Abstract
Maple sap is a complex nutrient matrix collected during spring to produce maple syrup. The characteristics of sap change over the production period and its composition directly impacts syrup quality. This variability could in part be attributed to changes in tree metabolism following dormancy release, but little is known about these changes in deciduous trees. Therefore, understanding the variation in sap composition associated with dormancy release could help pinpoint the causes of some defects in maple syrup. In particular, a defect known as “buddy”, is an increasing concern for the industry. This off-flavor appears around the time of bud break, hence its name. To investigate sap variation related to bud break and the buddy defect, we monitored sap variation with respect to a dormancy release index (Sbb) and syrup quality. First, we looked at variation in amino acid content during this period. We observed a shift in amino acid relative proportions associated with dormancy release and found that most of them increase rapidly near the point of bud break, correlating with changes in syrup quality. Second, we identified biological processes that respond to variation in maple sap by performing a competition assay using the barcoded Saccharomyces cerevisiae prototroph deletion collection. This untargeted approach revealed that the organic sulfur content may be responsible for the development of the buddy off-flavor, and that dormancy release is necessary for the appearance of the defect, but other factors such as microbial activity may also be contributing.
Introduction
Maple syrup is a natural sweetener obtained from the concentration of sap collected in the spring from sugar maple trees (Acer saccharum Marsh.) and related species (Acer rubrum, Acer nigrum). The sap composition is the product of a complex system involving tree metabolism and subsequent microbial activity during the collection and transformation process, which includes storage and membrane processing, such as reverse osmosis, prior to boiling. The sap is collected through networks of plastic tubing connected to hundreds of trees under a high vacuum and stored in a non-aseptic environment, where microbial activity can alter its composition1–4. Most maple syrup characteristics such as color, dominant flavors, pH and chemical composition vary over the production period5–7. Previous studies focusing on maple sap and syrup properties have mainly considered the cumulative percentage of sap flow or syrup color grade, corresponding to its light transmittance, as a reference to monitor variation5,8. Although maple sap composition has been investigated5, its complexity and variation is such that many compounds that can contribute to its properties are still unidentified6,9–13.
Maple sap composition is a critical factor for syrup quality since it is concentrated 35 to 40 fold during the production process. The production of maple syrup has doubled over the last two decades, reaching 207 million pounds in 201614. However, the variation of maple syrup quality has remained a constant concern, as on average 25% of the bulk production does not meet the standard quality14,15. Trace off-flavors are the most common problem, but other defects can depreciate the products and render them unsaleable, leading to significant economic losses for the producers and an increasing storage burden for the industry. The current system of bulk syrup classification is based on two distinctive parameters. One is based on physical properties such as light transmittance and soluble solids content (°Brix). The other is based on sensory appreciation by trained inspectors. How the various types of defects (Table 1) vary according to the collection period is unknown because the date of production is not systematically recorded. Sap composition variation can be in part attributed to metabolic changes occurring in deciduous trees during dormancy release or a stress response16–18.
Table 1.
The official maple syrup classification system in Quebec.
| Syrup quality | Description |
|---|---|
| Std | Standard quality, characteristic maple taste |
| V | Slight trace of unwanted taste or odor |
| 1 | Defect of natural origin such as woody, caramel, burnt, vegetal |
| 2 | Defect of microbial origin such as fermented or moldy |
| 3 | Defect of chemical origin for example contamination by cleaning products |
| 4a | Uncharacterized defects or combination of defects |
| 5 | Buddy defect |
| 6a | Viscous ropy syrup that runs 10 cm or more |
aSyrups in these classes can also present the buddy defect.
The annual cycle of deciduous trees is composed of several steps including growth cessation, bud set, dormancy induction over the winter period, and its release during spring. Dormancy release is defined as the removal of the growth-arresting physiological conditions in the bud19. Specific metabolic changes have been reported during these steps19–22. Several molecules that play a role in dormancy are phytohormones such as gibberellin, ethylene, abscisic acid, cytokinins, and auxin23–25. Some of them are known to be transported to the plant xylem through the sap as biological signals to trigger metabolic changes during the annual cycle23,24. Some studies investigated dormancy release through untargeted approach such as transcriptomic profiling analyses, improving knowledge about the genetic network involved26,27. However, little is known about the metabolic changes occurring during dormancy release in maple trees and their relationship with sap properties28–30. Hence, access to commercially harvested maple sap from hundreds of trees and multiple locations during spring offers a unique opportunity to study these changes. As the dormancy release period overlaps with the maple sap harvest period, molecules related to this metabolic change and that are transiting through the sap could directly or indirectly impact syrup quality. Moreover, the physiological requirement for sap flow and dormancy release may not be the same, such that these events are not necessarily synchronized. Sap flow has been reported to depend on temperature signals, cloud cover and rain29, while dormancy release depends on perception of photoperiodic and temperature signals19,20. For maple trees, a model was proposed by Raulier and Bernier28 based on winter chilling and spring warming records to predict the date of bud break, that is, the stage at which leaves are visible but not yet unfolded31. This particular decoupling between sap flow and dormancy release is a conundrum for maple syrup production, because some years, climatic conditions are such that sap flow is still heavy, but the tree physiology has reached a point where important quality defects appear in the syrup produced. In particular a characteristic “buddy” off-flavor, appearing around the time of bud break, has traditionally marked the end of maple syrup production30,32–34, but its relationship to dormancy release has not been scientifically documented35. The fact that the prevalence of this specific defect appears to be unrelated to new technologies introduced over the last decades such as membrane processing, plastic tubing and vacuum utilization further supports the hypothesis that this defect is of biological origin14,15. Moreover, the potential to extend sap collection beyond this period is substantial and would benefit maple syrup producers.
Here, we sought to identify the changes in maple sap with respect to dormancy release, associated with the appearance of quality defects, first with amino acid content profiling, then with a systems biology approach using a prototrophic yeast deletion collection as a tool for monitoring sap composition variation. We reasoned that the genomic tools available and the ecological association of Saccharomyces cerevisiae with deciduous trees makes them relevant and powerful biological reporters7,36. We adapted the approach by using the prototrophic yeast collection and a ureide-based control media to identify biological signals associated with sap composition, dormancy release and maple syrup quality. We found that changes in syrup quality coincide with the conversion of sulfate to organic sulfur in maple sap in the late stage of dormancy release.
Results
Relationship between sap quality and dormancy release
Our first aim was to improve our understanding of maple syrup defects with respect to the tree dormancy release. Our hypothesis was that a change in the tree metabolism is responsible for the buddy defect. Accordingly, we expected to see a relationship between a tree dormancy indicator and class 5 syrups, which corresponds specifically to the buddy defect (Table 1).
Here we refer to dormancy release as a period when the tree metabolism changes prior to bud break. We based our analysis on an index representing the remaining Sum of cumulative temperature necessary to reach the stage of Bud Break (Sbb), which we formulated using the model of Raulier and Bernier28, in which Sbb = 0 corresponds to bud break. High Sbb value are therefore found early in the season, prior to bud break. In our data, Sbb was highly correlated with the Julian date (JD) (Spearman ρ = −0.99, P-value = 2.2e−16) but for different years, the predicted bud break occurs at different dates (Supplementary Figs 1, 2a). Thus, the Sbb index was a better indicator to compare our samples, reducing the environmental effect associated with yearly variations (mostly associated with chill and temperature). Therefore, we expect that the index is more representative of the tree metabolic state than the Julian date or the percent of cumulative flow and would allow for better comparisons between studies.
We related this index to the quality of samples using two approaches. First, considering only the percentage of class 5 syrups produced on the sampling day. Second, considering each defect class independently.
In 2013, 66 sap samples were collected from nine production sites in the province of Quebec, Canada. For those samples, syrups barrels were inspected to identify potential defects. We observed a change of the syrup quality from standard (Std) to class 5 syrups for most of the production sites (Supplementary Fig. 3). The percentage of class 5 syrups was negatively correlated with the Sbb index (Spearman ρ = −0.57, P-value = 7.7e−7). Over the spring of 2016 and 2017, we obtained a total of 27 and 11 sap samples and 25 and nine syrups from 10 and eight locations, respectively (Supplementary data 1). For these samples, all types of defects classified by the industry (Table 1) were taken into account by matching each sample with their syrup and submitting them to a standardized quality assessment procedure. Comparison between quality and Sbb showed that standard syrups (Std) had significantly higher values than class 5 and class 6 syrups (Kruskal-Wallis P-value = 4.5e−3; Dunn test P-value = 0.01 (Std-class 5); 0.04 (Std-class 6), Supplementary Fig. 4 and data 1). No significant differences were observed among other defect classes. The results for both classification approaches are in agreement with our hypothesis and corroborate the previous observation that class 5 syrups appear shortly before the time of bud break (class 5 Sbb range = 13–41).
Amino acid profiling
Our second aim was to gather insight into maple sap composition variation over the dormancy release period to understand the origin of the buddy defect. We hypothesized that changes in amino acid composition are responsible for the defect appearance. Therefore, we quantified 36 amino acids or their derivatives in sap samples harvested in 2013 and searched for patterns that would associate their relative occurrence to the buddy defect (Supplementary Fig. 5a, and data 1). The total content increased over the harvest period and was correlated with the Sbb index (Spearman ρ = −0.52, P-value = 9.3e−6, Fig. 1a and b). An individual correlation with the Sbb index was found for 23 amino acids or derivative out of the 36 quantified (Spearman, adjusted P-value < 0.05) including 15 negative correlations (Supplementary data 1). The strongest correlations were found for valine, isoleucine, leucine and methionine (Spearman ρ = −0.81 (adjusted P-value = 1e−15; range: 0.01 to 66 µM), −0.77 (adjusted P-value = 6e−13; range: > 0.01 to 82 µM), −0.75 (adjusted P-value = 4e−12; range: 0.01 to 76 µM) and −0.69 (adjusted P-value = 2e−9; range: > 0.01 to 76 µM), respectively).
Figure 1.
Maple sap amino acid profiles shift over the dormancy release process. (a) Constellation plot of amino acid profiles from 2013 samples adjusted at 2° Brix, showing hierarchicalclustering (Ward’s method) of early and late spring saps. Branch length represents square root distances. Color and shape size represent the Sbb index and total amino acid content, respectively. Shapes reflect the proportion of class 5 syrup produced on the day of sampling. (b) Class 5 syrup occurrence increases in late spring. Dot size reflects the total amino acid concentration. Colors and shape size refer to clusters defined in (a) and total amino acid concentration, respectively. (c) Total amino acid contents increase in late spring. Dot size reflects class 5 syrup proportion.
The total amino acid and derivatives content was also correlated with class 5 occurrence (Spearman ρ 0.45, P-value = 1.4e−4). Correlations between the proportion of class 5 syrups and the 23 amino acids were also found (Spearman, adjusted P-value < 0.05) including 14 positive correlations (Supplementary data 1). The strongest correlations were found for sarcosine, methionine, isoleucine and leucine (Spearman ρ = 0.59 (adjusted P-value = 7e−6; range: 0.01 to 0.12 µM), 0.56 (adjusted P-value = 2e−5), 0.55 (adjusted P-value = 2e−5) and 0.54 (adjusted P-value = 2e−5), respectively). Given that sarcosine and methionine are part of the plant one-carbon metabolism37, these results point to a link between this biological process and the buddy defect. A closer look at the relationship between methionine, Sbb and quality indicates that standard syrups produced at Sbb < 41 contain very low amount of methionine (Supplementary Fig. 5b).
Since amino acids can compete as a substrate in Maillard reactions that occur during the transformation process of maple syrup and contribute to its flavors, their relative proportion was also considered. A hierarchical classification of amino acid proportion profiles shows that samples clustered according to the Sbb index (Wilcoxon P-value = 9.4e−7), but not by production sites (Fig. 1a, Supplementary Fig. 6). According to sample clustering, the amino acid profiles show a breaking point at around Sbb = 100 which is before the appearance of class 5 syrups (Fig. 1c). The class 5 associated samples were further split in two groups within the late spring harvest cluster, indicating that at least two subtypes of amino acid profiles can lead to the buddy defect, highlighting the complexity of this matrix.
Yeast fitness competition in sap
To further our aim to gather insight into maple sap composition variation over the dormancy release period and its relationship with maple syrup quality, we used the prototrophic version of the barcoded yeast deletion collection as a biological reporter. The collection represents a set of genome-wide non-essential individual gene deletions. Because the yeast genome is well annotated, an enhancement or diminution of growth defect for a particular strain or group of strains can indicate which molecules are present in the media. We measured variation in yeast growth in 27 saps from 2016 in the form of a yeast genome-scale fitness profile of 4090 non-essential genes (Supplementary data 2). We compared strain fitness in a minimal control media containing sucrose and allantoin as carbon and nitrogen source, respectively, to fitness in media in which sucrose was substituted with the sap samples. Since we have previously shown that allantoate, an intermediate in allantoin catabolism, is the main nitrogen source for yeast growth in maple sap during the spring flow period9 and sucrose is the main carbon source, we expected to emphasize the effect of other relevant compounds under these conditions. A principal component analysis (PCA) on correlations between the fitness profiles showed differences between sap samples and sucrose controls, and that standard (Std) quality sap can also be discriminated from buddy sap (class 5) with this approach (Supplementary Fig. 7). Hierarchical classification of fitness profiles shows that sap samples cluster according to the Sbb index (Wilcoxon P-value = 3.6e−4) (Fig. 2a) with a breaking point around 30–40 Sbb which coincides with the appearance of various defects, class 5 in particular (Fig. 2b).
Figure 2.
Yeast fitness profiles shift according to dormancy release and maple syrup quality. (a) Constellation plot (Hierarchical clustering, Ward’s method) of the yeast fitness collection profiles of sap samples from 2016 showing a partition between Std and class 5 samples. Branch length represents square root distances while color and shape represent Sbb and sample quality, respectively. (b) Sap defects are not randomly distributed over the dormancy release period. Buddy defects appear after a turning point coinciding with the clustering based on yeast fitness profiles. Color and shape represent clusters defined in (a).
Based on the fitness profiles, we selected three lists of deletion strains: (1) the sap list, reports strains whose fitness distribution significantly differs between the control media and the sap (Kolmogorov–Smirnov test, adjusted P-value < 0.05, Supplementary data 2), (2) the Sbb list contains strains whose fitness is correlated to Sbb (Spearman test, adjusted P-value < 0.05) and (3) the quality list, for which strain fitness differs between the Std and the class 5 samples (Welch test, adjusted P-value < 0.05 & Δfitness ± 0.02). The sap, Sbb and quality lists contained 1662, 1035 and 218 strains, respectively (Supplementary Fig. 8), including 204 strains common to the Sbb and quality lists. The three lists were sharing two Gene Ontology Biological Process (GO-BP) enrichments, electron transport and mitochondria translation. We found more common categories for the quality and the Sbb list such as amino acid activation, RNA processing, mitochondrial activity and sulfate assimilation (Permute score < 0.05) (Fig. 3, Supplementary data 3). Gene ontology enrichments were compared with results from VanderSluis et al.38, which report the response of the same Saccharomyces cerevisiae prototrophic deletion collection to sole carbon or nitrogen sources. Since our competition media was supplemented with allantoin to ensure sufficient growth in sap and we observed that other nitrogen sources such as amino acids become available before bud break (Fig. 1b), we expected biological processes involved in allantoin utilization to be enriched in our lists. This was indeed the case, 14 enriched categories were common between the results of VanderSluis and our lists (Supplementary data 3). Aside from these common categories, the sap list shares most of its enrichments with genes involved in the utilization of glutamate (Fig. 3), the most abundant amino acid in maple sap (Supplementary Fig. 5). The quality and Sbb lists share most of their enrichments with substrates that require mitochondrial functions for their utilization, namely ribose, galactose and proline39,40. The enrichment for sulfate transport appears to be unique to the Sbb index and sulfate assimilation to both the Sbb and quality list. An analysis of gene-metabolite association showed that our three lists were enriched for genes associated with specific metabolites (Supplementary data 3). In particular the Sbb was associated with sulfate, while the quality list was associated with sulfite, phosphoadenosine phosphosulfate (PAPS) and S-adenosylmethionine (SAM) (Fig. 4, Supplementary Fig. 9). Altogether these results indicate that changes in sulfur composition in maple sap is associated with dormancy release and maple syrup quality.
Figure 3.
Heatmap of the most enriched biological processes in Maple sap, Quality and Sbb lists (P-value < 0.01) alongside enrichments for genes involved in utilization of particular substrates37. We excluded the enrichments related to growth on allantoin common between our assay and the VanderSluis experiments (P-value < 0.01).
Figure 4.
The S. cerevisiae deletion strains annotated as involved in the sulfate metabolic pathway and its regulation are enriched in the Sbb and quality lists. (a) Schematic representation of the sulfate metabolic pathway. APS: Adenosine phosphosulfate; PAPS: Phosphoadenosine phosphosulfate; SAM; S-adenosyl-L-methionine; Met: Methionine; Hcy: Homocystein. (b) The detailed fitness effect of each gene deletion in relation to the Sbb index of maple samples. SUL1 and SUL2 gene deletion strains present opposite fitness as they are low- and high-affinity sulfate transporter, respectively. Line represent the loess regression curve between the fitness and the Sbb index.
To confirm the results of the yeast fitness competition, we performed individual liquid growth assay for four deletion strains (opi3, met14, met5 and skp2) involved in the sulfur metabolism pathway (Fig. 4, Supplementary Fig. 8) in the 2016 and additional 2017 samples. The opi3 strain growth profile matched that of the fitness competition experiment for the 2016 samples, but growth in additional samples from 2017 did not support the relationship with quality (Supplementary data 4). No clear pattern was found in met14 regarding the Sbb value or the quality indicating that interaction with other strains may have been key to the results obtained in the fitness competition experiment (Fig. 5a). However, the strains met5 and skp2 had minimal or no growth in all Std saps but were able to grow in all class 5 saps (Fig. 5a). Since the met5 deletion strain requires organic sulfur for growth, variation in its content in maple sap may explain the appearance of the buddy defect.
Figure 5.
Growth profile of Saccharomyces cerevisiae strains impaired in their sulfur metabolism pathway in a subset of 2016 and 2017 samples. (a) Intrinsic fitness of the four mutant strains met14, met5, opi3 and skp2 in 2016 and 2017 saps. Colors represent quality classes. The horizontal axis present samples ordered by decreasing Sbb. (b) Organic sulfur concentration in saps, as estimated by a standard curve of met5 intrinsic fitness in methionine, varies along the sample Sbb and coincides with quality. The line represents the loess regression curve between the estimated organic sulfur concentration and the Sbb index.
We used the met5 strain growth as a proxy to estimate the organic sulfur content based on a methionine standard curve (Fig. 5b). We found a significant correlation between the estimated organic sulfur concentration and the Sbb index (Spearman ρ = −0.70, P-value: 1.2e−05, Fig. 5b) and with the sap quality (Kruskal-Wallis P-value: 2.8e−3; Dunn test P-value = 0.02 (Std-class 5)), reflecting a change in the nutrient content of maple sap associated with dormancy release. Moreover, the increase in organic sulfur observed between 30 and 40 Sbb coincides with the occurrence of class 5 syrups in 2016 and 2017. Thus, at least for these samples, met5 appears to be an appropriate biomarker of maple sap quality with an interval concentration of 2.5 µM to >100 µM.
Sulfur compounds in maple sap
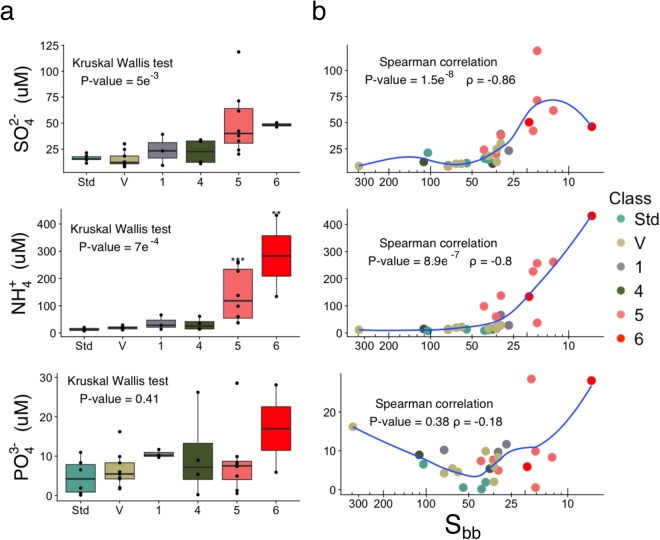
The yeast fitness competition revealed a specific association between dormancy release and sulfate, while the buddy defect was associated with sulfate assimilation products. Therefore, we hypothesized that the maple tree metabolism is releasing sulfate that can be metabolized by the sap microbiota in a variety of organic sulfur molecules, including amino acids such as methionine. We measured inorganic sulfur (SO42−), Ammonium (NH4+), and phosphorus (PO43−) in saps harvested in 2016 and 2017. Ammonium was measured because it is a catabolic product of allantoate and would therefore reflect microbial activity. Phosphorus was measured as a negative control, since the negative regulation of phosphorus metabolic process was specifically enriched in the sap list, but not in the Sbb or quality list. SO42− and NH4+ were indeed correlated wih each other (ρ = 0.78, P-value = 2.6e−6) and significantly higher in class 5 samples than in Std samples (SO42−: Kruskal-Wallis P-value = 7e−4; Dunn test P-value = 0.05 (Std-class 5); NH4+: Kruskal-Wallis P-value = 5e−3; Dunn test P-value = 4.8e−4 (Std-class 5); 6.7e−3 (Std-class 6); Fig. 6a; Supplementary data 4). We also found a correlation between NH4+ and the organic sulfur content (ρ = 0.61, P-value: 9.6e−4). Their concentrations were also highly correlated with the Sbb index (Spearman ρ = −0.80, 8.9e−7; ρ = 0.86, P-val = 1.5e−8, respectively) (Fig. 6b) with the correlation between SO42− and the Sbb index being the strongest reported in this study. As expected, the PO43− concentration was not correlated to the Sbb index or the quality (Fig. 6). Together, these results support the conclusion that the organic sulfur content in maple sap comes from microbial activity.
Figure 6.
Variation in NH4+, SO42−, and PO43− concentration in maple sap. (a) Boxplots of concentration by sample quality as defined in Table 1 showing that NH4+ and SO42− are significantly different between Std and class 5 syrups. Syrups with defects were compared to the Std group with a Dunn’s test after a Kruskal Wallis test (P-value < 0.05). * Denotes a significant difference (P-value < 0.05). Boxplots show the median, 1st and 3rd quantiles. (b) Sap variation in NH4+ and SO42− are correlated to dormancy release (Sbb) (Spearman P-value < 0.05) while PO43− is not.
As the concentration of organic sulfur in sap was discriminating between the Std and class 5 samples, and the range of concentration observed for sulfur amino acids in 2013 (methionine, cysteine (2 sulfur atoms), cystathionine and thiaproline) was lower (range 20 nM to 28.5 µM, Supplementary data 1) than the estimated range of total organic sulfur, we looked for additional organic sulfur compounds in maple sap. We targeted three molecules as likely candidates, SAM, S-Adenosyl Homocystein (SAH), and Methylthioadenosine (MTA). We based this choice on the enrichment results (Supplementary data 3), and the fact that in plants, SAM can be converted to MTA to produce ethylene, which has been shown to have a concentration that peaks in late spring in a maple tree species41. We quantified SAM, SAH and MTA, but the three molecule concentrations were below the limit of detection in all sap samples regardless of their quality (detection limits SAH & MTA: 2.6–2.9 nM; SAM: 33 nM; Supplementary data 4). We also measured SAM concentration by generating the double yeast mutant strain sam1 sam2 as this strain is auxotroph for this molecule. The auxotroph strain did not grow in any of the samples, suggesting the absence or very low concentration of SAM (<15 nM) (Supplementary data 4).
Discussion
Maple syrup is a complex natural product whose attributes result from biological, microbial, and human activity, which leads to variation in quality and functional properties that in turn can have drastic consequences for marketability3,6,12,32–34,42–44. However, little is known about the exact molecular compounds that explain this quality variation, and even less about the precursor compounds present in the sap, as most of the defects can only be detected once the syrup is produced35. The collection of sap and the transformation process are not standardized, making it challenging to identify factors impacting the final quality of the product. On the one hand, the classification currently in use is not well suited to investigate the origin of specific defects since some of them can be found in multiple classes. For instance, the buddy defect can be classified in class 4, 5 or 6 according to its intensity or combination of defects. On the other hand, the mechanisms of dormancy release and bud break in deciduous trees are beginning to come to light19,20,22,26,27,45. Here we devised a basic dormancy index to compare our samples. So far the index only takes into account climatic parameters from the nearest meteorological station. Therefore, there is opportunity for improvements with factors associated with dormancy release, such as considering the effect of photoperiod, altitude, age and the genetic background oftrees20. Empirical observations show that maple tree bud break differs according to elevation and location, but these have not been documented in the scientific litterature. Also, little is known about the genetic population structure of maple trees31. In this study, we observed that Std syrups were still obtained at Sbb < 41 for two production sites in 2013, and the sap samples contained little to no methionine. This discrepancy could be the result of a delayed response relative to our Sbb model. Despite these pitfalls, we showed a consistent relationship between the tree dormancy release and the defect appearance, as it recurrently appears at Sbb < 41, supporting earlier empirical observations35. Our model should prove more accurate than the harvest date to predict the appearance of the buddy defect regardless of the harvest year. Nevertheless, curative methods need to be developed to prevent the premature ending of the harvest season, therefore understanding the changes leading to the appearance of the defect is necessary.
Our first efforts to identify the underlying cause of the buddy defect were to profile 36 amino acids in maple sap samples collected over the spring. Amino acids are precursors for the Maillard reaction, a non-enzymatic browning reaction that occurs during the transformation process of syrup and contributes to the development of the flavors and colors of the product41. Our results confirmed a global increase of amino acids content in sap prior to bud break, which has been reported in prior work9. We found significant correlations between the Sbb index and amino acid contents, but correlations were overall weaker with class 5 syrup occurrence. Since we obtained information for all class 5 syrup produced on the sampling day and not precisely matching samples in 2013, the possibility remains that a more significant effect was missed. Considering that there could be competitive effects between amino acids as substrate in the Maillard reaction46, we also considered their relative proportion profiles. The results show a main clustering split at around Sbb = 100. These results indicate a first shift in amino acid profiles unrelated to the appearance of class 5 samples. Interestingly, a shift in microbial communities between early and mid-season has previously been reported1,8 and could be related to the pattern observed here. The class 5 samples were also grouped in two sub-clusters within the low Sbb cluster, indicating that at least two subtypes of amino acid profiles can be associated with the buddy defect. Hence, it is likely that class 5 syrups represent two types of defects both recognized as buddy or that the conditions leading to the same defect development in syrup are not unique.
To further understand changes in maple sap composition related to dormancy release and syrup quality, we used an untargeted approach using the yeast S. cerevisiae. Yeast fitness profiles clustered similarly to amino acid profiles, partitioning early and late harvest samples. These results show the usefulness of this approach to identify compounds that vary in coordination with dormancy release. In addition, the Sbb breaking point matched a shift in syrup quality, indicating that this approach revealed a different signal than amino acid profiling. When comparing biological process enrichments between our lists of responsive strains and lists of strains with altered growth rate on various substrates38, we found that we could explain most enrichments. However, enrichment for sulfate transport and sulfate assimilation were specific to our lists, hinting at the release of sulfate over dormancy release and its assimilation into organic compounds in buddy saps. Indeed, sulfur appears to be necessary for bud break as various sources of sulfur compounds are increasing prior to this event, depending on the tree species47–49. Several sulfur compounds (cysteine, glutathione, S-methyl-methionine, sulfate) transiting through the xylem prior to bud break have been reported in woody plants, however composition varies between species47–50. We confirmed the presence of sulfate in maple sap and showed an increase in sulfate towards bud break. Also, based on the growth of met5, a strain auxotroph for organic sulfur, we showed that organic sulfur is not available in Std saps and its appearance coincides with that of the buddy defect. For SO42− to be incorporated into organic compounds, a nitrogen source would also have to be available. Given that allantoate was shown to be the major source of nitrogen available to yeast in maple sap and increased in concentration over the harvest period, and that microbial contamination also increases2,6, we measured the allantoate degradation product NH4+ to assess microbial activity. We found that NH4+ and organic sulfur are correlated and associated with quality, which most likely indicates that microbial activity is responsible for the presence of organic sulfur and suggests that microbial activity plays a role in the buddy defect appearance.
These results, combined with the knowledge that the buddy defect cannot be detected before boiling the sap35, suggest that one or a combination of organic sulfur compounds produced by microorganisms are precursors to the reaction generating the buddy off-flavor during the transformation process. Sulfur compounds are known to be associated with bad flavors in various food51–53. The main sulfur amino acid measured in our assays was methionine and was also one of the most correlated to class 5 syrups. However, its concentration range was lower than the estimated organic sulfur content, suggesting the presence of additional molecules. Therefore, we looked for other sulfur compounds enriched in our results, namely SAM and SAH, and the related metabolite MTA. SAM is the precursor of ethylene, and MTA is a by-product of the reaction54. Ethylene is a phytohormone proposed to be involved in dormancy release and bud break through crosstalk with abscisic acid20,55. In Acer platanoides L, a seasonal increase in ethylene coinciding with bud break has been observed41. However, we did not detect the presence of these metabolites in maple sap. Given the central metabolic role of SAM in sulfur metabolism56, it is possible that the effects observed on yeast fitness are the result of a convergence of the metabolism of multiple organic sulfur sources. Furthermore, as it is collected, maple sap nutrient becomes available to microorganisms, combining the molecules produced by the tree and others resulting from microbial activity. It is reasonable to suspect that methionine, the main organic sulfur source in maple sap, is produced by microbial activity. Prior studies on maple sap microbial communities over the flow period did not report on buddy syrups nor nitrogen sources. Therefore, additional information should be gathered to attempt to capture microbial communities and their functions involving sulfur compounds. Interestingly, a method to prevent the production of buddy syrups has been patented in 1963 and involved incubation with an inoculum of Pseudomonas geniculata35. However, the biochemical mechanism of action was not reported and the method was not implemented in the industry.
Overall, our approach enabled us to contribute towards the existing knowledge of sap composition variation in relation to dormancy release and maple syrup quality. We devised a dormancy index that could be a useful tool to predict syrup quality and we report that the organic sulfur content, NH4+ and SO42− are potential markers to monitor sap quality. We conclude that while the dormancy release certainly contributes to a coordinated appearance of the buddy defect, we suspect a determining role for microbial activity. Are there specific microbial communities or community functions associated with the buddy defect? The interactions between microbial communities, environmental conditions and sap composition are a promising avenue of research to further understand maple syrup quality variation and develop biocontrol applications.
Methods
Maple sap and syrup samples
Maple sap concentrated by membrane processing and syrups were obtained from Québec producers located in a latitude range of 45.9 to 48.0 and longitude range of −72.5 to −68.5 during the spring of 2013, 2016 and 2017 with an emphasis on the end of the flow period. Distance range between weather stations and production sites were 4 to 56 km (Supplementary Fig. 2b).
Samples were kept frozen at −20 °C until analysis.
One sap sample was obtained on each processing day during the entire 2013 season from nine production sites. The concentration of solid content for each sap sample was measured with a digital refractometer (0–95 solids or °Brix, Reichert AR200). All barrels of maple syrup produced by nine production sites were subjected to sensory evaluation by certified inspectors following standard procedures used in the maple industry. The percentage of class 5 syrup barrels, corresponding specifically to the buddy defect, produced on each sampling day was calculated.
Samples harvested in 2016 and 2017 were provided on a voluntary basis, sap concentrates were obtained with the corresponding syrup produced in 11 and 7 production sites, respectively (Supplementary data 1). Sap concentrates were centrifuged for 5 min × 915 g at 4 °C and sterilized by 0.2 μM filtration. The concentration of each filtered sap was measured with a digital refractometer (°Brix). Syrups were subjected to sensorial evaluation by three certified inspectors and classified as of standard quality (Std) or otherwise with the defect types presented in Table 1.
Index of dormancy release
Meteorological records from the nearest stations were obtained from the Canadian government public records (http://climate.weather.gc.ca). Missing data were imputed using data from the nearest station. The observed temperature sum (Sw) was calculated by cumulating from December 1st the positive difference between the average daily temperature and 10 °C. Similarly, the number of chilling days (dC) was obtained by cumulating the number of days on which the average temperature was below 10 °C. A combined chilling and warming model28 was used to predict the temperature sum necessary for leaf emergence (Swp) given the dC observed for each sample. In this model, leaf emergence corresponds to a bud break state where leaf margins are visible but not yet unfolded29. As an index of dormancy release at the time of sampling, we used the difference between Swp and Sw to estimate the remaining temperature sum necessary to reach this stage of bud break (Sbb).
Culture media
The control media for our experiments consisted of 1.75 g.L−1 of yeast nitrogen base, 1.25 g.L−1 of allantoin and 2% sucrose. For the sap media, sucrose was substituted by sap concentrates diluted to a final density of 2°Brix.
Functional genomic screen
The yeast deletion collection used was obtained from VanderSluis et al.38. The functional genomic screen was performed as in Filteau et al.7, with the following modifications: liquid assays were carried out in 250 µL of control and sap media in 96 well plates, after an average of 18 generations, aliquots from eight replicate wells were pooled for DNA extractions. A total of 50 libraries, divided into two runs, were then constructed by PCR, including four from the initial strain pool, 18 sucrose controls and 27 saps from 2016, using the primers listed in Supplementary data 1. DNA extractions, PCR, library preparations and sequencing were performed as in Filteau et al.7.
Sequencing analysis
Sequencing results were analyzed as in Filteau et al.7, at the Plateforme d’Analyses Génomiques of the Université Laval (IBIS), with the following modifications: the sequence reads were mapped to the reference using Geneious R657, with the following parameters: trim including region from 1 to 80 first nucleotides, word length = 20, index word length = 15, 4% maximum mismatches per reads, allow 1% gap, maximum gap size = 1 and maximum ambiguity = 16. Fitness calculation was normalized using the following pseudogenes strains: “YLL017W”, “YIL170W”, “YCL075W”, “YFL056C”, “YIL167W”, “YIR043C”. After alignments, libraries with more than one million reads were used for further analysis, excluding one initial pool and three sucrose controls. We obtained an unambiguous assignment for 83% of reads and Spearman correlation coefficients of ρ = 0.92 to 0.93 between initial pool replicates and ρ = 0.92 to 0.97 between sucrose replicates.
There were 4772 strains present in the prepared pool, among which 4484 were detected. The 4090 strains that had a sum of more than 100 reads in the three initials pool replicates were considered for further analysis.
A principal component analysis (PCA) was performed on the strains fitness according to the sap quality using the R packages FactoMineR and factoextra. Two complementary statistical analyses were then performed on strain fitness. We first evaluated the correlation between the Sbb index and the strain fitness using a Spearman correlation test and a Welch’s test on the five Std saps and six buddy saps. Gene Ontology (GO) and metabolites enrichment of each list, including VanderSluis dataset38, on the S. cerevisiae prototrophic collection were performed using GoElite with the same parameters as in Filteau et al. [58] retrieved form Saccharomyces Genome Database and Yeast Metabonome DataBase, respectively59.
Growth experiments
We selected four mutant strains (met5, met14, skp2 and opi3) from the prototrophic deletion collection to validate the functional genomic screen results. Each strain was individually grown overnight at 30 °C without agitation in the control media, then, they were inoculated in fresh sap media. Initial Optical Density (OD) was adjusted for each strain at 0.05 in 200 µL in a 96 well microplate and measured in triplicates. OD was measured every 30 minutes for 36 hours at 30 °C using a Tecan plate reader (Zürich, Switzerland). For met5 strain, after modelling the growth curve for each well using Gompertz model fit in JMP13 software (SAS Institute, Cary, NC), the intrinsic fitness value was calculated by integrating the area under the curve up to 48 hours of growth and used to estimate the total organic sulfur based on a standard curve of methionine (1 mM to 10 nM).
Strain constructions
To generate the double yeast mutant strain sam1/sam2, the SAM2 locus was replaced with the HPH antibiotic resistance cassette on sam1 competent cells strain. The deletion cassette was designed and amplified with the plasmid pFA6-hphNT160, and the SAM2 flanking region with the following oligo sequences (Supplementary data 4). Cell regeneration was done on selected media YPD supplemented with the antibiotics 250 µg.mL−1 of hygromycin, 200 µg.mL−1 kanamycin and 60 µM of SAM (Sigma Aldrich A7007) since the double mutant strain is auxotroph for this molecule. To verify the cassette insertion, amplification was performed using the Forward primer and an internal cassette primer (Supplementary data 4).
SAM, SAH and MTA detection
Concentrated saps for the 2016 and 2017 harvests were filtrated by a micropore of 0.2 μm before quantification. SAH, SAM were purchased from Toronto research chemicals (Ontario, Canada) and MTA from MTA Cayman chemicals (Ann Arbor, MI). Acetonitrile LC-MS and acetic acid LC-MS of UPLC-MSMS grade were purchased from VWR International (Quebec, QC, Canada) and Fisher Scientific Ltd (Montreal, Quebec), respectively. UPLC-MSMS analysis was performed using a Waters Acquity H-Class Ultra-Performance LC system (Waters, Milford, MA, USA), equipped with a quaternary pump system. Samples were loaded on an Aquity BEH Column C18, 1.7 μm, 2.1 mm × 50 mm from Waters set at 30 °C. The mobile phase consisted of 100% aqueous acetic acid (glacial) pH 2.6 (eluent A) and acetonitrile 100% (eluent B). The flow rate was 0.35 ml.min−1 and the gradient elution was 0–1 min, from 0% to 0% B; 1–3.5 min, from 0% to 100% B; 3.5–4 min, from 100% to 100% B; 4–6 min, from 100% to 0% B.
The MS analyses were carried out on a Xevo TQD mass spectrometer (Waters) equipped with a Z-spray electrospray interface. The analysis was performed in positive mode. The ionization source parameters were capillary voltage 3.0 kV; source temperature 120 °C; cone gas flow rate 0 L.h−1 and desolvation gas flow rate 500 L−1; desolvation temperature 250 °C. Multiple reaction monitoring (MRM) transitions, individual cone voltages and collision energy voltages were summarized in Supplementary data 4.
Data acquisition was carried out with MassLynx 4.1 (Waters). Quantification was performed based on external calibration.
Statistical analysis
Statistical analysis was performed using R for Spearman correlation, Kolmogorov–Smirnov, Welsh, Anova, Tukey, Kruskal-Wallis and Dunn test. Strains from the Spearman correlation Sbb list and Kolmogorov–Smirnov test sap list were considered significant at adjusted P-value < 0.05. Strains from the Welsh test in the quality list were considered significant at adjusted P-value < 0.05 and Δfitness ± 0.02. P-value were adjusted with the Benjamini & Hochberg (FDR) method. To ensure that the correlations reported were not driven by outlier production sites, the Jackknife method was used. Correlation range for the Sbb − quality, Sbb − amino acid concentration and quality – amino acid concentrations were −0.48 to −0.57 (P-value < 0.001), −0.48 to −0.61 (P-value < 0.001) and 0.38 to 0.53 (P-value < 0.001), respectively, when samples from a single production site were removed from the dataset.
Amino acid quantification
Amino acids analysis was conducted on LC/MS/MS system according to EZ:faast protocol for free amino acids (Phenomenex, Torrance, CA, USA). The EZ:faast kit contains standard solutions, reagents, sorbents tips and LC column. Chemicals and additives such as methanol (LC-grade) and ammonium formate (Mass spectrometry grade) were purchased respectively from Fischer Scientific and Sigma Aldrich. Water used for analysis was obtained from MILLI-Q Water System. For samples analysis, 200 µL of maple sap was used for SPE extraction and derivatisation of amino acids following EZ:fasst protocol. Then, after solvent evaporation, the extract was reconstituted in 100 µL of the initial LC mobile phase, and 2 µL of this sample was injected on LC-MS/MS system. The LC system (Prominance, Shimadzu) is equipped with binary pumps LC-20AB, degasser DGU-20A5, column oven CTO-20 AC and an Autosampler SIL-2. The Qtrap3200 Mass Spectrometer Detector (Applied Biosystems, Sciex) was used for MS/MS analysis. The MS parameters were provided by Phenomenex (EZ:faast kit) and have been used without significant modifications. Amino acids content was then analyzed according to its concentration (µM) and relative mass proportion (mg.L−1) in each sample harvested in 2013 adjusted to 2°Brix. Profile visualization was performed using R.
Inorganic compounds quantification
Ammonium and sulfate were quantified with FIA Quikchem 8500 series2 using Quikchem method 10-107-06-2-B Ammonia in surface water, wastewater, and Quikchem method 10-116-10-1-C by FIA (tubidimetric method), respectively. Phosphate was analyzed with FIA Quikchem 8500 series2 with Tecator method ASN 60-01/83 in water by FIA (stannous chloride method). Data acquisition was carried out with Omnion 3.0 from Lachat Instruments. Quantification was performed based on external calibration.
Electronic supplementary material
Acknowledgements
We are grateful to the Club qualité acéricole Beauce-Appalache for providing the maple samples and to Raymond Nadeau for logistical help and discussions. We are also thankful to the fédération des producteurs acéricoles du Quebec for their financial support. This work was funded by a Mitacs accelerate grant to MF and CRL in partnership with the Centre ACER and NSERC Discovery grants to CRL and MF. CRL holds the Canada Research Chair in Evolutionary Cell and Systems Biology. NM work was supported by MAPAQ via the PAFRAPD program.
Author Contributions
Study conception and design: M.F., C.R.L., L.L., N.M. Acquisition of data: N.G., M.J., M.F., N.M. Analysis and interpretation of data: G.N., M.F. Drafting of manuscript: N.G., M.F. Critical revision: M.F., C.R.L., N.M., M.J., L.L., N.G.
Data Availability
Raw sequencing data are available at Bioproject number PRJNA473500 at http://www.ncbi.nlm.nih.gov/bioproject/.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32940-y.
References
- 1.Lagacé L, Pitre M, Jacques M, Roy D. Identification of the bacterial community of maple sap by using amplified ribosomal DNA (rDNA) restriction analysis and rDNA sequencing. Appl Environ Microbiol. 2004;70:2052–2060. doi: 10.1128/AEM.70.4.2052-2060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filteau M, Lagacé L, LaPointe G, Roy D. Seasonal and regional diversity of maple sap microbiota revealed using community PCR fingerprinting and 16S rRNA gene clone libraries. Syst Appl Microbiol. 2010;33:165–173. doi: 10.1016/j.syapm.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Morselli M, Whalen M. Aseptic tapping of sugar maple (Acer saccharum) results in light color grade syrup. Canadian Journal of Forest Research. 1991;21:999–1005. doi: 10.1139/x91-137. [DOI] [Google Scholar]
- 4.Naghski J, Reed L, Willits C. Maple sirup. X. Effect of controlled fermentation of maple sap on the color and flavor of maple sirup. Journal of Food Science. 1957;22:176–181. doi: 10.1111/j.1365-2621.1957.tb16998.x. [DOI] [Google Scholar]
- 5.Lagacé Luc, Leclerc Simon, Charron Carmen, Sadiki Mustapha. Biochemical composition of maple sap and relationships among constituents. Journal of Food Composition and Analysis. 2015;41:129–136. doi: 10.1016/j.jfca.2014.12.030. [DOI] [Google Scholar]
- 6.Filteau M, Lagacé L, LaPointe G, Roy D. Maple sap predominant microbial contaminants are correlated with the physicochemical and sensorial properties of maple syrup. International journal of food microbiology. 2012;154:30–36. doi: 10.1016/j.ijfoodmicro.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Filteau M, Charron G, Landry CR. Identification of the fitness determinants of budding yeast on a natural substrate. ISME J. 2017;11:959–971. doi: 10.1038/ismej.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filteau M, Lagacé L, LaPointe G, Roy D. Correlation of maple sap composition with bacterial and fungal communities determined by multiplex automated ribosomal intergenic spacer analysis (MARISA) Food Microbiol. 2011;28:980–989. doi: 10.1016/j.fm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Dumont, J. Caractéristiques chimiques et nutritives du sirop d’érable. Les acides aminés de la sève et du sirop d’érable Programme de développement des marchés des produits de l’érable Projet 3 (1994).
- 10.Legault J, Girard-Lalancette K, Grenon C, Dussault C, Pichette A. Antioxidant activity, inhibition of nitric oxide overproduction, and in vitro antiproliferative effect of maple sap and syrup from Acer saccharum. Journal of medicinal food. 2010;13:460–468. doi: 10.1089/jmf.2009.0029. [DOI] [PubMed] [Google Scholar]
- 11.Thériault M, Caillet S, Kermasha S, Lacroix M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chemistry. 2006;98:490–501. doi: 10.1016/j.foodchem.2005.05.079. [DOI] [Google Scholar]
- 12.Unno T. Antioxidant activity of different grades of maple syrup as determined by the hydrophilic oxygen radical absorbance capacity method. Food Science and Technology Research. 2015;21:495–498. doi: 10.3136/fstr.21.495. [DOI] [Google Scholar]
- 13.Yuan T, Li L, Zhang Y, Seeram N. Pasteurized and sterilized maple sap as functional beverages: chemical composition and antioxidant activities. J Funct Foods. 2013;5:1582–1590. doi: 10.1016/j.jff.2013.06.009. [DOI] [Google Scholar]
- 14.Fédération des producteurs acéricoles du Québec, Statistiques Acéricoles. (2016).
- 15.Fédération des producteurs acéricoles du Québec, Dossier économique. (2010).
- 16.Grassi G, Millard P, Wendler R, Minotta G, Tagliavini M. Measurement of xylem sap amino acid concentrations in conjunction with whole tree transpiration estimates spring N remobilization by cherry (Prunus avium L.) trees. Plant, Cell & Environment. 2002;25:1689–1699. doi: 10.1046/j.1365-3040.2002.00949.x. [DOI] [Google Scholar]
- 17.Ito A, Sugiura T, Sakamoto D, Moriguchi T. Effects of dormancy progression and low-temperature response on changes in the sorbitol concentration in xylem sap of Japanese pear during winter season. Tree Physiol. 2013;33:398–408. doi: 10.1093/treephys/tpt021. [DOI] [PubMed] [Google Scholar]
- 18.Loewenstein NJ, Pallardy SG. Drought tolerance, xylem sap abscisic acid and stomatal conductance during soil drying: a comparison of young plants of four temperate deciduous angiosperms. Tree Physiol. 1998;18:421–430. doi: 10.1093/treephys/18.7.421. [DOI] [PubMed] [Google Scholar]
- 19.Hanninen H, Tanino K. Tree seasonality in a warming climate. Trends Plant Sci. 2011;16:412–416. doi: 10.1016/j.tplants.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Cooke JEK, Eriksson ME, Junttila O. The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant, Cell & Environment. 2012;35:1707–1728. doi: 10.1111/j.1365-3040.2012.02552.x. [DOI] [PubMed] [Google Scholar]
- 21.Olmsted CE. Experiments on photoperiodism, dormancy, and leaf age and abscission in sugar maple. Botanical Gazette. 1951;112:365–393. doi: 10.1086/335673. [DOI] [Google Scholar]
- 22.Ruttink T, et al. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell. 2007;19:2370–2390. doi: 10.1105/tpc.107.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvim R, Hewett EW, Saunders PF. Seasonal variation in the hormone content of willow: I. Changes in abscisic Acid content and cytokinin activity in the xylem sap. Plant Physiol. 1976;57:474–476. doi: 10.1104/pp.57.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid DM, Burrows WJ. Cytokinin and gibberellin-like activity in the spring sap of trees. Experientia. 1968;24:189–190. doi: 10.1007/BF02146981. [DOI] [PubMed] [Google Scholar]
- 25.Tanino KK. Hormones and endodormancy induction in woody plants. Journal of Crop Improvement. 2004;10:157–199. doi: 10.1300/J411v10n01_08. [DOI] [Google Scholar]
- 26.Howe GT, et al. Extensive Transcriptome Changes During Natural Onset and Release of Vegetative Bud Dormancy in Populus. Front Plant Sci. 2015;6:989. doi: 10.3389/fpls.2015.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao X, et al. Comprehensive Transcriptome Analyses Reveal Differential Gene Expression Profiles of Camellia sinensis Axillary Buds at Para-, Endo-, Ecodormancy, and Bud Flush Stages. Front Plant Sci. 2017;8:553. doi: 10.3389/fpls.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raulier F, Bernier PY. Predicting the date of leaf emergence for sugar maple across its native range. Revue canadienne de recherche forestière. 2000;20:1429–1435. doi: 10.1139/x00-064. [DOI] [Google Scholar]
- 29.Kim Y, Leech R. Effects of climatic conditions on sap flow in sugar maple. The Forestry Chronicle. 1985;61:303–307. doi: 10.5558/tfc61303-4. [DOI] [Google Scholar]
- 30.Bergeron, N. & Sedjo, R. The impact of El Nino on northeastern forests: A case study on maple syrup production. (Citeseer, 1999).
- 31.Kriebel H, Wang C-W. The interaction between provenance and degree of chilling in bud-break of sugar maple. Silvae Genet. 1962;11:125–130. [Google Scholar]
- 32.Heiligmann, R. B., Koelling, M. R. & Perkins, T. D. North American maple syrup producers manual. (Ohio State University Extension, 2006).
- 33.Moore, H., Anderson, W. R. & Baker, R. Ohio maple syrup: Some factors influencing production. (1951).
- 34.Singh AS, Jones AMP, Saxena PK. Variation and correlation of properties in different grades of maple syrup. Plant foods for human nutrition. 2014;69:50–56. doi: 10.1007/s11130-013-0401-x. [DOI] [PubMed] [Google Scholar]
- 35.Wasserman, E. A. & Willits, C. Preparation of maple sirup from buddy sap. (1963).
- 36.Boynton PJ, Greig D. The ecology and evolution of non‐domesticated Saccharomyces species. Yeast. 2014;31:449–462. doi: 10.1002/yea.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson AD, Gage DA, Shachar-Hill Y. Plant one-carbon metabolism and its engineering. Trends Plant Sci. 2000;5:206–213. doi: 10.1016/S1360-1385(00)01599-5. [DOI] [PubMed] [Google Scholar]
- 38.VanderSluis B, et al. Broad metabolic sensitivity profiling of a prototrophic yeast deletion collection. Genome biology. 2014;15(15):R64. doi: 10.1186/gb-2014-15-4-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. The Plant Cell. 1996;8:1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossignol R, et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer research. 2004;64:985–993. doi: 10.1158/0008-5472.CAN-03-1101. [DOI] [PubMed] [Google Scholar]
- 41.Eklund L. Seasonal variations of O2, CO2, and ethylene in oak and maple stems. Canadian Journal of Forest Research. 1993;23:2608–2610S. doi: 10.1139/x93-324. [DOI] [Google Scholar]
- 42.Morselli, M. & Whalen, M. L. Maple chemistry and quality. North American maple syrup producers manual, The Ohio State University, Columbus, Ohio, 162–171 (1996).
- 43.Akochi-K E, Alli I, Kermasha S. Characterization of the pyrazines formed during the processing of maple syrup. Journal of agricultural and food chemistry. 1997;45:3368–3373. doi: 10.1021/jf960306i. [DOI] [Google Scholar]
- 44.Lagacé L, et al. Spectrofluorimetric determination of formaldehyde in maple syrup. J AOAC Int. 2002;85:1144–1147. [PubMed] [Google Scholar]
- 45.Horvath DP, Anderson JV, Chao WS, Foley ME. Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci. 2003;8:534–540. doi: 10.1016/j.tplants.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Ashoor S, Zent J. Maillard browning of common amino acids and sugars. Journal of Food Science. 1984;49:1206–1207. doi: 10.1111/j.1365-2621.1984.tb10432.x. [DOI] [Google Scholar]
- 47.Schneider A, Kreuzwieser J, Schupp R, Sauter JJ, Rennenberg H. Thiol and amino acid composition of the xylem sap of poplar trees (Populus × canadensis robusta) Canadian journal of botany. 1994;72:347–351. doi: 10.1139/b94-046. [DOI] [Google Scholar]
- 48.Schupp R, Glavac V, Rennenberg H. Thiol composition of xylem sap of beech trees. Phytochemistry. 1991;30:1415–1418. doi: 10.1016/0031-9422(91)84176-S. [DOI] [Google Scholar]
- 49.Rennenberg H, Schupp R, Glavac V, Jochheim H. Xylem sap composition of beech (Fagus sylvatica L.) trees: seasonal changes in the axial distribution of sulfur compounds. Tree physiology. 1994;14:541–548. doi: 10.1093/treephys/14.5.541. [DOI] [PubMed] [Google Scholar]
- 50.Prima-Putra D, Botton B. Organic and inorganic compounds of xylem exudates from five woody plants at the stage of bud breaking. Journal of plant physiology. 1998;153:670–676. doi: 10.1016/S0176-1617(98)80219-8. [DOI] [Google Scholar]
- 51.Vermeulen C, Gijs L, Collin S. Sensorial contribution and formation pathways of thiols in foods: a review. Food reviews international. 2005;21:69–137. doi: 10.1081/FRI-200040601. [DOI] [Google Scholar]
- 52.Mestres M, Busto O, Guasch J. Analysis of organic sulfur compounds in wine aroma. J Chromatogr A. 2000;881:569–581. doi: 10.1016/S0021-9673(00)00220-X. [DOI] [PubMed] [Google Scholar]
- 53.Landaud S, Helinck S, Bonnarme P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Applied microbiology and biotechnology. 2008;77:1191–1205. doi: 10.1007/s00253-007-1288-y. [DOI] [PubMed] [Google Scholar]
- 54.Adams DO, Yang SF. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ophir R, et al. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Molecular Biology. 2009;71:403–423. doi: 10.1007/s11103-009-9531-9. [DOI] [PubMed] [Google Scholar]
- 56.Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol. 2000;32:391–395. doi: 10.1016/S1357-2725(99)00139-9. [DOI] [PubMed] [Google Scholar]
- 57.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filteau M, et al. Systematic identification of signal integration by protein kinase A. Proc Natl Acad Sci USA. 2015;112:4501–4506. doi: 10.1073/pnas.1409938112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramirez-Gaona M, et al. YMDB 2.0: a significantly expanded version of the yeast metabolome database. Nucleic acids research. 2016;45:D440–D445. doi: 10.1093/nar/gkw1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data are available at Bioproject number PRJNA473500 at http://www.ncbi.nlm.nih.gov/bioproject/.