Abstract
Whether hedonism or eudaimonia are two distinguishable forms of well-being is a topic of ongoing debate. To shed light on the relation between the two, large-scale available molecular genetic data were leveraged to gain more insight into the genetic architecture of the overlap between hedonic and eudaimonic well-being. Hence, we conducted the first genome-wide association studies (GWAS) of eudaimonic well-being (N = ~108 K) and linked it to a GWAS of hedonic well-being (N = ~222 K). We identified the first two genome-wide significant independent loci for eudaimonic well-being and six independent loci for hedonic well-being. Joint analyses revealed a moderate phenotypic correlation (r = 0.53) and a high genetic correlation (rg = 0.78) between eudaimonic and hedonic well-being. This indicates that the genetic etiology of hedonic and eudaimonic well-being is substantially shared, with divergent (environmental) factors contributing to their phenotypic divergence. Loci regulating expression showed significant enrichment in the brain cortex, brain cerebellum, frontal cortex, as well as the cerebellar hemisphere for eudaimonic well-being. No significant enrichment for hedonic well-being is observed, although brain tissues were top ranked. Genetic correlations patterns with a range of positive and negative related phenotypes were largely similar for hedonic –and eudaimonic well-being. Our results reveal a large overlap between the genes that influence hedonism and the genes that influence eudaimonia.
Introduction
For centuries, people have asked themselves questions about well-being with hedonic well-being and eudaimonic well-being as its major philosophical schools of thoughts. Hedonic well-being concerns the balance of pleasure over pain, with Aristippus (c. 435–c. 356 BCE), as one of its founders1. Whereas the hedonic tradition focused on what is good for a person, the eudaimonic tradition took well-being to centre around virtuous activity, defined as knowledge (practiced over time) and the fulfilment of human capacities2. One of the important founders of eudaimonic well-being is Aristotele (c. 384–c. 322 BCE), who was a true opponent of the hedonistic school of thought describing it as “vulgar”3. According to Aristotle, eudaimonic well-being is more than being happy and is it about the actualization of the human potential4.
In contemporary behavioural and social sciences, the term hedonic well-being is used less frequently. A reason for this is that hedonism as a theoretical (data-free) concept is difficult to quantify. To redefine the hedonic line of thought in an operational construct, the subjective well-being (SWB) definition, as proposed by Diener5, is widely adopted. Herein, SWB consists of three hallmarks: (1) it is subjective; (2) it includes positive measures (not just the absence of negative measures), and (3) it includes a global assessment of all aspects of a person’s life. SWB has been repeatedly found to be associated with health and mortality e.g.6–9. Analogous to hedonism, the term eudaimonic well-being has gradually shifted towards psychological well-being (PWB) in contemporary science. To assess PWB, six core dimensions are widely used: self-acceptance, positive relations with others, autonomy, environmental mastery, purpose in life, and personal growth10. Several studies have found that people who believe their lives have meaning or purpose appear better off, with better mental and physical health and engagement in healthier life styles11–16.
Although, it is recognized that modern-day hedonism and eudaimonia are central concepts of well-being, the overlap and distinction between these two forms of well-being is a topic of an ongoing debate1,17–23. Factor analytic studies show that hedonic and eudaimonic aspects of well-being load on separate yet highly correlated factors, with correlations in the range of 0.81 to 0.9224–26. Application of less restrictive exploratory structural equation modelling, results in a correlation of 0.60 between hedonic and eudaimonic well-being22. A more in-depth overview of the reported correlation between hedonic and eudaimonic uncovers a wide spread in correlations resulting from differences in degree of centrality (if the hedonic measures are the core aspect of the analyses or if the correlation is based on correlates of the concepts), application of different categories of analyses (if hedonia and eudaimonia is considered an orientation, behavior, experience, or function) and level of measurement (state versus trait)20.
A way to provide more clarity on the overlap and distinction of hedonic and eudaimonic well-being is by exploring the underlying sources of overlap. Differences in both hedonic and eudaimonic well-being have been found to be partly genetic. Twin-family studies, which contrast the resemblance of monozygotic (MZ), dizygotic (DZ) twins and their non-twin siblings or other family members, report heritability estimates in the range of 30–64% for both hedonic and eudaimonic well-being27,28. Most molecular genetic work, so far, focused on hedonic measures of well-being. Initially a handful of studies attempted to associate specific candidate genes (e.g. 5-HTTLPR, MAOA, FAAH) to hedonic well-being29–32. However, these studies were most likely underpowered and results have not been replicated. More recent molecular genetic approaches revealed that 5–10% of the variation in responses to single-item survey hedonic measures (happiness) is accounted for by genetic variants measured on presently used genotyping platforms33. Additionally, a recent large genome-wide association study (GWAS; N = 298,420) identified the first three genetic variants (two at chromosome 5 (rs3756290 and rs4958581) and one at chromosome 20 (rs2075677)) associated with SWB, defined as a combination of hedonic measurements like happiness and satisfaction with life34.
There have only been two attempts to use molecular genetic data to reveal the overlap and distinction between hedonic and eudaimonic well-being35,36. The first study showed divergent transcriptome profiles between both measurements35. Hedonic well-being was associated with up-regulated gene expression of a conserved transcriptional response to adversity (CTRA), while eudaimonic well-being was associated with CTRA down-regulation. After substantial critiques and replies37–40, the authors of the initial finding replicated part of the results by showing a significant inverse relation between down-regulated CTRA expression and eudaimonic well-being36. Based on these results, the authors conclude that eudaimonic well-being might play a more significant role in the link between well-being and health, than hedonic well-being.
The availability of large-scale molecular data make it possible to gain more insight into the genetic factors underpinning overlap and distinction between hedonic and eudaimonic well-being. In the current paper, we therefore leverage data from the UK Biobank and estimate the molecular genetic based heritability and bivariate genetic correlation. To this end, we conduct the first genome-wide association study (GWAS) to identify genetic variants associated with eudaimonic well-being as well as a GWAS for hedonic well-being. For eudaimonic well-being we used the question: “To what extent do you feel your life to be meaningful” as a proxy phenotype. For hedonic well-being, we used”In general how happy are you” as a proxy phenotype. As the genetic architecture can be a reflection of common biology, we annotate the genome-wide association results using gene-mapping and tissue specific enrichment analyses. Finally, we estimate whether hedonic and eudaimonic well-being show different genetic correlations patterns with positively and negatively related traits.
Results
Descriptive statistics and phenotypic correlation
For eudaimonic well-being, females and males mean scores were similar (mean = 3.69, sd = 0.82 and 0.83, t = −0.79, P = 0.43). For hedonic well-being, males were significantly, but only slightly, happier (mean 4.52, sd = 0.74) than females (mean 4.51, sd = 0.72) (t = 4.00, P < 0.001). Eudaimonic and hedonic well-being were moderately correlated (r = 0.53, P < 0.001).
Genome-wide association analyses
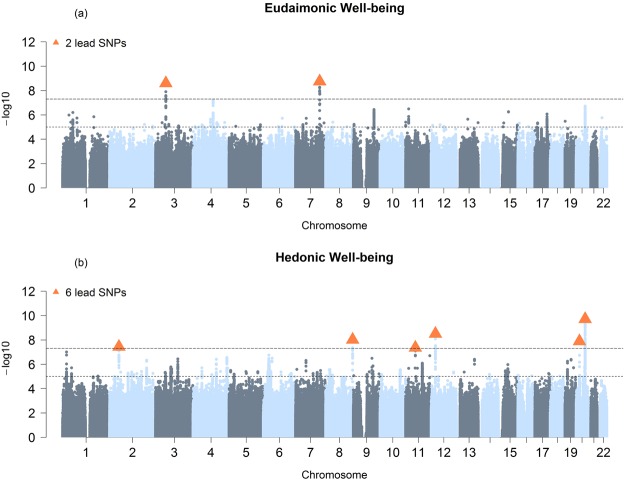
For eudaimonic well-being, 2 genetic variants reached genome-wide significance (Table 1 and Fig. 1a). The two univariate GWAS for hedonic well-being (UKB ID 4526 and UKB ID 20458) identified, respectively 1 and 2 genome-wide significant hits (Supplementary Table 2 and Supplementary Figs 1–2). The genomic inflation factor (lamda Genomic Control) of eudaimonic well-being (λGC = 1.14) and hedonic well-being (λGC_UKB ID 4526 = 1.13 and λGC_UKB ID 20458 = 1.13) were inflated. The estimated intercept from LD Score regression, though, did not exceed 1.02, indicating that nearly all the inflation is the GWAS analyses is due to polygenic signal rather than bias41 (Supplementary Table 3). Based on the high genetic correlation between the two hedonic well-being measures (rg = 0.99, P < 0.001), we performed a multivariate N-weighted GWAMA to increase the effective sample size. The multivariate N-weighted GWAMA for the two hedonic GWAS analyses yielded 6 genetic variants for hedonic well-being that reached genome-wide significance (λGC = 1.21, LD intercept = 1.00; Fig. 1b, Table 1 and Supplementary Table 3). The significant SNPs associated with eudaimonic well-being had low P-values (7.6 × 10−4 for rs79520962 and 3.4 × 10−5 for rs7618327) in the hedonic analyses. Three out of 6 significant SNPs associated with hedonic well-being had low P-values (P < 3.6 × 10−5) in the eudaimonic GWAS.
Table 1.
Genome-wide significant hits for eudaimonic -and hedonic well-being.
| Eudaimonic well-being | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | RS | CHR | BP | A1 | A2 | Z | P | N | EAF | BETA | SE |
| 7:127671511 | rs79520962 | 7 | 127671511 | A | G | −6.015 | 1.80E-09 | 108154 | 0.05 | −0.051 | 0.009 |
| 3:54376990 | rs7618327 | 3 | 54376990 | G | A | −5.961 | 2.52E-09 | 108154 | 0.12 | −0.033 | 0.006 |
| Hedonic well-being Multivariate | |||||||||||
| 20:47746974 | rs34841991 | 20 | 47746974 | C | T | 6.367 | 1.92E-10 | 221575 | 0.24 | 0.022 | 0.004 |
| 12:22874365 | rs261909 | 12 | 22874365 | C | G | 5.925 | 3.12E-09 | 221575 | 0.44 | 0.018 | 0.003 |
| 8:142617261 | rs746839 | 8 | 142617261 | G | C | −5.739 | 9.53E-09 | 221575 | 0.38 | −0.018 | 0.003 |
| 20:17445078 | rs4239724 | 20 | 17445078 | G | A | −5.689 | 1.28E-08 | 221575 | 0.22 | −0.021 | 0.004 |
| 2:49222872 | rs6732220 | 2 | 49222872 | C | G | 5.506 | 3.68E-08 | 221575 | 0.77 | 0.020 | 0.004 |
| 11:51477511 | rs146213057 | 11 | 51477511 | A | G | 5.476 | 4.36E-08 | 221575 | 0.01 | 0.084 | 0.015 |
CHR = chromosome, BP = Base Pair, A1 = Effect allele, A2 = Other allele, Z = Zscore, P = P-value, N = sample size, EAF = Estimated Allele Frequency, SE = Standard Error.
Figure 1.
Manhattan Plot for GWAS results. Result is shown for (a) Univariate GWAS of eudaimonic well-being and, (b) N-weighed GWAMA of hedonic well-being. The x axis shows chromosomal position, and the y axis shows association significance on a −log10 scale. The upper dashed line marks the threshold for genome-wide significance (P = 5 × 10−8), and the lower dashed line marks the threshold for nominal significance (P = 1 × 10−5). Each approximately independent genome-wide significant association (lead SNP) is marked by an orange Δ. Each lead SNP is the SNP with the lowest P value within the locus, as defined by our clumping algorithm.
Validation genome-wide significant results
To validate our analyses, we cross-checked our GWAS results against a published GWAS of multiple positive affect measurements (N ~ 133 K)34 omitting UK Biobank samples. For hedonic well-being we identified 5 genome-wide significant SNPs present in both our current results and the previous published GWAS. All betas showed a similar direction of effect in both studies (Supplementary Table 4). For eudaimonic well-being, the genome wide significant SNP (rs7618327) is also present in the previous published GWAs with similar direction of effect in both studies. From the 20 SNPs with a P-value < 1 × 10−5, eighty-five percent had similar direction of effects showing a significant relation (χ2(1) = 7.54, P = 0.006; Supplementary Table 5).
SNP heritability and Genetic Correlation
For eudaimonic well-being, SNP h2 was 6.2% (se = 0.005), while for hedonic well-being the SNP h2 was 6.2% (se = 0.005) (UKB ID 4526) and 6.4% (se = 0.005) (UKB ID 20458; Supplementary Table 3). The genetic correlation between the two measurements of hedonic wellbeing was –as expected- extremely high (0.99, P < 0.001). Additionally, the genetic correlation between eudaimonic and hedonic well-being was rg = 0.78, (P < 0.001, Fig. 2 and Supplementary Table 6).
Figure 2.
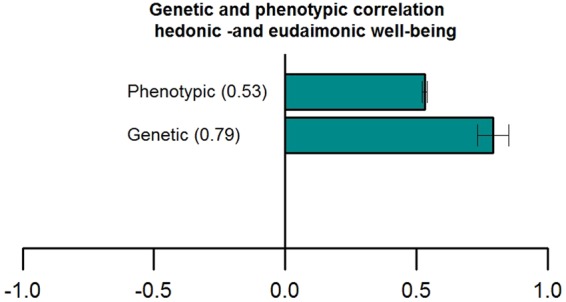
Phenotypic and genetic correlations between eudaimonic and hedonic well-being with their corresponding 95% confidence intervals.
Polygenic prediction
Polygenic scores were calculated for 10 P-value thresholds, using Caucasian UK Biobank participants with non-British ancestry as an independent sample. PRS based on the hedonic well-being GWAMA explained 0.83% (P = 2.81 × 10−18) of the variance in eudaimonic well-being whereas PRS based on the eudaimonic well-being GWAS explained 0.43% (P = 2.60 × 10−10) of the variance in hedonic well-being. A complete overview of the polygenic scores including all thresholds can be found in Supplementary Table 7 and Supplementary Fig. 3.
Functional annotation
Eudaimonic well-being
We searched the NHGI GWAS catalog to determine which of the lead SNP (P < 5 × 10−8, independent from each other at r2 < 0.1) associated with eudaimonic well-being have been previously reported. This search initially revealed that none of the variants are previously reported. However, if we look at the results of the gene-based test as computed by MAGMA including all SNPs with a P value below 0.05, genes associated with Educational attainment42 (ARFGEF2), Subjective Well-being34 (ARFGEF2, CSE1L) and height43 (STAU1, ZFAS1) were found.
Based on the eudaimonic well-being GWAS, 3 genes were found through positional mapping, 1 through eQTL mapping, and 13 through chromatine interaction-mapping (Supplementary Tables 8–10). Looking at the results of the gene-based test as computed by MAGMA including all SNPs with a P value below 0.05, 10 genes were associated with eudaimonic well-being (Supplementary Table 11). Of these 27 genes in total, one gene (SND1) was implicated in all four methods. The SND1 gene encodes a transcriptional co-activator that interacts with the acidic domain Epstein-Barr virus nuclear antigen (EBNA 2), a transcriptional activator that is required for B-lymphocyte transformation. Proteins encode by this gene are thought to be essential for normal cell growth (https://www.ncbi.nlm.nih.gov/gene/27044).
Hedonic well-being
We first searched the NHGI GWAS catalog to determine which of the lead SNP associated with hedonic well-being have been previously reported. Here we found that the variants have been reported in Educational attainment42 (ARFGEF2), Obesity-related traits44 (PCSK2, ARFGEF2), Subjective Well-being34 (ARFGEF2, CSE1L) and height43 (STAU1, ZFAS1) (Supplementary Table 12).
Based on the multivariate N-weighted GWAMA, 7 genes were implicated through positional mapping, 9 through eQTL mapping, and 50 through chromatine interaction-mapping (Supplementary Tables 13–15). Using the results of the gene-based test as computed by MAGMA including all SNPs with a P value below 0.05, 35 genes were associated with hedonic well-being (Supplementary Table 16). Of these 101 genes in total, 16 were found in more than one strategy. Of these, two genes (CSE1L, STAU1) were implicated by all four methods. Proteins encode by CSE1L, may play a role in apoptosis and in cell proliferation (https://www.ncbi.nlm.nih.gov/gene/1434?otool=inlvulib). The STAU1 gene is a member of the family of double stranded RNA (dsRNA)-binding proteins involved in the transport and/or localization of mRNAs to different subcellular compartments. STAU1 contains a microtubule-binding domain similar to that of microtubule-associated protein 1B (MAP1B) and bind tubulin (https://www.ncbi.nlm.nih.gov/gene/6780).
Tissue Specific expression
Tissue expression analysis, performed on GTEx RNA-sq data, showed significant enrichment in the brain cortex, brain cerebellum, frontal cortex, as well as the cerebellar hemisphere for eudaimonic well-being. In contrast, no significant results were found for hedonic well-being, although brain tissues were top ranked in their enrichment (Supplementary Tables 17 and 18, Supplementary Fig. 4).
Genetic Correlations
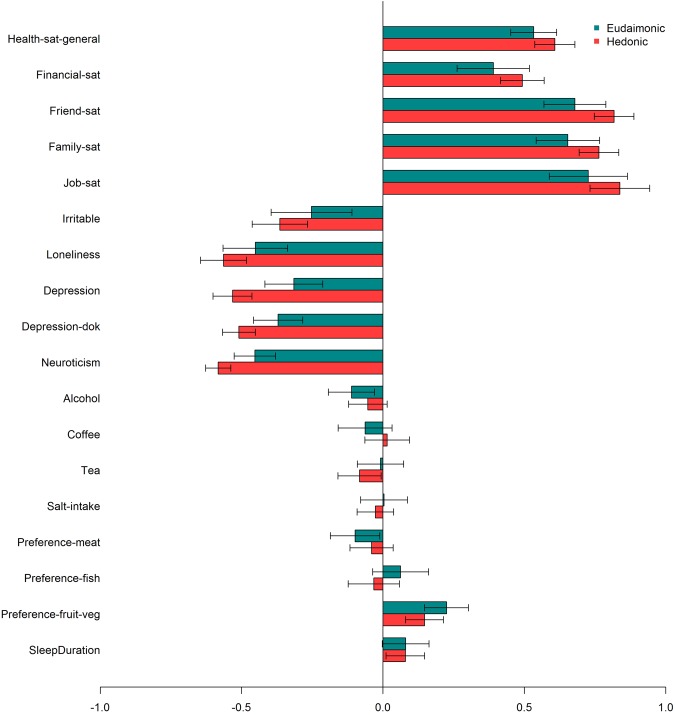
Another way to study the relationship between eudaimonic and hedonic well-being is by comparing their genetic correlation patterns with positive and negative related traits. Overall, we found a similar pattern for both eudaimoninc and hedonic well-being. Both were positively correlated with satisfaction with health (rgEUD = 0.53, rgHED = 0.61), financial satisfaction (rgEUD = 0.39, rgHED = 0.49), friendship satisfaction (rgEUD = 0.68, rgHED = 0.81), family Satisfaction (rgEUD = 0.65, rgHED = 0.76) and job satisfaction (rgEUD = 0.73, rgHED = 0.84). Negative correlations were found for irritable (rgEUD = −0.25, rgHED = −0.36), loneliness (rgEUD = −0.45, rgHED = −0.56), depressive symptoms (rgEUD = −0.32, rgHED = −0.53), depression diagnosed by doctor (rgEUD = −0.37, rgHED = −0.51), and neuroticism (rgEUD = −0.45, rgHED = −0.58; Fig. 3 and Supplementary Table 19). These similar patterns support the finding of a large overlap between the genetic determinants of eudaimonic and hedonic well-being.
Figure 3.
Genetic correlations between eudaimonic (blue) –and hedonic well-being (red) with (from top to bottom): satisfaction with health, financial satisfaction, friendship satisfaction, familial satisfaction, job satisfaction, irritable, loneliness, depression, depression diagnosed by a doctor, neuroticism, alcohol use, coffee use, tea use, salt intake, meat preference, fish preference, fruit preference and sleep duration. 95% confidence intervals are provided.
Discussions
In this article, we provide evidence for a strong overlap between the genetic contributions to hedonic and eudaimonic wellbeing. Our analyses revealed a moderate phenotypic correlation (r = 0.53), but a high correlation in the portion attributable to genetic variation (rg = 0.78), suggesting a large shared genetic etiology. Our results include the first two genome-wide significant independent loci for eudaimonic well-being and six independent loci for hedonic well-being. Biological annotation points to a central role for the central nervous system in both forms of well-being. Loci regulating expression showed significant enrichment in the brain cortex, brain cerebellum, frontal cortex, as well as the cerebellar hemisphere for eudaimonic well-being. No significant enrichment for hedonic well-being is observed, although brain tissues were top ranked.
To validate our genome-wide analyses, we performed a direction of effect test with a previous GWAS study including multiple positive affect measurements (N = ~133 K). Significant SNPs for both hedonic -and eudaimonic well-being have similar directions in the previous published GWAS of positive affect, whereas 17 out of 20 SNPs (eighty-five percent) of the suggestive eudaimonic SNPs had similar direction of effects. Moreover, we obtained significant polygenic score predictions for both eudaimonic –eudaimonic well-being. Although the explained variance is small (<1%), due to the small effect sizes of the genetic variants, our results are in line with previous studies34,45. Given these results, together with the multiple robustness checks (e.g. LD Score intercept of one, large genetic correlation with each other and similar patterns of genetic correlation with related traits), we are, beyond reasonable doubt, convinced that our genome-wide associations findings are credible findings.
The overlapping genetic etiology of the two forms of well-being can be a product of a causal relationship between the two traits. The direction of effect between hedonic –and eudaimonic well-being can be assessed using a two-sample Mendelian Randomization (MR) design. However, given the relatively small sample size and limited genetic variants reaching genome-wide significance, we are not able to construct strong instrumental variables that are needed for trustworthy interpretations of the direction of effect between hedonic –and eudaimonic well-being. However, recent-non-genetic- studies investigating the relationship between subjective well-being (SWB) and psychological well-being (PWB) found stronger evidence for a causal relation from PWB to SWB than vice versa46–48. It would be very interesting for future studies to investigate the causal relationship between hedonic-and eudaimonic well-being in a genetically informed dataset to be able to investigate causality and (genetic) pleiotropy.
Further evidence for a shared genetic architecture between hedonic and eudamonic well-being is provided by the similar patterns of genetic correlations with other traits. Largest correlations were found for job satisfaction followed by friendship –and family satisfaction and general health satisfaction. Remarkably, in contrast to job satisfaction, financial satisfaction showed the lowest correlation with both eudaimonic –and hedonic well-being. Genetic correlations with negative related phenotypes were for both measures largest for neuroticism followed by loneliness, depression (2X) and irritable. Thus, genetic correlations showed similar patterns for both measures of well-being, with largely overlapping confident intervals (CIs). However, point estimates for hedonic well-being were systematically larger compared to eudaimonic well-being, which is unlikely due to chance. Therefore, it would be interesting for future studies with larger samples to test whether hedonic well-being indeed a shows stronger associations with related phenotypes. Moreover, the lower phenotypic correlation suggests that there are divergent (environmental) factors having an effect on hedonic –and eudaimonic well-being. It would be very interesting to identify these factors in future studies. In this light our results are supportive of a two-factor model with highly correlated constructs.
Besides adding to the ongoing debate on the overlap and distinction between hedonic and eudaimonic well-being the current study provides novel insight into the genetics of well-being by identifying genome-wide significant genetic variants that explain differences in eudaimonic well-being. These variants have not been associated with a complex trait before, and thus warrant replication. Robustness of the current findings, though, is reflected by our validation analyses. Moreover, the genome-wide significant genetic variant at chromosome 20 identified in the hedonic well-being GWAMA lies in close proximity (<50 kb) to a genetic variants previously associated with subjective well-being34. We do however, not directly replicate the significant hits on chromosome 5, as reported by Okbay et al. This is most probably due to the fact that the current analyses are based on a single item in a very homogenous population, while the analyses in Okbay et al. are based on the intersection of many different well-being measures in less homogenous populations. The one item happiness question as used to define hedonic well-being in the current paper could be considered to be part of the overall umbrella of well-being as has been studied by Okbay et al. The large genetic correlation between both GWASs (rg ~ 0.8) and the concordance in direction of effect (reflected in the sign test results) nevertheless imply a strong overlap of the results in both studies given the many variants involved in well-being on the genome-wide scale.
The findings of this study should be interpreted in light of the following limitations. One is that eudaimonic and hedonic well-being are based on single item measurements. Ideally, measurements with multi-item measurements would be included. For eudaimonic well-being, principal factor analysis of the 8-item Flourishing scale49 showed that all items of this scale, which included our included question: “To what extent do you feel your life to be meaningful”, load all on one single factor. Moreover, our question showed the highest correlation with all other items as well as with the total score. For Hedonic well-being, Bartels and Boomsma27 have shown that both multi-item and single-item questionnaires load on a single well-being factor. We, however, have explicitly chosen not to include all other available hedonic results of our previous work34,50, to leverage the power of homogeneity of the UK Biobank dataset and to ease the interpretation of our findings. Research studying higher-quality measures of the various facets of well-being is a critical next step. Our results can help facilitate such work because, if the variants we identify are used as candidates, studies conducted in the smaller samples in which more fine-grained phenotype measures are available can be well powered. Additionally, it is known that participants of the UK Biobank have a specific age range (40–70 years). In previous work we, however, showed that the variance explained by genetic factors for well-being over time is stable51 and that genetic innovation is not likely to take place in adulthood52. Therefore, we are confident that this characteristic of the UKbiobank sample will not have a large effect on the results.
In conclusion, we found a moderate phenotypic correlation between eudaimonic and hedonic well-being, but identified a more substantial overlap in the genetic variants that contribute to each. Future studies should acknowledge that eudaimonic and hedonic well-being share overlapping genetic contributions and include both to increase our understanding of the etiology of well-being.
Methods
Participants
We analyzed data from the UK Biobank project53. The UK Biobank is a prospective study designed to be a resource for research into the causes of disease in middle and old age. The study protocol and information about data access are available online (http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf) and more details on the recruitment and study design have been published elsewhere53. The UK Biobank study was approved by the North West Multi-Centre Research Ethics Committee (reference number 06/ MRE08/65), and at recruitment all participants gave informed consent to participate in UK Biobank and be followed-up, using a signature capture device. All experiments were performed in accordance with guidelines and regulations from these committees. In brief, all participants were registered with the UK National Health Service (NHS) and lived within 25 miles (40 km) of one of the assessment centres. The UK Biobank invited 9.2 million people to participate through postal invitation with a telephone follow-up, with a response rate of 5.7%. A total of 503,317 men and women aged 40–70 years were recruited in assessment centres across England, Wales and Scotland, between 2006 and 2010. In total, 608 participants have subsequently withdrawn from the study and their data were not available for analysis. Participants attended 1 of 22 assessment centers across the UK, at which they completed a touch-key questionnaire, had a face-to-face interview with a trained nurse, and underwent physical assessments. Participants completed sociodemographic questionnaires, which included questions on financial satisfaction and income as well as questionnaires about their physical and mental health.
Data access permission was granted under UKB application 25472 (PI Bartels). For the discovery genome-wide association analyses we used data of ≈110 K UK-habitant Caucasian individuals only. A full overview of the included participants with valid phenotypic measurements as well as genetic data is presented in Supplemental Table 1.
Phenotypic data
Eudaimonic well-being was assessed in the online follow-up with its core element meaning in life (“To what extent do you feel your life to be meaningful?”; UKB Data-Field 20460). Answers were provided on a 5-item likert scale that ranged from “Not at all” (score 1) to “An extreme amount” (score 6). Information on eudaimonic well-being and genotypic data were available for 108,154 UK Biobank participants (56% female).
Hedonic Well-being was assessed with its core element general happiness (“In general how happy are you?”; UKB Data-Field 4526 & UKB Data-Field 20458). Answers were provided on a 6-item likert scale that ranged from “Extremely happy” (score 1) to “Extremely unhappy” (score 6). Scores were reversed so that a higher score was associated with higher levels of happiness. Hedonic well-being, as part of the touchscreen questionnaire on psychological factors and mental health (data-field 4526), was available for 111,470 individuals. Hedonic well-being was also assessed in the online follow-up (data-field 20458) and this measure is available for 110,105 individuals. Almost forty thousand individuals (n = 39,999) participated in both assessments. In total, information on hedonic well-being and genotypic data were was available for 181,578 unique UK Biobank participants (49% female; Supplementary Table 1).
Because the online follow-up questionnaire (ID 20458) of hedonic well-being took place at a later stage (~4 years later), there is a possible discrepancy between the genetic and psychological assessment. To study whether this has an effect on the genetic analyses we will calculate the genetic correlation between both measurements of hedonic well-being. Doing so allows us to investigate whether the same genes have an effect on both measurements.
Genotypic data
Participants were genotyped using one of two platforms: The affymetrix UK BiLEVE Axiom array or the Affymetrix UK Biobank Axiom array. The genetic data underwent rigorous quality control and was phased and imputed against a reference panel of Haplotype Reference Consortium (HRC), UK10K and 1000 Genomes Phase 3 haplotypes54. Due to an issue with the imputation of UK10K and 1000 Genomes variants, analyses were restricted to HRC variants only. Samples were excluded based on the following genotype-based criteria; non-European ancestry, relatedness, mismatch between genetic sex and self-reported gender, outlying heterozygosity, and excessive missingness54. For more details on the UK Biobank genotyping, imputation, and quality control procedures see55.
Descriptive statistics and phenotypic correlation
Descriptive statistics and spearman’s rank correlation between eudaimonic and hedonic well-being were calculated in R. We, furthermore, tested for sex and age effects on mean levels.
Univariate Genome-wide association analyses
Univariate genome-wide association analyses for eudaimonic well-being and for hedonic well-being (touchscreen measure and online follow-up separately) were performed in PLINK56,57 using a linear regression model of additive allelic effects. Standard pre-GWAS- quality control filters were applied, which included removing SNPs with minor allele frequency < 0.005 and/or with an INFO-score < 0.8 for imputed SNPs, and removing individuals with ambiguous sex and/or non-British ancestry. We, furthermore, randomly selected 1 individual from each closely related pair (i.e. parent offspring pairs, sibling pairs). The GWAS included 40 principal components, age, sex, and a chip dummy as covariates. Additionally, following a pre-specified analysis plan, we conducted a stringent post-GWA quality control (QC) protocol based on the paper of Winkler and colleagues58.
Multivariate Genome-wide association analyses
To increase the effective sample size, we conducted multivariate N-Weighted genome-wide association meta-analyses (GWAMA) by leveraging the association between the two hedonic well-being univariate GWAS analyses (UKB Data-field 4526 and 20458, nobs total = 221,575). The dependence between effect sizes (error correlation) induced by sample overlap in both these GWAMAs was estimated from the genome-wide summary statistics of the univariate GWAS analyses using LD score regression59,60. Knowledge of the error correlation between the univariate GWAS analyses allowed us to meta-analyze them together, providing a gain in power while guarding against inflated type I error rates. For a detailed description on performing N-weighted GWAMA, please see Baselmans and colleagues50.
Validation genome-wide significant results
To validate our analyses, we cross-checked our GWAS results against a published GWAS of multiple positive affect measurements (N ~ 133 K)34 omitting UK Biobank samples. The positive affect GWAS used the HapMap2 CEU as reference sample (~2.2 million SNPs), which contains considerable less SNPs compared to the roughly 8.6 million SNPs (1000 G, phase 3) present in the UK Biobank analyses. We used the following strategy to identify proxy genome-wide significant SNPs present in both datasets. First, we extracted the genome-wide significant (P < 5 × 10−8 from the GWAS of hedonic well-being) and suggestive SNPs (P < 1 × 10−5, GWAS of eudaimonic well-being) and matched these to the corresponding positive affect SNPs of the published GWAs. Next, using a clumping procedure (250 kb window and R2 > 0.1), we identified the independent SNPs present in both datasets, which will be used for testing the direction of effect. When there is a discrepancy in direction of effect between the two datasets, a Chi-square test of independence was calculated to test the significance of the relation.
SNP heritability and Genetic Correlation
SNP heritability for eudaimonic and hedonic well-being separately was estimated using bivariate LD Score Regression59,60. The same methodology was used to estimate the genetic correlation between the two measures of hedonic well-being and between eudaimonic and hedonic well-being. LD scores regression produces unbiased estimates even in the presence of sample overlap and only requires summary statistics and a reference panel from which to estimate each SNP’s “LD score” (the amount of genetic variation tagged by a SNP). We used the file of LD scores computed by Finucane et al.61 using genotypic data from a European-ancestry population (see https://github.com/bulik/ldsc/wiki/Genetic-Correlation, accessed September 8, 2017).
Polygenic prediction
We performed polygenic risk score prediction (PRS) using Caucasian UK Biobank participants with non-British ancestry as independent prediction sample (nobs = 28,582). For eudaimonic well-being, polygenic prediction was performed in 9,088 individuals. For hedonic well-being, we used phenotypic measurements closest to genotype-collection (UKB Data-Field 20458) for polygenic scores and scores were available for 9,276 individuals. The weights used for the polygenic scores are based on the univariate GWAS (eudaimonic) and multivariate GWAMA (hedonic well-being). Polygenic scores were based on the genotyped SNPs (nobs = 619,049). To calculate the incremental R2, the phenotypes (eudaimonic and hedonic well-being) were standardized and regressed on sex and age as well as principal components, which were included to correct for ancestry. Next, the same analysis was repeated with inclusion of the polygenic scores. The differences in R2 between both regression is referred to as incremental R2. To obtain 95% confidence intervals (CI) around the incremental R2’s, bootstrapping was performed with 2000 repetitions.
Functional annotation
Functional annotation was performed in FUMA62 (http://fumactglab.nl) for the eudaimonic well-being GWAS and the hedonic well-being GWAMAs. Lead SNPs were defined as having a genome-wide significant P values (5 × 10−8) and being independent from each other (r2 < 0.1). Functional annotation was performed on these lead SNPs and SNPs with P < 0.05, MAF < 0.01, and in high LD (r2 > 0.6) with those lead SNPs.
Gene-mapping
This set of SNPs was mapped to genes in FUMA using three strategies. The SNPs were mapped to genes based on (1) their physical distance (i.e. within 10 kb window), (2) significant eQTL association (i.e. the expression of that gene is associated with allelic variation at the SNP). eQTL mapping in FUMA uses information from the GTEx, Blood eQTL browser, and BIOS QTL browser, and is based on cis-eQTLs that can map SNPs to genes up to 1MB apart. A false discovery rate (FDR) of 0.05 was applied to define significant eQTL associations. 3) a significant chromatin interaction between a genomic region and promoter regions of genes (250 bp up and 500 bp downstream of transcription start site (TSS)). Chromatine interaction mapping can involve long-range interaction as it does not have a distance boundary as in eQTL mapping. We used a FDR p-value of 1 × 10−5 to define significant interactions.
Finally, given our modest sample size and expected polygenicity of our phenotypes, we added an extra strategy in which all SNPs (P < 0.05) were included and mapped to genes based on physical distance (i.e. within 10 kb window) from known protein coding genes (GRCh37/hg19). Genome-wide significance for this test was defined at P = 0.05/18187 = 2.74 × 10−6.
Tissue Expression Analysis (MAGMA)
To test the relationship between highly expressed genes in a specific tissue and genetic associations, gene-property analysis is performed using average expression of genes per tissue type as a gene covariate. Gene expression values are log2 transformed average RPKM (Reads Per Kilobase Million) per tissue type after winsorized at 50 based on GTEx RNA-seq data. Tissue expression analysis is performed for 53 specific tissue types separately. The result of the gene analysis (gene-based P value) were used in MAGMA to test for one-side increased expression conditioned on average expression across all tissue types.
Genetic Correlation
To test whether hedonic or eudaimonic well-being are genetically differently correlated with a set of related phenotypes, bivariate LD Score regression was applied with both measures of well-being and the following UK Biobank summary statistics: satisfaction with health (UKB ID 20459), financial satisfaction (UKB ID 4581), friendship satisfaction (UKB ID 4570), family satisfaction (UKB ID 4559), job satisfaction (UKB ID 4537), irritable (UKB ID 4653), loneliness (UKB ID 2020), depressive symptoms (UKB ID 2100), depression diagnosed by doctor (UKB ID 2090), neuroticism (UKB ID 20127). To test the relationship between hedonic/eudaimonic well-being with less established phenotypes we included the following phenotypes: alcohol (UKB ID 1558), coffee (UKB ID 1498), tea (UKB ID 1488), salt (UKB ID 1478), food preference meat (UKB ID 1349), food preference fish (UKB ID 1329), food preference fruit/vegetarian (UKB ID 1289), sleep duration (UKB ID 1160). For every genetic correlation 95% confident intervals were calculated.
Electronic supplementary material
Acknowledgements
M. Bartels and B.M.L Baselmans are financially supported by the University Research Chair position of M. Bartels. This work is supported by an ERC consolidation grant (well-being 771057 PI Bartels). This research has been conducted using UK Biobank resource (application number 25472 (PI Bartels). We would like to thank the participants and researches who collected and contributed to the data. We would like to thank Michel Nivard, Matthijs van der Zee, and Hill Fung Ip for their contributions in processing the UK Biobank genetic data. Computational facilities were supplied by NWO via the grant: “Population scale genetic analysis” 2018/EW/00408559.
Author Contributions
M.B. supervised the entire project. M.B. and B.B. wrote the main manuscript text. M.B. was responsible for data curation. M.B. and B.B. performed formal analyses. B.B. prepared all (supplementary) figures and tables. M.B. and B.B. took care of the project administration.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32638-1.
References
- 1.Biswas-Diener R, Kashdan TB, King LA. Two traditions of happiness research, not two distinct types of happiness. J. Posit. Psychol. 2009;4:208–211. doi: 10.1080/17439760902844400. [DOI] [Google Scholar]
- 2.McMahon DM. Living well in light of science. Ann. N. Y. Acad. Sci. 2016;1384:36–38. doi: 10.1111/nyas.13064. [DOI] [PubMed] [Google Scholar]
- 3.Waterman AS. The relevance of Aristotle’s conception of eudaimonia for the psychological study of happiness. Theor. Philos. Psychol. 1990;10:39–44. doi: 10.1037/h0091489. [DOI] [Google Scholar]
- 4.Huta V, Ryan RM. Pursuing Pleasure or Virtue: The Differential and Overlapping Well-Being Benefits of Hedonic and Eudaimonic Motives. J. Happiness Stud. 2010;11:735–762. doi: 10.1007/s10902-009-9171-4. [DOI] [Google Scholar]
- 5.Diener E. Subjective well-being. Psychological Bulletin. 1984;95:542–575. doi: 10.1037/0033-2909.95.3.542. [DOI] [PubMed] [Google Scholar]
- 6.Martín-María N, et al. The Impact of Subjective Well-being on Mortality. Psychosom. Med. 2017;79:565–575. doi: 10.1097/PSY.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A, Deaton A, Stone A. Subjective wellbeing, health, and ageing. Lancet. 2014;385:640–648. doi: 10.1016/S0140-6736(13)61489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 9.Petrie KJ, et al. Which Aspects of Positive Affect Are Related to Mortality? Results From a General Population Longitudinal Study. Ann. Behav. Med. 2018;55:110–121. doi: 10.1093/abm/kax018. [DOI] [PubMed] [Google Scholar]
- 10.Ryff CD, Keyes CLM. The structure of psychological well-being revisited. J. Pers. Soc. Psychol. 1995;69:719–727. doi: 10.1037/0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 11.Czekierda K, Banik A, Park CL, Luszczynska A. Meaning in life and physical health: systematic review and meta-analysis. Health Psychol. Rev. 2017;11:387–418. doi: 10.1080/17437199.2017.1327325. [DOI] [PubMed] [Google Scholar]
- 12.Pearson PR, Sheffield BF. Psychoticism and purpose in life. Pers. Individ. Dif. 1989;10:1321–1322. doi: 10.1016/0191-8869(89)90245-6. [DOI] [Google Scholar]
- 13.Marco JH, Guillén V, Botella C. The buffer role of meaning in life in hopelessness in women with borderline personality disorders. Psychiatry Res. 2017;247:120–124. doi: 10.1016/j.psychres.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Marco JH, Pérez S, García-Alandete J, Moliner R. Meaning in Life in People with Borderline Personality Disorder. Clin. Psychol. Psychother. 2017;24:162–170. doi: 10.1002/cpp.1991. [DOI] [PubMed] [Google Scholar]
- 15.Steger MF, Oishi S, Kashdan TB. Meaning in life across the life span: Levels and correlates of meaning in life from emerging adulthood to older adulthood. J. Posit. Psychol. 2009;4:43–52. doi: 10.1080/17439760802303127. [DOI] [Google Scholar]
- 16.Steger MF, Mann JR, Michels P, Cooper TC. Meaning in life, anxiety, depression, and general health among smoking cessation patients. J. Psychosom. Res. 2009;67:353–358. doi: 10.1016/j.jpsychores.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Keyes Corey L. M., Shmotkin Dov, Ryff Carol D. Optimizing well-being: The empirical encounter of two traditions. Journal of Personality and Social Psychology. 2002;82(6):1007–1022. doi: 10.1037/0022-3514.82.6.1007. [DOI] [PubMed] [Google Scholar]
- 18.Deci EL, Ryan RM. Hedonia, eudaimonia, and well-being: An introduction. J. Happiness Stud. 2006;9:1–11. doi: 10.1007/s10902-006-9018-1. [DOI] [Google Scholar]
- 19.Keyes CLM, Annas J. Feeling good and functioning well: Distinctive concepts in ancient philosophy and contemporary science. J. Posit. Psychol. 2009;4:197–201. doi: 10.1080/17439760902844228. [DOI] [Google Scholar]
- 20.Huta V, Waterman AS, Waterman AS. Eudaimonia and Its Distinction from Hedonia: Developing a Classification and Terminology for Understanding Conceptual and Operational Definitions. J Happiness Stud. 2014;15:1425–1456. doi: 10.1007/s10902-013-9485-0. [DOI] [Google Scholar]
- 21.Proctor C, Tweed R, Morris D, Proctor C, Morris D. The Naturally Emerging Structure of Well-Being Among Young Adults: ‘“Big Two”’ or Other Framework? J Happiness Stud. 2015;16:257–275. doi: 10.1007/s10902-014-9507-6. [DOI] [Google Scholar]
- 22.Joshanloo M. Revisiting the Empirical Distinction Between Hedonic and Eudaimonic Aspects of Well-Being Using Exploratory Structural Equation Modeling. J. Happiness Stud. 2016;17:2023–2036. doi: 10.1007/s10902-015-9683-z. [DOI] [Google Scholar]
- 23.Kashdan TB, Biswas-Diener R, King LA. Reconsidering happiness: the costs of distinguishing between hedonics and eudaimonia. J. Posit. Psychol. 2008;3:219–233. doi: 10.1080/17439760802303044. [DOI] [Google Scholar]
- 24.Bobowik M, Basabe N, Páez D. The bright side of migration: hedonic, psychological, and social well-being in immigrants in Spain. Soc. Sci. Res. 2015;51:189–204. doi: 10.1016/j.ssresearch.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher Matthew W., Lopez Shane J., Preacher Kristopher J. The Hierarchical Structure of Well-Being. Journal of Personality. 2009;77(4):1025–1050. doi: 10.1111/j.1467-6494.2009.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keyes CLM. The mental health continuum: from languishing to flourishing in life. J. Health Soc. Behav. 2002;43:207–22. doi: 10.2307/3090197. [DOI] [PubMed] [Google Scholar]
- 27.Bartels M, Boomsma DI. Born to be happy? the etiology of Subjective well-being. Behav. Genet. 2009;39:605–615. doi: 10.1007/s10519-009-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartels M. Genetics of Wellbeing and Its Components Satisfaction with Life, Happiness, and Quality of Life: A Review and Meta-analysis of Heritability Studies. Behav. Genet. 2015;45:137–156. doi: 10.1007/s10519-015-9713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, et al. The MAOA gene predicts happiness in women. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:122–125. doi: 10.1016/j.pnpbp.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Neve JE. Functional polymorphism (5-HTTLPR) in the serotonin transporter gene is associated with subjective well-being: evidence from a US nationally representative sample. J Hum Genet. 2011;56:456–459. doi: 10.1038/jhg.2011.39. [DOI] [PubMed] [Google Scholar]
- 31.De Neve, J. E., Christakis, N. A., Fowler, J. H. & Frey, B. S. Genes, Economics, and Happiness. J. Neurosci. Psychol. Econ. 5 (2012). [DOI] [PMC free article] [PubMed]
- 32.Minkov, M. & Bond, M. H. A Genetic Component to National Differences in Happiness. J. Happiness Stud. (2016).
- 33.Rietveld CA, et al. Molecular genetics and subjective well-being. Proc. Natl. Acad. Sci. USA. 2013;110:9692–7. doi: 10.1073/pnas.1222171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okbay, A. et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet.48, 624–633 (2016). [DOI] [PMC free article] [PubMed]
- 35.Fredrickson BL, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110:13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredrickson Barbara L., Grewen Karen M., Algoe Sara B., Firestine Ann M., Arevalo Jesusa M. G., Ma Jeffrey, Cole Steve W. Psychological Well-Being and the Human Conserved Transcriptional Response to Adversity. PLOS ONE. 2015;10(3):e0121839. doi: 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown NJ, MacDonald DA, Samanta MP, Friedman HL, Coyne JC. A critical reanalysis of the relationship between genomics and well-being. Proc Natl Acad Sci USA. 2014;111:12705–12709. doi: 10.1073/pnas.1407057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coyne JC. Highly correlated hedonic and eudaimonic well-being thwart genomic analysis. Proc Natl Acad Sci USA. 2013;110:E4183. doi: 10.1073/pnas.1315212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole SW, Fredrickson BL. Reply to Coyne: Genomic analyses are unthwarted. Proc Natl Acad Sci USA. 2013;110:E4184. doi: 10.1073/pnas.1316887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole SW, Fredrickson BL. Errors in the Brown et al. critical reanalysis. Proc Natl Acad Sci USA. 2014;111:E3581. doi: 10.1073/pnas.1413316111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, et al. Genomic inflation factors under polygenic inheritance Peter M Visscher 1 and the GIANT Consortium. Eur. J. Hum. Genet. 2011;1939:807–812. doi: 10.1038/ejhg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okbay A, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533:539–542. doi: 10.1038/nature17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Justice AE, et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat. Commun. 2017;8:14977. doi: 10.1038/ncomms14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Moor MHM, et al. Meta-analysis of genome-wide association studies for neuroticism, and the polygenic association with Major Depressive Disorder. JAMA Psychiatry. 2015;72:642–650. doi: 10.1001/jamapsychiatry.2015.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshanloo M, Sirgy MJ, Park J. Directionality of the relationship between social well-being and subjective well-being: evidence from a 20-year longitudinal study. Qual. Life Res. 2018;0:0. doi: 10.1007/s11136-018-1865-9. [DOI] [PubMed] [Google Scholar]
- 47.Joshanloo, M. Investigating the Relationships Between Subjective Well- Being and Psychological Well-Being Over Two Decades. Emotion (2018). [DOI] [PubMed]
- 48.Joshanloo M. Longitudinal associations between subjective and psychological well-being in Japan: A four-year cross-lagged panel study. Pers. Individ. Dif. 2018;134:289–292. doi: 10.1016/j.paid.2018.06.033. [DOI] [Google Scholar]
- 49.Diener E, et al. New Well-being Measures: Short Scales to Assess Flourishing and Positive and Negative Feelings. Soc. Indic. Res. 2010;97:143–156. doi: 10.1007/s11205-009-9493-y. [DOI] [Google Scholar]
- 50.Baselmans BML, et al. Multivariate Genome-wide and integrated transcriptome and epigenome-wide analyses of the Well-being spectrum. bioRxiv. 2017;115915:1–27. [Google Scholar]
- 51.Baselmans BML, et al. Unraveling the Genetic and Environmental Relationship Between Well-Being and Depressive Symptoms Throughout the Lifespan. Front. Psychiatry. 2018;9:261. doi: 10.3389/fpsyt.2018.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nivard, M. G. et al. Stability in symptoms of anxiety and depression as a function of genotype and environment: a longitudinal twin study from ages 3 to 63 years. Psychol. Med. 1–11 (2015). [DOI] [PubMed]
- 53.Sudlow C, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12:1–10. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.UK Biobank. UK Biobank. Genotype imputation and genetic association studies of UK Biobank. May 2015. (2015).
- 55.Bycroft, C. et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv166298 (2017).
- 56.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng, Y. et al. Genome-wide Regional Heritability Mapping Identifies a Locus Within the TOX2 Gene Associated With Major Depressive Disorder. Biol. Psychiatry 1–10 (2016). [DOI] [PMC free article] [PubMed]
- 58.Winkler TW, et al. Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc. 2014;9:1192–212. doi: 10.1038/nprot.2014.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–41. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finucane HK, et al. Partitioning heritability by functional category using GWAS summary statistics. Nat. Genet. 2015;47:1228–1235. doi: 10.1038/ng.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. FUMA: Functional mapping and annotation of genetic associations. bioRxiv110023 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.