Abstract
In this study we conducted a cross sectional study to comprehensively evaluated the risk factors of chronic kidney disease (CKD) in a large sample of Chinese adults under primary care for type 2 diabetes mellitus (T2DM). We investigated the risk factors associated with the prevalence of CKD in adults with T2DM, who were enrolled in the Risk Factor Assessment and Management Programme for Patients with Diabetes Mellitus (RAMP-DM) of Hong Kong from July 2014 to June 2017. We collected the individual data of 31,574 subjects, with mean age of 63.0 (±10.8) years and mean DM duration of 7.4 (±6.4) years. Of them 9,386 (29.7%) had CKD and 7,452 (23.6%) had micro- or macro-albuminuria. After adjustment for multiple demographic and lifestyle confounders, we identified several modifiable risk factors associated with higher rate of CKD: obesity (OR = 1.54), current smoking (OR = 1.33), higher systolic blood pressure (OR = 1.01), dyslipidemia (OR = 1.32 and 0.61 for triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C)), hyperglycemia (OR = 1.11 for HbA1c), diabetic retinopathy (OR = 1.36 and 2.60 for non-sight and sight threatening retinopathy), and stroke (OR = 1.43). The risk factors of lower dialytic blood pressure and coronary heart disease were identified only in men, whereas peripheral arterial disease only in women. In conclusion, several modifiable and gender specific risk factors were significantly associated with higher prevalence of CKD in Chinese adults with T2DM. The high-risk populations identified in this study shall receive regular screening for renal functions to achieve better patient management in primary care settings.
Introduction
Diabetes Mellitus (DM) is one of the most common chronic diseases worldwide. Globally, in 2014 there were 8.5% of people living with DM which nearly doubled the prevalence rate of 4.7% in 19801. A clear rising trend was observed in Hong Kong in the past decade, and type 2 (T2DM) accounts for over 90% of all the persons with diabetes2. The International Diabetes Federation estimated that in 2011 the prevalence of diabetes was 9.4% in adults aged 20–79 years of Hong Kong, and this rate could reach 11.9% by 20302.
Chronic kidney diseases (CKD) is one of common complications associated with both type 1 and type 2 DM. In the US, 40% of patients with CKD also had DM3. The mortality in DM complicated with CKD was estimated as high as 48 deaths per 1,000 patient-years at risk, and this rate tripled if patients also had cardiovascular conditions3. Previous studies have identified several risk factors for CKD, such as male, older age, obesity, hypercholesterolemia, hypertension and hyperglycemia4–7. However, most studies used glomerular filtration rate to identify CKD (GFR < 60 ml/min per 1.73 m2) or end-stage kidney disease (ESKD, GFR < 15 ml/min per 1.73 m2)8. Few have combined GFR and albuminuria categories, both of which were required for the diagnosis of CKD9,10. Furthermore, data are relatively limited in Chinese population under primary care.
In Hong Kong, the Risk Factor Assessment and Management Programme for Patients with Diabetes Mellitus (RAMP-DM) was implemented in the General Outpatients Clinics (GOPC) managed by the Hospital Authority (HA) since 2009, to conduct a comprehensive risk assessment and screening for diabetic complications in the primary care setting. Another objective of RAMP-DM was to educate DM patients by involving multi-disciplinary healthcare professionals including doctors, nurses and optometrists. Previous studies in Hong Kong have extensively evaluated the risks of cardiovascular diseases and diabetic retinopathy in patients of RAMP-DM11–13. Two recent publications have assessed the risk of developing end-stage renal disease (ESRD) among RAMP-DM participants14,15. Here we conducted a cross-sectional study using the RAMP-DM data, with the aim to explore the risk factors associated with CKD in Chinese adults. The literature on the risk factors of CKD is less for T2DM than for type 1, and 99% of patients registered to RAMP-DM were diagnosed with T2DM; hence, type 1 and gestational DM were excluded in this study.
Results
Subject characteristics
There were 40,781 people with DM enrolled into RAMP-DM of NTWC from 2 July 2014 to 16 June 2017, and 39,652 diagnosed with T2DM were eligible in this study. Of them, 8,078 (20%) subjects were further excluded from subsequent analyses because of missing data in estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (UACR). The missing data of other variables ranged 0–1.4%, with the only exception of diabetic retinopathy (DR) status, which had 4.4% and 3.3% missing in the subjects with or without CKD. The demographic characteristics of these subjects with missing data were similar to those included in the analysis (data not shown). A final sample size of 31,574 adults with T2DM were eligible for analysis. Table 1 summarizes the demographic and clinical characteristics of patients. The mean age of the study cohort is 63.0 ± 10.8 years with mean DM duration 7.4 ± 6.4 years. The number of female and male patients was nearly equal. The prevalence of CKD was 29.7% (9386/31574) at their first RAMP-DM assessment (Supplementary Table S1). 3,650 (11.6%) patients with T2DM were classified as the eGFR stages of G3a through G5, 7,452 (23.6%) as the UACR stages of A2 through A3 (micro- and macroalbuminuria), and three (<0.01%) already reached ESKD that required dialysis.
Table 1.
Descriptive statistics of demographic and clinical characteristics of all the people with type 2 diabetes, those with and without chronic kidney disease (CKD).
| Variables | Overall n = 31574 | With CKD n = 9386 | Without CKD n = 22188 | P-valuea |
|---|---|---|---|---|
| Socio-demographic (n, %) | ||||
| Sex (n, %) | <0.001 | |||
| Female | 15649 (49.6%) | 4882 (52.0%) | 10767 (48.5%) | |
| Male | 15925 (50.4%) | 4504 (48.0%) | 11421 (51.5%) | |
| Age (Years, mean ± SD) | 63.0 ± 10.8 | 67.0 ± 11.7 | 61.3 ± 9.9 | <0.001 |
| Education (n, %) | <0.001 | |||
| No formal education | 3993 (12.6%) | 1802 (19.2%) | 2191 (9.9%) | |
| Primary | 11754 (37.2%) | 3687 (39.3%) | 8067 (36.4%) | |
| Secondary | 14016 (44.4%) | 3447 (36.7%) | 10569 (47.6%) | |
| Tertiary | 1682 (5.3%) | 414 (4.4%) | 1268 (5.7%) | |
| Receiving CSSA (n, %) | 3018 (9.6%) | 1232 (13.1%) | 1786 (8.0%) | <0.001 |
| BMI category (n, %) | <0.001 | |||
| Underweight | 376 (1.2%) | 113 (1.2%) | 263 (1.2%) | |
| Normal | 6357 (20.1%) | 1659 (17.7%) | 4698 (21.2%) | |
| Overweight | 6723 (21.3%) | 1867 (19.9%) | 4856 (21.9%) | |
| Obese | 18073 (57.2%) | 5718 (60.9%) | 12355 (55.7%) | |
| Smoking (n, %) | 0.010 | |||
| Non-smoker | 21572 (68.3%) | 6319 (67.3%) | 15253 (68.7%) | |
| Current Smoker | 4402 (13.9%) | 1299 (13.8%) | 3103 (14.0%) | |
| Ex-smoker | 5574 (17.7%) | 1761 (18.8%) | 3813 (17.2%) | |
| Alcohol (n, %) | <0.001 | |||
| Non-drinker | 23251 (73.6%) | 7093 (75.6%) | 16158 (72.8%) | |
| Current Drinker | 1465 (4.6%) | 399 (4.3%) | 1066 (4.8%) | |
| Ex-drinker | 1751 (5.5%) | 594 (6.3%) | 1157 (5.2%) | |
| Social drinker | 4950 (15.7%) | 1243 (13.2%) | 3707 (16.7%) | |
| Clinical characteristics | ||||
| DM Duration (Years, mean ± SD) | 7.4 ± 6.4 | 8.9 ± 7.1 | 6.7 ± 5.9 | <0.001 |
| CHD (n, %) | 764 (2.4%) | 315 (3.4%) | 449 (2.0%) | <0.001 |
| Stroke (n, %) | 1302 (4.1%) | 608 (6.5%) | 694 (3.1%) | <0.001 |
| PAD (n, %) | <0.001 | |||
| Yes | 299 (0.9%) | 143 (1.5%) | 156 (0.7%) | |
| Suspected | 139 (0.4%) | 48 (0.5%) | 91 (0.4%) | |
| DR Status (n, %) | <0.001 | |||
| No DR | 20276 (64.2%) | 5041 (53.7%) | 15235 (68.7%) | |
| Non-sight threatening | 6333 (20.1%) | 1990 (21.2%) | 4343 (19.6%) | |
| Sight threatening | 3131 (9.9%) | 1575 (16.8%) | 1556 (7.0%) | |
| Ungradable | 90 (0.3%) | 31 (0.3%) | 59 (0.3%) | |
| Biomedical measurements (mean ± SD) | ||||
| SBP (mmHg), n = 31559 | 131.7 ± 16.2 | 134.4 ± 17.2 | 130.5 ± 15.5 | <0.001 |
| DBP (mmHg), n = 31559 | 74.8 ± 10.2 | 74.0 ± 10.9 | 75.2 ± 9.9 | <0.001 |
| UACR (mg/mmol), n = 31574 | 6.4 ± 26.5 | 18.8 ± 46.3 | 1.2 ± 0.6 | <0.001 |
| eGFR (ml/min/1.73 m2), n = 31574 | 84.1 ± 19.7 | 72.3 ± 23.1 | 89.0 ± 15.6 | <0.001 |
| HbA1c (mmol/mol), n = 31574 | 54 ± 11 | 55 ± 13 | 53 ± 10 | <0.001 |
| (%) | 7.1 ± 1.2 | 7.2 ± 1.4 | 7.0 ± 1.1 | |
| TG (mmol/L), n = 31574 | 1.5 ± 1.0 | 1.6 ± 1.1 | 1.4 ± 0.9 | <0.001 |
| LDL-C (mmol/L), n = 31162 | 2.3 ± 0.7 | 2.3 ± 0.7 | 2.3 ± 0.7 | 0.003 |
| HDL-C (mmol/L), n = 31572 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.3 | <0.001 |
aP-Value of Chi-square test for categorical data and of Mann-Whitney test for continuous data.
Due to the missing data of some covariates, there were 28,596 participants included in regression analysis (Fig. 1). Women, those without former education, receiving comprehensive social security assistance (CSSA), ex-smokers, ex-/nondrinkers and obese people had a higher prevalence of CKD than the other people did. Compared to those without CKD, patients with CKD were older, had longer duration of diabetes, worse glycemic control and lipid profile (higher HbA1c, TG, and lower HDL-C), and higher likelihood of other diabetic complications (Table 1). Subjects with CKD were more likely to have stage 2 hypertension, with higher systolic blood pressure (SBP) but slightly lower diastolic blood pressure (DBP). The probability of receiving insulin treatment and other medications was also significantly higher in those with CKD than those without (data not shown).
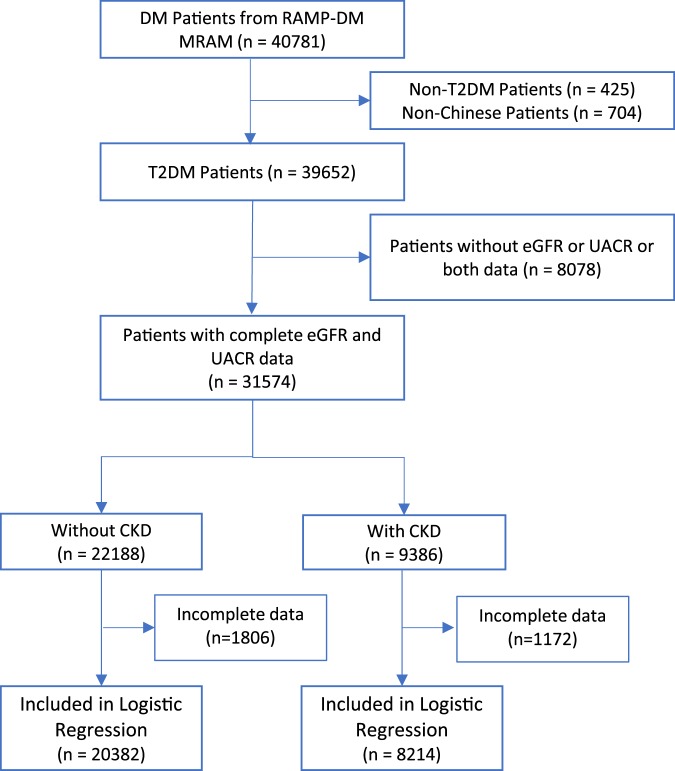
Figure 1.
Flow chart of data extraction and analysis. Incomplete data refer to missing in at least one of the following variables: sex, age, DM duration, education, smoking, alcohol drinking, SBP, DBP, hypertension, BMI categories, CHD, stroke, PAD, DR status, HbA1c, HDL-C, LDL-C, TG, receiving CSSA.
Risk factors of CKD
The crude ORs of CKD associated with different factors were estimated from univariate logistic regressions (Table 2). All the estimates were significant except for underweight (vs normal), current smokers (vs non-smokers), and suspected peripheral arterial disease (PAD) (vs no PAD). All the variables that were significant in univariate model remained significant after adjustment, but generally had smaller magnitude than the crude estimates, except for body mass index (BMI) categories, smoking, TG, and HDL-C. After adjustment, low-density lipoprotein cholesterol (LDL-C) became positively associated with CKD, but no longer significant. The association of coronary heart disease (CHD) and CKD became marginally significant with a smaller effect estimate. The adjusted OR of SBP and DBP were similar to the crude OR, with a significant inverse association found between DBP and CKD.
Table 2.
Crude and adjusted odds ratio (OR) of chronic kidney disease (CKD) associated with different factors.
| Variable | Crude OR (95% CI)a | p-valueb | Adjusted OR (95% CI)c | p-valueb |
|---|---|---|---|---|
| Sex: Male | 0.87 (0.83, 0.91) | <0.001 | 0.82 (0.76, 0.88) | <0.001 |
| Age (Years) | 1.05 (1.05, 1.06) | <0.001 | 1.05 (1.04, 1.05) | <0.001 |
| DM Duration (Years) | 1.05 (1.05, 1.06) | <0.001 | 1.02 (1.02, 1.03) | <0.001 |
| Education | ||||
| Primary | 0.56 (0.52, 0.60) | <0.001 | 0.86 (0.79, 0.94) | 0.001 |
| Secondary | 0.40 (0.37, 0.43) | <0.001 | 0.83 (0.76, 0.92) | <0.001 |
| Tertiary | 0.40 (0.35, 0.45) | <0.001 | 0.81 (0.69, 0.94) | 0.006 |
| BMI category | ||||
| Underweight | 1.22 (0.97, 1.52) | 0.091 | 1.28 (0.97, 1.68) | 0.071 |
| Overweight | 1.09 (1.01, 1.18) | 0.031 | 1.15 (1.05, 1.25) | 0.003 |
| Obesity | 1.31 (1.23, 1.40) | <0.001 | 1.54 (1.42, 1.66) | <0.001 |
| Smoking | ||||
| Current smoker | 1.01 (0.94, 1.08) | 0.773 | 1.33 (1.22, 1.46) | <0.001 |
| Ex-smoker | 1.11 (1.05, 1.19) | <0.001 | 1.10 (1.01, 1.20) | 0.023 |
| Alcohol drinking | ||||
| Current drinker | 0.85 (0.76, 0.96) | 0.008 | / | |
| Social drinker | 0.76 (0.71, 0.82) | <0.001 | / | |
| Ex-drinker | 1.17 (1.05, 1.30) | 0.003 | / | |
| Receiving CSSA | 1.73 (1.60, 1.86) | <0.001 | 1.23 (1.12, 1.35) | <0.001 |
| CHD | 1.68 (1.45, 1.95) | <0.001 | 1.17 (0.99, 1.39) | 0.068 |
| Stroke | 2.15 (1.92, 2.40) | <0.001 | 1.43 (1.26, 1.63) | <0.001 |
| PAD | ||||
| Suspected | 1.26 (0.88, 1.78) | 0.198 | 1.12 (0.75, 1.65) | 0.572 |
| Yes | 2.19 (1.74, 2.75) | <0.001 | 1.66 (1.28, 2.16) | <0.001 |
| DR Status | ||||
| Non-sight threatening | 1.38 (1.30, 1.47) | <0.001 | 1.36 (1.27, 1.46) | <0.001 |
| Sight threatening | 3.06 (2.83, 3.30) | <0.001 | 2.60 (2.38, 2.84) | <0.001 |
| Ungradable | 1.59 (1.02, 2.44) | 0.038 | 1.08 (0.67, 1.72) | 0.740 |
| SBP | 1.01 (1.01, 1.02) | <0.001 | 1.01 (1.01, 1.01) | <0.001 |
| DBP | 0.99 (0.99, 0.99) | <0.001 | 0.99 (0.99, 0.99) | <0.001 |
| Hypertension | ||||
| Elevated | 1.24 (1.15, 1.34) | <0.001 | / | |
| Stage 1 | 1.20 (1.12, 1.29) | <0.001 | / | |
| Stage 2 | 1.65 (1.54, 1.77) | <0.001 | / | |
| HbA 1c | 1.17 (1.14, 1.19) | <0.001 | 1.11 (1.09, 1.14) | <0.001 |
| TG | 1.21 (1.18, 1.24) | <0.001 | 1.32 (1.27, 1.38) | <0.001 |
| LDL-C | 0.96 (0.92, 0.99) | 0.016 | 1.03 (0.99, 1.07) | 0.152 |
| HDL-C | 0.51 (0.47, 0.55) | <0.001 | 0.61 (0.55, 0.68) | <0.001 |
Reference level for sex = female, education = no formal education, BMI category = normal, smoking = non-smoker, alcohol drinking = non-drinker, receiving CSSA = no, hypertension = normal, CHD = no, stroke = no, PAD = no, DR status = no.
aCrude OR estimated from the univariate logistic regression models.
bp-value of Wald test for individual factors.
cAdjusted OR estimated from the stepwise multivariate logistic regression model with all the above variables added except hypertension stages. The variable of alcohol drinking was eliminated from the final model during stepwise selection.
Sensitivity analysis
We did a sensitivity analysis by replacing the continuous variables SBP and DBP with hypertension stages, which gave similar effect estimates to the main analysis for all the covariates (Supplementary Table S2). The adjusted OR showed an increasing trend over hypertension stages, but no significant difference was found between elevated and normal stages. We also filled in the missing values of LDL-C by multiple imputation and the estimates were similar to those from main analysis (Supplementary Table S2).
Stratified analysis by gender
We compared the characteristics of subjects with or without CKD for men and women, respectively (Supplementary Table S3). Among the subjects with CKD, compared to men, women were older, living longer with DM, less likely obese or having other diabetic complications, with lower levels of UACR and eGFR. The gender difference in HbA1c, TG, LDL-C and HDL-C was small, although all reached statistical significance. Similar patterns were found in male and female subjects with CKD.
The interaction terms of gender with BMI categories, DR status and HbA1c were significant (data not shown); we therefore did a stratified analysis of female and male subjects. The effect estimates after adjustment were similar between female and male, except education, DBP, CHD and BMI categories (Supplementary Table S4). An inverse association between DBP and CKD, as well as a positive association between CHD and CKD, were found significant in men but not in women. PAD was associated with a higher rate of CKD, but only significant in women.
Discussion
We conducted a large-scale cross-sectional study in 31,574 participants with individual medical records in primary care setting, to identify the risk factors of CKD and potential gender differences. We found that the prevalence of micro- and macro-albuminuria in Chinese adults with T2DM was 23.6%, which is within the range of prevalence rates reported in a global cross sectional study (9.8–38.8%)16, and also comparable to the rates of 28.9% and 25.2% that were reported by two cross-sectional studies in mainland China6,7. The prevalence of CKD defined by both eGFR and albuminuria was 29.7% in our subjects, which is also close to the prevalence rate of 27.1% in another study of Chinese population7 but slightly lower than the rates (34.5–36.4%) in the U.S.17. It has been under debate that eGFR may not accurately reflect the real renal function of T2DM adults18. Our study found that the prevalence of CKD dramatically decreased if only eGFR or albuminuria criteria were applied (29.7% vs 11.6% and 23.6%). This highlights the need of incorporating routine urine albumin tests into the management program of persons with diabetes for early detection of CKD.
It is surprising that in our study men had a lower rate of CKD than women, which is contrary to the previous findings4. This could be explained by the fact that female subjects in our study were older and had a longer duration of DM than males. The stratified analysis identified some common risk factors of CKD shared by both female and male, and gender-specific factors such as DBP and CHD that were only significant in men, and PAD only in women. Gender difference has been well documented in the previous studies on ESRD and mortality in populations with diabetes19,20. Our findings further support that different prediction models shall be separately established for women and men.
The association of CHD and CKD has been widely demonstrated21. This association is particularly strong in persons with diabetes, as both are caused by accumulating damages to vascular systems during the disease progress22. We found that the CKD subjects were more likely to have CHD as compared to those without CKD. Similarly, several cross-sectional studies in Chinese adults with T2DM also demonstrated significant association of CHD with CKD23,24. However, we could not establish the temporal sequence of these adverse events due to the limitation of cross-sectional study design. In a cohort study of T2DM subjects in Hong Kong, CHD was associated with a higher risk of CKD25. It will be of great interest to conduct prospective cohort studies with the aim to explore the possibility of using CHD as an early predictor for CKD events or vice versa.
The results from the multivariate model showed that SBP was significantly associated with CKD, whereas an inverse association was found between DBP and CKD. This finding echoes a study that were conducted in mainland China with smaller sample size, which also found that SBP, retinopathy, TC, TG and anemia were risk factors of CKD7. In sensitivity analysis, we adopted the new AHA classification of hypertension and found that compared to subjects with normal BP, those at hypertension stage 1 and 2 had a significantly higher prevalence of CKD, but no significant difference was found between normal and elevated groups. This finding is consistent with one previous study in mainland China that found hypertension could significantly increase the risk of CKD5.
There are several limitations in this study. First, we used a cross-sectional study design, therefore could not establish the causal relationship between risk factors and CKD. Nevertheless, a large sample size still allowed us to have statistical power large enough to identify the significant risk factors for CKD. The findings of this study could also shed light on future cohort studies when more follow-up data become available. Second, it was uncertain that CKD in our subjects was diabetic nephropathy, since they might also have abnormal kidney structures or other diseases that deposited them to a higher risk of CKD. Third, in this study we used creatinine-based UACR estimates which have been criticized for lack of accuracy especially within the normal or high range of GFR10. Nevertheless, creatinine-based UACR is still widely adopted in current clinical practice. Fourth, this study population were the Chinese adults enrolled in NTWC; hence, the findings might not be applicable to all the Chinese populations. Last but not least, although we have adjusted for multiple variates in regression models, there were potential confounding factors that were not routinely collected in clinical practice still remained unadjusted.
In conclusion, we found a high prevalence rate of CKD in Chinese adults with T2DM in this large-scale cross-sectional study. We also identified several modifiable risk factors associated with CKD, including obesity, current smoking, higher SBP, lower DBP (only in men), dyslipidemia, hyperglycemia, the presence of diabetic retinopathy, stroke, PAD (only in women) and CHD (only in men). Given the increasing trend of T2DM prevalence and ageing population, the high-risk populations identified in this study shall receive regular screening for renal functions to achieve better patient management in primary care settings. Future studies on risk or beneficial factors of CKD incidence and disease progress could be conducted in this large patient cohort when more follow-up data become available, in order to achieve better prognosis for Chinese adults with diabetes.
Methods
Data sources
Individual data of people who were enrolled in the RAMP-DM from 2 July 2014 to 16 June 2017 were obtained from the New Territories West Cluster (NTWC) of the Hospital Authority (HA). The study period was chosen because of the good data completeness. This cluster serves the population of 1.09 million living in the northwest region of Hong Kong Special Administrative Region. Participation into RAMP-DM is voluntary. After 2014, over 90% of people with diabetes have been enrolled to RAMP-DM (Jun Liang, data not published). The details of RAMP-DM have been described elsewhere11,26. Briefly, individual data of demographics, education, lifestyle (smoking, alcohol and physical activity), family history, blood pressure, body mass index (BMI) and waist-hip ratio (WHR) were collected during their first visits to clinics. The participants were assessed for diabetic neurological, macrovascular and microvascular complications, coronary heart disease, stroke, malignancy and diabetic retinopathy. Fasting blood samples were taken during each visit to test for HbA1c, fasting glucose, total cholesterol, LDL-C, HDL-C, triglyceride (TG) and serum creatinine. Urine samples were also taken to test for albumin to calculate urine albumin to creatinine ratio (UACR), urine protein to creatinine ratio (PCR). The estimated glomerular filtration rate (eGFR) was calculated using the following formula27:
Individual data collected at their first RAMP-DM assessment were analyzed in this study. Additional laboratory data of eGFR, UACR and HbA1c during hospitalized episodes and outpatient visits were retrieved from the Clinical Data Analysis and Reporting System (CDARS) for the period of 180 days before to 180 days after the first RAMP-DM assessment, by matching the unique patient reference numbers. The laboratory data on the dates closest to the first RAMP-DM assessment were selected for subsequent analyses.
Chronic kidney diseases
The CKD diagnosis followed the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guideline28, which combines eGFR and UACR to define CKD. The eGFR stages include G1 normal and high (≥90 ml/min/1.73 m2), G2 mild reduction (60–89), G3a mild-moderate reduction (45–59), G3b moderate-severe reduction (30–44), G4 severe reduction (15–29) and G5 kidney failure (<15). The UACR stages are A1 normal to miLDL-Cy increased (<3 mg/mmol), A2 moderately increased (microalbuminuria) (3–30) and A3 severely increased (macroalbuminuria) (>30). CKD is defined as the eGFR stages of G3a through G5 and/or the UACR stages of A2 through A3.
Risk factors and covariates
Individual demographic data, such as age, gender, duration of T2DM, occupation, education and whether receiving Comprehensive Social Security Assistance (CSSA), were included in the model as potential risk factors. Smoking status was classified into current, ex- and non-smokers. Alcohol drinking was grouped into current, social, ex- and non-drinkers. Duration of DM was the number of years between the first RAMP-DM assessment date and the first year of DM diagnosis. Obesity status was categorized according to the Asian criteria: underweight as BMI < 18.5, normal as 18.5 ≤ BMI < 23, overweight as 23 ≤ BMI < 25, and obese as BMI ≥ 25. Individual data of blood pressure will be included in the model as continuously variables or alternatively as categorical variable indicating the level of hypertension status. Hypertension status of each subject was classified by systolic blood pressure (SBP) and diastolic blood pressure (DBP) that were measured at their first assessment in RAMP-DM, according to the 2017 Guidelines for High Blood Pressure in Adults from the American College of Cardiology/American Heart Association29. Patients are classified into four categories: normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated (120 mmHg ≤ SBP < 130 mmHg and DBP < 80 mmHg), stage 1 (130 mmHg ≤ SBP < 140 mmHg or 80 mmHg ≤ DBP < 90 mmHg), and stage 2 (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg). The diagnosed diabetic complications of coronary heart disease (CHD), stroke, peripheral arterial disease (PAD) were also retrieved for the first RAMP-DM assessment. Diabetic retinopathy was classified into four categories: (1) sight threatening retinopathy were those who had laser, vitrectomy or anti-vascular endothelia growth factor (anti-VEGF) therapy, or were classified as severe non-proliferative DR, proliferative DR or maculopathy); (2) non sight threatening retinopathy were those who are classified as mild/moderate non-proliferative DR, and no maculopathy and no laser for both eyes; (3) No DR are those who were classified as no-DR without any maculopathy, laser, vitrectomy or anti-VEGF therapy; (4) Ungradable were those with at least one eye classified as “Not known” grade of DR, maculopathy and laser, and not classified as severe pre-proliferative DR, proliferative DR, and also without vitrectomy nor anti-VEGF therapy. We also retrieved lipid profiles of TG, HDL-C and LDL-C cholesterol measured at the first RAMP-DM assessment for all the subjects. Individual records of HbA1c, eGFR and UACR were retrieved from the electronic health records of CDARS by matching the unique patient reference numbers.
Statistical analysis
The participants with and without CKD were compared for their demographic and clinical characteristics using Chi-square test for categorical variables and t-test for continuous variables (normally distributed) or Mann-Whitney (not normally distributed). Each variable was assessed for proportion of missing data, and the subjects with complete and incomplete data were compared for other variables, in order to determine whether data imputation was necessary. Univariate logistic regressions were performed to identify the potential covariates and multivariate logistic regression to estimate the effects after adjustment for covariates. We fit a stepwise multivariate logistic regression model, which adjusted for sex, age, DM duration, education, BMI, smoking, alcohol drinking, receiving CSSA, CHD, stroke, PAD, DR status, HbA1c, TG, LDL-C, HDL-C, and both SBP and DBP as continuous variables. Only alcohol drinking was eliminated from the final multivariate model. Adjusted odd ratios (OR) and the corresponding 95% confidence interval (CI) were calculated for the variables in the regression model. The product variables of gender with other significant covariates were respectively added into interaction models to assess the gender difference. If any significance was found in these interaction terms, stratified analysis by gender was also conducted to estimate the age-specific effect estimates. Statistical analysis was conducted by using R version 3.4.2 (The R Foundation for Statistical Computing). A P-value less than 0.05 was considered as statistically significant.
Sensitivity analysis
We replaced the linear covariates of SBP and DBP by hypertension categories defined according to the new AHA classification of hypertension29. We did another sensitivity analysis by filling the missing values of LDL-C with multiple imputation on age, sex, DM duration, BMI, HDL-C, TG and comorbidity, and the above multivariate regression was repeated using the new dataset.
Ethical approval and informed consent
The ethical approval has been obtained from the New Territory West Cluster Clinical and Research Ethics Committee. All research was performed in accordance with relevant guidelines. Consent forms were exempted because all the data were extracted from the computerized data system of Hospital Authority, and no personal data were collected for this study.
Electronic supplementary material
Acknowledgements
We thank Mr. Jason So for helpful discussions on data analysis.
Author Contributions
L.Y. and J.Y. designed the study; T.K.C. and C.W.L. collected the data; P.K.L. conducted the data analysis; J.L., L.Y., J.X.L. and H.R.N. interpreted the results and drafted the manuscript; all the authors finalized the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the Hospital Authority of Hong Kong Special Administrative Region, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Hospital Authority of Hong Kong Special Administrative Region.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32983-1.
References
- 1.WHO. Global reports on diabetes. (2016).
- 2.Federation, I. D. IDF Diabetes Atlas. 8th edn (2017).
- 3.Saran R, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69:S1–S688. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. Journal of Nephropharmacology. 2015;5:49–56. [PMC free article] [PubMed] [Google Scholar]
- 5.Jia W, et al. Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Shanghai diabetic complications study (SHDCS) Nephrology Dialysis Transplantation. 2009;24:3724–3731. doi: 10.1093/ndt/gfp349. [DOI] [PubMed] [Google Scholar]
- 6.Lou Q-L, et al. Chronic Kidney Disease and Associated Cardiovascular Risk Factors in Chinese with Type 2 Diabetes. Diabetes Metabolism Journal. 2012;36:433–442. doi: 10.4093/dmj.2012.36.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo K, et al. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. Journal of Diabetes and Its Complications. 2016;30:803–810. doi: 10.1016/j.jdiacomp.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Luk AOY, et al. The Clinical Utility of SUDOSCAN in Chronic Kidney Disease in Chinese Patients with Type 2 Diabetes. PLoS ONE. 2015;10:e0134981. doi: 10.1371/journal.pone.0134981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerums G, Panagiotopoulos S, Premaratne E, MacIsaac RJ. Integrating albuminuria and GFR in the assessment of diabetic nephropathy. (glomerular filtration rate) (Report) Nature Reviews Nephrology. 2009;5:397–406. doi: 10.1038/nrneph.2009.91. [DOI] [PubMed] [Google Scholar]
- 11.Fung CS, et al. Evaluation of the quality of care of a multi-disciplinary risk factor assessment and management programme (RAMP) for diabetic patients. BMC Fam Pract. 2012;13:116–124. doi: 10.1186/1471-2296-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao FF, et al. Effects of the Multidisciplinary Risk Assessment and Management Program for Patients with Diabetes Mellitus (RAMP-DM) on biomedical outcomes, observed cardiovascular events and cardiovascular risks in primary care: a longitudinal comparative study. Cardiovascular diabetology. 2014;13:127–136. doi: 10.1186/s12933-014-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan EY, et al. Effectiveness of a multidisciplinary risk assessment and management programme-diabetes mellitus (RAMP-DM) on patient-reported outcomes. Endocrine. 2017;55:416–426. doi: 10.1007/s12020-016-1124-1. [DOI] [PubMed] [Google Scholar]
- 14.Yang XL, et al. End-stage renal disease risk equations for Hong Kong Chinese patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetologia. 2006;49:2299–2308. doi: 10.1007/s00125-006-0376-3. [DOI] [PubMed] [Google Scholar]
- 15.Wan EYF, et al. Prediction of new onset of end stage renal disease in Chinese patients with type 2 diabetes mellitus - a population-based retrospective cohort study. BMC Nephrology. 2017;18:257–265. doi: 10.1186/s12882-017-0671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parving HH, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 17.de Boer IH, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIsaac RJ, et al. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of Glomerular Filtration Rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol. 2015;16:198–210. doi: 10.1186/s12882-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitsch D., Grams M., Sang Y., Black C., Cirillo M., Djurdjev O., Iseki K., Jassal S. K., Kimm H., Kronenberg F., Oien C. M., Levey A. S., Levin A., Woodward M., Hemmelgarn B. R. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346(jan29 1):f324–f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan EYF, Fong DYT, Fung CSC, Lam CLK. Incidence and predictors for cardiovascular disease in Chinese patients with type 2 diabetes mellitus – a population-based retrospective cohort study. Journal of Diabetes and Its Complications. 2016;30:444–450. doi: 10.1016/j.jdiacomp.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Sarnak MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease - A statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.Cir.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 22.Anavekar NS, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. New Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh M-C, et al. Chronic Kidney Disease as a Risk Factor for Coronary Artery Disease in Chinese with Type 2 Diabetes. American Journal of Nephrology. 2008;28:317–323. doi: 10.1159/000111388. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, et al. Association of Chronic Kidney Disease with Coronary Heart Disease and Stroke Risks in Patients with Type 2 Diabetes Mellitus: An Observational Cross-sectional Study in Hangzhou, China. Chinese Medical Journal. 2017;130:57–63. doi: 10.4103/0366-6999.196564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong PCY, et al. Hematocrit, Independent of Chronic Kidney Disease, Predicts Adverse Cardiovascular Outcomes in Chinese Patients With Type 2 Diabetes. Hematocrit, Independent of Chronic Kidney Disease, Predicts Adverse Cardiovascular Outcomes in Chinese Patients With Type 2 Diabetes. 2006;29:2439–2444. doi: 10.2337/dc06-0887. [DOI] [PubMed] [Google Scholar]
- 26.Luk AOY, et al. Declining Trends of Cardiovascular-Renal Complications and Mortality in Type 2 Diabetes: The Hong Kong Diabetes Database. Diabetes care. 2017;40:928–932. doi: 10.2337/dc16-2354. [DOI] [PubMed] [Google Scholar]
- 27.Ma YC, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 28.(KDIGO), K. D. I. G. O. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International3, 1–150 (2013). [DOI] [PubMed]
- 29.Whelton Paul K., Carey Robert M., Aronow Wilbert S., Casey Donald E., Collins Karen J., Dennison Himmelfarb Cheryl, DePalma Sondra M., Gidding Samuel, Jamerson Kenneth A., Jones Daniel W., MacLaughlin Eric J., Muntner Paul, Ovbiagele Bruce, Smith Sidney C., Spencer Crystal C., Stafford Randall S., Taler Sandra J., Thomas Randal J., Williams Kim A., Williamson Jeff D., Wright Jackson T. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. Journal of the American College of Cardiology. 2018;71(19):2199–2269. doi: 10.1016/j.jacc.2017.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Hospital Authority of Hong Kong Special Administrative Region, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Hospital Authority of Hong Kong Special Administrative Region.