Recombinant urate oxidase (rasburicase) was approved for the prevention and treatment of malignancy-associated hyperuricaemia in paediatrics by the US Food and Drug Administration in 2002. However, since then, there has been limited data to inform evidence-based practice for its administration. Current guidelines rely on expert opinion, and multiple published guidelines provide conflicting recommendations.(Agrawal and Feusner 2011, Bertrand, et al 2008, Cairo, et al 2010, Howard, et al 2011) As a first step in addressing this data gap we sought to understand rasburicase prescribing practices at children's hospitals throughout the United States.
We performed a retrospective cohort study using the Pediatric Health Information System database (PHIS), an administrative database containing inpatient data from 48 children’s hospitals throughout the United States. Available data include demographics, diagnosis codes and daily resource utilization data (including pharmaceuticals, laboratory/imaging studies and procedures). A data subset (PHIS+) also contains laboratory results from six hospitals from 2007–2012.
Subjects were drawn from previously validated cohorts of paediatric patients with acute lymphoblastic leukaemia (ALL) or non-Hodgkin lymphoma (NHL) within PHIS.(Citrin, et al 2017, Fisher, et al 2014) Patients were included in this study if their first admission associated with the malignant diagnosis code (“index admission”) was between 2004 and 2015. They were followed until discharge or for a maximum of 30 days. Patients with contraindications to rasburicase were excluded.
Demographics were summarized using frequencies (proportions). Higher severity of presentation was defined as utilization of any Intensive Care Unit level care resource within the first two days of admission.(Maude, et al 2014) Multivariable logistic regression models of rasburicase use controlled for hospital, demographics, diagnosis, and presentation severity. The primary outcome was the receipt of rasburicase during the index admission. Prescribing variability was assessed by comparing the adjusted proportion of rasburicase-exposed patients at each institution, limited to hospitals treating at least 20 patients during the study period. Sub-analyses were performed in PHIS+, with further adjustment for presenting white blood cell (WBC) and uric acid values (utilized as multi-level categorical variables based on age and gender norms) (Flerlage and Engorn 2015). Analyses were conducted using Stata version 14.0 (StataCorp LLC, College Station, TX).
A total of 13,112 patients with ALL and NHL were identified. Twenty-nine patients were excluded due to contraindications to rasburicase, leaving 11,682 patients with ALL and 1,401 patients with NHL for analyses (Table I). We identified 2,078 (17.8%) patients with ALL and 459 (32.8%) patients with NHL who received rasburicase. The odds of receiving rasburicase was significantly higher for older patients (>10 years) with ALL and younger patients (<10 years) with NHL. Additionally, regardless of disease group, males were more likely to receive rasburicase (Odds ratio [OR] 1.41; 95% confidence interval [CI] 1.28–1.56; p<0.0001). This gender effect differed by age, with no significant association in children <5 years (OR 0.96; 95% CI 0.82–1.13 p=0.66) and increasing discrepancies in the three older age categories. The magnitude of this discrepancy varied, but was present at a majority of institutions.
Table I.
Adjusted use of rasburicase by demographic category*
| ALL (n = 11,682) | NHL (n = 1,401) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Prevalence of rasburicase use |
Crude OR of rasburicase use (95% CI) |
Adjusted OR of rasburicase use (95% CI)* |
P (adjusted) | N | Prevalence of rasburicase use |
Crude OR of rasburicase use (95% CI) |
Adjusted OR of rasburicase use (95% CI)* |
P (adjusted) |
|
| Age | ||||||||||
| 0 – <5 years | 5,579 | 748 (13.4%) | 183 | 72 (39.3%) | ||||||
| 5 – <10 years | 2,997 | 484 (16.2%) | 1.24 (1.10–1.41) | 1.29 (1.13–1.47) | <0.0001 | 374 | 148 (39.6%) | 1.01 (0.70–1.45) | 0.93 (0.60–1.42) | 0.724 |
| 10 – <15 years | 1,904 | 503 (26.4%) | 2.32 (2.04–2.63) | 2.36 (2.06–2.72) | <0.0001 | 429 | 143 (33.3%) | 0.77 (0.54–1.10) | 0.70 (0.46–1.06) | 0.095 |
| 15 – <22 years | 1,202 | 343 (28.5%) | 2.58 (2.23–2.99) | 2.85 (2.43–3.34) | <0.0001 | 415 | 96 (23.1%) | 0.46 (0.32–0.67) | 0.33 (0.21–0.52) | <0.0001 |
| Sex | ||||||||||
| Female | 5,169 | 776 (15.0%) | 340 | 84 (24.7%) | ||||||
| Male | 6,513 | 1,302 (20.0%) | 1.41 (1.28–1.56) | 1.33 (1.20–1.48) | <0.0001 | 1,061 | 375 (35.3%) | 1.67 (1.26–2.20) | 1.57 (1.13–2.18) | 0.007 |
| Race | ||||||||||
| Caucasian | 8,377 | 1,448 (17.3%) | 960 | 327 (34.1%) | ||||||
| African American | 867 | 216 (24.9%) | 1.59 (1.35–1.87) | 1.18 (0.97–1.42) | 0.093 | 126 | 39 (31.0%) | 0.87 (0.58–1.30) | 0.81 (0.49–1.32) | 0.392 |
| Other | 2,202 | 385 (17.5%) | 1.01 (0.90–1.15) | 1.00 (0.87–1.16) | 0.956 | 296 | 73 (29.7%) | 0.82 (0.62–1.09) | 0.81 (0.56–1.16) | 0.252 |
| Missing | 236 | 29 (12.3%) | 0.67 (0.45–0.99) | 0.99 (0.64–1.53) | 0.970 | 19 | 5 (26.3%) | 0.69 (0.25–1.94) | 1.07 (0.29–4.03) | 0.917 |
| Insurance | ||||||||||
| Private | 5,059 | 848 (16.8%) | 778 | 254 (32.7%) | ||||||
| Public | 5,012 | 951 (19.0%) | 1.16 (1.05–1.29) | 1.09 (0.97–1.23) | 0.136 | 491 | 161 (32.8%) | 1.01 (0.79–1.28) | 0.83 (0.61–1.12) | 0.226 |
| Other | 1,611 | 279 (17.3%) | 1.04 (0.90–1.21) | 0.89 (0.75–1.05) | 0.176 | 132 | 44 (33.3%) | 1.03 (0.70–1.53) | 1.08 (0.66–1.75) | 0.768 |
| ICU level care resources in first 48 h | ||||||||||
| No | 11,252 | 1,789 (15.9%) | 1,344 | 418 (31.1%) | ||||||
| Yes | 430 | 289 (67.2%) | 10.74 (8.81–13.34) | 13.43 (10.67–16.89) | <0.0001 | 57 | 41 (71.9%) | 5.68 (3.15–10.23) | 5.85 (2.93–11.68) | <0.0001 |
| Time to first rasburicase exposure, days | ||||||||||
| Range | 1 – 21 | 1 – 27 | ||||||||
| Mean | 1.99 | 3.51 | ||||||||
| Cumulative rasburicase exposure, days | ||||||||||
| Range | 0 – 9 | 0 – 8 | ||||||||
| Mean (in exposed patients) | 1.83 | 1.98 | ||||||||
Based on logistic regression model adjusted for hospital indicator, age, sex, race, insurance and presentation severity.
95% CI: 95% confidence interval; ICU: intensive care unit; OR: odds ratio.
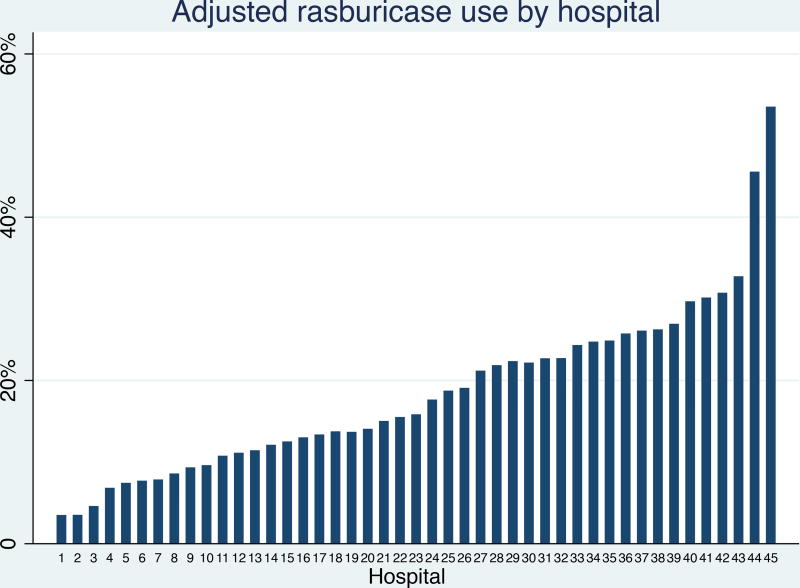
There was substantial inter-hospital variability in rasburicase use that persisted after adjustment for covariates (Figure 1). This variability was more pronounced among patients with NHL, with adjusted rasburicase exposure ranging from 6.8% to 70.7% among 32 hospitals. In patients with ALL, this variability ranged from 3.1% to 50.0% at 45 hospitals. We found no trend in rasburicase usage over time, and therefore time was excluded from all models.
Figure 1.
Adjusted* inter-hospital variation in rasburicase use among children with acute lymphoblastic leukaemia and non-Hodgkin lymphoma
*Adjusted for individual level age, gender, race, insurance status, diagnosis, diagnosis year and severity of presentation
The PHIS+ sub-analysis included 1,090 ALL patients and 129 NHL patients (Table SI), with similar demographics to the larger cohort. Patients with higher WBC and uric acid values were more likely to receive rasburicase. However, variation in exposure based on gender persisted (Figure S1), with trends toward significance in the overall cohort (OR 1.7; 95% CI 1.0–2.8; p=0.063) and when restricted to patients with ALL (OR 1.8; 95% CI 1.0–3.1; p=0.054). Corresponding analyses were not performed on the PHIS+ NHL cohort due to the small sample size. Inter-hospital variability also persisted in PHIS+, with adjusted rasburicase rates of 0.8% to 28.7%.
This study sought to describe patterns of rasburicase use for paediatric patients with ALL and NHL. We found significant centre-level variation in rasburicase use, consistent with published studies of other supportive care practices.(Fisher, et al 2013, Walker, et al 2013) These differences may be due to the inconsistent recommendations of current rasburicase use guidelines.(Agrawal and Feusner 2011, Bertrand, et al 2008, Cairo, et al 2010, Howard, et al 2011) Hospital level factors may also drive this variability, and additional studies are ongoing to evaluate these factors.
One notable finding was the difference in rasburicase exposure by gender, with males more likely to receive rasburicase. This difference persisted after adjusting for demographics and presentation severity, with trends toward significance after incorporating laboratory values. One possible explanation is that uric acid reference ranges for males ≥12 years are higher than corresponding ranges for females, with males potentially receiving rasburicase based upon absolute values rather than age-based reference ranges. This explanation is consistent with the data showing a more substantial disparity for older patients. However, there are other documented gender disparities in oncology care (Walker, et al 2013), and further studies are needed to confirm this association and identify its underlying cause.
As with all studies using administrative/billing data, certain limitations exist. Most notably, if a patient is transferred to a PHIS hospital, no data from the initial institution will be captured. Therefore, it is possible that a subject may receive rasburicase prior to transfer and be misclassified as non-exposed. Presenting uric acid values below the lower limit of the reference range may indicate uncaptured rasburicase exposure. However, this was the case for only 3% of patients in our cohort (Harriet Lane Service (Johns Hopkins Hospital), et al), suggesting that misclassification related to transfer is minimal.
Even with this limitation, this observational study shows marked variation in rasburicase use across paediatric hospitals. This variation is probably, at least in part, related to guidelines that are inconsistent and not evidence-based. Finally, the finding of decreased rasburicase use in female patients may represent an addressable gender disparity. Future work will focus on assessing the comparative effectiveness of rasburicase with the ultimate goal of informing evidence-based practice. Such work is of particular importance as several published treatment algorithms are the result of projects funded by the rasburicase manufacturer. Sanofi-Aventis (Cairo, et al 2010, Howard, et al 2011), raising concern that these guidelines may not be entirely free from conflicts of interest.
Supplementary Material
Acknowledgments
This study was supported by Eagles Fly for Leukemia, the NIH Grant R01 CA133881 (R.A.), and by Alex’s Lemonade Stand Foundation.
Footnotes
Author Contributions
All authors were involved in the study design and result synthesis. RC, YL, and KG conducted the primary data analyses. RC wrote the initial draft, with editing and final approval from all authors.
Competing interests: The authors have no competing interests.
References
- Agrawal AK, Feusner JH. Management of tumour lysis syndrome in children: what is the evidence for prophylactic rasburicase in non-hyperleucocytic leukaemia? Br J Haematol. 2011;153:275–277. doi: 10.1111/j.1365-2141.2010.08506.x. [DOI] [PubMed] [Google Scholar]
- Bertrand Y, Mechinaud F, Brethon B, Mialou V, Auvrignon A, Nelken B, Notz-Carrer A, Plantaz D, Patte C, Urbieta M, Baruchel A, Leverger G. SFCE (Societe Francaise du Lutte contre les Cancers et Leucemies de l'Enfant et de l'Adolescent) recommendations for the management of tumor lysis syndrome (TLS) with rasburicase: an observational survey. J Pediatr Hematol Oncol. 2008;30:267–271. doi: 10.1097/MPH.0b013e318162bd41. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- Citrin R, Horowitz JP, Reilly AF, Li Y, Huang YS, Getz KD, Seif AE, Fisher BT, Aplenc R. Creation of a pediatric mature B-cell non-Hodgkin lymphoma cohort within the Pediatric Health Information System Database. PLoS One. 2017;12:e0186960. doi: 10.1371/journal.pone.0186960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BT, Gerber JS, Leckerman KH, Seif AE, Huang YS, Li Y, Harris T, Torp K, Douglas R, Shah A, Walker D, Aplenc R. Variation in hospital antibiotic prescribing practices for children with acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:1633–1639. doi: 10.3109/10428194.2012.750722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BT, Harris T, Torp K, Seif AE, Shah A, Huang YV, Bailey LC, Kersun LS, Reilly AF, Rheingold SR, Walker D, Li Y, Aplenc R. Establishment of an 11-year cohort of 8733 pediatric patients hospitalized at United States free-standing Children's Hospitals with de novo acute lymphoblastic leukemia from heath care administrative data. Medical Care. 2014;52:e1–e6. doi: 10.1097/MLR.0b013e31824deff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flerlage J, Engorn B, editors. The Harriet Lane handbook : a manual for pediatric house officers/the Harriet Lane Service, Children's Medical and Surgical Center of the Johns Hopkins Hospital. 20. Saunders; Philadelphia: 2015. Children's Medical and Surgical Center. [Google Scholar]
- Howard SC, Jones DP, Pui C. The tumor lysis syndrome. New England Journal of Medicine. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Fitzgerald JC, Fisher BT, Li Y, Huang YS, Torp K, Seif AE, Kavcic M, Walker DM, Leckerman KH, Kilbaugh TJ, Rheingold SR, Sung L, Zaoutis TE, Berg RA, Nadkarni VM, Thomas NJ, Aplenc R. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr Crit Care Med. 2014;15:112–120. doi: 10.1097/PCC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Fisher BT, Seif AE, Huang YS, Torp K, Li Y, Aplenc R. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013;60:616–620. doi: 10.1002/pbc.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.