Abstract
Introduction:
Non-invasive telemonitoring programmes detecting deterioration of heart failure are increasingly used in heart failure care.
Aim:
The aim of this study was to compare different monitoring algorithms used in non-invasive telemonitoring programmes for patients with chronic heart failure.
Methods:
We performed a systematic literature review in MEDLINE (PubMed) and Embase to identify published reports on non-invasive telemonitoring programmes in patients with heart failure aged over 18 years.
Results:
Out of 99 studies included in the study, 20 (20%) studies described the algorithm used for monitoring worsening heart failure or algorithms used for titration of heart failure medication. Most frequently used biometric measurements were bodyweight (96%), blood pressure (85%) and heart rate (61%). Algorithms to detect worsening heart failure were based on daily changes in bodyweight in 20 (100%) studies and/or blood pressure in 12 (60%) studies. In 12 (60%) studies patients were contacted by telephone in the case of measurements outside thresholds.
Conclusion:
Only one in five studies on telemonitoring in chronic heart failure reported the algorithm that was used to detect worsening heart failure. Standardised description of the telemonitoring algorithm can expedite the identification of key components in telemonitoring algorithms that allow accurate prediction of worsening heart failure.
Keywords: Heart failure, telemonitoring programmes, monitoring algorithms
Introduction
Chronic heart failure is a progressive clinical syndrome characterised by recurrent episodes of worsening heart failure. Patients with chronic heart failure benefit from regular follow-up and monitoring of biometric measurements and heart failure symptoms to detect deterioration of heart failure and to ensure the safety and optimal dosing of heart failure medication.1 However, deterioration of heart failure is unpredictable and often not fully captured in the fixed visits the patient has with his or her healthcare professional.
Telemonitoring is a promising technique that can detect early deteriorations in heart failure in order to avoid hospital admissions. However, clinical trials regarding the effectiveness of telemonitoring in managing heart failure care have produced mixed results, in part because of differences in the telemedicine approaches used.2–4
In the last decades, non-invasive telemonitoring in heart failure has moved from structured telephone calls to remote management systems. In the case of remote management systems, data from telemonitoring devices is linked into a telemedical platform and send to the telemedical centre, hospitals or primary care provider.5
One of the key components in non-invasive telemonitoring are biometric values (e.g. bodyweight, blood pressure and heart rate), measured on a regularly basis by patients at home.6 The use of biometric measurements in non-invasive telemonitoring are based on the clinical presentation of patients with deterioration of heart failure, such as fluid overload (increased bodyweight), hypo- or hypertension, or brady- or tachyarrhythmias.
Besides biometric measurements, heart failure status questions are used for detection of deterioration and early intervention to prevent (re)hospitalization. The topics are mostly based on the clinical presentation of symptoms of deterioration of heart failure (e.g. dyspnoea, oedema).
To monitor the values in non-invasive telemonitoring programmes, there are several methods. The most commonly used method is based on manually reviewing biometric measurements by a healthcare provider, who is often helped by a protocol including thresholds and treatment plans. Another method is automated algorithms integrated in telemonitoring programmes.
At present, there is little evidence what combination of biometric measurements and questionnaires of self-reported symptoms is most effective in predicting deterioration of heart failure. Besides the combination, thresholds for these measurements are also less well studied. It stands to reason that when set too wide, then there is a high risk that the patient is decompensated before an alert trigger is generated whereas thresholds which are set too tightly could lead to many ‘false’ alert triggers.6
The aim of this systematic review was, first, to assess how algorithms in non-invasive telemonitoring programmes were reported and, secondly, which subsequent thresholds for biometric parameters (bodyweight, blood pressure, heart rate) and symptom questions were used to monitor symptoms of heart failure and/or guide titration of heart failure medication.
Methods
We undertook this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. See the PRISMA checklist in Supplementary Material Appendix 1.
Search strategy for identification of relevant studies
A comprehensive search was conducted through the MEDLINE (PubMed) and Embase (www.embase.com) databases published until September 2017. Searched terms expressing heart failure were used in combination with terms identifying telemedicine. The full search strategy can be found in Supplementary Material Appendix 2. References of included studies were checked for additional relevant articles.
Review methods and selection criteria
Two reviewers (MB and SK) independently screened all titles and abstracts and made decisions regarding potential eligibility after full-text review. Discrepancies in judgment were resolved by a third reviewer (FA). We searched for articles written in English containing published reports of non-invasive telemedicine programmes (including telephone support) using biometric data performed in patients with heart failure aged over 18 years. We excluded publications: (a) addressing tele-rehabilitation only; (b) addressing tele-education only; (c) addressing telemonitoring programmes continuously collecting biometric data; (d) not monitoring patients at home; (e) not addressing an intervention.
Data extraction
For each included study, the following information was extracted: data source/study period, study location, number of hospitals, number of patients, study outcome, intervention, internal and external validity as reported by the authors.
Risk of bias in individual studies
We performed risk of bias assessments using the ‘Risk of bias’ tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (available from http://handbook.cochrane.org).7 We assessed each study according to the following quality domains: random sequence generation, allocation concealment, participants and personnel were aware/unaware of the treatment assignments, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases (Supplementary Material Appendix 3).
Summary measures and synthesis of results
The process of data synthesis was performed through a descriptive analysis of the selected studies.
Results
Article selection and risks of bias assessment
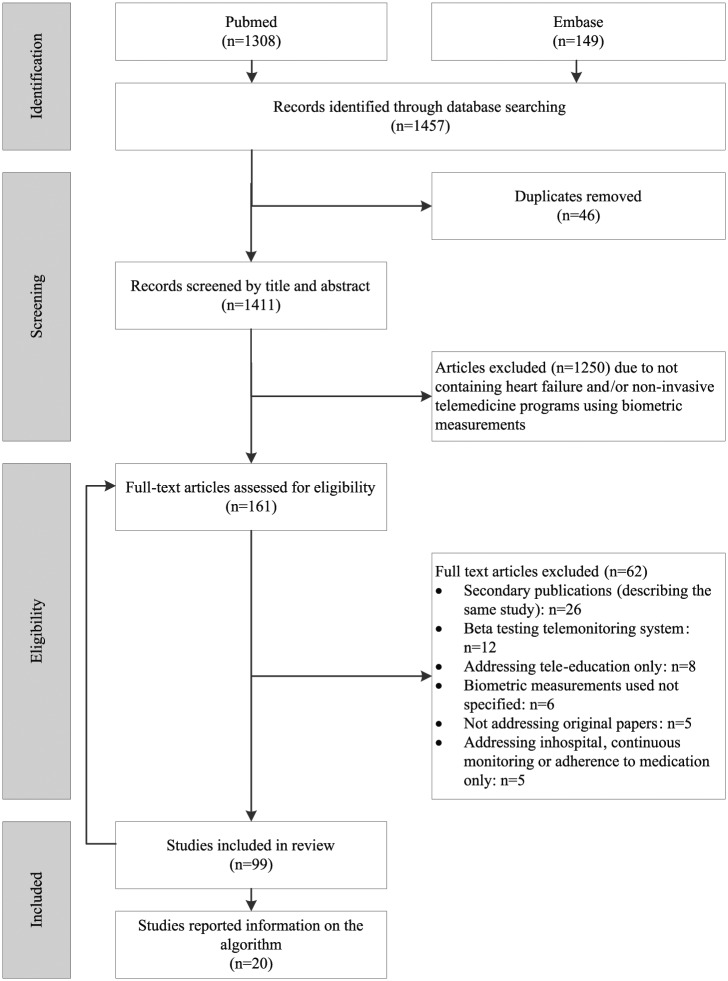
The literature search (performed on 1 September 2017) identified 1457 publications in MEDLINE (n=1308) and Embase (n=149). Out of 99 studies that met the inclusion and exclusion criteria, 20 (20%) studies reported information on the algorithm used (Figure 1). The detailed characteristics of the 20 studies that reported on the telemonitoring algorithm are presented in Supplementary Material Appendix 4. In 16 studies, data were automatically transferred to a telemonitoring service centre or trial site, and in four studies a mobile phone-based monitoring system was used.
Figure 1.
Flow of studies through the review process.
No study was judged to be at low risk of bias (Supplementary Material Appendix 3).
Biometric measurements and heart failure status questions in non-invasive telemonitoring programmes
The most frequently used biometric parameters in telemonitoring in heart failure were bodyweight (96%), blood pressure (85%), heart rate (61%), oxygen saturation (23%), and heart rhythm (17%) (Figure 2). An electrocardiogram (ECG) (15%), holter (1%) or 15-second heart rhythm strip (1%) were used to detect the patient’s heart rhythm. Less frequently used biometric parameters were heart and lungs auscultation (via electronic stethoscope) (7%), body temperature (6%), blood glucose (5%), respiration rate (2%), international normalized ratio (INR) (1%), measurement of ankle circumference with tape (1%), a six-minute walk test using a telemedical accelerometer (1%) and measuring steps a day with a pedometer (1%). In 42% of the articles, heart failure status questions were used in the algorithms such as ‘Do you feel breathing is more difficult?’, ‘Have you felt more short of breath in the last day?’ and ‘Do you have any swelling in your hands or feet?’ (Supplementary Material Appendix 5. Heart failure status questions in telemonitoring systems). In all heart failure status questionnaires at least one question concerning dyspnoea was included.8–15 A question concerning oedema was included in six questionnaires.8,9,11,13–15
Figure 2.
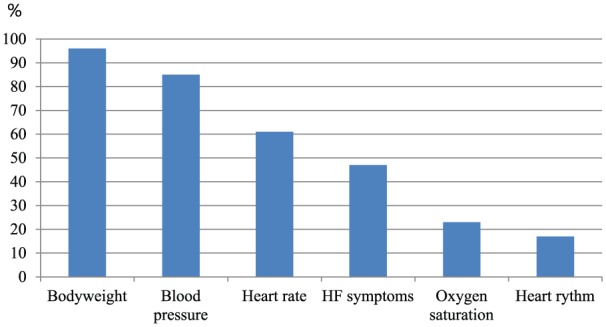
Biometric parameters and heart failure (HF) symptoms measured in articles (%).
Only Kurtz et al., in 2011, described the use of an algorithm based on a decision tree with numeric scores and a total score.12 The algorithm contained three heart failure questions concerning bodyweight chance (answer possibilities: no change (zero points), change <2 kg (one point), change >2 kg (two points)), dyspnoea (answer possibilities: breath unchanged (zero points), more short of breath (one point)) and general health status (stable (zero points), deteriorated (one point)).12
The Chronic Heart Failure Assessment by Telephone (CHAT) study used an algorithm including bodyweight and questions with regard to heart failure clinical status, medical management of the patient’s condition and social questions relevant to the patient’s heart failure status.8 The algorithm contained the following modules: exercise, medicine adherence, dehydration, arrhythmia, appointment, excess body fluid.8
In the study of Black et al., published in 2014, the thresholds set for biometric measurements were combined with alerts in heart failure status questions.11 For example when a patient had a systolic blood pressure of <90 mm Hg or >160 mm Hg with symptoms, then an urgent alert was generated. Without symptoms, the systolic blood pressure threshold was <80 mm Hg or >170 mm Hg.11
Biometric measurements and alert triggers
The biometric measurements and the use of heart failure symptom questionnaires in the algorithm are shown in Table 1. In the CHAT trial, heart failure status questions were specified but not the alert variance triggers of bodyweight (the only biometric parameter used in the study).8 In 2007, in the study of Gambetta et al., alert triggers were set for absolute and relative changes in signs, reporting of symptoms, and noncompliance to medication.9
Table 1.
Articles and alert triggers defined.
| Author | Study | Weight | Blood pressure | Heart rate | Symptom questions | Medication adjustments and alert triggers bodyweight, blood pressure, heart rate |
|---|---|---|---|---|---|---|
| Biddis et al., 2009 10 | Yes | Yes | Yes | Yes | – | |
| Black et al., 2014 11 | BEAT-HF | Yes | Yes | Yes | Yes | – |
| Chaudhry et al., 2007 13 | Tele-HF | Yes | – | – | Yes | – |
| Cleland et al., 2005 20 | TEN-HMS | Yes | Yes | Yes | – | Yes, beta-blocker |
| Dendale et al., 2012 22 | TEMA-HF | Yes | Yes | Yes | – | – |
| Gambetta et al., 2007 9 | Yes | – | – | Yes | – | |
| Giordano et al., 2009 23 | Yes | Yes | – | – | – | |
| Hofmann et al., 2015 27 | CardioBBEAT | Yes | Yes | Yes | – | – |
| Krum et al., 2013 | CHAT | Not specified | – | – | Yes | – |
| Kurtz et al., 2011 12 | Yes | – | – | Yes | – | |
| Lyngå et al., 2012 18 | WISH | Yes | – | – | – | – |
| Maric et al., 2010 14 | Yes | – | – | Yes | – | |
| Martín-Lesende et al., 2011 17 | TELBIL | Yes | Yes | Yes | – | – |
| Morguet et al., 2008 26 | Yes | Yes | Yes | – | – | |
| Piette et al., 2015 25 | Yes | – | – | – | – | |
| Roth et al., 2004 19 | Yes | Yes | – | – | – | |
| Scherr et al., 2009 24 | MOBITEL | Yes | – | – | – | – |
| Spaeder et al 2006 21 | – | Yes | Yes | – | Yes, carvedilol | |
| Wagenaar et al., 2015 16 | E-Vita HF trial | Yes | Yes | Yes | – | – |
| White-Williams et al., 2015 15 | Yes | Yes | Yes | Yes | – |
BEAT-HF: Better Effectiveness After Transition – Heart Failure; CHAT: Chronic Heart Failure Assessment by Telephone; MOBITEL: MOBIle TELemonitoring in Heart Failure Patients; Tele-HF: Telemonitoring to Improve Heart Failure Outcomes; TEMA-HF: Telemonitoring in the Management of Heart Failure; TEN-HMS: Trans-European Network-Home-Care Management System; WISH: Weight monitoring in patients with severe heart failure.
In four articles authors noted physicians could change the biometric parameters, depending on the patient’s status.10,11,16,17
In most studies participants were asked to measure the biometric parameters daily (Table 2). The biometric alert triggers used in the articles are listed in Table 2, the used heart failure status questions are in Supplementary Material Appendix 5.
Table 2.
Alert triggers of bodyweight, blood pressure, heart rate.
| Author | Study | Bodyweight variance alert triggers | Blood pressure (mm Hg) | Heart rate, beats/min | Frequency | |
|---|---|---|---|---|---|---|
| Systolic | Diastolic | |||||
| Biddis et al., 2009 10 | Increase 0.91 kga since previous day or if weight increases by 1.36 kga in a rolling 7 days | <100 or drops by 20 from previous reading | – | <55 or >120 | Daily | |
| Black et al., 2014 11 | BEAT-HF | With symptomsa
Daily gain >1.36 kga or weekly gain >2.72 kga |
With symptomsa <90 or >160 Without symptoms <80 or >170 |
– | With symptomsa <50 or >100 Without symptoms <40 or >110 |
Daily |
| Chaudhry et al., 2007 13 | Tele-HF | >1.36 kga above or below first bodyweight entered | – | – | – | Daily |
| Cleland et al., 2005 20 | TEN-HMS | >2 kg weight gain | <90 or >140 | – | Resting heart rate <50 or >80 | Twice daily |
| Dendale et al., 2012 22 | TEMA-HF | +2 kg from discharge bodyweight | <90 or >140 | – | <50 or >90 | Daily |
| Gambetta et al., 2007 9 | A weight change of 1.36 kga in 3 days | – | – | – | Daily | |
| Giordano et al., 2009 23 | Increase >2 kg | Dizziness with <90 | – | – | Daily | |
| Hofmann et al., 2015 27 | Cardio- BBEAT |
>2 kg within 3 days | <90 or >140 | – | Resting heart rate <50 or >80 | Daily |
| Krum et al., 2013 8 | CHAT | Not specified | – | – | – | ⩾Monthly |
| Kurtz et al., 2011 12 | Weight change <2 kg or >2 kg |
– | – | – | Weekly | |
| Lyngå et al., 2012 18 | WISH | >2 kg weight gain in 3 days | – | – | – | Daily |
| Maric et al., 2010 14 | Weight changed ⩾2 kg in 2 days, ⩾5 kg in 7 days | – | – | – | Daily | |
| Martín-Lesende et al., 2011 17 | TELBIL | >1 kg within 3 days | <100 or >160 | <60 or >90 | <50 or >100 | Daily |
| Morguet et al., 2008 26 | >1.5 kg | <90 or >180 | >110 | – | Daily | |
| Piette et al., 2015 25 | Increase of 2.27 kga over 1 or 2 weeks, a 3.18 kga increase over 3 weeks, or an average gain of 0.91 kga per week since the last automated call if more than 3 weeks had elapsed | – | – | – | Daily | |
| Roth et al., 2004 19 | >1.5 kg compared to baseline | <90 or >180 | >110 | – | Daily | |
| Scherr et al., 2009 24 | MOBITEL | Increased or decreased more than 2 kg in 2 days | – | – | – | Daily |
| Spaeder et al., 2006 21 | Carvedilol titration | - | Between 85–99 | – | Between 55–64 | Daily |
| Wagenaar et al., 2015 16 | E-Vita HF trial | +1 kg in 1 day, +2 kg in three consecutive days, –3 kg in 1 day and +2 kg or −2 kg from baseline bodyweight) | Average <90 or >140 for 3 consecutive days | Average <50 or >100 for 3 consecutive days | <50 or >100 | Daily |
| White-Williams et al., 2015 15 | >2.27 kga increase | Vary >10 mm Hg | Vary >10 mm Hg | <60 or >120 | Daily | |
BEAT-HF: Better Effectiveness After Transition – Heart Failure; CHAT: Chronic Heart Failure Assessment by Telephone; MOBITEL: MOBIle TELemonitoring in Heart Failure Patients; Tele-HF: Telemonitoring to Improve Heart Failure Outcomes; TEMA-HF: Telemonitoring in the Management of Heart Failure; TEN-HMS: Trans-European Network-Home-Care Management System; WISH: Weight monitoring in patients with severe heart failure.
Pounds to kilogram conversion (1 lb=0.45359237 kg).
Monitoring of data by healthcare professionals
In 11 studies, alert triggers were monitored by a nurse.8,9,11,14, 16–22 In one study both the general practitioner and healthcare professionals at the heart failure clinic monitored the data.23 In five studies it was less specifically described who monitored the data, because of the use of the general terms ‘clinician’ and ‘physician’.10,13,15,24,25 In three studies the healthcare professional (e.g. physician, nurse) who monitored the biometric values remained unclear.12,26,27 The frequency of monitoring biometric measurements varied between daily and three times a week.
Actions of healthcare professionals on alert triggers
In 12 studies, patients were contacted by telephone in the case of measurements outside thresholds.11,13–19,23,24,26,27 In the CardioBBEAT trial, the study site called the patient if questionnaires revealed potential deterioration.27 The telemedicine centre called the patient if biometric measurements were outside of the established limits. In six studies, it remained unclear how health professionals contacted patients (visits, telephone calls).8–10,20,21,25 In the Telemonitoring in the Management of Heart Failure (TEMA-HF) study a general practitioner visited or contacted the patient if the general practitioner considered it necessary. The heart failure nurse called the patient 1–3 days after the alert.22 Kurtz et al., in 2011, used an automated self-monitoring system.12 When measurements were stable (zero points) the patients were asked to repeat the measurements after a week, in the case of minor worsening (one point) after three days, in the case of suspected deterioration of heart failure (two points) to proceed a medical visit or to increase the frequency of telephone contacts and inform the patient’s physician, in the case of high risk of hospitalization (three points) according to the algorithm the patient were directly connected to a heart failure clinic healthcare professional.12 The actions taken by healthcare professionals who contacted the patients after an alert trigger varied between changes in medication treatment (in consultation with a cardiologist or with a general practitioner), further investigations or a scheduled consult of a cardiologist, nurse or general practitioner (deterioration of heart failure). The telemedicine system was checked again on schedule within 2–3 days. In five studies (25%) it remained unclear which actions were taken after contacting the patient in the case of stable or deterioration of heart failure.8–10,17,25
Medication protocols
Changes in heart failure medication in the case of alert triggers remained unclear in most of the studies. In the WISH trial patients were asked to take an additional dose of their diuretics in the case of deterioration of heart failure.18 The CHAT trial used a diuretic protocol, but did not further specify the protocol.8 In the Trans-European Network-Home-Care Management System (TEN-HMS) trial a heart rate of ⩽65 bpm during the titration phase was an indication to delay further increase in dose of beta-blocker.20 The study of Spaeder et al., in 2006, consisted of rapid titration of carvedilol using a telemedical system versus frequent outpatient heart failure clinic visits.21
Discussion
In the present study, we assessed thresholds of algorithms in non-invasive telemonitoring programmes for symptoms of heart failure. First and foremost, we found that only one in five telemonitoring studies specified the algorithm used to monitor heart failure symptoms. The thresholds used for alert triggers in bodyweight, blood pressure and heart rate varied. Furthermore, the level of precision in measurement of bodyweight, blood pressure and heart rate remained unclear. With regard to heart failure status questionnaires, there was a wide range of topics and number of questions asked. Lastly, in the included articles, there was considerable heterogeneity in describing telemonitoring programmes and protocols.
The most common biometric measurement in telemonitoring was bodyweight and was mostly combined with other biometric measurements such as blood pressure or with heart failure status questions.
In most studies nurses interpreted the data.8,9,11,14,16–22 Some articles used general terms as ‘clinician’ or ‘physician’ to indicate the healthcare professional monitoring the data and in three studies it remained unclear who interpreted the data. In most cases little information was given about the educational background of the healthcare professional monitoring the data concerning heart failure and interpretation of telemonitoring values. Thereby the relationship between knowledge and experience at monitoring data and effectiveness of a telemonitoring programme remains unclear. It may be valuable to report in articles the profession of the healthcare professional and in the case of nurses if they were specialised in heart failure and if he/she was educated in monitoring biometric data. Even though there is a wide diversity in nursing education in heart failure and in organization of heart failure care, within and between countries, this could help to interpret the findings of scientific research.28,29
Furthermore, the description of the actions taken in the case of an alert trigger can be informative. In six studies it remained unclear how healthcare professionals contacted patients in the case of an alert trigger (visits, telephone calls, video consult), which subsequent actions were taken (fixing a new scheduled appointed (stable conditions) and if medication were changed (e.g. in consultation with cardiologist or with general practitioner).8–10,20,21,25 In these studies it remained unclear how the actions taken on alert triggers affected the findings of the trial.
Underreported in articles were medication titration protocols used in telemonitoring programmes in heart failure. In one study, the TEN-HMS study, a threshold for heart rate was reported to delay further increase in dose of betablocker.20 Medication titration algorithms for angiotensin-converting-enzyme inhibitor (ACEi), angiotensin II inhibitor (ARB), beta-blockers, mineralocorticoid receptor antagonists (MRAs) were not identified. The WISH and CHAT studies reported loop diuretic adjustments, but did not specify them.8,18 As medication protocols remain underexposed in telemedicine articles, the effects of medication changes on the results of telemedicine studies remain to be elucidated.
In the field of telemonitoring of heart failure, the view is arising that it is of paramount importance to increase comparability between telemonitoring studies.2–4 Not only the description of both the intervention (e.g. telemedical system used, contacts with healthcare professionals) and usual care provide important information, but also algorithms, level of precision in measurement of biometric equipment, information about monitoring data and actions taken on values outside thresholds provide meaningful insights. The importance of specifying telemonitoring algorithms may be seen to be particularly helpful for the monitoring of bodyweight. Monitoring of daily bodyweight is recommended in chronic heart failure and there is substantial heterogeneity in published rule-of-thumb thresholds that define clinically important bodyweight gain that can indicate deterioration of heart failure.30 Several research groups reported that simple rules of sudden bodyweight change in patients with heart failure were demonstrated to generate many alerts with poor sensitivity.6,30–32
Different methods are being used to interpret values outside thresholds in telemonitoring chronic heart failure. Some telemonitoring programmes depend only on know-ledge of the healthcare professional interpreting the data. Other telemonitoring programmes use automated algorithms to monitor data and issue an alarm in the case of values outside the established thresholds. The healthcare professional subsequently interprets the alarm and decides which actions to take (with or without use of a protocol).
Most of the automated algorithms used in monitoring early deterioration of heart failure are decision-tree and threshold-based. In the nearby future, more personalised self-learning automated algorithms (machine-learning methods) will likely be used in monitoring early deterioration of heart failure.
Study limitations
The systematic review was limited by the amount and nature of the data available. We limited the review to fully published studies.
Conclusions
Only one in five studies on non-invasive telemonitoring of chronic heart failure reported the algorithm that was used to detect worsening heart failure. The most commonly used biometric parameter in non-invasive telemonitoring in heart failure is bodyweight. Standardised description of the telemonitoring algorithm can expedite the identification of key components in telemonitoring algorithms that allow accurate prediction of worsening heart failure.
Supplemental Material
Supplemental material, Supplementary_Material for Algorithms used in telemonitoring programmes for patients with chronic heart failure: A systematic review by Maaike Brons, Stefan Koudstaal and Folkert W Asselbergs in European Journal of Cardiovascular Nursing
Footnotes
Authors contribution: All authors wrote, reviewed and edited the manuscript before submission.
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: Folkert W Asselbergs is supported by University College London (UCL) Hospitals National Institute for Health Research (NIHR) Biomedical Research Centre.
Implications for practice
- Little is known about algorithms used to detect deterioration of heart failure. Standardised reporting of algorithms used and the actions taken on alarms can help to compare telemonitoring programmes more effectively.
- It may be valuable to report in articles the profession of the healthcare professional and in the case of nurses whether they were specialised in heart failure and if educated in monitoring biometric data.
- As medication protocols remain underexposed in telemedicine articles, the effect of medication changes on the results of telemedicine studies remains to be elucidated.
References
- 1. Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 2016: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Inglis SC, Clark RA, Dierckx R, et al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015; 10: CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The Telemedical Interventional Monitoring in Heart Failure study. Circulation 2011; 123: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med 2010; 363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet 2011; 378: 731–739. [DOI] [PubMed] [Google Scholar]
- 6. Cuba Gyllensten I, Crundall-Goode A, Aarts RM, et al. Simulated case management of home telemonitoring to assess the impact of different alert algorithms on work-load and clinical decisions. BMC Med Inform Decis Mak 2017; 17: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
- 8. Krum H, Forbes A, Yallop J, et al. Telephone support to rural and remote patients with heart failure: The Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc Ther 2013; 31: 230–237. [DOI] [PubMed] [Google Scholar]
- 9. Gambetta M, Dunn P, Nelson D, et al. Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort. Prog Cardiovasc Nurs 2007; 22: 196–200. [DOI] [PubMed] [Google Scholar]
- 10. Biddiss E, Brownsell S, Hawley MS. Predicting need for intervention in individuals with congestive heart failure using a home-based telecare system. J Telemed Telecare 2009; 15: 226–231. [DOI] [PubMed] [Google Scholar]
- 11. Black JT, Romano PS, Sadeghi B, et al. A remote monitoring and telephone nurse coaching intervention to reduce readmissions among patients with heart failure: Study protocol for the Better Effectiveness After Transition – Heart Failure (BEAT-HF) randomized controlled trial. Trials 2014; 15: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurtz B, Lemercier M, Pouchin SC, et al. Automated home telephone self-monitoring reduces hospitalization in patients with advanced heart failure. J Telemed Telecare 2011; 17: 298–302. [DOI] [PubMed] [Google Scholar]
- 13. Chaudhry SI, Barton B, Mattera J, et al. Randomized trial of telemonitoring to improve heart failure outcomes (tele-HF): Study design. J Card Fail 2007; 13: 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maric B, Kaan A, Araki Y, et al. The use of the Internet to remotely monitor patients with heart failure. Telemed J E Health 2010; 16: 26–33. [DOI] [PubMed] [Google Scholar]
- 15. White-Williams C, Unruh L, Ward K. Hospital utilization after a telemonitoring program: A pilot study. Home Health Care Serv Q 2015; 34: 1–13. [DOI] [PubMed] [Google Scholar]
- 16. Wagenaar KP, Broekhuizen BD, Dickstein K, et al. Effectiveness of an interactive platform, and the ESC/HFA heartfailurematters.org website in patients with heart failure: Design of the multicentre randomized e-Vita heart failure trial. Eur J Heart Fail 2015; 17: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 17. Martin-Lesende I, Orruno E, Cairo C, et al. Assessment of a primary care-based telemonitoring intervention for home care patients with heart failure and chronic lung disease. The TELBIL study. BMC Health Serv Res 2011; 11: 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lyngå P, Persson H, Hagg-Martinell A, et al. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur J Heart Fail 2012; 14: 438–444. [DOI] [PubMed] [Google Scholar]
- 19. Roth A, Kajiloti I, Elkayam I, et al. Telecardiology for patients with chronic heart failure: The ‘SHL’ experience in Israel. Int J Cardiol 2004; 97: 49–55. [DOI] [PubMed] [Google Scholar]
- 20. Cleland JG, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: The Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol 2005; 45: 1654–1664. [DOI] [PubMed] [Google Scholar]
- 21. Spaeder J, Najjar SS, Gerstenblith G, et al. Rapid titration of carvedilol in patients with congestive heart failure: A randomized trial of automated telemedicine versus frequent outpatient clinic visits. Am Heart J 2006; 151: 844–845. [DOI] [PubMed] [Google Scholar]
- 22. Dendale P, De Keulenaer G, Troisfontaines P, et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: The TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail 2012; 14: 333–340. [DOI] [PubMed] [Google Scholar]
- 23. Giordano A, Scalvini S, Zanelli E, et al. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol 2009; 131: 192-199. [DOI] [PubMed] [Google Scholar]
- 24. Scherr D, Kastner P, Kollmann A, et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: Randomized controlled trial. J Med Internet Res 2009; 11: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piette JD, Striplin D, Marinec N, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: A randomized comparative effectiveness trial. J Med Internet Res 2015; 17: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morguet AJ, Kuhnelt P, Kallel A, et al. Impact of telemedical care and monitoring on morbidity in mild to moderate chronic heart failure. Cardiology 2008; 111: 134–139. [DOI] [PubMed] [Google Scholar]
- 27. Hofmann R, Voller H, Nagels K, et al. First outline and baseline data of a randomized, controlled multicenter trial to evaluate the health economic impact of home telemonitoring in chronic heart failure – CardioBBEAT. Trials 2015; 16: 343–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riley JP, Astin F, Crespo-Leiro MG, et al. Heart Failure Association of the European Society of Cardiology heart failure nurse curriculum. Eur J Heart Fail. 2016; 18: 736–743. [DOI] [PubMed] [Google Scholar]
- 29. Working Group of the Education Committee of the ESC Council on Cardiovascular Nursing and Allied Professions; Working Group of the Education Committee of the ESC Council on Cardiovascular Nursing and Allied Professions. Education for nurses working in cardiovascular care: A European survey. Eur J Cardiovasc Nurs. 2014; 13: 532–540. [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Goode KM, Cuddihy PE, et al. Predicting hospitalization due to worsening heart failure using daily weight measurement: Analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur J Heart Fail 2009; 11: 420–427. [DOI] [PubMed] [Google Scholar]
- 31. Ledwidge MT, O’Hanlon R, Lalor L, et al. Can individualized weight monitoring using the HeartPhone algorithm improve sensitivity for clinical deterioration of heart failure? Eur J Heart Fail 2013; 15: 447–455. [DOI] [PubMed] [Google Scholar]
- 32. Anand IS, Tang WHW, Greenberg BH, et al. Design and performance of a multisensor heart failure monitoring algorithm: Results from the Multisensor Monitoring in Congestive Heart Failure (MUSIC) study. J Card Fail 2012; 18: 289–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material for Algorithms used in telemonitoring programmes for patients with chronic heart failure: A systematic review by Maaike Brons, Stefan Koudstaal and Folkert W Asselbergs in European Journal of Cardiovascular Nursing