Abstract
Background
Neonatal sepsis is a leading cause of child morbidity and mortality, especially in premature and low-birth-weight (LBW) infants. Prompt antibiotic therapy is warranted, but its inappropriate use leads to bacterial resistance and adverse outcomes. Our objective is to describe the antibiotic use for late onset sepsis (LOS) in Peruvian premature infants.
Methods
Prospective study as a secondary analysis of a clinical trial in three Neonatal Care Units in Peru. We included infants in the first 72 hours of life, with birth weight (BW) <2000g. We described the antibiotic use as length of therapy (LOT) per 1000 patient days (PD) and antibiotic courses.
Results
We included 408 neonates, with 12204 PD of follow-up; 253 infants (62%) had a BW ≤1500 g. Total antibiotic use for LOS was 2395 LOT (196 LOT/1000PD). 271 patients (66.4%) did not receive antibiotics for LOS during their hospitalization. In total, 204 antibiotic courses were administered; 92 infants (22.5%) received 1 course, and 45 (11.0%) received 2 to 5 antibiotic courses. Mean duration of antibiotic course was 10.8 days (SD: ±7.3). We found a significant association between a lower BW and increased antibiotic use per day (p<0.001). The most commonly used antibiotics were vancomycin (143 LOT/1000PD), carbapenems (115 LOT/1000PD), aminoglycosides (72 LOT/1000PD), and ampicillin (41 LOT/1000PD).
Conclusions
Premature infants receive antibiotics for longer than recommended periods of time. Antibiotic overuse is greater in neonates with lower BW. Vancomycin is the most used antibiotic. There is an urgent need to develop antimicrobial stewardship programs in our setting.
Keywords: Antibiotics, neonates, antimicrobial stewardship
INTRODUCTION
The impact of sepsis on newborn mortality is well known: 15% of all neonatal deaths are attributable to infections.1 Low-birth-weight (LBW) and premature infants are more susceptible to severe infections. Sepsis rates are around 4–6% in late preterm infants versus 60% in very-low-birth-weight and extremely premature newborns.2,3 Neonatal sepsis has detrimental effects that can be seen even after the infection has subsided, such as a higher incidence of periventricular leukomalacia and, later in life, low scores on the Bayley Scales of Infant Development,4,5 and increased rates of cerebral palsy6.
Diagnosing sepsis in premature infants is still a challenge.7,8 The combination of non-specific clinical signs, altered laboratory results, and the high vulnerability of these patients establish a need for a prompt start of empiric antibiotic therapy. Blood cultures are performed and monitored for 48–72 hours to determine if treatment needs to be stopped or modified according to the results9 and, in optimal conditions, they have good sensitivity and specificity, detecting bacteria in concentrations of 1–10 CFU/ml.10 However, there are some limitations specific to the neonatal population that can affect the performance of this test.11 On the other hand, the risk of inappropriate use of antibiotics is high. Long-term use of broad-spectrum agents is related to a higher incidence of antibiotic resistance, necrotizing enterocolitis, Candida colonization, invasive candidiasis, alteration of gut microbiota and death.12–15
Developing countries face additional challenges in the diagnosis and management of neonatal sepsis. Differences with developed countries, such as in causative pathogens and patterns of resistance to antimicrobial agents,7 and the lack of regional studies with a standardized methodology,16 increase the need for reports specific to these settings. Our goal is to describe the use of antibiotics for late-onset sepsis (LOS) in a cohort of premature Peruvian infants and to compare it by birth weight (BW) and clinical indication. This study represents a first step toward creating adequate practices of antimicrobial stewardship.
METHODS
Study Design and Setting
We conducted a prospective study as a secondary analysis of the NEOLACTO study (Lactoferrin for prevention of sepsis in infants - NCT01525316), a randomized, double-blinded, placebo-controlled clinical trial of bovine lactoferrin supplementation for prevention of LOS in premature infants with a birth weight <2000 g. This study was conducted in the neonatal care units (Intensive and intermediate care) of three tertiary care hospitals in Lima, Peru: “Hospital Cayetano Heredia”, “Hospital Nacional Almenara” and “Hospital Nacional Sabogal”. Subject enrollment was carried out from May 2012 to September 2014. The Institutional Review Boards of the National Institute of Health of Peru in Lima, the “Universidad Peruana Cayetano Heredia”, the University of Texas Health Science Center at Houston and the three hospitals mentioned above, approved the study. The clinical trial is registered on www.clinicaltrials.gov.
Study Subjects
We included infants with a BW of <2000 grams who were born in or transferred to the three mentioned hospitals and were within the first 72 hours of life. Infants with congenital malformations that impaired oral intake, family history of cow milk intolerance and severe neurological defects were excluded from the initial study. We enrolled 414 subjects in the clinical trial and 6 participants were excluded for this analysis because they withdrew from the study before any data could be gathered. Since the treatment intervention for our clinical trial (lactoferrin) did not have an effect on sepsis, subjects from the treatment and control groups were included in this analysis. We obtained consent from both parents before enrollment.
Antibiotic use and variable definitions
Patient characteristics and follow-up information were obtained from the clinical trial records. We registered the use of medications daily until discharge, specifying the antibiotics that were used. For this analysis, we excluded oral and topical antibiotics as well as anti-fungal drugs. We defined an antibiotic course as a period in which a patient receives at least one IV antibiotic, preceded and followed by at least two days without any antimicrobial treatment. The length of therapy (LOT) is defined as the number of calendar days in which a patient receives at least one antibiotic. To better describe and compare the antibiotic use by type and by indication, we used the concept of LOT/1000 patient-days (PD), which is calculated by dividing LOT by the total patient-days of follow-up and multiplying it by 1000.
Indications for antibiotic use
A “suspected LOS” episode was registered and followed each time a patient older than 72 hours was started on any IV antibiotic treatment. Blood cultures were sampled as standard of care in each center using the BACTEC and/or Bact/ALERT automated microbial detection systems. Antibiotic resistance was determined by disk diffusion and minimal inhibitory concentration (MIC). All episodes were retrospectively discussed with the principal investigator, the study coordinator and the neonatologists involved in the care of the patient.
To classify them, we divided the suspected LOS episodes into three categories as described by Haque’s “Definition of bloodstream infection in the newborn” 17. The first is culture-proven sepsis, which included episodes with positive results in one or more blood and/or cerebrospinal fluid cultures in the presence of clinical signs and symptoms of infection. In the case of coagulase-negative staphylococci infections, they were considered as culture-proven if the episode had two positive blood cultures within two days of each other, or one positive blood culture and an elevated C-reactive protein (CRP) (>10 mg/L) within two days of the blood culture. The concepts of probable sepsis (clinical signs and symptoms of infection plus two or more abnormal laboratory findings when blood culture is negative) and possible sepsis (clinical signs and symptoms of infection and an elevated CRP when blood culture is negative) were grouped as culture-negative sepsis. “No sepsis” grouped all the episodes where antibiotics were used but that did not clinically meet the criteria for the definitions mentioned above, and was further sub-divided into 2 categories: “Other infections”, for episodes in which an alternative infectious etiology was identified, and ruled-out sepsis, for episodes with no other infectious etiology where antimicrobial therapy was used. “Other infections” included necrotizing enterocolitis (NEC), defined as Modified Bell’s criteria of IIA or higher; urinary tract infection (UTI), diagnosed with a urine culture from a catheterized sample with >103/ml colony-forming units; pneumonia and skin infections, as diagnosed by the primary team.
Statistical Analysis
We performed descriptive analyses. Categorical variables were expressed as absolute and relative frequencies; continuous variables were expressed as a mean and standard deviation (SD) or as a median and interquartile range (IQR) when appropriate. Bivariate analysis was performed on antibiotic use and sepsis characteristics, depending on the case, Chi-square, Fisher exact test, Mann–Whitney test or the Student’s t-test was used to testing for association between different birth weights (≤1500 g and > 1500 g), and p <0.05 was considered significant. Statistical analysis was performed using Epi Info software, version 7.
RESULTS
We included 408 neonates with a BW <2000 g. There were 253 (62%) infants with a BW ≤1500 g. Mean gestational age (GA) was 30.8 ± 3 weeks (see Table, Supplemental Digital Content 1). The median (IQR) of total hospitalization was 29 (18 – 46) days duration and 13 (5 – 26) days for ICU stay. There were 12204 PD of follow-up.
Antibiotic use
During the observation period, 137 patients received antibiotics for suspected LOS, with a higher percentage among ≤1500 g (80.2%). We registered a total LOT of 2395 days (196 LOT/1000 PD), distributed in 204 antibiotic courses. The mean (SD) duration of an antibiotic course was 10.8 (±7.3) days, with significantly longer duration among infants ≤1500 g (Table 1). Patients that died during the initial hospitalization had a LOT/1000 PD of 616, and those who survived received 176 LOT/1000 PD.
TABLE 1.
Characteristics of antibiotic use by birth weight
| Birth weight | ≤1500 g (n = 253) | >1500 g (n = 155) | p value | |
|---|---|---|---|---|
|
|
|
|||
| Use of any antibiotic, n (%) | 110 (43.5%) | 27 (17.4%) | < 0.001 | |
| Antibiotic course duration in days, mean ± SD (min - max) | 11.04 ± 7.57 (1 – 64) | 9.52 ± 5.52 (1 – 22) | < 0.001 | |
|
|
|
|||
| 95% CI | ||||
|
| ||||
| Number of antibiotic courses, n (%)* | 0 | 143 (56.5%) | 128 (82.6%) | 0.88 – 1.42 |
| 1 | 70 (27.7%) | 22 (14.2%) | 1.97 – 5.14 | |
| 2 | 29 (11.5%) | 4 (2.6%) | 2.55 – 20.62 | |
| >3 | 11 (4.3%) | 1 (0.6%) | 1.42 – 85.20 | |
|
| ||||
| Total number of different antibiotics used during hospitalization, n (%)* | 0 | 143 (56.5%) | 128 (82.6%) | 0.88 – 1.42 |
| 2 | 8 (3.2%) | 6 (3.9%) | 0.46 – 3.84 | |
| 3 | 7 (2.7%) | 7 (4.5%) | 0.35 – 2.85 | |
| >4+ | 95 (37.5%) | 14 (9.0%) | 3.87 – 11.89 | |
|
| ||||
| Max. number of antibiotic used per day, n (%)* | 0 | 143 (56.5%) | 128 (82.6%) | 0.88 – 1.42 |
| 1 | 1 (0.4%) | - | - | |
| 2 | 56 (22.1%) | 23 (14.8%) | 1.50 – 3.96 | |
| 3 | 33 (13.0%) | 3 (1.9%) | 3.37 – 35.87 | |
| 4 | 20 (7.9%) | 1 (0.7%) | 2.68 – 149.02 | |
Up to 10 different antibiotics,
SD: Standard deviation, CI: Confidence interval
p<0.001 Chi-square for trend
The number of different antibiotics received during the hospitalization varied between 0 and 10, with a higher number used in those with BW ≤1500g (p<0.001). A total of 3 or more different antibiotics were used in 30% of the patients. Among those who were treated with antibiotics, almost all received 2 or more different agents in a single day. The maximum number of different antibiotics given in one day was 4, which was the case for 21 patients (5.1%). We found a statistical difference between weight groups for specific antibiotics used; however, there was no difference in treatment duration among each antibiotic (Table 2).
TABLE 2.
Frequency and duration of antibiotic use
| Antibiotics | ≤1500 g (n = 253)
|
>1500 g (n = 155)
|
||
|---|---|---|---|---|
| Patients that received antibiotic n (%) | Treatment duration, daysmedian (IQR) | Patients that received antibiotic n (%) | Treatment duration, daysmedian (IQR) | |
| Gent/Amika* | 101 (39.9%) | 5 (3 – 8) | 26 (16.7%) | 4 (7 – 10) |
| Ampicillin* | 95 (37.5%) | 3 (2 – 6) | 20 (12.9%) | 4 (3 – 7) |
| Vancomycin* | 100 (39.5%) | 13 (8 – 20) | 15 (9.7%) | 6 (3 – 25) |
| Imipenem* | 64 (25.3%) | 10 (6 – 14) | 9 (5.8%) | 8 (4 – 12) |
| Meropenem* | 46 (18.2%) | 10 (6 – 17) | 5 (3.2%) | 11 (7 – 14) |
| Ceftazidime* | 32 (12.6%) | 6 (4 – 9) | 10 (6.5%) | 6 (5 – 10) |
| Metronidazole* | 21 (8.3%) | 8 (3 – 14) | 1 (0.6%) | 23 |
| Cefotaxime | 18 (7.1%) | 3.5 (2 – 7) | 4 (2.6%) | 2.5 (2 – 5) |
| Ciprofloxacin | 16 (6.3%) | 6.5 (5 – 10) | 1 (0.6%) | 12 |
| Pip/Tazo* | 15 (5.9%) | 8 (3 – 19) | 0 | - |
| Cefepime* | 10 (4.0%) | 7 (5 – 8) | 2 (1.3%) | 5.5 (1 – 10) |
| Linezolid* | 8 (3.2%) | 14.5 (5 – 21) | 0 | - |
There was no significant difference in treatment duration between BW groups for all antibiotics analyzed.
p<0.05 when comparing number of patients that received antibiotic between both BW groups
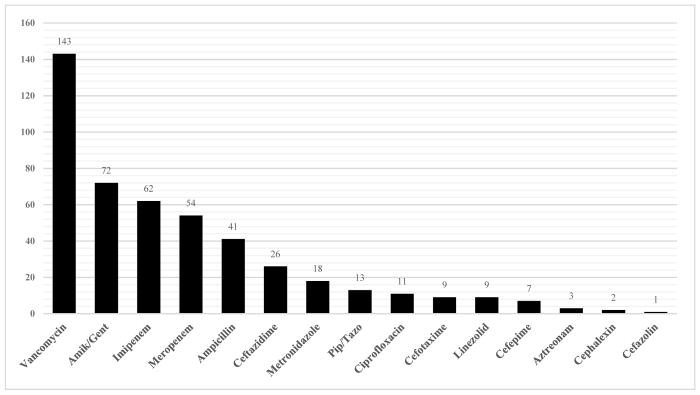
The most commonly used antibiotics were aminoglycosides, which were administered to 127 patients. These were followed by carbapenems (124), ampicillin (115), and vancomycin (115). However, to normalize our results by follow-up time, we expressed antibiotic use in LOT/1000 PD. The highest value corresponded to vancomycin (143 LOT/1000 PD), followed by carbapenems (115 LOT/1000 PD), gentamicin/amikacin (72 LOT/1000 PD), and ampicillin (41 LOT/1000 PD) (Figure 1).
FIGURE 1.
Length of therapy per 1000 patient-days (LOT/1000 PD) by antibiotic type
Our study subjects presented 204 episodes of suspected LOS. These were divided in culture-proven (48), culture-negative sepsis (71) and no sepsis (85). Most were found in the ≤1500 g group. Within the no sepsis group, 17 patients were diagnosed with NEC, 12 with pneumonia, 8 with UTI, and 3 with skin infections. The most common Gram-positive isolated pathogens were Staphylococcus (coagulase-negative and aureus), with 20 confirmed episodes, and a 90% resistance rate to methicillin. In the Gram-negative group, the most common bacteria were E.coli and Klebsiella, with a total of 13 cases and a 46% ceftriaxone resistance rate (Table 3). In 6 culture-proven sepsis episodes, the isolated pathogens were Candida species. Given the etiology was unknown at the beginning of the episodes, these patients received antibiotics for a period of time.
TABLE 3.
Antibiotic resistance patterns of isolated pathogens
| Pathogen* | n (%) of resistance | ||
|---|---|---|---|
|
|
|
||
| Gram positives | n | Oxacillin | Vancomycin |
|
|
|
||
| Coagulase-negative Staphylococcus | 17 | 15 (88%) | 1 (6%) |
| Staphylococcus aureus | 3 | 3 (100%) | 0 (0%) |
| Enterococcus | 4 | 2 (50%) | 1 (25%) |
| Streptococcus sp. | 1 | - | - |
|
|
|
||
| Gram negatives | n | Ceftriaxone | Carbapenems |
|
|
|
||
| Klebsiella pneumoniae | 7 | 5 (71%) | 0 (0%) |
| Escherichia coli | 6 | 1 (17%) | 0 (0%) |
| Enterobacter | 3 | 2 (67%) | 0 (0%) |
| Pseudomona aeruginosa | 2 | 2 (100%) | 2 (100%) |
| Acinetobacter iwoffii | 1 | 1 (100%) | 1 (100%) |
| Empedobacter brevis | 1 | 1 (100%) | 1 (100%) |
6 cultures were positive for Candida, susceptibility tests were not done.
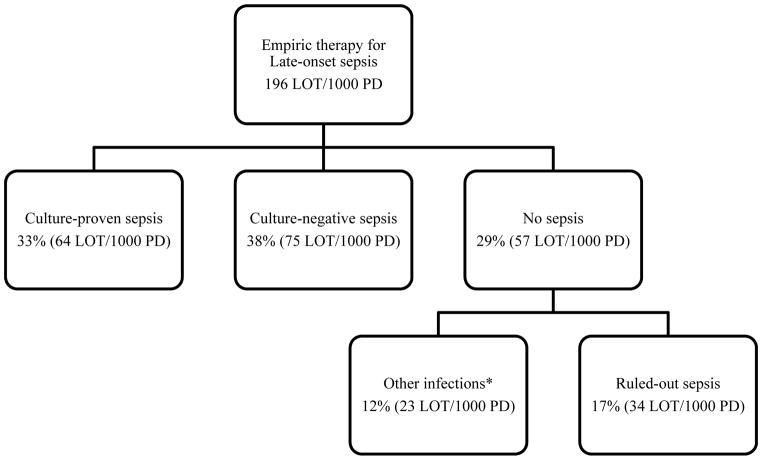
The distribution of antibiotic treatment by indication is shown in Figure 2. 67% of all antibiotics given for LOS were administered despite negative blood cultures. The duration of the antibiotic treatment varied according to the definition, with a median of 14 (RIQ: 11–19) days for culture-proven sepsis, 10 (RIQ: 8–15) days for culture-negative sepsis and 7 (RIQ: 5–9) days for no sepsis. Within this last group, ruled-out sepsis episodes received a median of 6 (3–9) days of antimicrobial therapy.
FIGURE 2. Length of therapy per 1000 PD (LOT/1000 PD) according to clinical indication.
*Other infections that require antibiotic therapy, which include pneumonia, urinary tract infections, necrotizing enterocolitis, and skin infections.
Lumbar puncture and meningitis
A total of 45 (20.9%) lumbar punctures were performed for all cases of suspected LOS. When divided by type of sepsis, lumbar punctures were performed in 27.1% of culture-proven (13/48), 24.7% of culture-negative (18/73), and 15.3% of cases that did not meet criteria for sepsis (14/93). Meningitis was diagnosed in 8 cases, which constitutes 3.7% of all suspected sepsis episodes and 17.8% of all the lumbar punctures performed. These patients received a median of 16 (IQR: 10–21) days of antibiotic treatment.
DISCUSSION
Our study shows that in Peruvian neonatal care units, broad-spectrum antibiotics are commonly used in very low birth weight (VLBW) neonates, and they are continued for prolonged periods of time. There is a lack of similar reports detailing the magnitude of antimicrobial use in our setting, and we consider these findings to be fundamental for optimizing the treatment of neonatal infections.
The total LOT/1000 PD in our study was 196, which is similar to what was found by Cantey et al.18 They conducted a study in a Level III NICU in Dallas, TX (USA) and included 1607 infants with a median BW of 2793g and GA of 37.4 weeks. They found a total of 195.3 LOT/1000 PD, including early and late-onset neonatal sepsis. Additionally, 85% of their total antibiotic use corresponded to agents that are most often used for the treatment of early-onset sepsis, which allows us to speculate that there may be greater use of antibiotics for LOS in our population. This difference was expected given that they included patients with a higher BW and GA, which may account for a lower prevalence of severe infections. Our research complements their findings and suggests that we can achieve a more significant impact with interventions aimed at the VLBW population.
On the other hand, when observing the duration of the antibiotic courses, Cantey reported that, in 32% of cases with ruled-out sepsis, antibiotics were stopped within 48 hours, and the rest continued to no more than five days.18 In our case, 78% of ruled-out sepsis episodes were treated for more than two days after negative cultures, with a median duration of 6 days. This is above the recommended length of therapy as described by Kaiser et al., who found that >98% of positive cultures grew in the first 48 hours.19 This prolongation can carry terrible consequences according to Ting et al., who analyzed the association between antibiotic use and morbidity/mortality among neonates without culture-proven sepsis or NEC. They found that a 10% increase in antibiotic use was associated with a significant increase in mortality and a worse overall composite outcome that included major morbidities or death.20
The prolonged antibiotic use in our NICUs may be related to limited access to acute phase reactants: only 81% of ruled-out sepsis episodes had at least one CRP measurement, and none had procalcitonin or other more advanced methods. Acute-phase reactants are recognized tools that aid in the prompt diagnosis of sepsis.7,11 This situation can create more resilience toward stopping antibiotic therapy, even though retrospectively, the treating neonatologists decided to catalog the episodes as ruled-out sepsis. Another potential cause for prolonged treatment may be the insufficient sampling for blood cultures, which gives less credibility to the results. Reports from the literature recommend the use of two bottles for aerobic and anaerobic cultures with a volume of at least 0.5 ml in each one of them.11,20 Unfortunately, we did not register the exact amounts of the blood culture samples. Ultimately, given there is no antimicrobial stewardship program implemented in our hospitals, the decision to continue or to stop antibiotics is made by the attending physicians based on their clinical judgment. Peruvian clinicians distrust sterile blood cultures despite the evidence.10 In addition, preliminary results can sometimes take longer than 48 hours to be available.
Culture-negative sepsis treatment also accounts for a significant proportion of antibiotic use in our study (38%). In contrast to other reports,13,18,21 our definition was standardized, given that it was part of the outcome of our clinical trial. Each episode was reviewed with the primary neonatologists after the necessary information was gathered. The episodes characterized as culture-negative sepsis raised high suspicion and warranted the initiation of antimicrobials. Despite this, the duration of treatment in this cases exceeds the recommended 5–7 days of therapy.22 Moreover, when comparing the LOT/1000 PD for neonates that died during the initial hospitalization and those that didn’t, we found a 3.5 fold increase. This shows that, when presented with newborns with more severe conditions, clinicians are more likely to prescribe prolonged courses of antibiotics. The duration may be additionally extended in patients with a lower birth weight and gestational age, but it has also been reported that continuation of antibiotics in culture-negative sepsis may not have a correlation with clinical features or risk factors and can be only related to institutional decisions.21
Of interest, we noted a limited number of cases in which a lumbar puncture was performed: 27.1% of culture-proven sepsis and 20.9% of episodes in which empiric therapy for LOS was initiated. Lumbar puncture is considered an essential tool in determining the duration of treatment in sepsis, as a meningoencephalitis diagnosis can prolong it to 3 weeks or even more.22 The absence of this information may add to the uncertainty of when treatment should be stopped and lead to its unnecessary continuation.
Vancomycin was the antibiotic with the highest LOT/1000 PD and the second longest duration of treatment. Similarly, carbapenems were used in 36% of our population and had the third longest duration of treatment. We can attribute this to the higher prevalence of resistant bacteria isolated in our study. Additionally, a recent publication showed high rates of oxacillin-resistant Staphylococci (up to 90%) and extended-spectrum beta-lactamase producing gram-negative bacteria (75%) in a Peruvian NICU.23 This adds up to the known emergence of resistant pathogens in other developing regions.24 These situations may partially justify the use of broad-spectrum antibiotics, but given that adverse outcomes have been related to their prolonged utilization, there is a need for more studies analyzing the consequences of the excessive use of these agents in our setting.
The creation of antimicrobial stewardship programs is a growing worldwide initiative, as its positive impact in reducing bacterial resistance and optimizing costs is well-known.25 Since 2011, the Centers for Disease Control and Prevention (CDC) has been promoting an initiative for optimizing antibiotic utilization in all hospitalized patients.26 Patel and Saiman recognized the challenges of applying the CDC’s recommendations to the neonatal population and concluded that the general principles of rational antibiotic use apply to the NICU patients.27 The main obstacle for the implementation of pediatric antimicrobial stewardship programs in the US is the lack of resources 28 and this limitation can become an even bigger problem in low-middle income countries like ours. Straightforward and coordinated interventions to approach antibiotic overuse can achieve a considerable impact as demonstrated in the SCOUT study.29 Currently, there are initiatives for controlling antibiotic resistance in our region,30 but we are missing measures focused on premature LBW infants and, as shown in our results, they continue to be exposed to multiple broad-spectrum agents.
Our study has some limitations. For the description of antibiotic use, we did not have a full registry that included dosage amounts and intervals. This action would have allowed us to comply with WHO recommended measures of medication use, represented by the defined daily dose (DDD), which is the assumed mean dose of maintenance given in a day of a drug used for its main indication.31 However, DDD has a limitation, as it uses adult doses which limits its applicability to pediatric populations. Our decision to use LOT/1000 PD is supported by a study published by Valcourt et al. which found that that “days of drug use”, a concept equivalent to our LOT/1000 PD, may be the most precise and uncomplicated method for describing and comparing antibiotic use between different facilities.32 Finally, our population may not be representative of all patients in the NICU, given that our patients were part of a clinical trial. Nevertheless, to our knowledge, this is the first study on daily antimicrobial use in NICUs in a developing country.
CONCLUSION
Broad-spectrum antibiotics are overused in Peruvian NICUs for LOS sepsis management in LBW infants and are continued for longer than recommended periods, especially in VLBW newborns. Vancomycin is the most commonly used antibiotic, followed by carbapenems. Physicians and policymakers should focus their efforts toward the creation and implementation of antimicrobial stewardship programs that can help reduce the burden of antibiotic use in countries like ours, where bacterial resistance is rising and vulnerable populations are at risk for serious complications.
Acknowledgments
Funding Source: Funded by the National Institute of Child Health & Human Development (NICHD), grant number: R01-HD067694.
We want to express out most sincere gratitude to all the infants that participated in our study, as well as their parents and guardians, for their commitment to the project.
Special thanks to the neonatologists of the NEOLACTO Research Group: Maria Rospigliosi MD, Veronica Webb MD, Geraldine Borda MD, and Ericka Bravo MD, from Hospital Cayetano Heredia, Lima, Peru; Ana Lino MD and Augusto Cama MD from Hospital Nacional Alberto Sabogal Sologuren, Lima, Peru; Raul Llanos MD, Oscar Chumbes MD, Liliana Cuba MD, Julio Tresierra MD, and Carmen Chincaro MD, from Hospital Nacional Guillermo Almenara Irigoyen, Lima, Peru; and the research nurses: Lourdes Tucto RN, Mayela Huanay RN, Cristina Suarez RN, and Nadia Rojas RN, from Universidad Peruana Cayetano Heredia, Lima, Peru.
Footnotes
Clinical Trial Registration: Lactoferrin for Prevention of Sepsis in Infants (NEOLACTO), NCT01525316, web link: https://clinicaltrials.gov/ct2/show/NCT01525316
References
- 1.Wardlaw T, You D, Hug L, et al. UNICEF Report: Enormous progress in child survival but greater focus on newborns urgently needed. Reprod Health. 2014;11(1) doi: 10.1186/1742-4755-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen-Wolkowiez M, Moran C, Benjamin DK, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28(12):1052–1056. doi: 10.1097/inf.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 5.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128(2):e348–357. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- 6.Mitha A, Foix-L’Hélias L, Arnaud C, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2):e372–380. doi: 10.1542/peds.2012-3979. [DOI] [PubMed] [Google Scholar]
- 7.Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61(1):1–13. doi: 10.1093/tropej/fmu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn JL, Wong HR, Shanley TP, et al. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2014;15(6):523–528. doi: 10.1097/PCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivanandan S, Soraisham AS, Swarnam K. Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Int J Pediatr. 2011;2011:712150. doi: 10.1155/2011/712150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129(2):275–278. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 11.Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60(2):367–389. doi: 10.1016/j.pcl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuppala VS, Meinzen-Derr J, Morrow AL, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi N, Cotten CM, Smith PB. Antibiotic use and misuse in the neonatal intensive care unit. Clin Perinatol. 2012;39(1):61–68. doi: 10.1016/j.clp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaver D, Zaidi AKM. Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr Infect Dis J. 2009;28(1 Suppl):S3–9. doi: 10.1097/INF.0b013e3181958755. [DOI] [PubMed] [Google Scholar]
- 17.Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(3 Suppl):S45–49d. doi: 10.1097/01.PCC.0000161946.73305.0A. [DOI] [PubMed] [Google Scholar]
- 18.Cantey JB, Wozniak PS, Sánchez PJ. Prospective Surveillance of Antibiotic Use in the Neonatal Intensive Care Unit: Results From the SCOUT Study. Pediatr Infect Dis J. 2015;34(3):267–72. doi: 10.1097/INF.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser JR, Cassat JE, Lewno MJ. Should antibiotics be discontinued at 48 hours for negative late-onset sepsis evaluations in the neonatal intensive care unit? J Perinatol Off J Calif Perinat Assoc. 2002;22(6):445–447. doi: 10.1038/sj.jp.7210764. [DOI] [PubMed] [Google Scholar]
- 20.Ting JY, Synnes A, Roberts A, et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016;170(12):1181–1187. doi: 10.1001/jamapediatrics.2016.2132. [DOI] [PubMed] [Google Scholar]
- 21.Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2003;24(9):662–666. doi: 10.1086/502270. [DOI] [PubMed] [Google Scholar]
- 22.Pammi M, Weisman LE. Late-onset sepsis in preterm infants: update on strategies for therapy and prevention. Expert Rev Anti Infect Ther. 2015;13(4):487–504. doi: 10.1586/14787210.2015.1008450. [DOI] [PubMed] [Google Scholar]
- 23.Alvarado-Gamarra G, Alcalá-Marcos KM, Abarca-Alfaro DM, et al. Microbiological and therapeutic characteristics of confirmed neonatal sepsis at a hospital in Lima, Peru. Rev Peru Med Exp Salud Pública. 2016;33(1):74–82. [PubMed] [Google Scholar]
- 24.Zaidi AKM, Huskins WC, Thaver D, et al. Hospital-acquired neonatal infections in developing countries. Lancet Lond Engl. 2005;365(9465):1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 25.Nash C, Simmons E, Bhagat P, et al. Antimicrobial Stewardship in the NICU: Lessons We’ve Learned. NeoReviews. 2014;15(4):e116–e122. [Google Scholar]
- 26.Centers for Disease Control and Prevention. [Accessed Nov 28, 2016];Get Smart for Healthcare [CDC website] 2016 Nov 15; Available from: https://www.cdc.gov/getsmart/healthcare/index.html.
- 27.Patel SJ, Saiman L. Principles and Strategies of Antimicrobial Stewardship in the Neonatal Intensive Care Unit. Semin Perinatol. 2012;36(6):431–436. doi: 10.1053/j.semperi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hersh AL, Beekmann SE, Polgreen PM, et al. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol. 2009;30(12):1211–1217. doi: 10.1086/648088. [DOI] [PubMed] [Google Scholar]
- 29.Cantey JB, Wozniak PS, Pruszynski JE, et al. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16(10):1178–1184. doi: 10.1016/S1473-3099(16)30205-5. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed Sep 29, 2016];ReAct - ReAct Latin America [ReAct Latin America web site] Available from: http://www.reactgroup.org/who-we-are/organisation/react-latin-america.html.
- 31.WHO Collaborating Centre for Drug Statistics Methodology. [Accessed Jul 28, 2016];Guidelines for ATC Classification and DDD Assignment 2016. [WHO web site] 2016 Available from: http://www.whocc.no/filearchive/publications/2016_guidelines_web.pdf.
- 32.Valcourt K, Norozian F, Lee H, et al. Drug use density in critically ill children and newborns: analysis of various methodologies. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2009;10(4):495–499. doi: 10.1097/PCC.0b013e3181a3101e. [DOI] [PubMed] [Google Scholar]