions of the vivarium. White noise (70 dB) was present during acclimation and testing.
For testing, food (either PREF or chow, per group assignment) was present and intake was measured during the first two hours in the box. The second hour, after 1-hr of reacclimation, was considered the “baseline” for motor activity, for which beam breaks were analyzed separately (Table S2). Food was removed, and the number of beam breaks was compared for 2h beginning 2h after withdrawal of the food, as in plus-maze testing. Groups were compared using Kruskal-Wallis H-tests due to non-parametric distribution of the data (Fig 9). All rats received chow after each motor test until the next scheduled PREF access period.
Figure 9: Anxiety-like behavior and psychomotor arousal.
Effect of acute withdrawal from PREF on (a) time spent in the open arm of an elevated plus maze during a 5min test period and (b) motor activity during a 2h test period. Both tests occurred following 2h of PREF access for 2h, 4h, and 8h groups. #different than 4h and 8h (p < .05); *Chow different than 4h (Mann-Whitney p < .01).
2.8. Defensive withdrawal
To test the hypothesis that rats receiving the intermittent access to the palatable food showed enhanced anxiety-like behavior, a defensive withdrawal test also was performed after 7–8 weeks on the feeding schedule. As in the plus-maze, on the test day, all rats received 2-h access to their normal food (either PREF or chow, per group assignment) in their home cages beginning at 0900. Food was switched to chow at 1100, when all rats were moved to the anteroom (which matched the lighting conditions of the vivarium) for acclimation. Testing began at 1300 under bright room light (~300 lux) with 70 dB white noise present.
To start each session, the rat was placed in a cylindrical chamber (a 2-L cylindrical Pyrex beaker) wrapped in dark brown packing tape that was situated within a walled test arena (a 106 × 92 × 77 cm open field made of black foamed polyvinylchloride). The chamber was open at one end and centrally located 15 cm from a wall of the open field, aligned lengthwise, with the open end facing the center. Each rat underwent one 8-min session, which was video-recorded for later analysis by a rater naïve to treatment condition. Primary dependent variables were the following: initial latency to leave the chamber (i.e., placement of all four paws in the open field), the total time spent in the open arena, the number and duration of subsequent withdrawals back into the chamber, and the number and duration of subsequent emergences back out of the chamber. Anxiolytics and anxiogenics, respectively, decrease and increase the duration of time spent withdrawn in the chamber in this model (see (Takahashi, Kalin, & Baker, 1990), and references therein). Due to technical issues with video, data from 6 (of 48) rats were not available. All rats received chow after each locomotor test until the next scheduled PREF access period.
2.9. Data analysis
Food intake and BW data were analyzed and are presented as weekly averages (± SE). Days on which experimental testing was performed (e.g., behavioral testing, blood sampling, preparatory food removal) were omitted from weekly averages. Unless otherwise specified, between-group comparisons were performed using one-way ANCOVA with Cohort as a covariate, which did not interact with Group on any measure. For weekend chow intake analyses, 4 (Group) x 2 (Day) mixed-design ANOVA was used with Day (Sat vs. Sun) as a repeated-measure. Weekends 3 and 4 were omitted from analyses due to the altered feeding schedule of plus-maze testing on the preceding Friday. Initial BW was used as a covariate to analyze weight change independent of starting values(Senn, 2006). Newman Keuls post hoc comparisons were used following significant omnibus tests unless otherwise specified. To detect a linear relationship between duration of PREF access and intake, linear contrast analysis was performed and linear contrast estimate (LCE) ± SE are reported accordingly. Correlation analyses were performed using Pearson correlation.
3. Results
3.1. Food intake
3.1.1. Individual differences in food intake across time:
Table 1 shows that all 3 PREFexposed feeding groups showed strong stable individual differences in their first 2h (binge-like) intake, whereas Chow rats did not (Table 1). Furthermore, all feeding groups showed stable individual differences in total energy intake on access days. However, per Cicchetti et al.’s criteria(Cicchetti & Sparrow, 1981), the stability of individual differences in total daily energy intake was “excellent” in Chow and 2h rats, but only “good” in 4h rats and “fair” in 8h rats (Table 1). Similarly, the stability of individual differences in weekend chow intake was “excellent” in Chow, “good” in 2h rats, but not significant in 4h and 8h rats (Table 1).
Table 1:
Individual differences in intake. Stability of individual differences in 24h weekday, 2h weekday, and 48h weekend energy intake within each experimental group across weeks 2–6 of diet schedule exposure.
| Food intake measure | Group | Single measure ICC |
|---|---|---|
| 24h weekday energy intake (PREF + chow) | Chow | 0.74a |
| 2h | 0.68a | |
| 4h | 0.47a | |
| 8h | 0.44a | |
| 2h energy intake (PREF or chow) | Chow | 0.09 |
| 2h | 0.78a | |
| 4h | 0.75a | |
| 8h | 0.56a | |
| 48h weekend chow intake (cohort 1 only; n = 6/group) | Chow | 0.85a |
| 2h | 0.58b | |
| 4h | 0.21 | |
| 8h | 0.21 | |
Intraclass correlation coefficient (ICC):
p< .001;
p< .01
3.1.2. Access day intake:
PREF daily intake.
Figure 1a shows that weekday PREF intake increased each day during the first week of exposure for all PREF groups, but the increase was steeper for 2h and 4h, compared to 8h, rats [Day x Group, F(6, 96) = 5.1, p < .01). For each of the first four days of access, groups differed from one another in PREF intake (Fig 1a; Group F(2, 32) = 40.8, p < .01). By the end of Week 1, PREF intake stabilized at levels that persisted through the 6-week period (compare Fig 1a vs Fig 2a).
When considering weekly averages across 6 weeks, as expected, a Group main effect (F(2,32) = 21.7 p < .001) showed that greater duration of access directly and consistently predicted greater daily PREF intake (Fig 2a & c, LCE: F(1, 35) = 37.0, p < .001 for absolute intake and LCE: F(1, 35) = 52.8, p < .001 for % total intake, averaged across weeks 2–6,); thus, 8h rats ate the most PREF (~78 kcal, ~71% of total daily energy intake), 4h rats consumed less (~ 59.0 kcal, ~55% total daily intake), and 2h rats consumed the least (~48.5 kcal, ~44% total daily intake, Figs 2a & c). There were no Group x Week interactions for PREF intake in calories (F(10,160) = 0.7, p > .05) or as % of daily energy intake (F(10,160) = 0.8, p > .05, Figs 2a & c). Week main effects for absolute (F(5,160) = 2.4, p < .05) and % of daily energy intake (F(5, 160) = 2.8, p < .05) reflected that all groups ate less PREF during week 1, when they were still escalating, than subsequent weeks (ps < .05).
Chow daily intake.
Conversely, Figure 1b shows that, among PREF groups, weekday chow intake decreased each day during the first week of exposure, whereas it increased slightly among chow controls [Day x Group, F(7.3, 102.3) = 8.4, p < .01]. A Group main effect across the 6-week period (F(3, 43) = 141.0, p < .001) showed that greater duration of PREF access predicted less chow intake on access days (LCE: F(1, 47) = 328.5, p < .001, averaged across week 2–6); thus, 8h rats consumed the least chow (~32.7 kcal over 16h), 4h rats consumed more (~49.0 kcal over 20h) and 2h rats consumed the most (~62.7 kcal over 22h, Fig 2b). All PREF access groups ate significantly less chow than Chow controls (~107 kcal over 24h). A Group X Week interaction (F (15, 215) = 2.0, p < .05, Fig 2b), reflected that rats with access to PREF further decreased their chow intake after week 1, whereas Chow rats slightly increased it (Fig 2b).
Total daily intake:
Figure 3 shows that, despite the large differences in acceptance of the specific diets, total energy intake on access days did not differ across groups, nor was it linearly related to PREF access duration [Group: F(3,43) = 0.32, p > .05, Group X Time: F(14.6, 209.5) = 0.65, p > .05), LCE: F(1, 47) = 0.004, p > .05].
Figure 3: Average total daily energy intake.
Total energy (chow + PREF) intake; ns = not significant.
First 2h food intake:
Food intake during the first 2 h of the dark phase was compared as a measure of binge-like intake upon renewed access (Figs 1c and 4) and to allow comparison to analogous analysis of initial self-administration in drug abuse models of extended access (S. H. Ahmed, 2011). Figure 1c shows that, among PREF groups, intake within the first 2-hr greatly increased on day 2 compared to day 1, and less so thereafter, whereas it only slightly increased among chow controls [Day x Group, F(4.8, 77.4) = 7.4, p < .001]. During the overall 6-week period, rats with time-limited access to PREF ate more within the first 2 h than did Chow controls (Group: F(3, 43) = 33.0, p < .001). Linear contrasts indicated that briefer duration of PREF access was related to greater 2-h PREF intake (LCE: F(1, 35) = 13.3, p = .001, average of weeks 2–6). A Group X Week interaction (F(14.4, 206.3) = 3.7, p < .001) reflected that, whereas all PREF access groups ate similar amounts during the first week of access (30–35 kcal within 2h, p > .05 for each comparison, Fig 4), 2h rats ate significantly more than 8h rats in all weeks thereafter, with 4h rats intermediate.
Figure 4: Two-hour energy intake.
Energy intake during first 2h of dark cycle (chow for Chow group and PREF for 2h, 4h, and 8h groups); inset: change in 2h energy intake from week 1 to 2; *main effect of Group; &Group x Week interaction; different letters indicate differences between groups within that week.
3.1.3. Inverse correlation between chow and PREF intake
During weeks 2–6, on access days, cumulative chow intake was inversely correlated with cumulative PREF intake both across all PREF-exposed groups collectively (Pearson r = −.88, p < .001, see Fig 5) and within each PREF-exposed group separately (rs = −.64 to −.79, ps < .03). These correlations did not simply reflect an inverse relation of intake across times of day because among Chow controls, there was no similar inverse correlation between 0–2h vs. 2–24h chow intake (r = 0.21, p > .05, not shown).
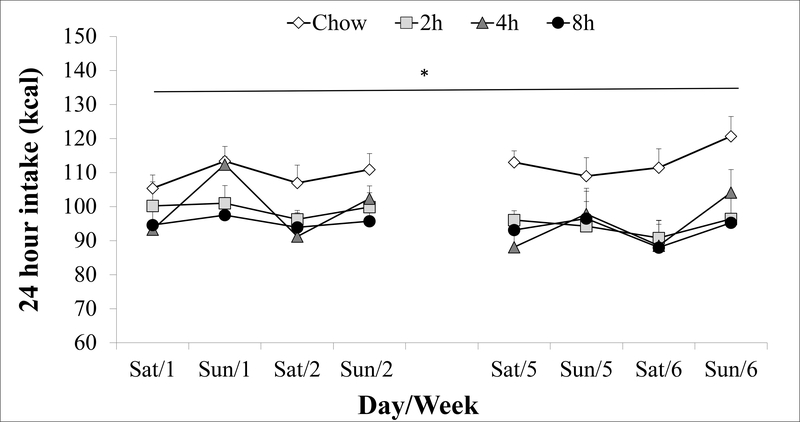
3.1.4. Weekend chow intak
Weekends 3–4 were not included in statistical analysis because of (potentially disruptive) behavioral and physiological testing that occurred immediately prior to them. As Figure 6 shows, the 2h (~97 kcal/day), 4h (~97 kcal), and 8h PREF (~94 kcal) rats ate similar amounts of chow to each other on weekends (ps > .05), and all ate significantly less than chow controls (~111 kcal/day, Group: F (3,20) = 5.7, p < .01). PREF groups, especially the 4 h group, tended to consume more chow on Sundays than on Saturdays, but the Group X Day interaction did not reach significance (p = .07). Unlike the inverse relation of chow with PREF intake on access days, there was no significant relationship within PREF rats between Week 6 weekend chow intake (Saturday, Sunday, or total 48-hour) and average daily PREF intake on weekdays (rs = −0.18 – 0.07, ps > .05, not shown).
Figure 6: Weekend chow intake.
Daily chow intake on weekends (weekends 3–4 not included due to behavioral testing); *main effect of Group.
3.2. BW gain
3.2.1. BW gain on access days
Fig. 7a shows that, during PREF access days, PREF rats of each access duration comparably gained more body weight than Chow controls (~4.2 vs. 3.5g daily M-Th; Group: F (3,43) = 3.62, p < .05; pairwise ps < .01 vs. Chow). In cumulative BW gain from study onset through week 6 (F (3,43) = 4.60, p < .01); the 2h and 8h PREF groups (~167g) comparably gained more weight than Chow controls (~140g), and the 4h group (~157g) trended toward a significant difference from Chow controls (p < .10, Fig 7b).
3.2.2. BW gain on weekend non-access days
Over the 48h weekend period, when all groups received chow only, the 8h group gained significantly less BW each day (~1.1g) than the Chow (2.5g) and 2h (2.2g), (F (3,19) = 4.72, p < .05] and trended toward a difference from the 4h (2.2g) group (p < .10, Fig 7c). Linear contrasts showed that greater duration of weekday PREF access related to less BW gain during non-access weekends (LCE: F(1,23) =13.7, p < .05, Fig 7c).
3.3. Carcass composition
As shown in Figure 8a, there were trends for Group differences in relative fat and water in carcass composition analysis (F (3, 23) = 2.8, p = .07 and F (3,23) = 2.5, p = .09, respectively). Dunnett’s test, which controls the familywise error rate when comparing multiple experimental groups with a single control (Dunnett, 1964), revealed that 2h rats had significantly higher fat composition (22.3% ± 1.3, p < .05) and lower water composition (54.1% ± 1.0, p < .05) vs. Chow controls (17.2% ± 1.5 fat and 58.0% ± 1.3 water, Fig 8a).
Figure 8: Body composition and fasting blood glucose.
Effect of diet schedules on (a) relative fat, water, and fat free dry (ash + protein) mass at termination of experiment (after 12 weeks of diet schedules, cohort 2 only); and (b) fasting blood glucose In b, different letters indicate differences between groups (p < .05); *different than Chow (Dunnett T, p < .05).
3.4. Fasting blood glucose
Significant group differences were seen in fasting blood glucose levels by the second blood sampling (6–8 weeks of feeding, F (3,44) = 6.5, p < .01, Fig. 8b), but not the first (4 weeks, not shown). Specifically, glucose levels were significantly higher in the 2h (82.2 mg/dL ± 2.6) and 8h groups (85.1 mg/dL ± 2.2) vs. Chow controls (73.1 mg/dL ± 2.3). Fasting glucose in 4h rats (76.3 ± 1.5 mg/dL) did not differ from that of Chow or 2h rats, but also was lower than that of 8h rats.
3.5. Elevated plus maze
Table S1 shows that 2h rats descriptively spent less time in the open arms (~10 sec, or ~ 4% of the 5-min test session) than any other group (~38–52 sec, ~16–24% of the time). This difference trended toward, but did not reach, statistical significance (F (3, 23) = 2.6, p < .10, Fig 9a). There also were no significant ANOVA effects for closed or total arm entries, putative measures of locomotor activity, or center time, a putative measure of risk assessment/decision-making(Anseloni & Brandão, 1997; Boguszewski & Zagrodzka, 2002; Doremus et al., 2006; Zhao et al., 2007).
3.6. Motor activation
3.6.1. Baseline intake and activity
As expected from their home cage behavior, a Group effect (F (3,43) = 10.1, p < .05) reflected that all three PREF groups (2h, 4h, 8h) ate significantly more than Chow controls (21.1 ± 1.5 kcal) prior to withdrawal in the motor activation study. The 2h (46.5 ± 1.5 kcal) and 4h (44.4 ± 5.1 kcal) rats also ate more than 8h rats (32.8 ± 3.3 kcal, ps < .05, not shown). As desired, no group differences in motor activation were seen by the last hour of baseline observation (Kruskal-Wallis, p > .05, Table S2).
3.6.2. Food withdrawal
Figure 9b shows significant group differences in the motor activity of rats beginning 2-hr after removal of their respective diets (Kruskal Wallis χ2 (3) = 9.28, p < .05). Mann-Whitney comparisons revealed that 4h rats (median = 677.5) were more active than chow rats (median = 445, U = 13.0, p < .01, Fig 9b). Differences between 2h rats (median = 637) and 8h rats (median = 604.5) vs. Chow rats also trended toward statistical significance (U = 30.0, p = .08, and U = 34.5, p = .09, respectively, Fig 9b).
3.7. Defensive withdrawal
There were no group differences in the latency, frequency, or duration of emerging from the withdrawal chamber or in the frequency or duration of withdrawals (Table S3).
4. Discussion
In the present study, restricted access to PREF produced rapidly-induced binge-like intake with stable individual differences, as well as undereating of the less preferred chow in male rats. Intake cycling was related to duration of availability of the preferred food and the magnitude of consumption of each diet type reflected magnitude of consumption of the other. Only the most extended (8h) PREF access group showed reduced BW gain during chow-only access, despite similar total BW gain over the entire course of the experiment. Only 2h and 8h rats displayed increased fasting glucose whereas all restricted access rats tended to show increased psychomotor arousal when the diet was withdrawn.
4.1. More restricted access elicits more binge-like intake with individual vulnerability
Similar to previous studies, rats with time-limited access to a preferred food rapidly increased their intake of that food within the first week of access(R. L. Corwin, 2004; Cottone P, Sabino V, Steardo L, 2008; Cottone, Sabino, Steardo, et al., 2009; Parylak et al., 2012; Wojnicki, Johnson, & Corwin, 2008). Within the range of durations studied (2–8 hr/day), longer access led to greater daily PREF intake (Fig 2a), but briefer access led to greater initial (2-hr) intake (Fig 4). This result resembles our recent findings that intermittent, brief access (Monday-Wednesday-Friday, 30 min/day) elicits ~40–50% greater 30-min intake than intermittent, extended access (MWF, 24 hr/day), but that extended access rats ate 3–4× more preferred diet daily than brief access rats. However, when rats were given ad lib access to preferred diet in that study, they did not eat more than chow controls, emphasizing the importance of intermittency rather than palatability alone in escalated intake. Together, these findings with free-feeding rats differ from operant drug abuse models in which more extended access led not only to escalated daily self-administration, but also greater self-administration within the same brief timeframe received by short access groups(S. H. Ahmed, 2011).
Restricted access also led to strong, stable individual differences in the degree of overeating within the first 2h of access. Descriptively, these individual differences were greater in rats with more restricted access (2-hr: ICC=0.78, 4-hr: ICC=0.75), though 8-hr rats showed them as well (ICC=0.56), in sharp contrast to chow controls (ICC=0.09). Translationally, these and similar previous results(Cottone et al., 2008b; Kreisler et al., 2017; Parylak et al., 2012; Sinclair et al., 2015) may model individual differences in risk for developing binge-feeding behavior in people that follow diet patterns with restriction of food reward(Feeney et al., 2011; Garcia-Bailo, Toguri, Eny, & El-Sohemy, 2009).
4.2. Intake cycling
Restricted access also decreased consumption of the chow diet. On days when the preferred food was available, greater duration of access to PREF (Fig 2b) and how much PREF was eaten (Fig. 2a), were related to greater underconsumption of chow. As a result, total energy intake on access days was unchanged vs. chow controls. Thus, homeostatic caloric compensation cannot be ruled out as an explanation for decreased chow intake on access days in this model.
Unlike the inverse relation seen on access days here, several limited access studies have reported a dissociation between the degree of binge-like intake of preferred food vs. the degree of chow hypophagia(Cottone, Sabino, Steardo, et al., 2009; Kreisler et al., 2017). One key procedural difference is that previous studies involved female subjects. Female rats show greater fixed- and progressive-ratio self-administration of this preferred diet than males(Spierling et al., 2018), suggesting that it may be more reinforcing for them. Thus, perhaps there was greater rewardrelated tolerance or diet contrast in previous studies, leading to greater devaluation of the chow. Further, there are sex differences in how males vs. females metabolically adapt to cycling on this diet(Spierling et al., 2018).
Another procedural difference is that previous studies involved more extended durations of access (e.g., 1–2 days) and non-access (1–4 days) to preferred food. As a result, the undereating described in previous studies was not measured on access days, but rather on non-access days, akin to weekends of the present study. Here, restricted access groups also underate chow on weekends (less so than on weekdays; Fig. 2b vs 6). In contrast to what was seen on access days, neither duration of access on weekdays nor weekday PREF intake, predicted the degree of weekend chow hypophagia. Further, PREF rats underate on weekends despite their weekday energy intake being the same as that of controls. Thus, similar to reviewed studies(Cottone et al., 2008a, 2008b; Flaherty & Rowan, 1986; Johnson & Kenny, 2010; Teegarden & Bale, 2007), weekend undereating here is not easily explained as homeostatic caloric compensation. Rather, it may reflect devaluation of less preferred food due to recent experience with or the prospect of a more preferred alternative(Cottone et al., 2008a, 2008b; Flaherty & Rowan, 1986; Johnson & Kenny, 2010; Teegarden & Bale, 2007).
4.3. Anxiety-like behavior and arousal
Restricted access rats did not show significant increases in anxiety-like behavior in the elevated plus-maze or defensive withdrawal test. A trend was observed for 2h access rats to show less open arm time in the plus-maze, an anxiogenic-like effect (Fig. 9a), perhaps suggesting a greater effect of more restricted access but this did not reach statistical significance. Several previous studies have observed spontaneously increased anxiety-like behavior following longer durations of withdrawal to chow from preferred food(Cottone, Sabino, Roberto, et al., 2009; Cottone et al., 2008b; Cottone, Sabino, Steardo, et al., 2009; Iemolo et al., 2013). Anxiogenic-like behavior in the above studies was not observed if intermittent access subjects were currently receiving the preferred diet. Thus, perhaps 2h without preferred food, as in the present study, was not a sufficient duration to elicit withdrawal-like anxiety; however, a briefer exposure to a frustrative non-rewarded food cue in other models can elicit stress-like effects and binge-like eating via orexin and CRF-dependent mechanisms(Alcaraz-Iborra & Cubero, 2015; Piccoli et al., 2012). Longer withdrawal periods also do not always elicit anxiety-like behavior(Rossetti, Spena, Halfon, & Boutrel, 2014). Analogously, anxiogenic-like effects are sometimes(Valdez et al., 2002), but not always(Macey, Schulteis, Heinrichs, & Koob, 1996; Zhang, Morse, Koob, & Schulteis, 2007), seen within 2h of alcohol withdrawal, even though decreased brain reward function is already present(Schulteis, Markou, Cole, & Koob, 1995). Rather, they peak later (e.g., 6–24 hr post-withdrawal(Macey et al., 1996; Zhang et al., 2007; Zhao et al., 2007)). In addition, others have observed increased anxiety-like behavior following palatable food withdrawal, but only after total food deprivation or pretreatment with opioid(Colantuoni et al., 2002) or CB1 receptor antagonists(Blasio et al., 2013). Thus, more work is needed to understand better the time course and conditions under which food withdrawal-associated anxiety-like behavior is seen.
Similar to our(Cottone, Sabino, Steardo, et al., 2009; Parylak et al., 2012) and other(Teegarden & Bale, 2007) previous work, subjects did show increased motor activity during withdrawal from preferred food. Though results only reached statistical significance for the 4h group, all durations of access showed descriptively similar elevated levels of motor arousal, and multiple subjects in each restricted access group exceeded activity of the most active control. Thus, an interpretation relevant to negative results in the exploration-based anxiety models is that the motor arousal may reflect food-seeking behavior. In this interpretation, the recent (2h) or otherwise expected availability (4h and 8h groups) of palatable food might supersede the anxiogenic-like plus-maze and defensive withdrawal environments, leading to food-seeking behavior despite the threat of open spaces (Micioni Di Bonaventura et al., 2017; Parylak et al., 2012). Such a result was seen in open-field conflict reinstatement tests(Dore et al., 2014; Teegarden & Bale, 2007).
4.4. Body weight gain
Despite comparable total energy intake as controls (Fig 3), all restricted access groups showed similarly increased weekly weight gain on access days (Fig 7a). This result is consistent with our and other prior work showing greater weight gain of diet-cycled(Bake, Morgan, & Mercer, 2014; Cottone, Sabino, Roberto, et al., 2009; Cottone, Sabino, Steardo, et al., 2009) or food restricted/meal-scheduled(Cottone et al., 2008b; Parylak et al., 2012) rats, despite similar or less energy intake. Novelly, increased BW gain was accompanied by disproportionately higher fat mass in 2h rats, but not 4h or 8h rats (Fig 8a), suggesting that, within this range of access, more restricted access promotes greater fat accumulation. Perhaps this reflects metabolic adaptation to the longer duration of self-restriction by 2h rats on access days. Consistent with this possibility, despite undereating as much as 8h rats did on weekends, 2h rats nonetheless still gained as much body weight as controls on weekends and more than 8h rats. We similarly recently reported that rats with intermittent 30-min access to preferred food showed greater feed-efficiency on non-access days than rats with intermittent 24 hr access to preferred food(Kreisler et al., 2017). A previous study in rats with daily 2-hr access to palatable vegetable shortening did not elicit changes in body fat composition(R. L. Corwin et al., 1998). Potentially-relevant procedural differences between the present study and Corwin et al., include: 1) the greater sucrose-composition of the current diet, 2) the greater proportional intake of the current diet than was seen for shortening in the daily Corwin et al. access condition. 3) the absence of a longer period of restriction in the Corwin et al daily access condition than the weekly 48h non-access period in the present study and/or 4) less frequent bingeing in the MWF access rats in Corwin et al., than in the current study (daily M-F).
Alternatively, 2h rats learned to consume the preferred diet the quickest among all groups (Fig 4), so perhaps the increased rate of consuming the preferred diet when accessible or endocrine adaptations that enable binge-like eating of palatable food(Woods, 1991; Woods & Ramsay, 2000) were enough to promote more fat deposition in 2h rats. Indeed, self-reported quick eating is positively associated with higher BMI in clinical populations(Ohkuma et al., 2015), and binge-like eating behavior is more prevalent among obese adults than the general population(Long, Blundell, & Finlayson, 2015). Altogether, the results support a link between more restricted access to preferred food and fat accumulation.
4.5. Blood glucose
Fasting glucose was increased in the most restricted access group (2h). Insofar as they developed the most binge-like episodes of intake, this result is consistent with findings in humans that distributing caloric load throughout the day, rather than consuming it all at once, improves fasting glucose levels(Carlson et al., 2007; Jenkins et al., 1992; Taylor et al., 1999) and also that the prevalence of type II diabetes is much higher in patients with binge-eating disorder than in those without(Raevuori et al., 2015; Thornton et al., 2017). Less expected, however, was the finding that 8h rats, who do not develop as marked binge-like intake as 2h rats, also showed increased fasting glucose levels while 4h rats did not. This result raises the hypothesis that the greater cumulative excess intake of PREF diet by 8h rats may play an independent role. Alternatively, whereas 2h rats experienced the longest duration of self-restriction, 8h rats experienced the most severe degree of chow self-restriction (~2 kcal/hr vs. ~2.5–3 kcal/hr for the other groups). Perhaps these greater histories of self-restriction alter glucose regulation during fasting.
4.6. Conclusion
In sum, the duration of restricted access to palatable sucrose-rich food can directly influence its intake and related outcomes. All restricted access conditions increased weight gain and shifted diet acceptance, leading to cycling intake according to the preferredness of the available diet. Undereating of the less preferred alternative on weekends could not easily be explained by homeostatic caloric compensation. Rats with the briefest access developed the most binge-like intake, evidence of increased feed efficiency when preferred food was not available and disproportionately greater fat accumulation. In contrast, more extended access rats showed greater daily intake of preferred food, slower weight gain when preferred food was not available, and greater individual variability of their daily energy intake from week to week. Increased fasting glucose was seen only in the briefest and most extended access conditions, whereas, across all durations, restricted access rats tended to show increased psychomotor arousal when the diet was withdrawn. Thus, intermittent access broadly promoted weight gain, fasting hyperglycemia and psychomotor arousal during early withdrawal. More restricted access promoted greater binge-like intake and fat accumulation, whereas longer access promoted evidence of greater food reward tolerance.
Supplementary Material
Table S1: Elevated plus maze. Effect of acute withdrawal from PREF on various measures during a 5 min test period on the elevated plus maze. Gray bars indicate parameters for which only data from cohort 2 were available.
Table S2: Baseline motor activation. Motor activation during 1h baseline period prior to testing.
Table S3: Defensive withdrawal. Effect of acute withdrawal from PREF on various measures during a 2h defensive withdrawal test. *data missing from some subjects
Acknowledgments
Research reported in this publication was supported by the Pearson Center for Alcoholism and Addiction Research and the National Institute on Alcohol Abuse and Alcoholism and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under awards T32 AA007456, P60 AA006420, and R01 DK070118. We also acknowledge Maria Johnson and Tim Nagy at the Small Animal Phenotyping Core at University of Alabama Birmingham for the chemical analysis of body composition. Their work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Nutrition & Obesity Research Center (P30DK056336) and Diabetes Research Center (P30DK079626). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH (2011). Escalation of Drug Use In Olmstead M (Ed.), Animal Models of Drug Addiction, Neuromethods (pp. 267–292). New York: Humana Press; Retrieved from http://stoa.usp.br/vahs/files/−1/16174/Animal+models+of+Drug+Addiction+-+Omstead [Google Scholar]
- Ahmed S, Walker JR, & Koob GF (2000). Persistent Increase in the Motivation to Take Heroin in Rats with a History of Drug Escalation. Neuropsychopharmacology, 22(4), 413–421. 10.1016/S0893-133X(99)00133-5 [DOI] [PubMed] [Google Scholar]
- Alcaraz-Iborra M, & Cubero I (2015). Do Orexins contribute to impulsivity-driven binge consumption of rewarding stimulus and transition to drug/food dependence? Pharmacology Biochemistry and Behavior, 134, 31–34. 10.1016/j.pbb.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Anseloni VZ, & Brandão ML (1997). Ethopharmacological analysis of behaviour of rats using variations of the elevated plus-maze. Behavioural Pharmacology, 8(6–7), 533–40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9832967 [DOI] [PubMed] [Google Scholar]
- Avena NM, Gold JA, Kroll C, & Gold MS (2012). Further developments in the neurobiology of food and addiction: Update on the state of the science. Nutrition, 28(4), 341–343. 10.1016/j.nut.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, & Hoebel BG (2008). Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience & Biobehavioral Reviews, 32(1), 20–39. 10.1016/j.neubiorev.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake T, Morgan DGA, & Mercer JG (2014). Feeding and metabolic consequences of scheduled consumption of large, binge-type meals of high fat diet in the Sprague–Dawley rat. Physiology & Behavior, 128, 70–79. 10.1016/J.PHYSBEH.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Iemolo A, Sabino V, Petrosino S, Steardo L, Rice KC, … Cottone P (2013). Rimonabant Precipitates Anxiety in Rats Withdrawn from Palatable Food: Role of the Central Amygdala. Neuropsychopharmacology, 38(12), 2498–2507. 10.1038/npp.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguszewski P, & Zagrodzka J (2002). Emotional changes related to age in rats--a behavioral analysis. Behavioural Brain Research, 133(2), 323–32. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12110466 [DOI] [PubMed] [Google Scholar]
- Brink PJ, & Ferguson K (1998). The decision to lose weight. Western Journal of Nursing Research, 20(1), 84–102. 10.1177/019394599802000106 [DOI] [PubMed] [Google Scholar]
- Calder RK, & Mussap AJ (2015). Factors influencing women’s choice of weight-loss diet. Journal of Health Psychology, 20(5), 612–24. 10.1177/1359105315573435 [DOI] [PubMed] [Google Scholar]
- Cannon G (2005). Dieting. Makes you fat? The British Journal of Nutrition, 93(4), 569–70. 10.1079/BJN20041382 [DOI] [PubMed] [Google Scholar]
- Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, … Mattson MP (2007). Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism: Clinical and Experimental, 56(12), 1729–34. 10.1016/j.metabol.2007.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, & Koob GF (2006). Unlimited Access to Heroin Self-Administration: Independent Motivational Markers of Opiate Dependence. Neuropsychopharmacology, 31(12), 2692–2707. 10.1038/sj.npp.1301008 [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, & Sparrow SA (1981). Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. American Journal of Mental Deficiency, 86(2), 127–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7315877 [PubMed] [Google Scholar]
- Cohen A, Koob GF, & George O (2012). Robust Escalation of Nicotine Intake with Extended Access to Nicotine Self-Administration and Intermittent Periods of Abstinence. Neuropsychopharmacology, 37(9), 2153–2160. 10.1038/npp.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, & Hoebel BG (2002). Evidence That Intermittent, Excessive Sugar Intake Causes Endogenous Opioid Dependence. Obesity Research, 10(6), 478–488. 10.1038/oby.2002.66 [DOI] [PubMed] [Google Scholar]
- Corwin RL (2004). Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite, 42(2), 139–142. 10.1016/j.appet.2003.08.010 [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, & Boggiano MM (2011). Feeding and reward: perspectives from three rat models of binge eating. Physiology & Behavior, 104(1), 87–97. 10.1016/j.physbeh.2011.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RLW, & Babbs RK (2012). Rodent Models of Binge Eating: Are They Models of Addiction? ILAR Journal, 53(1), 23–34. 10.1093/ilar.53.1.23 [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FHE, Fisher JO, Dimitriou SG, Rice HB, & Young MA (1998). Limited Access to a Dietary Fat Option Affects Ingestive Behavior But Not Body Composition in Male Rats. Physiology & Behavior, 65(3), 545–553. 10.1016/S0031-9384(98)00201-7 [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, E. Z (2008). Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. American Journalfof Physiology - Regulatory, Integrative and Comparative Physiology, 295(4). Retrieved from https://www.physiology.org/doi/pdf/10.1152/ajpregu.90309.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, … Zorrilla EP (2009). CRF system recruitment mediates dark side of compulsive eating. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20016–20. 10.1073/pnas.0908789106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, & Zorrilla EP (2008a). Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 295(4), R1066–76. 10.1152/ajpregu.90309.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, & Zorrilla EP (2008b). Opioid-Dependent Anticipatory Negative Contrast and Binge-Like Eating in Rats with Limited Access to Highly Preferred Food. Neuropsychopharmacology, 33(3), 524–535. 10.1038/sj.npp.1301430 [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, & Zorrilla EP (2009). Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology, 34(1), 38–49. 10.1016/j.psyneuen.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore R, Valenza M, Wang X, Rice KC, Sabino V, & Cottone P (2014). The inverse agonist of CB 1 receptor SR141716 blocks compulsive eating of palatable food. Addiction Biology, 19(5), 849–861. 10.1111/adb.12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, & Spear LP (2006). Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior, 83(4), 570–7. 10.1016/j.pbb.2006.03.019 [DOI] [PubMed] [Google Scholar]
- Dunnett CW (1964). New Tables for Multiple Comparisons with a Control. Biometrics, 20(3), 482 10.2307/2528490 [DOI] [Google Scholar]
- Feeney E, O’Brien S, Scannell A, Markey A, Gibney ER, Berghöfer A, … Meyerhof W (2011). Genetic variation in taste perception: does it have a role in healthy eating? Proceedings of the Nutrition Society, 70(01), 135–143. 10.1017/S0029665110003976 [DOI] [PubMed] [Google Scholar]
- Flaherty CF, & Rowan GA (1986). Successive, simultaneous, and anticipatory contrast in the consumption of saccharin solutions. Journal of Experimental Psychology. Animal Behavior Processes, 12(4), 381–93. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3772302 [PubMed] [Google Scholar]
- Garcia-Bailo B, Toguri C, Eny KM, & El-Sohemy A (2009). Genetic Variation in Taste and Its Influence on Food Selection. OMICS: A Journal of Integrative Biology, 13(1), 69–80. 10.1089/omi.2008.0031 [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Wall M, Loth KA, Le Grange D, & Neumark-Sztainer D (2012). Which Dieters Are at Risk for the Onset of Binge Eating? A Prospective Study of Adolescents and Young Adults. Journal of Adolescent Health, 51(1), 86–92. 10.1016/j.jadohealth.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, … Koob GF (2009). Corticotropin-releasing factor-1 receptor antagonists decrease heroin selfadministration in long- but not short-access rats. Addiction Biology, 14(2), 130–143. 10.1111/j.1369-1600.2008.00142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, & Koob GF (n.d.). The α 1 Adrenergic Receptor Antagonist Prazosin Reduces Heroin Self-Administration in Rats with Extended Access to Heroin Administration. 10.1016/j.pbb.2008.07.012 [DOI] [PMC free article] [PubMed]
- Harris RB, & Martin RJ (1984). Recovery of body weight from below "set point" in mature female rats. The Journal of Nutrition, 114(6), 1143–50. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6726478 [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, & Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism, Clinical and Experimental Research, 35(11), 1938–47. 10.1111/j.1530-0277.2011.01545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, & Cottone P (2013). CRF-CRF1 receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 38(12), 2456–66. 10.1038/npp.2013.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, … Cottone P (2012). Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behavioural Pharmacology, 23(5–6), 593–602. 10.1097/FBP.0b013e328357697f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Ocana A, Jenkins AL, Wolever TM, Vuksan V, Katzman L, … Patten R (1992). Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. The American Journal of Clinical Nutrition, 55(2), 461–7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1734685 [DOI] [PubMed] [Google Scholar]
- Johnson PM, & Kenny PJ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience, 13(5), 635–41. 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, & Markou A (2005). Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology, 179(1), 247–254. 10.1007/s00213-004-2069-2 [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, & Pulvirenti L (2006). Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology, 186(1), 48–53. 10.1007/s00213-006-0353-z [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine S, Hildebrandt B, & Sisk CL (2013). Sex differences in binge eating patterns in male and female adult rats. International Journal of Eating Disorders, 46(7), 729–736. 10.1002/eat.22139 [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, … George O (2014). Addiction as a stress surfeit disorder. Neuropharmacology, 76, 370–382. 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2005). Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nature Neuroscience, 8(11), 1442–1444. 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- Kreisler AD, Garcia MG, Spierling SR, Hui BE, & Zorrilla EP (2017). Extended vs. brief intermittent access to palatable food differently promote binge-like intake, rejection of less preferred food, and weight cycling in female rats. Physiology & Behavior, 177, 305–316. 10.1016/j.physbeh.2017.03.039 [DOI] [PubMed] [Google Scholar]
- Lenoir M, & Ahmed SH (2008). Supply of a Nondrug Substitute Reduces Escalated Heroin Consumption. Neuropsychopharmacology, 33(9), 2272–2282. 10.1038/sj.npp.1301602 [DOI] [PubMed] [Google Scholar]
- Long CG, Blundell JE, & Finlayson G (2015). A Systematic Review of the Application And Correlates of YFAS-Diagnosed “Food Addiction” in Humans: Are Eating-Related “Addictions” a Cause for Concern or Empty Concepts? Obesity Facts, 8(6), 386–401. 10.1159/000442403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR (2015). Dieting: proxy or cause of future weight gain? Obesity Reviews, 16(S1), 19–24. 10.1111/obr.12252 [DOI] [PubMed] [Google Scholar]
- Lowe MR, Doshi SD, Katterman SN, & Feig EH (2013). Dieting and restrained eating as prospective predictors of weight gain. Frontiers in Psychology, 4, 577 10.3389/fpsyg.2013.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes J, & Tiggemann M (2003). Body dissatisfaction, dieting awareness and the impact of parental influence in young children. British Journal of Health Psychology, 8(2), 135–147. 10.1348/135910703321649123 [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, & Koob GF (1996). Time-dependent quantifiable withdrawal from ethanol in the rat: Effect of method of dependence induction. Alcohol, 13(2), 163–170. 10.1016/0741-8329(95)02030-6 [DOI] [PubMed] [Google Scholar]
- Mase T, Miyawaki C, Ohara K, & Nakamura H (2015). The Relationships among Perception of Body Image, a Desire for Thinness, and Dieting Behavior in Young Females in Japan. Health, 07(01), 112–118. 10.4236/health.2015.71013 [DOI] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, & Bulik CM (2009). The biology of binge eating. Appetite, 52(3), 545–53. 10.1016/j.appet.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Lutz TA, Romano A, Pucci M, Geary N, Asarian L, & Cifani C (2017). Estrogenic suppression of binge-like eating elicited by cyclic food restriction and frustrative-nonreward stress in female rats. International Journal of Eating Disorders, 50(6), 624–635. 10.1002/eat.22687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani J-P, Schutz Y, & Dulloo AG (2015). Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obesity Reviews, 16(S1), 7–18. 10.1111/obr.12251 [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, … Koob GF (2007). Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. The Journal of Pharmacology and Experimental Therapeutics, 320(1), 180–93. 10.1124/jpet.106.105270 [DOI] [PubMed] [Google Scholar]
- Ohkuma T, Hirakawa Y, Nakamura U, Kiyohara Y, Kitazono T, & Ninomiya T (2015). Association between eating rate and obesity: a systematic review and meta-analysis. International Journal of Obesity, 39(11), 1589–1596. 10.1038/ijo.2015.96 [DOI] [PubMed] [Google Scholar]
- Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, & Koob GF (2013). Corticotropin-releasing factor (CRF) and α 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. The International Journal of Neuropsychopharmacology, 16(08), 1867–1875. 10.1017/S1461145713000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, & Zorrilla EP (2012). Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiology & Behavior, 107(2), 231–42. 10.1016/j.physbeh.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, & Zorrilla EP (2011). The dark side of food addiction. July, 25(1041), 149–156. 10.1016/j.physbeh.2011.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJA, Massagrande M, Montanari D, … Corsi M (2012). Role of Orexin-1 Receptor Mechanisms on Compulsive Food Consumption in a Model of Binge Eating in Female Rats. Neuropsychopharmacology, 37(9), 1999–2011. 10.1038/npp.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, & Herman CP (1985). Dieting and binging: A causal analysis. American Psychologist, 40(2), 193–201. 10.1037/0003-066X.40.2.193 [DOI] [PubMed] [Google Scholar]
- Raevuori A, Suokas J, Haukka J, Gissler M, Linna M, Grainger M, & Suvisaari J (2015). Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. International Journal of Eating Disorders, 48(6), 555–562. 10.1002/eat.22334 [DOI] [PubMed] [Google Scholar]
- Rogers PJ (1985). Returning ‘cafeteria-fed’ rats to a chow diet: Negative contrast and effects of obesity on feeding behaviour. Physiology & Behavior, 35(4), 493–499. 10.1016/0031-9384(85)90129-5 [DOI] [PubMed] [Google Scholar]
- Rossetti C, Spena G, Halfon O, & Boutrel B (2014). Evidence for a compulsive-like behavior in rats exposed to alternate access to highly preferred palatable food. Addiction Biology, 19(6), 975–85. 10.1111/adb.12065 [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, & Koob GF (1995). Decreased brain reward produced by ethanol withdrawal. Proceedings of the National Academy of Sciences of the United States of America, 92(13), 5880–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7597046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn S (2006). Change from baseline and analysis of covariance revisited. Statistics in Medicine, 25(24), 4334–4344. 10.1002/sim.2682 [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Fleiss JL (1979). Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin, 86(2), 420–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18839484 [DOI] [PubMed] [Google Scholar]
- Sinclair EB, Culbert KM, Gradl DR, Richardson KA, Klump KL, & Sisk CL (2015). Differential mesocorticolimbic responses to palatable food in binge eating prone and binge eating resistant female rats. Physiology & Behavior, 152, 249–256. 10.1016/j.physbeh.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, & Koob GF (2008). CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology, 196(3), 473–82. 10.1007/s00213-007-0983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling SR, Kreisler AD, Williams CA, Fang SY, Pucci SN, Kines KT, & Zorrila EP (2018). Intermittent, extended access to preferred food leads to escalated food reinforcement and cyclic whole-body metabolism in rats: sex differences and individual vulnerability. Physiology & Behavior, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, & Baker EW (1990). Corticotropin-releasing factor antagonist attenuates defensive-withdrawal behavior elicited by odors of stressed conspecifics. Behavioral Neuroscience, 104(2), 386–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2346630 [DOI] [PubMed] [Google Scholar]
- Taylor AE, Hubbard J, & Anderson EJ (1999). Impact of Binge Eating on Metabolic and Leptin Dynamics in Normal Young Women 1. The Journal of Clinical Endocrinology & Metabolism, 84(2), 428–434. 10.1210/jcem.84.2.5502 [DOI] [PubMed] [Google Scholar]
- Teegarden SL, & Bale TL (2007). Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biological Psychiatry, 61(9), 1021–9. 10.1016/j.biopsych.2006.09.032 [DOI] [PubMed] [Google Scholar]
- Thornton LM, Watson HJ, Jangmo A, Welch E, Wiklund C, von Hausswolff-Juhlin Y, … Bulik CM (2017). Binge-eating disorder in the Swedish national registers: Somatic comorbidity. International Journal of Eating Disorders, 50(1), 58–65. 10.1002/eat.22624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, & Koob GF (2002). Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism, Clinical and Experimental Research, 26(10), 1494–501. 10.1097/01.ALC.0000033120.51856.F0 [DOI] [PubMed] [Google Scholar]
- Vandervoort J, Aimé A, & Green-Demers I (2015). The monster in the mirror: reasons for wanting to change appearance. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 20(1), 99–107. 10.1007/s40519-014-0160-1 [DOI] [PubMed] [Google Scholar]
- Vendruscolo JCM, Tunstall BJ, Carmack SA, Schmeichel BE, Lowery-Gionta EG, Cole M, … Vendruscolo LF (2017). Compulsive-Like Sufentanil Vapor Self-Administration in Rats. Neuropsychopharmacology. 10.1038/npp.2017.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, & Koob GF (2011). Escalation patterns of varying periods of heroin access. Pharmacology Biochemistry and Behavior, 98(4), 570–574. 10.1016/J.PBB.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Tomasi D, & Baler RD (2013). Obesity and addiction: neurobiological overlaps. Obesity Reviews : An Official Journal of the International Association for the Study of Obesity, 14(1), 2–18. 10.1111/j.1467-789X.2012.01031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AS, Read NW, Laugharne JDE, & Ahluwalia NS (1998). Alterations in mood after changing to a low-fat diet. British Journal of Nutrition, 79(01), 23 10.1079/BJN19980005 [DOI] [PubMed] [Google Scholar]
- Wojnicki FHE, Johnson DS, & Corwin RLW (2008). Access conditions affect binge-type shortening consumption in rats. Physiology & Behavior, 95(5), 649–57. 10.1016/j.physbeh.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC (1991). The eating paradox: how we tolerate food. Psychological Review, 98(4), 488–505. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1961770 [DOI] [PubMed] [Google Scholar]
- Woods SC, & Ramsay DS (2000). Pavlovian influences over food and drug intake. Behavioural Brain Research, 110(1–2), 175–82. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10802313 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morse AC, Koob GF, & Schulteis G (2007). Dose- and Time-Dependent Expression of Anxiety-Like Behavior in the Elevated Plus-Maze During Withdrawal From Acute and Repeated Intermittent Ethanol Intoxication in Rats. Alcoholism: Clinical and Experimental Research, 31(11), 1811–1819. 10.1111/j.1530-0277.2007.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, & Zorrilla EP (2007). Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism, Clinical and Experimental Research, 31(9), 1505–15. 10.1111/j.1530-0277.2007.00456.x [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Wee S, Zhao Y, Specio S, Boutrel B, Koob GF, & Weiss F (2012). Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addiction Biology, 17(2), 300–8. 10.1111/j.1369-1600.2011.00329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Elevated plus maze. Effect of acute withdrawal from PREF on various measures during a 5 min test period on the elevated plus maze. Gray bars indicate parameters for which only data from cohort 2 were available.
Table S2: Baseline motor activation. Motor activation during 1h baseline period prior to testing.
Table S3: Defensive withdrawal. Effect of acute withdrawal from PREF on various measures during a 2h defensive withdrawal test. *data missing from some subjects