Abstract
Background
Approaches to changing providers’ behavior around Clostridium difficile (CD) management are needed. We hypothesized that case-specific teaching points and face-to-face discussions with prescribers and nurses would improve management of patients with a positive CD test.
Methods
Charts of patients age ≥18 years with positive CD tests hospitalized July 2016 to May 2017 were prospectively reviewed to assess CD practices and generate management recommendations. The study had 4 periods: baseline (pre-intervention), intervention #1, observation, and intervention #2. Both interventions consisted of an in-person, real-time, case-based discussion and education by a CD Action Team (CDAT). Assessment occurred within 24 hours of a positive CD test for all periods; during the intervention periods, management was also assessed within 48 hours after CDAT-delivered recommendations. Outcomes included proportion of patients receiving optimized treatment and incidence rate ratios of practice changes (both CDAT-prompted and CDAT-independent).
Results
Overall, the CDAT made recommendations to 84 of 96 CD cases during intervention periods, and providers accepted 43% of CDAT recommendations. The implementation of the CDAT led to significant improvement in bowel movement (BM) documentation, use of proton pump inhibitors, and antibiotic selection for non-CD infections. Selection of CD-specific therapy improved only in the first intervention period. Laxative use and treatment of CD colonization cases remained unchanged. Only BM documentation, a nurse-driven task, was sustained independent of CDAT prompting.
Conclusions
A behavioral approach to changing the management of positive CD tests led to self-sustained practice changes among nurses but not physicians. Better understanding of prescribers’ decision-making is needed to devise enduring interventions.
Keywords: behavioral, management, C. difficile
Effective implementation of evidence-based Clostridium difficile (CD) practices remains a challenge in acute care settings. C. difficile infections (CDIs) are overdiagnosed in hospitals that use nucleic acid amplification tests (NAATs) that detect the CD toxin gene rather than the toxin itself [1]. Colonization with CD and noninfectious diarrhea are common in hospitalized patients [2, 3], and several reports have documented frequent use of laxatives in patients undergoing CD testing or with positive results [4–6]. Review of the necessity of proton pump inhibitor (PPI) use and shortening or discontinuation of antibiotics for non-CD infections whenever possible are among the recommended strategies to prevent and limit complications from CDI [7, 8].
Solutions to tackle nonadherence to CD guidelines are not well established. Most studies have focused on optimizing testing (mostly through electronic support tools) or prevention of CDI rather than management of positive cases. Electronic hard stops and prompts have helped improve CD testing by rejecting samples from patients without significant diarrhea, patients with concomitant laxative use, or prior recent CD testing [6, 9–11]. Such a strategy could be considered to improve management once a CD test comes back positive (eg, an electronic prompt to review the need for antibiotics or PPIs). However, “alert fatigue” in the current highly computerized clinical environment may limit electronic tool effectiveness, and behavioral interventions may be desirable. Feedback of antibiotic use to doctors has been used successfully to reduce select antibiotic prescriptions and reduce CDI rates [12, 13]. Data on nontechnical strategies leading to sustained improvements in the management of CD-positive cases are lacking. In response to a continued trend of treatment of CD-positive cases without significant diarrhea and despite an electronic hard stop at our hospital to limit CD testing in patients receiving laxatives, we developed a quality improvement project involving creation of a Clostridium difficile Action Team (CDAT) with the goal of engaging primary providers (prescribers and nurses) in discussions around CD management. We hypothesized that an in-person intervention in real time involving current cases, along with case-specific teaching points by a multidisciplinary team led by an infectious diseases (ID) physician, would result in a significant and sustained improvement in the management of inpatients with a positive CD NAAT.
METHODS
Patient Population and Data Collection
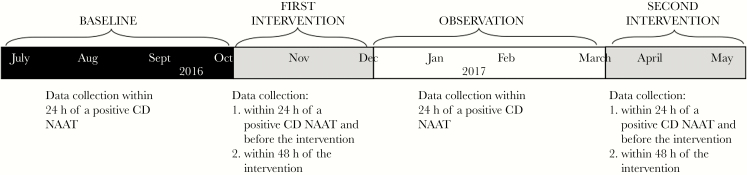
All patients age ≥18 years admitted to The Johns Hopkins Hospital (JHH), a 1078-bed tertiary care hospital in Baltimore, Maryland, with a positive CD NAAT between July 2016 and May 2017 were included in the study. All data were prospectively collected by manual chart review. The study included 4 periods (Figure 1): baseline (July 17, 2016–October 8, 2016), first intervention (October 9, 2016–December 18, 2016), observation (December 19, 2016–March 30, 2017), and second intervention (March 31, 2017–May 5, 2017); and 6 data collection time points: before initiation of the CDAT intervention (baseline), within 24 hours of a positive CD NAAT case and before the intervention and within 48 hours after the CDAT discussed management and provided education to the treating teams during the first and second intervention periods, and within 24 hours of a positive CD NAAT case during the observation period. The purpose of recording CD practices before the intervention during the intervention periods was to assess whether the intervention resulted in independent and sustained practice changes by front-line providers.
Figure 1.
Timeline of the study and data collection points.
CDAT and Target Interventions
We used a quasi-experimental removed-treatment design evaluating the impact of face-to-face feedback and education for nurses and prescribers. The CDAT focused on 1 nurse-driven task (bowel movement documentation) and 5 prescriber-led tasks (not treating cases likely to represent colonization, optimizing CDI treatment, stopping high-risk or any unnecessary antibiotics for non-CD infections, discontinuing laxatives in patients being evaluated for CDI, and discontinuing unnecessary PPI). The study was conducted over 11 months.
The CDAT was composed of 3 ID physicians, 3 pharmacists, and 2 infection preventionists. One ID physician (T.M.) reviewed all CD-positive cases from a list generated in the electronic medical record (EMR) to identify suboptimal CD management and developed written recommendations to improve CD management and teaching points specific to the case during the intervention periods, which were reviewed by the rest of the team before in-person discussion with the primary team by the same ID physician (T.M.) throughout the project (Supplementary Data). The CDAT provided recommendations and education in the afternoon, when rounds and associated decision-making regarding positive cases would have been expected to occur, and when staff generally have more time for education or to engage in discussions. Interaction with prescribers lasted on average 10 minutes and occurred more commonly in documentation rooms, whereas interactions with nurses lasted 3 minutes on average and occurred at the nurse’s workstation. The following definitions were used to determine suboptimal management: (a) CD colonization: positive CD NAAT without ≥3 daily BMs or with an alternative cause of diarrhea and without clinical or laboratory evidence of CDI (eg, no fever, abdominal pain, leukocytosis); (b) inappropriate antibiotic therapy for CDI based on noncompliance with local guidelines for CDI therapy developed by the JHH Antimicrobial Stewardship Program (https://www.hopkinsmedicine.org/amp/index.html); (c) inappropriate antibiotic therapy for concomitant non-CD infections: continuing antibiotic therapy if not still indicated, lack of selection of an agent less associated with CDI chosen (ie, fluoroquinolones or clindamycin chosen), or excess duration of therapy given or planned; (d) suboptimal BM documentation: absent documentation or discrepant documentation between physicians’ notes and daily recording of vital signs (eg, physician documents diarrhea in the progress note, but there is no recording of the number of BMs in vital signs sheet); (e) inappropriate PPI use: no documented indication; (f) any laxative use was considered suboptimal.
Outcome Measures and Data Analyses
The outcome measures were (a) CDAT-prompted changes and (b) CDAT-independent changes. CDAT-prompted changes were assessed by comparing the proportion of patients receiving optimized CD management within 48 hours after CDAT intervention with the proportion of patients receiving optimized CD management during the baseline period. CDAT-independent changes were assessed by comparing the proportion of patients receiving optimized CD management during the observation period and before CDAT intervention during the intervention periods with the proportion of patients receiving optimized CD management during the baseline period. Independent rate ratios with 95% confidence intervals (CIs) were calculated for all practice measures and compared by 2-sided Poisson regression. Categorical variables were compared by the chi-square test, and means of continuous variables by the Student t test. A 2-sided P value <.05 was considered statistically significant for all tests. All analyses were performed using Stata 13 (StataCorp). This was acknowledged by the JHH Institutional Review Board as a quality improvement project.
RESULTS
There were 100 patients with a positive CD NAAT in the baseline period, 62 patients in the first intervention period, 88 patients in the observation period, and 34 patients in the second intervention period. Patient characteristics were similar between nonintervention and intervention periods (Table 1).
Table 1.
Clinical Characteristics of the Cohort
| Patient Characteristics | Observation Periods (n = 188) |
Intervention Periods (n = 96) |
P Value |
|---|---|---|---|
| Median age, range 18–98 (IQR), y | 61 (47–69) | 63 (52–69) | .2 |
| History of Clostridium difficile infection, No. (%) | 44 (23) | 24 (25) | .8 |
| Immunocompromised host, No. (%) | 39 (21) | 15 (16) | .2 |
| Admitted to intensive care unit, No. (%) | 25 (13) | 13 (13) | .4 |
| Colitis on imaging, No. (%) | 37 (20) | 27 (28) | .1 |
| Fever at presentation, No. (%) | 33 (17) | 21 (22) | .3 |
| Acute renal failure at presentation, No. (%) | 47 (25) | 30 (33) | .2 |
| Mean daily bowel movement at time of C. difficile testing | 3.1 | 3.7 | .07 |
| Infectious diseases consult, No. (%) | 52 (28) | 26 (27) | .9 |
Abbreviation: IQR, interquartile range.
The CDAT identified at least 1 opportunity to improve CD management in 84 of 96 patients and provided recommendations for 76 cases during the intervention periods (Figure 2). Compared with baseline practice, significant improvements in response to direct interventions by the CDAT were seen for (a) BM documentation (suboptimal documentation decreased by 68% during the first intervention and by 92% during the second intervention period; P = .02 and < .01 respectively), (b) antibiotics for non-CD infections (suboptimal use was reduced by 57% and 76% during first and second intervention periods, respectively; P < .01 for both), and (c) PPI use (inappropriate PPI use decreased by 63% in both interventions periods; P < .05 for both) (Figure 2; Supplementary Table 1). CD-specific antibiotic therapy improved significantly during the first but not the second intervention period (64% improvement for the first intervention period; P > .001; and 52% for the second intervention; not statistically significant). Treatment of CD colonization cases improved by 53% and 45% in the first and second intervention periods, respectively, although changes were not statistically significant. Laxative use remained similar throughout the study.
Figure 2.
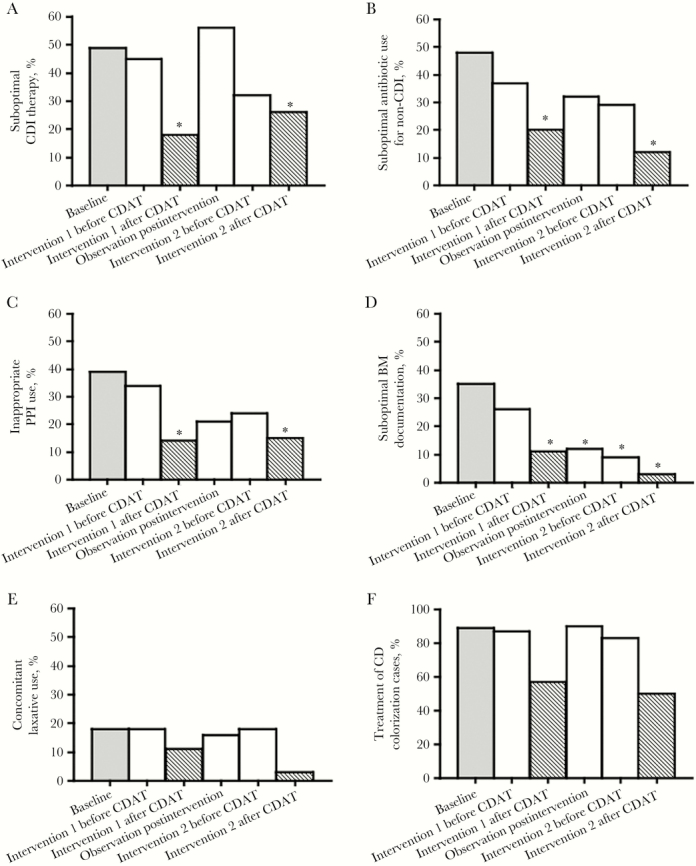
Practice changes in Clostridium difficile (CD) management over time: (A) proportion of patients with suboptimal CD infection (CDI) therapy, (B) proportion of patients with suboptimal antibiotic use for non-CDI indications, (C) proportion of patients with inappropriate proton pump inhibitor (PPI) use, (D) proportion of patients with suboptimal bowel movement documentation, (E) proportion of patients with concomitant laxative use, (F) proportion of patients likely colonized who received CD treatment. Baseline practices before CD Action Team (CDAT) was initiated are shown in the black bar. CDAT-independent practice changes (CD practices measured: (1) before CDAT intervention during intervention periods and (2) during the observation period) are shown in the white bars. CDAT-prompted practice changes (CD practices measured within 48 hours of the intervention) are shown in the gray bars. Statistically significant changes (P < .05) between the baseline period before CDAT was started and postbaseline are marked with an asterisk.
For CDAT-independent practice changes (ie, practices during the observation period and before CDAT intervention during the intervention periods), only BM documentation was sustained after the first intervention and independent of daily contact with the CDAT during the second intervention (Figure 2; Supplementary Table 2). There was a trend toward prescribers demonstrating self-sustained change in antibiotic use for non-CD infections and PPI use. There was no significant change in CD-specific therapy, concomitant laxative use, or treatment of CD colonization cases after the first CDAT intervention or independent of daily contact with the CDAT during the second intervention period.
Overall, acceptance of practice change recommendations varied based on the category assessed: stop unnecessary PPIs in 15/29 (52%) of cases, stop laxatives in 9/26 (53%), improve BM documentation in 11/19 (57%), change or stop CD therapy in 24/41 (58%), and change or stop antibiotics for non-CD infections in 16/33 (48%) of cases. In the whole cohort, 125/284 CD NAAT–positive cases were adjudicated as colonization by the CDAT (a positive result without evidence of CDI).
DISCUSSION
In-person discussion of CD management recommendations, along with case-specific education of nurses and prescribers by a dedicated CD team, resulted in significant improvement in some but not all suboptimal CD practices. Nurses but not prescribers sustained practice changes on their own. To our knowledge, this is the first report describing the immediate and sustained effects of an intervention targeting adaptive changes in practice of nurses and prescribers in the management of CD NAAT–positive cases.
Optimal CD testing and management remains a challenge in many hospitals. CDI overdiagnosis due to inappropriate testing, inaccurate BM documentation that limits interpretation of clinical picture, and unnecessary concomitant antibiotic, laxative, and PPI use in CDI are some of the areas that need improvement in the management of CD-positive patients in acute care settings [1, 6, 11, 14, 15]. At our institution, efforts to improve CD testing and management have been ongoing for several years. Education via didactic sessions, electronic short communications, screensavers, and an electronic hard stop to limit testing of patients with concomitant laxative use or recent prior CD testing were implemented before the CDAT. Behavioral interventions to improve management of CD NAAT–positive cases have not been well studied. We conducted a quality improvement project with the goal of raising awareness of the important need to accurately evaluate patients before and after testing for CD to avoid unnecessary treatment of asymptomatic carriers and to ensure best practices in patients with symptomatic CDI. An opportunity to improve CD care was found in 87% of patients included in the intervention periods. Overall, CDAT recommendations were accepted in 43% of cases, with practice improvements ranging from 50% to 90% of cases. Our intervention seems to be more effective than feedback via telephone by an ID pharmacist, where an overall practice improvement was reported in 30% of cases [4]. Like others [15], we observed a high proportion of CD NAAT–positive cases representing “asymptomatic carriers.” Most of these patients received CD-specific therapy, although teams were willing to discontinue therapy after CDAT discussion in half of the cases. However, the proportion of CD colonization remained similar over time despite the CDAT. It is possible that in hospitals using a 2-step algorithm for CDI diagnosis (NAAT followed by toxin testing), prescribers would be more willing to stop CD-specific therapy in cases representing colonization rather than infection. We did not formally capture the reasons behind lack of compliance to recommendations among the providers who did not accept them; however, expectation that a test will detect subclinical disease, poor understanding of the limitations of the test, defensive medicine, and overestimation of signs and symptoms and subsequent classification of patients as having severe disease are some explanations for medical overdoing [16, 17]; these could have contributed to our findings. In agreement with other reports [5], we also observed a significant number of CD NAAT–positive patients with concomitant laxative use, a practice that did not change over time despite the CDAT’s advice to discontinue laxatives and the electronic hard stop limiting CD testing in patients with concomitant laxative use. We suspect that there is a disconnect in communication among prescribers and nurses regarding laxative use because laxatives are regularly ordered as “as necessary” medications upon admission, given by nurses without communication back to prescribers and without prescribers screening for their use to discontinue them if a patient develops diarrhea. Interventions to decrease laxative use will require additional evaluation of these dynamics.
The CDAT’s face-to-face interaction with providers led to significant improvement in BM documentation, antibiotic use for concomitant non-CD infections, and PPI use. The CDAT improved CD-specific therapy during 1 but not both intervention periods. When we investigated whether providers self-sustained CD practice changes, we found that only BM documentation was improved independently of the CDAT. This observation with nurses but not prescribers may be explained by a more consistent exposure to the CDAT by nurses (the CDAT delivered the intervention around the same time every day; nurses have minimal variability in shift schedule) or by the higher complexity of the prescribers’ team workflow, where a resident or advanced practitioner may “accept” a recommendation by the CDAT but there may be reasons preventing the change from being implemented (eg, the prescriber receiving the recommendation may be covering, may not be the one “entering orders” for the patient, or may need to discuss changes with the rest of the team). Our results add to the emerging literature supporting a role for nurses in antibiotic and diagnostic stewardship efforts [18]. Nurses perform many antibiotic functions in their daily practice (eg, they review and administer antibiotics and other medications that can impact the course of an infection, they obtain specimens for microbiology testing); however, these activities are not formally aligned with antibiotic stewardship (AS) efforts. Studies to better characterize ways to embrace nursing in AS efforts are needed.
We did not document which providers received education; hence, it is possible that some clinicians may have received feedback and education only once, which may explain the variability in acceptance of recommendations throughout the study. However, prescribers work in 2- to 4-week blocks, and there is a limited pool of physicians, so prescribers would have been likely to interact with the CDAT more than once. It is possible that the reported changes in non-CDI antibiotics are overestimated, as we compared practices at different times (practices within 48 hours of CDAT intervention were compared with practices within 24 hours of CD NAAT results). This was a nonrandomized trial, and the sample size did not allow for a time series analysis; however, we utilized a removed-treatment design. The intervention was conducted at a single center; thus, differences in the culture of medical practice between institutions may result in greater or lesser benefit from a similar intervention. Finally, the intervention was resource intensive (total 45 minutes per case for preparation and intervention), requiring daily involvement of the CDAT (average 1.3 cases per day during intervention periods) and potentially limiting its reproducibility at other sites.
In summary, in-person, case-specific, real-time feedback and education resulted in improved management of CD; however, prescribers were dependent on the education and prompts by CDAT and did not appear to change practice independently. For some CD practices (non-CDI antibiotic therapy and BM documentation), we observed a cumulative effect after a second intervention, suggesting a potential long-term benefit of repetitive interventions. Further research is needed to better understand decisions around CD and devise enduring interventions to optimize CD testing and management that do not depend on daily expert supervision.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Andres Matoso, MD, for critical review of the manuscript.
Financial support. None.
Potential conflict of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rock C, Pana Z, Leekha S, et al. ; CDC Prevention Epicenters Program National Healthcare Safety Network laboratory-identified Clostridium difficile event reporting: a need for diagnostic stewardship. Am J Infect Control 2018; 46:456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riggs MM, Sethi AK, Zabarsky TF, et al. . Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007; 45:992–8. [DOI] [PubMed] [Google Scholar]
- 3. Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis 2012; 55:982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckel WR, Avdic E, Carroll KC, et al. . Gut check: Clostridium difficile testing and treatment in the molecular testing era. Infect Control Hosp Epidemiol 2015; 36:217–21. [DOI] [PubMed] [Google Scholar]
- 5. Ahmad SM, Blanco N, Dewart CM, et al. . Laxative use in the setting of positive testing for Clostridium difficile infection. Infect Control Hosp Epidemiol 2017; 38:1513–5. [DOI] [PubMed] [Google Scholar]
- 6. Yen C, Holtom P, Butler-Wu SM, et al. . Reducing Clostridium difficile colitis rates via cost-saving diagnostic stewardship. Infect Control Hosp Epidemiol 2018; 39:734–6. [DOI] [PubMed] [Google Scholar]
- 7. Dubberke ER, Carling P, Carrico R, et al. . Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35(Suppl 2):S48–65. [DOI] [PubMed] [Google Scholar]
- 8. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 9. Madden GR, German Mesner I, Cox HL, et al. . Reduced Clostridium difficile tests and laboratory-identified events with a computerized clinical decision support tool and financial incentive. Infect Control Hosp Epidemiol 2018; 39:737–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Truong CY, Gombar S, Wilson R, et al. . Real-time electronic tracking of diarrheal episodes and laxative therapy enables verification of Clostridium difficile clinical testing criteria and reduction of Clostridium difficile infection rates. J Clin Microbiol 2017; 55:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo RF, Spradley S, Banaei N. Alerting physicians during electronic order entry effectively reduces unnecessary repeat PCR testing for Clostridium difficile. J Clin Microbiol 2013; 51:3872–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrowsky B, Ruiz R, Brown S, et al. . Lessons learned from implementing Clostridium difficile-focused antibiotic stewardship interventions. Infect Control Hosp Epidemiol 2014; 35(Suppl 3):S86–95. [DOI] [PubMed] [Google Scholar]
- 13. Fowler S, Webber A, Cooper BS, et al. . Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother 2007; 59:990–5. [DOI] [PubMed] [Google Scholar]
- 14. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med 2015; 175:784–91. [DOI] [PubMed] [Google Scholar]
- 15. Polage CR, Gyorke CE, Kennedy MA, et al. . Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg J, Green JB. Over-testing: why more is not better. Am J Med 2014; 127:362–3. [DOI] [PubMed] [Google Scholar]
- 17. Avorn J. The psychology of clinical decision making - implications for medication use. N Engl J Med 2018; 378:689–91. [DOI] [PubMed] [Google Scholar]
- 18. Raybardhan S, Chung B, Ferreira D, et al. . Nurse prompting for prescriber-led review of antimicrobial use in the critical care unit: a quality improvement intervention with controlled interrupted time series analysis. Open Forum Infect Dis 2017; 4(Suppl 1):S278. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.