Abstract
Objective
To use the minimal important difference (MID) versus standardized mean difference (SMD) approach to provide more robust and clinically relevant information regarding the association between land-based aerobic exercise and changes in self-reported fatigue among adults with rheumatoid arthritis (RA).
Methods
Data from a previous meta-analysis of five randomized controlled trials representing up to 298 participants per study were used to calculate nine effect sizes (ES) using the MID approach. Minimal important difference data were derived from previously reported anchor-based values specific to each fatigue instrument in adults with RA. Results were pooled using a random-effects model.
Results
Aerobic exercise resulted in statistically significant reductions in self-reported fatigue (MID ES, −0.34, 95% CI, −0.58 to −0.10, p = 0.006). Findings were similar when ESs were collapsed so that only one ES represented each study (MID ES, −0.39, 95% CI, −0.76 to −0.03, p = 0.04). However, using previous cut points, it may be unlikely that a large number of participants with RA could derive clinically relevant reductions in fatigue.
Conclusions
Land-based aerobic exercise is associated with statistically significant reductions in fatigue. However, it may be unlikely that a substantial number of participants with RA could obtain clinically-relevant reductions in fatigue. A need exists for additional studies, especially in RA participants with elevated levels of fatigue.
Keywords: exercise, fatigue, rheumatoid arthritis, minimal important difference, meta-analysis
INTRODUCTION
Rheumatoid arthritis (RA) is a common autoimmune disease that affects approximately 1% of the population worldwide (1). More common in women than men (2), the total annual costs attributed to RA in the United States in 2005 were estimated at $19.2 billion (3). One of the most common patient-reported outcomes reported by participants with RA is fatigue, a condition which may be described as a “sensation of exhaustion during or after usual activities, or a feeling of inadequate energy to begin these activities” (4). However, this definition does not capture the sensation that RA participants report in feeling that fatigue is unrelated to energy expenditure and unpredictable. The prevalence of fatigue in RA participants has been reported to be between 41% and 80% (5–9), with differing findings most likely the result of variations in the definitions and instruments used to assess fatigue as well as the lack of a gold standard instrument for assessing such. One potential non-pharmacological therapy for the treatment of fatigue in adults with RA is exercise. A recent meta-analysis by Rongen-van Dartel, et al., limited to randomized controlled trials of supervised land-based aerobic exercise in adults with RA found a statistically significant, standardized mean difference (SMD) effect size (ES) improvement of −0.31 (p = 0.02) in fatigue after ≤ 12 weeks of exercise but a non-statistically significant decrease after ≥ 24 weeks (ES, −0.15, p = 0.9) (10). While these results are informative, the use of the SMD approach for calculating ESs is influenced by factors such as the heterogeneity of the population and is not easily interpreted by clinicians and other healthcare providers (11). Alternatively, reporting ES results in minimal important difference (MID) units has been suggested as a potential solution to address problems associated with existing methods such as the SMD approach (11). Given the potential benefits of exercise on fatigue in adults with RA as well as the need for potentially more valid and easily interpretable information for decision-makers, the purpose of this paper was to apply the MID approach for the calculation of ESs to the recent meta-analysis of Rongen-van Dartel, et al., in order to determine the practically-relevant effects of exercise on fatigue in adults with RA (10).
MATERIALS AND METHODS
Data source
Data for this brief report were derived from a recently published systematic review with meta-analysis on the association between exercise and fatigue in adults with RA (10) as well as examining the original five studies that were included in the meta-analysis (12–16). Briefly, studies were limited to randomized controlled trials of supervised, land-based aerobic exercise lasting at least four weeks in adults with RA and in which fatigue was assessed as an outcome (10). The five studies (12–16) included in the meta-analysis (10) yielded 9 ESs representing 29 to 281 participants per study (17 to 136 exercise and 12 to 145 control). Ages of the eligible participants within each study ranged from 20 to 80 years while the percentage of females ranged from 59% to 82.7% (12–16). Training modalities included cycling, running, and circuit training 2 to 3 times per week for ≥ 15 minutes per session (10). Length of training ranged from 4 to 104 weeks (10). This included 104 weeks of exercise in one study (12), 12 and 24 weeks in two studies (13, 14), 8 and 24 weeks in one study (15), and 4 and 24 weeks in another (16). Assessment was conducted using the Multidimensional Assessment of Fatigue (MAF) Instrument in 3 studies (13–15), and either the Vitality subscale of the SF-36 (SF-36-V) (12) or Visual Analog Scale for Fatigue (VAS-F) (16) in one study each (10). Effect sizes in the original meta-analysis (10) were calculated using the SMD and pooled using the original random-effects, method-of-moments approach of Dersimonian and Laird (17). The overall findings were reported according to short-term (≤ 12 weeks) and long-term (≥ 24 weeks) land-based aerobic exercise (10). However, no rationale was provided to support this dichotomization (10).
Data synthesis
Given that fatigue is measured using different instruments with different scales, results need to be standardized so they can be pooled in a meta-analysis. For the current meta-analysis, the SMD ES was calculated using the same procedure as the original meta-analysis (10). Briefly, and as described in more detail elsewhere (11), this consisted of taking the mean difference in fatigue for each study, dividing by the standard deviation in each study, and then pooling across studies. The MID ES was calculated by replacing the denominator of the SMD, i.e., the standard deviation, with the MID. Thus, the MID ES was calculated by dividing the mean difference by the MID established for that instrument. The MID was defined as “the smallest difference in the outcome of interest that informed patients or informed proxies perceive as important, either beneficial or harmful, and which would lead the patient or clinician to consider a change in management” (18). Instrument-specific MIDs were obtained from a previous systematic review (19). Finally, when results are standardized by dividing the mean difference by the MID, the scale in which the meta-analysis is conducted is changed. To account for this change, previously recommended formulas were used (11).
The a priori plan was to pool ESs similar to the approach used in the meta-analysis of Rongen-van Dartel, et al.(10), in which results were pooled using a random-effects model and partitioned according to short term (≤ 12 weeks) and long term (≥ 24 weeks) changes in fatigue. However, a post hoc decision was made to not analyze the data according to short and long-term exercise given the lack of research to support such cut points. In addition, a two-tailed, z-distribution, random-effects, method-of-moments meta-regression was conducted in which no statistically significant association was found between length of training, in weeks, and SMD ES changes in fatigue (β1 = 0.0027, 95%, −0.0006, 0.0060, z = 1.62, p = 0.11). Consequently, data were initially analyzed by treating all 9 results from the 5 studies (12–16) as separate ESs. However, because multiple ES results for fatigue from the same RA participants were nested in four studies (13–16), resulting in a lack of independence, analyses were also conducted by pooling multiple results from the same study into one ES so that only one ES represented each study. Non-overlapping 95% confidence intervals and alpha values ≤0.05 were considered statistically significant. Based on prior suggestions, ESs >1 based on the MID were indicative of numerous participants deriving important benefits from the effects of land-based aerobic exercise on fatigue, an ES between 0.5 and 1.0 indicative of a considerable number of participants benefitting, while an ES <0.5 suggested that it was progressively less likely that a substantial number of participants would benefit (11). Heterogeneity was examined using the Q statistic, with alpha values ≤ 0.10 considered statistically significant. Inconsistency was examined using the I2 statistic and the same cut points used in the original meta-analysis: <25% (low), 25% to 75% (moderate), ≥75% (large) (10). Absolute between-study variance was calculated using tau-squared (τ2). Because the number of ESs was low (n = 9), results were also calculated with each ES deleted from the model once given the possibility that one ES could have a significant impact on the overall pooled results. Based on current guidelines, small-study effects (publication bias, etc.) were not examined because the number of ESs in each subgroup was less than 10 (20). Effect sizes were calculated using Microsoft Excel 2013 while data were analyzed using MetaXL, version 5.3 as well as Comprehensive Meta-Analysis, version 3.3.
RESULTS
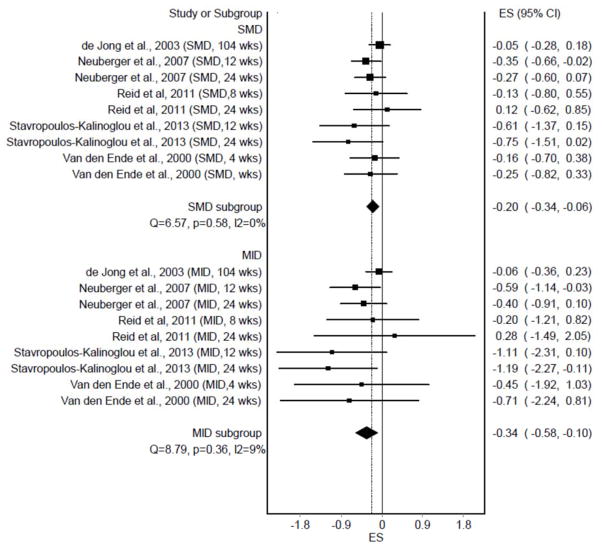
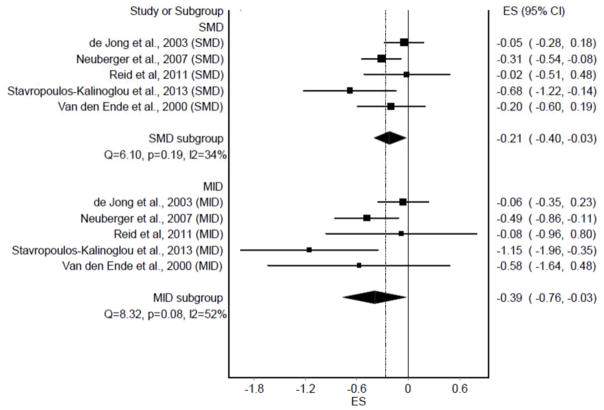
Individual ES results (n = 9) from each study, partitioned according to the SMD and MID ES approaches, are shown in Figure 1. As can be seen, statistically significant and non-overlapping 95% CI’s were observed for both the SMD (p = 0.004) and MID (p = 0.006). No statistically significant heterogeneity was observed and inconsistency was considered low. Tau-squared (τ2 ) was 0 for the SMD approach and 0.01 for the MID approach. The overall MID ES of −0.34 suggests that it is unlikely that a large number of participants with RA would derive clinically important reductions in fatigue by participating in land-based aerobic exercise (11). When ES results were collapsed so that only one ES represented each study (n = 5), similar results were observed (Figure 2). Statistically significant and non-overlapping 95% CI’s were again observed for both the SMD (p = 0.02) and MID (p = 0.04). No statistically significant heterogeneity was observed for SMD results and inconsistency was considered moderate. For the MID approach, statistically significant heterogeneity and moderate inconsistency were observed. Tau-squared (τ2 ) was 0.02 for SMD ESs and 0.08 for MID ESs. The overall MID ES of −0.39 again suggests that it is unlikely that a large number of participants with RA would derive clinically-relevant reductions in fatigue from land-based aerobic exercise (11).
Figure 1.
Forest plot representing individual effect sizes (ES) for fatigue, grouped according to the standardized mean difference (SMD) and minimal important difference (MID) approaches.
Figure 2.
Forest plot representing study-level effect sizes (ES) for fatigue, grouped according to the standardized mean difference (SMD) and minimal important difference (MID) approaches.
With each result deleted from the model once, ES changes in fatigue based on the SMD ranged from −0.29 to −0.17 when treating each ES separately as well as when results were collapsed so that only one ES represented each study. For MID ESs, changes ranged from −0.54 to −0.26 when treating each ES separately to −0.55 to −0.25 when results were collapsed so that only one ES represented each study.
DISCUSSION
This study provides clinically relevant information regarding the effects of supervised land-based aerobic exercise on self-reported fatigue in adults with RA. Specifically, based on both the SMD and MID ES approaches, the overall findings suggest that land-based aerobic exercise can yield statistically significant reductions in self-reported fatigue in those with RA. However, based on the MID ES and recommended cut points (11), it may be unlikely that a large number of participants with RA would derive clinically-relevant benefits. Importantly however, land-based aerobic exercise did not appear to increase fatigue. This is noteworthy given the numerous other benefits that can be derived from exercise in those with chronic conditions (21, 22). Specific to RA participants, these include, but are not necessarily limited to, improvements in physical function (23–26), disease activity (23), quality-of-life (24), and pain (24). In addition, previous research has reported no serious adverse events (26) or increases in disease activity as a result of exercise (23, 24).
The direction of findings are in general agreement with the overall results reported in the original meta-analysis of Rongen-van Dartel et al.(10), and in which ESs were based on using the pooled standard deviation versus MID in the denominator (10).
From the authors’ perspective, there are several strengths to the current study. First, the current study included the calculation of ESs using the MID approach (11). Such an approach helps to circumvent the problems associated with between-study variances based on the pooled standard deviation, i.e., the SMD approach (11). Specifically, these include the influence of heterogeneity in the population as well as the lack of intuitiveness for clinicians and other healthcare providers (11). A second and related strength was the generation of findings that provide more robust and clinically relevant information for decision-makers (11). The rationale for the MID ES being more clinically relevant is grounded in the fact that it is based on scores that clinicians, patients or their proxies perceive as important and which could result in a decision to make a change in the management of the participant’s condition (11, 18). A third strength was the availability of anchor-based MID data specific to each instrument in RA participants. As a result, the use of alternative approaches such as relying on MID data from other populations (cancer patients, etc.) or the use of distribution-based methods was avoided (11).
While the current study has several strengths, there are also potential limitations. First, and as pointed out in the original meta-analysis, none of the original studies included in the meta-analysis limited participants to those with elevated levels of fatigue (10). As a result, the value of exercise may not have been realized in those who may have the most to gain from such. Second, some may consider the MID ES cut points used for determining clinical relevance as somewhat arbitrary (11). However, the same can be true for the suggested cut points used for the SMD ES (27–29). Therefore, given the greater clinical relevance, it is suggested that the MID ES be the metric of choice when instruments using two or more different scales are used to assess the same outcome. This is of course assuming that MID data are available for each instrument and in the participant population of interest. For example, in the current study, MID values were available for each instrument that assessed fatigue in participants with RA (19). In contrast, unpublished results from the authors have shown that no MID data exist for instruments that assess anxiety in participants with RA.
In conclusion, the results of the current study suggest that land-based aerobic exercise is associated with statistically significant reductions in fatigue. While it may be unlikely that a large number of participants with RA could obtain clinically-relevant reductions in fatigue as a result of land-based exercise, they are likely to have meaningful improvements in other symptoms with some improvement in fatigue. A need exists for additional studies on this topic, especially in RA participants with elevated levels of fatigue.
SIGNIFICANCE AND INNOVATIONS.
This is the first meta-analysis to use the more robust and clinically relevant minimal important difference (MID) approach to examine the effects of supervised, land-based aerobic exercise on self-reported fatigue in adults with rheumatoid arthritis.
The findings suggest that participation in land-based aerobic exercise results in statistically significant reductions in fatigue but it may be unlikely that a large number of participants with rheumatoid arthritis could derive clinically important reductions in fatigue.
Supervised, land-based aerobic exercise did not increase self-reported fatigue in adults with rheumatoid arthritis.
Because they may have the most to gain, future exercise research should focus on rheumatoid arthritis participants with elevated levels of self-reported fatigue.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR061346 (GA Kelley, Principal Investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
George A. Kelley, School of Public Health, Department of Biostatistics, West Virginia University, Morgantown, WV, USA, 26506-9190, Office Phone: 304-293-6279, Fax: 304-293-5891.
Kristi S. Kelley, Research Instructor, School of Public Health, Department of Biostatistics, Robert C. Byrd Health Sciences Center, West Virginia University, PO Box 9190, Morgantown, WV, USA, 26506-9190, Office Phone: 304-293-6280, Fax: 304-293-5891
Leigh F. Callahan, Mary Link Briggs Distinguished Professor of Medicine, Professor, Departments of Social Medicine and Orthopaedics, Adjunct Professor, Department of Epidemiology, 3300 Thurston Bldg, Campus Box 7280, University of North Carolina, Chapel Hill, NC 27599-7280, Office Phone: 919-966-0564.
References
- 1.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18(13 Suppl):S295–302. [PubMed] [Google Scholar]
- 2.van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med. 2009;7:12. doi: 10.1186/1741-7015-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90. doi: 10.1185/03007990903422307. [DOI] [PubMed] [Google Scholar]
- 4.Chen MK. The epidemiology of self-perceived fatigue among adults. Prev Med. 1986;15(1):74–81. doi: 10.1016/0091-7435(86)90037-x. [DOI] [PubMed] [Google Scholar]
- 5.Overman CL, Kool MB, Da Silva JAP, Geenen R. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin Rheumatol. 2016;35:409–15. doi: 10.1007/s10067-015-3035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995;22(4):639–43. [PubMed] [Google Scholar]
- 7.Belza BL, Henke CJ, Yelin EH, Epstein WV, Gilliss CL. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res. 1993;42(2):93–9. [PubMed] [Google Scholar]
- 8.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23(8):1407–17. [PubMed] [Google Scholar]
- 9.Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981;24(10):1308–15. doi: 10.1002/art.1780241012. [DOI] [PubMed] [Google Scholar]
- 10.Rongen-van Dartel SA, Repping-Wuts H, Flendrie M, Bleijenberg G, Metsios GS, van den Hout WB, et al. Effect of aerobic exercise training on fatigue in rheumatoid arthritis: A meta-analysis. Arthritis Care Res. 2015;67(8):1054–62. doi: 10.1002/acr.22561. [DOI] [PubMed] [Google Scholar]
- 11.Johnston BC, Thorlund K, Schunemann HJ, Xie F, Murad MH, Montori VM, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes. 2010;8:116. doi: 10.1186/1477-7525-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jong Z, Munneke M, Zwinderman AH, Kroon HM, Jansen A, Ronday KH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum. 2003;48(9):2415–24. doi: 10.1002/art.11216. [DOI] [PubMed] [Google Scholar]
- 13.Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57(6):943–52. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 14.Stavropoulos-Kalinoglou A, Metsios GS, van Zanten JJJC, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Annals of Rheumatic Disease. 2013;72(11):1819–25. doi: 10.1136/annrheumdis-2012-202075. [DOI] [PubMed] [Google Scholar]
- 15.Reid A, Brady A, Blake C, Mongey AB, Veale DJ, FitzGerald O, et al. Randomised controlled trial examining the effect of exercise in people with rheumatoid arthritis taking anti-TNFalpha therapy medication. BMC Musculoskelet Disord. 2011;12:11. doi: 10.1186/1471-2474-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Ende CHM, Breedveld FC, le Cessie S, Dijkmans BAC, de Mug AW, Hazes JMW. Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2000;59(8):615–21. doi: 10.1136/ard.59.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Schunemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic respiratory disease questionnaire (CRQ) COPD. 2005;2(1):81–9. doi: 10.1081/copd-200050651. [DOI] [PubMed] [Google Scholar]
- 19.Nordin Å, Taft C, Lundgren-Nilsson Å, Dencker A. Minimal important differences for fatigue patient reported outcome measures—a systematic review. BMC Med Res Methodol. 2016;16:62. doi: 10.1186/s12874-016-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(s1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 23.Baillet A, Vaillant M, Guinot M, Juvin R, Gaudin P. Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2012;51(3):519–27. doi: 10.1093/rheumatology/ker330. [DOI] [PubMed] [Google Scholar]
- 24.Baillet A, Zeboulon N, Gossec L, Combescure C, Bodin LA, Juvin R, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: Meta-analysis of randomized controlled trials. Arthritis Care Res. 2010;62(7):984–92. doi: 10.1002/acr.20146. [DOI] [PubMed] [Google Scholar]
- 25.Hammond A, Prior Y. The effectiveness of home hand exercise programmes in rheumatoid arthritis: a systematic review. Br Med Bull. 2016;119(1):49–62. doi: 10.1093/bmb/ldw024. [DOI] [PubMed] [Google Scholar]
- 26.Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van den Ende EC. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. 2009;(4):CD006853. doi: 10.1002/14651858.CD006853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1988. [Google Scholar]
- 28.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8(2):597–9. [Google Scholar]