Abstract
In the northeastern United States, chronic wasting disease has recently been detected in white-tailed deer (Odocoileus virginianus) populations, and understanding the relationship between landscape configuration and home range may improve disease surveillance and containment efforts. The objectives of our study were to compare size of home range for deer occupying a continuum of forested landscapes and to investigate relationships between size of home range and measures of landscape configuration. We used a movement-based kernel density estimator to estimate home range at five spatial scales among deer across study areas. We developed 7 linear regression models that used measures of the configuration of the forested landscape to explain size of home range. We observed differences in size of home range between sexes among areas that differed based on landscape configuration. We documented size of home range changed with various metrics that identifying connectivity of forested patches. Generally, size of home range increased with an increasing proportion of homogenous forest. Our results suggest that deer in our region occupy a landscape at hierarchically-nested scales that is controlled by the connectivity of the forested landscape across local or broad geographical regions.
Introduction
Use of Global Positioning System (GPS) technology for tracking wildlife has resulted in estimators of home range that account for large numbers of locations and animal-specific data (e.g., habitat use, duration between locations) that was not possible with traditional estimators1–3. Traditional estimators of home range used outer-boundary polygons, kernel densities, or relatively sparse data collected using very high frequency (VHF) telemetry that only estimated the utilization distribution with location-based parameters (i.e., no temporal component included). In comparison, the movement-based kernel density estimator has the advantage of incorporating temporal components and habitat-specific movement vectors available with GPS technology4. Movement-based kernel density estimators (hereafter referred to as MKDE) incorporate serially correlated locations, duration between locations, positional error of GPS technology, and habitat to provide estimates of home range that better account for movements that relate to the landscape4. Applied use of MKDE to estimate home range can provide more refined shapes and scales of home range for analysis of landscape configuration and complexity in mammals1,5.
Spatial processes, including spread of disease, are affected by the scale at which deer establish home range and this can be related to landscape6–8. Understanding how landscape heterogeneity influences space use by cervids can assist wildlife managers with alleviating issues that include forest regeneration, crop damage, and disease transmission9–11. For example, the spatial distribution of chronic wasting disease was related to features of the relatively open landscapes in the West and Midwest in mule deer (Odocoileus hemionus)12–14 but has only recently been investigated in a predominantly forested ecosystem for white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease8. Furthermore, it is likely that the spatial distribution of chronic wasting disease throughout North America is related to both movements of infected species and landscapes that relate to these movements. Therefore, understanding the configuration and complexity of landscapes at which deer establish home range can provide a basis for disease surveillance and containment.
To address the spatial complexities of disease transmission, spatial processes can be influenced by scale of study area and demographic composition of the species studied and must be understood7,15,16. These spatial processes, however, are rarely assessed in predominately non-migratory species occupying restricted geographic range or with limited dispersal/migratory distances17–20. Studies assessing spatial scale have routinely documented the influence, or lack thereof, for landscape features (e.g., roads, rivers, and forest cover) or demographic composition of the population sampled (e.g., sex, age) to influence size of home range for various cervid species7,21,22. The foundation for previous assessments of spatial heterogeneity and how they relate to size of home range, however, is based on primarily VHF datasets utilizing arbitrarily-defined buffer sizes or estimators of home range that are no longer viable with GPS datasets1,2.
Understanding the relationship between use of heterogenous landscapes by cervids and spatial distribution of disease is related to the configuration and complexity of landscapes at which home ranges are established and can provide insights to address disease surveillance and containment. Because there has been no standardized method to assess this relationship, we propose the use of a relatively new estimator of home range on a species that exhibits various movement behaviors and seasonal shifts over a limited geographic range. Our specific objectives were to (1) compare size of home range between sexes and among study areas for white-tailed deer occupying a continuum of forested landscapes from fragmented to homogenous, (2) investigate relationships between size of home range and measures of landscape composition and configuration, and (3) determine differences in this relationship across spatial scales as determined from percentages of utilization distributions as opposed to arbitrarily-defined buffers or estimators.
Study Area
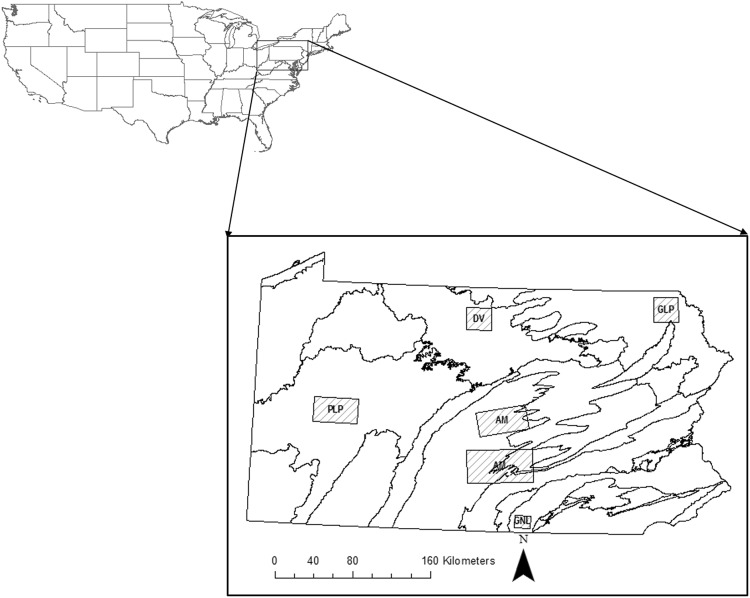
We estimated size of home range for white-tailed deer in distinct geographical areas that represented a physiographic province in Pennsylvania, USA (Fig. 1)23,24. Each area represented a continuum of forested landscapes ranging from fragmented to homogeneous (Table 1; Fig. 2). Gettysburg-Newark Lowland was located in the Gettysburg National Military Park in central Adams County and elevation ranged from 87 m to 236 m. Pasture and cropland were dominant classes of land cover throughout the area with a largest patch size of 627 m2 (Table 1). Forest cover was sparse (22%) and fragmented in this area due to the dominant presence of anthropogenically-modified habitats. The Pittsburgh- and Glaciated-Low Plateaus were located in the western and northeastern region of the state, respectively (Table 1). These areas represented rural and moderately fragmented landscapes where open (e.g., pasture, cropland) and forested classes created a mosaic with a largest patch size of 929 m2 and elevation ranging from 225 m to 819 m (Table 1). The Appalachian Mountain study areas were located in central Pennsylvania and were characterized by contiguous forests along ridgelines and contiguous distributions of pasture and cropland in valleys with a largest patch size of 779 m2 (Table 1). Elevation in these areas ranged from 111 m to 737 m. The Deep Valley area was located in the north-central region of the state in homogeneous northern hardwood forest that represented the dominant class of land cover with a largest patch size of 2,657 m2 (Table 1). Although sparse, other classes (e.g., pasture) were present and elevation ranged from 406 m to 785 m.
Figure 1.
General location of study areas used to assess the relationship between heterogeneity of landscape and size of home range for white-tailed deer (Odocoileus virginianus) from 2009 to 2015 in Pennsylvania, USA. Cross stitched polygons represent physiographic province of study areas that included: Gettsyburg-Newark Lowland (GN), Pittburgh Low Plateau (PLP), Glaciated Low Plateau (GLP), Appalachian Mountains (AM), and Deep Valleys (DV). Generated with ArcMap 10.2, www.esri.com.
Table 1.
Forest type, physiographic provincea, and proportions of 3 classes of land cover summarized within the extent of each study area used in analysis of home range for white-tailed deer (Odocoileus virginianus) from 2009 to 2015 in Pennsylvania, USA.
| Forest type | Physiographic province | Developed | Forested | Open |
|---|---|---|---|---|
| Appalachian oak | Gettysburg-Newark Lowland | 0.162 (62) | 0.221 (84) | 0.617 (639) |
| Appalachian oak | Pittsburgh Low Plateau | 0.100 (33) | 0.623 (390) | 0.277 (125) |
| Northern hardwoods | Glaciated Low Plateau | 0.037 (17) | 0.717 (929) | 0.246 (143) |
| Appalachian oak | Appalachian Mountain | 0.064 (27) | 0.723 (660) | 0.213 (252) |
| Appalachian oak | Appalachian Mountain | 0.073 (21) | 0.676 (779) | 0.251 (366) |
| Northern hardwoods | Deep Valleys | 0.018 (15) | 0.893 (2657) | 0.089 (110) |
The average land cover patch area is in parenthesis next to each land cover type.
aBureau of Topographic and Geologic Survey, Commonwealth of Pennsylvania Department of Conservation and Natural Resources.
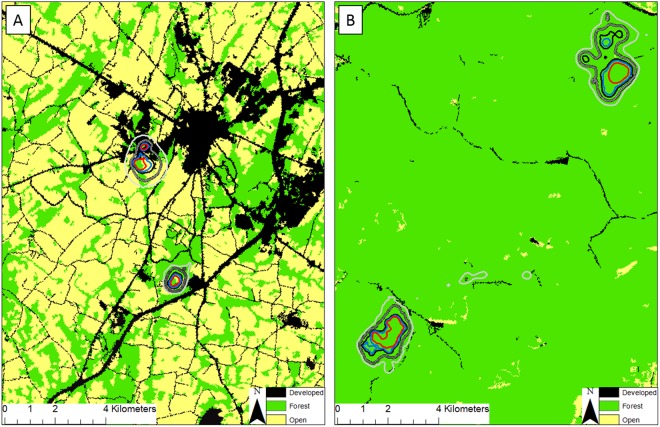
Figure 2.
Habitat categories with isopleth polygons to assess the relationship between heterogeneity of landscape and size of home range for white-tailed deer (Odocoileus virginianus) from 2009 to 2015 in (A) Gettsyburg-Newark Lowland and (B) Deep Valleys in Pennsylvania, USA. Polygons reflects isopleths of home range at 50% (red), 70% (blue), 80% (black), 95% (black-gray), 99% (gray). Generated with ArcMap 10.2, www.esri.com.
Results
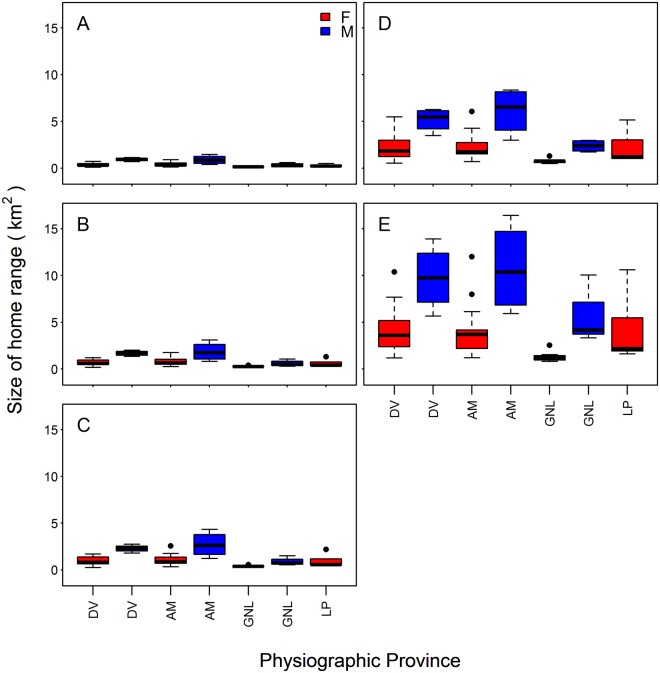
Size of 95% home range for females ranged from 0.50 km2 in the Gettysburg-Newark Lowland to 5.49 km2 in the Deep Valleys (Fig. 3). Size of 95% home range for males ranged from 1.73 km2 in the Gettysburg-Newark Lowland to 8.36 km2 in the Low Plateau regions (Fig. 3). Males established mean 95% home ranges (4.55 km2) that were more than twice the size of mean home ranges established by females (1.92 km2) across all study areas (F1,54 = 32.15, P < 0.001). We observed no sex-area interaction (P = 0.939) but we observed differences in mean 95% home range in Gettysburg-Newark Lowland (1.26 km2) and Deep Valleys (2.79 km2; P < 0.001; Fig. 3). Mean size of 99% home range also varied between males (8.66 km2) and females (3.63 km2; F1,54 = 30.12, P < 0.001). We observed no sex-area interaction (P = 0.599) but we observed differences in mean 99% home range in Gettysburg-Newark Lowland (2.57 km2) and Deep Valleys (5.30 km2; P < 0.001).
Figure 3.
Boxplot of mean size of home range (km2) by sex and habitat heterogeneity for white-tailed deer (Odocoileus virginianus) from 2009 to 2015 in Pennsylvania, USA for (A) 50%, (B) 70%, (C) 80%, (D) 95%, and (E) 99% isopleths representing spatial scales of study. Mean size (±SD) was by physiographic provinces: Gettysburg-Newark Lowland (GNL), Pittsburgh and Glaciated Low Plateau (LP), Appalachian Mountain (AM), and Deep Valley (DV). Generated with Rstudio (version 1.0.153, www.rstudio.com).
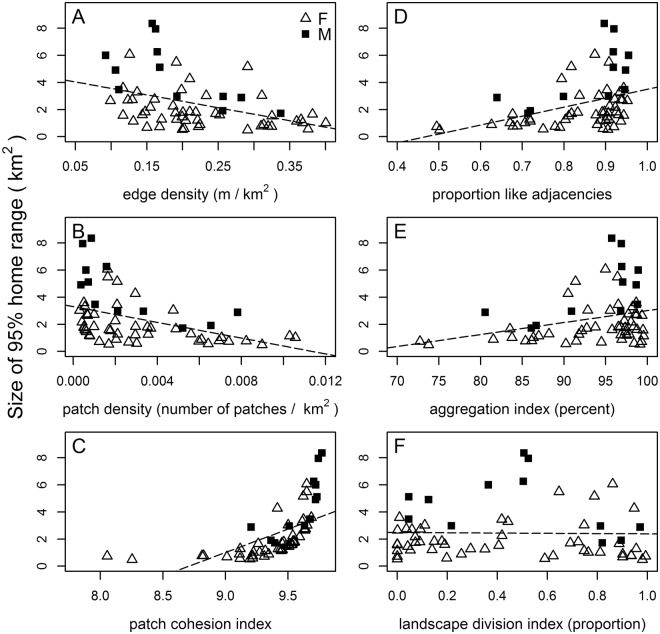
Increased levels of edge density and patch density were indicative of more fragmented landscapes across our study areas and corresponded with a decrease in size of home range (Fig. 4A,B). Patch cohesion index, proportion of like adjacencies, and aggregation index were high across our study sites indicating considerable connectivity of homogeneous forested areas. An increase in these three aforementioned metrics corresponded with an increase in size of home range (Fig. 4C–E). Landscape division index varied across our study areas with values closer to zero reflecting landscapes consisting of a single patch compared to values closer to one indicating a decrease in forest and forest patch size. Size of home range exhibited no relationship with landscape division index across our study areas suggesting this metric may not be suitable for describing size of home range (Fig. 4F).
Figure 4.
Relationship between size of 95% home range (km2) by sex of white-tailed deer (Odocoileus virginianus) from 2009 to 2015 in Pennsylvania, USA and various area, edge and aggregation metrics: (A) edge density, (B) patch density, (C) patch cohesion index, (D) proportion like adjacencies, (E) aggregation index, and (F) landscape division index. Generated with Rstudio (version 1.0.153, www.rstudio.com).
The model containing patch cohesion index was the most supported model of our landscape metrics and size of home range at all spatial scales. This model accounted for all the AICc weights regardless of spatial scale of analysis with no other models within 2.0 ΔAIC from the top model. Parameter estimates were 0.677 and 2.028 at our smallest (50% isopleth) and largest (99% isopleth) scales, respectively. Patch cohesion index was positively related to size of home range (Fig. 4C).
Discussion
Although our samples size was limited for males, we expected size of home range to vary between sexes, given associations of larger home range with males, as well as increased movements by males for dispersal, migration, and breeding25–28. Larger home range for males in our study area was consistent with findings in similar studies on white-tailed deer that identified several factors that influenced differences in size between sexes, including increased nocturnal movement by males during the breeding season (e.g., October–November), decreased diurnal movement by females during the growing season (e.g., June–July), and higher site fidelity by females across all seasons26,29,30. Increased rates and distances of dispersal were observed for males in the Northeast and Midwest27,28 and supported our findings of larger home range and greater daily distance traveled by males than females in each study area, regardless of habitat configuration, in which both sexes were included for analysis8.
Home ranges for males and females were smaller in the more fragmented landscapes (e.g. high edge density, low proportion like adjacencies) than home ranges in all other study areas. Dechen-Quinn et al.19 observed smaller home ranges in more fragmented areas for female white-tailed deer. It is possible that home ranges that were smaller in more fragmented areas of our study were related to the landscape comprised of small woodlots and high intensities of open and developed classes that may provide forage for white-tailed deer11,19,31. One of our study areas more closely resembled an agricultural landscape than any other study area, and size of annual home range for females in this area (0.63 km2) were comparable to mean annual home ranges for female white-tailed deer in agricultural landscapes of the Midwest (0.99–1.47 km2)15 but less than in an agricultural-forested landscape in the Northeast (2.03 km2; Fig. 2)19. In areas where open and developed classes were as prominent as they were in Gettysburg-Newark Lowland, deer likely were able to obtain suitable forage without being required to traverse large distances19,32.
Deer located in Deep Valleys study area established home ranges that were largest of all deer in our analysis. Areas that were predominantly forested may be viewed as less productive, because deer that rely on food sources (e.g., mast and browse) that can vary on a seasonal or annual basis may be required to establish home ranges that are larger to ensure access to sufficient resources10,33,34. In areas of Virginia that were predominantly forested, mast can comprise >76% of the diet of deer during fall and winter months35. Female deer in Virginia also expanded seasonal home ranges into oak (Quercus spp.) stands during years in which production of mast exceeded 300 kg/ha, whereas size of home range remained unchanged during years of poor mast production (<100 kg/ha)33. Production in these areas of Virginia varied greatly from year to year (e.g., 396 kg/ha to 3 kg/ha) and suggested that shifts in home range by deer in predominantly forested landscapes, that are similar to our study regions, are in response to availability of forage.
In some study areas, changing the spatial scale from 50% to 99% encompassed greater amounts of edge and other patches that were occupied less often by deer. Conversely, changing the scale in areas with larger forested patches encompassed only greater amounts of forest rather than other classes of land cover in the homogeneously forested landscape in the Deep Valleys. Our model selection results, however, remained the same regardless of the scale that was used. These findings were inconsistent with findings in female mule deer in the West and white-tailed deer in the Northeast, where measures of landscape that influenced size of home range changed as the scale of analysis was increased from 250 m to 5,000 m from the centroid location of each home range30,36. In a similar study of female white-tailed deer in the Midwest, measures of landscape that influenced size of home range changed as the spatial scale was modified15. Although changing spatial scale yielded similar results in our study, we are the first to report on spatial scales as determined by home ranges estimated using MKDE with GPS data in North America. The inherent nature of MKDE as a more refined estimator of home range (i.e., contours that follow the shape of non-stationary location data) compared to traditional KDE1 requires further evaluation to determine if our selection of estimator influenced similarities in spatial scale and if this would be similar for other cervid species.
A caveat of our study was that we were unable to assess deer densities across our study areas. High deer densities in Gettysburg-Newark Lowland (>40 deer/km2)37 were linked to crop damage11, and similar densities (47–51 deer/km2) were identified as the cause of forest regeneration issues on a predominantly forested landscape in Connecticut38. Deer in high-density areas also have been shown to exhibit greater site fidelity, especially during winter months39. Reduction in deer density appears to have varying effects on expansion of home range. Seasonal home range expanded by 30% in a developed area in South Carolina following a 50% reduction in herd size40, however, home ranges remained unchanged in Connecticut following reduction of the deer herd from 88 to 17 deer/km2)41. Although assessments of home range expansion in response to herd reduction were not feasible for our study, lower densities (~18 deer/km2)42 in our Deep Valleys area identified by the largest tracts of homogeneous forest (Table 1) may require males to traverse greater distances in search of females during the breeding season. Therefore, landscape is one of many factors that likely play a role in differences in size of home range between more fragmented and homogeneously forested landscapes.
Relatedly, spread of disease likely is related to the scale at which deer establish home range, and our findings show that this scale (i.e., size of home range) varies considerably between more fragmented versus homogeneously forested landscapes. Disease surveillance efforts in more fragmented areas could be concentrated locally to reflect concentrated movements and higher contact rates between social groups43,44, or conversely, surveillance in homogeneously forested areas could be conducted at a broader scale to reflect home ranges that are more expansive and dispersed due to limitations in foraging and breeding opportunities. Regardless of impetus for research, variability in size of home range, dispersal, and movements have been documented between fragmented, heterogeneous landscape in comparison to more homogenous landscapes for a variety of cervids16,36,45. Although methods to determine spatial scale varies considerably depending on objectives or researcher preferences, relationships of smaller home ranges within more heterogeneous and fragmented landscapes appears consistent across cervid species. Further evaluation of methodologies to measure metrics for consistency across studies and species at the landscape-scale (e.g., buffered circles) and local scale (e.g., home ranges isopleths) appears warranted.
Methods
Size of Home range
We captured and equipped 61 white-tailed deer with GPS collars across the study areas for various projects on white-tailed deer movements and survival between 2009 and 201517,37,46. All GPS collars had accuracy <10 m and fix success rates of >96% based on manufacturer recommendations (Supplemental Table 1). We captured deer using a combination of rocket nets47, single-gate Clover traps48, and drop nets (modified from49). Only adult deer (>2 years of age) were included in our analysis because dispersal occurs between 1 and 2 years of age in both sexes17,50 and these populations lack migratory movements to winter deer yards or similar habitat shifts19. All capture and handling methods were in accordance with protocols approved by the Pennsylvania State University Institutional Animal Care and Use Committee (IACUC No. 29677 and 34910) and within guidelines of the American Society of Mammalogists51. We estimated mean home range for each deer using the adehabitatHR package in program R (52,53 R Foundation for Statistical Computing, Vienna, Austria).
We incorporated duration of time between recorded locations, a minimum distance of 30 m that needed to be traveled between consecutive locations to be considered active, and landscape-specific diffusion coefficients into MKDE to estimate 50%, 70%, 80%, 95%, and 99% isopleths of annual home range for each deer (Supplemental Table 2). We defined annual as late winter of one year through late winter of the following year with no overlapping dates (e.g., 1 February–31 January) because dates of capture varied for each deer. We grouped deer according to sex and physiographic province and used a two-way ANOVA with a confidence level of 95% (α = 0.05) to assess differences in size of home range between sexes and landscape with post-hoc differences determined by Tukey’s multiple comparison test.
Land cover reclassification
We reclassified the 2011 National Land Cover Database with 30 m resolution into 5 classes: developed, forested, open, water, and wetland54. Prominent water sources and wetlands were present in only 2 study areas and represented less than 1% of the landscape in home ranges of 5 deer in these areas. Therefore we did not consider these 2 classes in our analysis and reclassified water as open (e.g., pastures, grasslands and croplands) and wetland as forested, given the association of woody wetlands with forest vegetation. The developed class contained roads and all intensities of development, including urban, suburban, and exurban. We extracted the forested class from the data for analysis because forested habitat would be the most influential given the association of forest with movement and disease epidemiology in white-tailed deer in the Northeast and Midwest28,50,55.
Landscape metrics
We calculated metrics of landscape configuration and connectivity for the forested class of land cover for each deer within 5 isopleths or spatial scales (50%, 70%, 80%, 95% and 99% isopleths of home range) using the SDMTools package in program R. We calculated 6 metrics for forest class of land cover across a variety of landscapes that reflected edges and aggregation of like habitats known to influence size and shape of home ranges based on previous research linking landscape heterogeneity to size of home range for white-tailed deer, red deer (Cervus elaphus) and roe deer (Capreolus capreolus)15,16,19. Our analysis included edge density (m/ha) because white-tailed deer are known to respond to edge habitats. Our analysis also included five aggregation metrics: patch density (number of patches/km2), patch cohesion index (measure of physical connectivity of forest), proportion of like adjacencies (measures the degree of aggregation of patch types accounting for patch size and shape), aggregation index (area-weighted mean class aggregation index), and landscape division index (probability that two randomly chosen pixels are not in the same patch). We selected these covariates due to their relationships with definitions of spatial heterogeneity and landscape configuration and connectivity56,57. We also limited our analysis to aggregation metrics of only the forest class because white-tailed deer are known to respond to amount and configuration of forested landscapes in the eastern and Midwestern U.S. and because we were comparing landscapes with varying extents of configuration and connectivity15,19,50.
Statistical analysis
We created 7 linear models a priori with the six covariates as independent variables and natural log of home range as the response variable for each spatial scale along with an intercept only model. We used covariates that corresponded to each spatial scale to determine if changing the scale of analysis from 50% to 99% would influence model selection results. We selected home range contours15,19 over buffered circles due to the arbitrary nature of defining radii of buffered circles in previous research30,36,45. We used Akaike’s Information Criterion with correction for small sample size to evaluate the set of models at each spatial scale (AICc)58.
Electronic supplementary material
Acknowledgements
We would like to thank the many field technicians who captured deer, and private landowners who allowed us to trap on their property. We would like to thank 2 anonymous reviewers for their considerable effort and the thoughtfulness of their review of this manuscript. Funding for this research was provided by the National Park Service and Pennsylvania Game Commission. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author Contributions
D.R.D., C.S.R. and W.D.W. conceived and designed the study. D.S., B.D.W. conducted field data collection and monitoring of animals. T.S.E. and W.D.W. performed data management. All authors contributed to analysis of results, writing the manuscript, and approval of the submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32937-7.
References
- 1.Walter WD, Onorato DP, Fischer JW. Is there a single best estimator? Selection of home range estimators using area-under-the-curve. Movement Ecology. 2015;3:10. doi: 10.1186/s40462-015-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemson G, et al. Are kernels the mustard? Data from global positioning systems (GPS) collars suggests problems for kernel home-range analyses with least-squares cross-validation. Journal of Animal Ecology. 2005;74:455–463. doi: 10.1111/j.1365-2656.2005.00944.x. [DOI] [Google Scholar]
- 3.Wells AG, et al. The Brownian bridge synoptic model of habitat selection and space use for animals using GPS telemetry data. Ecological Modelling. 2014;273:242–250. doi: 10.1016/j.ecolmodel.2013.11.008. [DOI] [Google Scholar]
- 4.Benhamou S, Cornelis D. Incorporating movement behavior and barriers to improve kernel home range space use estimates. Journal of Wildlife Management. 2010;74:1353–1360. doi: 10.1111/j.1937-2817.2010.tb01257.x. [DOI] [Google Scholar]
- 5.Benhamou S. Dynamic approach to space and habitat use based on biased random bridges. PLoS ONE. 2011;6:e14592. doi: 10.1371/journal.pone.0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borger L, Dalziel BD, Fryxell JM. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecology Letters. 2008;11:637–650. doi: 10.1111/j.1461-0248.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 7.Morellet N, et al. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. Journal of Animal Ecology. 2013;82:1326–1339. doi: 10.1111/1365-2656.12105. [DOI] [PubMed] [Google Scholar]
- 8.Evans TS, Kirchgessnerr MS, Eyler B, Ryan CW, Walter WD. Habitat influences distribution of chronic wasting disease in white-tailed deer. Journal of Wildlife Management. 2016;80:284–291. doi: 10.1002/jwmg.1004. [DOI] [Google Scholar]
- 9.Conner MM, Miller MW. Movement patterns and spatial epidemiology of a prion disease in mule deer population units. Ecological Applications. 2004;14:1870–1881. doi: 10.1890/03-5309. [DOI] [Google Scholar]
- 10.Alverson WS, Waller DM, Solheim SL. Forests Too Deer: Edge Effects in Northern Wisconsin. Conservation Biology. 1988;2:348–358. doi: 10.1111/j.1523-1739.1988.tb00199.x. [DOI] [Google Scholar]
- 11.Vecellio GM, Yahner RH, Storm GL. Crop damage by deer at Gettysburg park. Wildlife Society Bulletin. 1994;22:89–93. [Google Scholar]
- 12.Farnsworth ML, Hoeting JA, Hobbs NT, Miller MW. Linking chronic wasting disease to mule deer movement scales: a hierarchical bayesian approach. Ecological Applications. 2006;16:1026–1036. doi: 10.1890/1051-0761(2006)016[1026:LCWDTM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Walter WD, Walsh DP, Farnsworth ML, Winkelman DL, Miller MW. Soil clay content underlies prion infection odds. Nature Communications. 2011;2:1–6. doi: 10.1038/ncomms1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storm DJ, et al. Deer density and disease prevalence influence transmission of chronic wasting disease in white-tailed deer. Ecosphere. 2013;4:art10. doi: 10.1890/ES12-00141.1. [DOI] [Google Scholar]
- 15.Walter WD, et al. Regional assessment on influence of landscape configuration and connectivity on range size of white-tailed deer. Landscape Ecology. 2009;24:1405–1420. doi: 10.1007/s10980-009-9374-4. [DOI] [Google Scholar]
- 16.Bevanda M, Fronhofer EA, Heurich M, Müller J, Reineking B. Landscape configuration is a major determinant of home range size variation. Ecosphere. 2015;6:1–12. doi: 10.1890/ES15-00154.1. [DOI] [Google Scholar]
- 17.Lutz CL, Diefenbach DR, Rosenberry CS. Population density influences dispersal in female white-tailed deer. Journal of Mammalogy. 2015;96:494–501. doi: 10.1093/jmamma/gyv054. [DOI] [Google Scholar]
- 18.Nicholson MC, Bowyer RT, Kie JG. Habitat selection and survival of mule deer: tradeoffs associated with migration. Journal of Mammalogy. 1997;78:483–504. doi: 10.2307/1382900. [DOI] [Google Scholar]
- 19.Dechen-Quinn AC, Williams DM, Porter WF. Landscape structure influences space use by white-tailed deer. Journal of Mammalogy. 2013;94:398–407. doi: 10.1644/11-MAMM-A-221.1. [DOI] [Google Scholar]
- 20.Lovari S, Serrao G, Mori E. Woodland features determining home range size of roe deer. Behavioural Processes. 2017;140:115–120. doi: 10.1016/j.beproc.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Borger L, et al. An integrated approach to identify spatiotemporal and Individual-level determinants of animal home range size. The American Naturalist. 2006;168:471–485. doi: 10.1086/507883. [DOI] [PubMed] [Google Scholar]
- 22.van Beest FM, Rivrud IM, Loe LE, Milner JM, Mysterud A. What determines variation in home range size across spatiotemporal scales in a large browsing herbivore? Journal of Animal Ecology. 2011;80:771–785. doi: 10.1111/j.1365-2656.2011.01829.x. [DOI] [PubMed] [Google Scholar]
- 23.Omernik JM, Griffith GE. Ecoregions of the Conterminous United States: Evolution of a Hierarchical Spatial Framework. Environmental Management. 2014;54:1249–1266. doi: 10.1007/s00267-014-0364-1. [DOI] [PubMed] [Google Scholar]
- 24.Vogelman JE, Sohl T, Howard SM. Regional characterization of landcover using multiple sources of data. Photogrammetric Engineering and Remote Sensing. 1998;64:45–57. [Google Scholar]
- 25.Long ES, Diefenbach DR, Wallingford BD, Rosenberry CS. Influence of roads, rivers, and mountains on natal dispersal of white-tailed deer. Journal of Wildlife Management. 2010;74:1242–1249. doi: 10.1111/j.1937-2817.2010.tb01244.x. [DOI] [Google Scholar]
- 26.Beier P, McCullough DR. Factors influencing white-tailed deer activity patterns and habitat use. Wildlife Monographs. 1990;109:1–51. [Google Scholar]
- 27.Long ES, Diefenbach DR, Rosenberry CS, Wallingford BD. Multiple proximal and ultimate causes of natal dispersal in male white-tailed deer. Behavioral Ecology. 2008;19:1235–1242. doi: 10.1093/beheco/arn082. [DOI] [Google Scholar]
- 28.Nixon CM, et al. White-tailed deer dispersal behavior in an agricultural environment. American Midland Naturalist. 2007;157:212–220. doi: 10.1674/0003-0031(2007)157[212:WDDBIA]2.0.CO;2. [DOI] [Google Scholar]
- 29.Walter WD, et al. Factors affecting space use overlap by white-tailed deer in an urban landscape. International Journal of Geographical Information Science. 2011;25:379–392. doi: 10.1080/13658816.2010.524163. [DOI] [Google Scholar]
- 30.Williams DM, Dechen-Quinn AC, Porter WF. Landscape effects on scales of movement by white-tailed deer in an agricultural–forest matrix. Landscape Ecology. 2012;27:45–57. doi: 10.1007/s10980-011-9664-5. [DOI] [Google Scholar]
- 31.Grund MD, McAninch JB, Wiggers EP. Seasonal movements and habitat use of female white-tailed deer associated with an urban park. Journal of Wildlife Management. 2002;66:123–130. doi: 10.2307/3802878. [DOI] [Google Scholar]
- 32.Nixon CM, Hansen LP, Brewer PA, Chelsvig JE. Ecology of white-tailed deer in an intensively farmed region of Illinois. Wildlife Monographs. 1991;118:1–77. [Google Scholar]
- 33.McShea WJ, Schwede G. Variable acorn crops: responses of white-tailed deer and other mast consumers. Journal of Mammalogy. 1993;74:999–1006. doi: 10.2307/1382439. [DOI] [Google Scholar]
- 34.Rooney TP, Waller DM. Direct and indirect effects of white-tailed deer in forest ecosystems. Forest Ecology and Management. 2003;181:165–176. doi: 10.1016/S0378-1127(03)00130-0. [DOI] [Google Scholar]
- 35.Harlow RF, Whelan JB, Crawford HS, Skeen JE. Deer foods during years of oak mast abundance and scarcity. The Journal of Wildlife Management. 1975;39:330–336. doi: 10.2307/3799910. [DOI] [Google Scholar]
- 36.Kie JG, Bowyer RT, Nicholson MC, Boroski BB, Loft ER. Landscape heterogeneity at differing scales: effects on spatial distribution of mule deer. Ecology. 2002;83:530–544. doi: 10.1890/0012-9658(2002)083[0530:LHADSE]2.0.CO;2. [DOI] [Google Scholar]
- 37.Stainbrook, D. P. Methods of estimating white-tailed deer abundance at Gettysburg National Military Park: Testing Assumptions of Distance Sampling. Thesis, Pennsylvania State University, University Park, PA, USA (2011).
- 38.Kilpatrick HJ, Spohr SM, Chasko GG. A controlled deer hunt on a state-owned coastal reserve in Connecticut: controversies, strategies, and results. Wildlife Society Bulletin. 1997;25:451–456. [Google Scholar]
- 39.Lesage L, Crete M, Huot J, Dumont A, Ouellet J. Seasonal home range size and philopatry in two northern white-tailed deer populations. Canadian Journal of Zoology. 2000;78:1930–1940. doi: 10.1139/z00-117. [DOI] [Google Scholar]
- 40.Henderson DW, Warren RJ, Cromwell JA, Hamilton RJ. Responses of urban deer to a 50% reduction in local herd density. Wildlife Society Bulletin. 2000;28:902–910. [Google Scholar]
- 41.Kilpatrick HJ, Spohr SM, Lima KK. Effects of population reduction on home ranges of female white-tailed deer at high densities. Canadian Journal of Zoology. 2001;79:949–954. doi: 10.1139/z01-057. [DOI] [Google Scholar]
- 42.Tilghman NG. Impacts of White-Tailed Deer on Forest Regeneration in Northwestern Pennsylvania. The Journal of Wildlife Management. 1989;53:524–532. doi: 10.2307/3809172. [DOI] [Google Scholar]
- 43.Schauber EM, Storm DJ, Nielsen CK. Effects of joint space use and group membership on contact rates among white-tailed deer. Journal of Wildlife Management. 2007;71:155–163. doi: 10.2193/2005-546. [DOI] [Google Scholar]
- 44.Tosa MI, Schauber EM, Nielsen CK. Localized removal affects white-tailed deer space use and contacts. The Journal of Wildlife Management. 2017;81:26–37. doi: 10.1002/jwmg.21176. [DOI] [Google Scholar]
- 45.Anderson DP, et al. Factors influencing female home range sizes in elk (Cervus elaphus) in North American landscapes. Landscape Ecology. 2005;20:257–271. doi: 10.1007/s10980-005-0062-8. [DOI] [Google Scholar]
- 46.Buderman FE, Diefenbach DR, Rosenberry CS, Wallingford BD, Long ES. Effect of hunter selectivity on harvest rates of radio-collared white-tailed deer in Pennsylvania. The Journal of Wildlife Management. 2014;78:1456–1465. doi: 10.1002/jwmg.779. [DOI] [Google Scholar]
- 47.Beringer J, Hansen LP, Wilding W, Fischer J, Sheriff SL. Factors affecting capture myopathy in white-tailed deer. Journal of Wildlife Management. 1996;60:373–380. doi: 10.2307/3802238. [DOI] [Google Scholar]
- 48.Clover MR. Single-gate deer trap. California Fish and Game. 1956;42:199–201. [Google Scholar]
- 49.Ramsey CW. A drop-net deer trap. Journal of Wildlife Management. 1968;32:187–190. doi: 10.2307/3798257. [DOI] [Google Scholar]
- 50.Long ES, Diefenbach DR, Rosenberry CS, Wallingford BD, Grund MD. Forest cover influences dispersal distance of white-tailed deer. Journal of Mammalogy. 2005;86:623–629. doi: 10.1644/1545-1542(2005)86[623:FCIDDO]2.0.CO;2. [DOI] [Google Scholar]
- 51.Sikes R, Gannon W, Animal Care and Use Committee of the American Society of Mammalogists Guidelines of the American Society Mammalogists for the use of wild mammals in research. Journal of Mammalogy. 2011;92:235–253. doi: 10.1644/10-MAMM-F-355.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calenge, C. adehabitatlt: Analysis of animal movements. R package (Version 0.3.16). (2014).
- 53.Calenge, C. adehabitatHR: Home range Estimation. R package (Version 0.4.11). (2011).
- 54.Homer CG, et al. Completion of the 2011 National Land Cover Database for the conterminous United States – Representing a decade of land cover change information. Photogrammetric Engineering and Remote Sensing. 2015;81:345–354. [Google Scholar]
- 55.Kelly AC, et al. Genetic assessment of environmental features that influence deer dispersal: implications for prion-infected populations. Population Ecology. 2014;56:327–340. doi: 10.1007/s10144-013-0427-9. [DOI] [Google Scholar]
- 56.Li H, Reynolds JF. A simulation experiment to quantify spatial heterogeneity in categorical maps. Ecology. 1994;75:2446–2455. doi: 10.2307/1940898. [DOI] [Google Scholar]
- 57.FRAGSTATS: spatial pattern analysis program for quantifying landscape structure (General Technical Report PNW-351, U.S. Forest Service, Corvallis, 1995).
- 58.Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical information-theoretic approach. Vol. 2nd (Springer-Verlag, 2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.