The oxidative phosphorylation system, involved in cellular adenosine triphosphate production, is composed of 5 complexes (complexes I–V). The complex III catalyzes the transfer of electrons from ubiquinol to cytochrome c and contains 11 subunits among which only cytochrome b is encoded by a mitochondrial gene: MT-CYB. Nonsense, missense, and frameshift mutations in MT-CYB have been reported in patients mainly presenting with an exercise intolerance associated with encephalomyopathy, cardiomyopathy, or multisystemic disorder.1–4 We report an in-frame 21-base pair (bp) deletion in MT-CYB responsible for a severe clinical presentation with an atypical brain MRI of mitochondrial diseases.
Case report
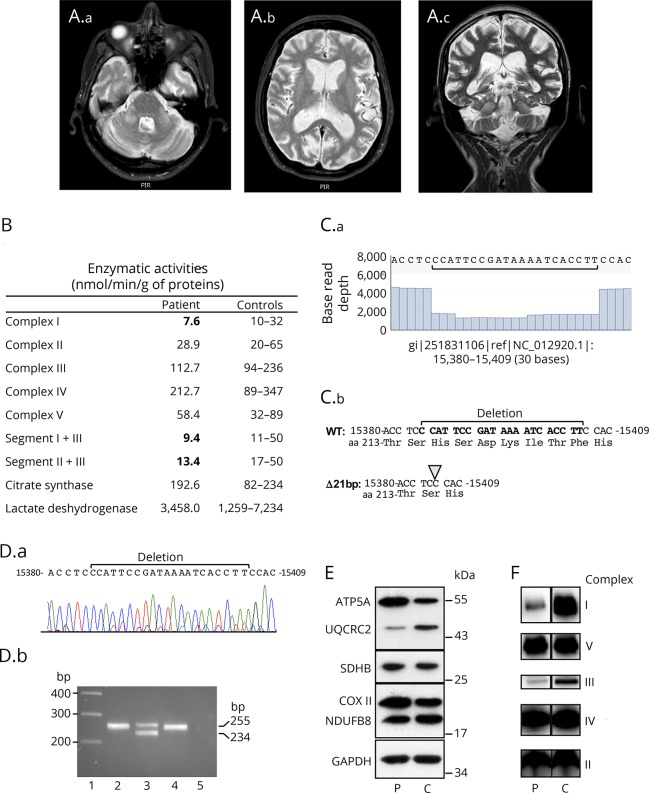
The patient was a 43-year-old woman who presented with recurrent episodes of coma with lactic acidosis since her infancy. Crises were reversible but the symptoms slowly progressed over time leading to cognitive decline, and spastic tetraparesis (walk with bilateral help) with muscle weakness predominantly affecting the proximal muscles of the upper limbs. Visual disturbances started during infancy with cataracts, and the patient became blind at 18 years of age. At age 43, the ophthalmologic examination revealed retinitis pigmentosa and glaucoma. The patient had isolated sinus tachycardia without cardiomyopathy. She presented choreiform movements involving the distal limbs and the tongue. Brain MRI showed bilateral symmetrical T2 hyperintensity of middle cerebellar peduncles with mild cerebellar atrophy and severe cortical and subcortical cerebral atrophy (figure, A). Lactic acid was elevated (2.62 mmoL/L; normal value, 0.50–2.20 mmoL/L) and the patient also had high levels of serum creatine phosphokinase (387 U/L; normal value, 20–167 U/L). There was no familial history. The patient died suddenly, at age 45, of unknown etiology.
Figure. Brain MRI and characterization of the 21-bp MT-CYB deletion.
(A) Brain MRI. Axial T2-weighted (A.a) and coronal T2-weighted fast spin-echo (FSE) (A.c) MRI showing bilateral symmetrical hyperintensity of middle cerebellar peduncles. Axial T2-weighted (A.b) and coronal T2-weighted FSE (A.c) showing severe cortical and subcortical cerebral atrophy and mild cerebellar atrophy. (B) Mitochondrial respiratory enzyme activities of muscle from the patient and controls. (C.a) Read coverage analysis from nucleotide 15380 to 15409 showing the base read depth of each nucleotide. (C.b) Sequence from nucleotide 15380 with deduced amino acid sequence showing the 21-bp deletion. (D.a) Sanger sequence chromatogram from patient's muscle. (D.b) PCR products separated on a 2% agarose gel obtained with primers 15238-forward (5′-CTGAGGAGGCTACTCAGTAG-3′) and 15493-reverse (5′-GAGGTCTGGTGAGAATAGTGT-3′) that encompass the 21-bp deletion. PCR was performed on genomic DNA extracted from patient's tissues (blood, lane 2; muscle, lane 3) and blood of a control (lane 4). The wild-type and deleted amplicons are 255- and 234-bp, respectively. Lane 1: molecular weight marker 1 kb Plus (ThermoFisher Scientific), lane 5: no template negative PCR control. (E) Western-blot analysis from equal amounts (10 μg) of muscle homogenates of patient (P) and control (C) using oxidative phosphorylation system antibodies cocktail detecting NDUFB8 (complex I), SDHB (complex II), UQCRC2 (complex III), COXII (complex IV), ATP5A (complex V), and anti-GAPDH antibody for loading control. (F) Blue native polyacrylamide gel analysis from muscle homogenates of patient (P) and control (C). Equal amounts (15 μg) of proteins were separated on a 4%–13% acrylamide gradient gel and electroblotted onto a polyvinylidene fluoride membrane prior to incubation with specific antibodies against GRIM19 (subunit of complex I), SDHA (subunit of complex II), UQCRC2 (subunit of complex III), MTCO1 (subunit of complex IV), and ATP5A (subunit of complex V).
Muscle biopsy was performed after written informed consent was obtained. Histochemical analysis revealed the presence of typical features of mitochondrial myopathy with numerous ragged red fibres. Spectrophotometric analysis of respiratory chain enzyme activities showed complex I and combined segment I + III and II + III defects (figure, B). The Coenzyme Q10 content, measured by liquid chromatography coupled with tandem mass spectrometry detection, was normal. We studied the entire mitochondrial genome from muscle using next generation sequencing (NGS), and we identified a heteroplasmic mitochondrial DNA (mtDNA) deletion affecting the MT-CYB gene. We quantified the mtDNA deletion level from NGS experiments (figure, C) and found a mutation load of 72%. This in-frame deletion is 21-bp long and encompasses nucleotide positions 15385–15405, causing the loss of 7 amino acids (His-Ser-Asp-Lys-Ile-Thr-Phe, p.His215_Phe221del) in the protein. Sanger sequencing confirmed the presence of the deletion in patient's muscle but we did not find deleted molecules in blood (figure, D). PCR amplification of a short fragment of 255-bp (nucleotide positions 15238–15493) confirmed the high level of deleted mtDNA in muscle and the absence of deletion in blood (figure, D). Consistent with the pathogenicity of this deletion, Western blot analysis showed a reduction in complex III protein levels in muscle (figure, E) and blue native-polyacrylamide gel assay revealed assembly defects of both complexes I and III (figure, F). The deletion removes a β-strand of the cytochrome b subunit and this structural modification is likely responsible for complex III assembly deficiency.
Discussion
Mutations in the MT-CYB gene are most commonly responsible for myopathy and exercise intolerance associated with complex III deficiency. Only a few MT-CYB deletions have been described, and this is the fourth report of an in-frame intragenic deletion of this gene.2,4,5 This in-frame 21-bp deletion is associated with a severe multisystemic disorder but without prominent exercise intolerance, reminding the absence of genotype-phenotype correlation in mtDNA diseases. Of interest, the brain MRI shows a bilateral symmetrical T2 hyperintensity of middle cerebellar peduncles, which is an atypical neuroimaging finding in mitochondrial diseases. This specific MRI sign is usually associated with adrenoleukodystrophy or fragile X-associated tremor ataxia syndrome. Mitochondrial dysfunction has been already described in FXTAS,6 and our observation raises the question of the possible role of mitochondria in brain damage associated with fragile X premutation.
Several previous studies reported MT-CYB mutations associated with deficiency of both complex I and complex III. This observation is due to a structural dependence among these 2 complexes, a fully assembled complex III being needed for the stability and the assembly of complex I.7 Our observation is consistent with these data because we found a complex I activity deficiency with a complex III activity in low values in muscle, whereas complex III levels were highly decreased with an assembly defect of both complexes I and III.
The MT-CYB deletion is found at a high percentage in our patient's muscle and absent in leukocytes as it has been frequently reported. Our findings highlight the interest of both muscle biopsy and NGS to detect small deletions responsible for mtDNA disease. They also expand the neuroimaging and clinical spectrum associated with MT-CYB mutations.
Acknowledgment
The authors thank Gaëlle Augé, Charlotte Cochaud, and Sandra Foustoul for technical help.
Author contributions
A. Chaussenot: clinical investigation, analysis of data, and critical revision of the manuscript. C. Rouzier: analysis of NGS data and critical revision of the manuscript. K. Fragaki: analysis of biochemical experiments and critical revision of the manuscript. S. Sacconi: clinical investigation. S. Ait-El-Mkadem: critical revision of the manuscript for intellectual content. V. Paquis-Flucklinger: study concept and design, critical revision of the manuscript. S. Bannwarth: study concept and design, acquisition, analysis and interpretation of data.
Study funding
Study funded by the French Ministry of Health and the Association Française contre les Myopathies (AFM).
Disclosure
A. Chaussenot, C. Rouzier, and K. Fragaki report no disclosures. S. Sacconi has received speaker honoraria from Sanofi Genzyme, Alnylam, and Biogen. S. Ait-El-Mkadem reports no disclosures. V. Paquis-Flucklinger has received research support from the French Ministry of Health and the Association Française contre les Myopathies (AFM). S. Bannwarth reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
References
- 1.Keightley JA, Anitori R, Burton MD, Quan F, Buist NR, Kennaway NG. Mitochondrial encephalomyopathy and complex III deficiency associated with a stop-codon mutation in the cytochrome b gene. Am J Hum Genet 2000;67:1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carossa V, Ghelli A, Tropeano CV, et al. A novel in-frame 18-bp microdeletion in MT-CYB causes a multisystem disorder with prominent exercise intolerance. Hum Mutat 2014;35:954–958. [DOI] [PubMed] [Google Scholar]
- 3.Emmanuele V, Sotiriou E, Rios PG, et al. A novel mutation in the mitochondrial DNA cytochrome b gene (MTCYB) in a patient with mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes syndrome. J Child Neurol 2013;28:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori M, Goldstein J, Young SP, Bossen EH, Shoffner J, Koeberl DD. Complex III deficiency due to an in-frame MT-CYB deletion presenting as ketotic hypoglycemia and lactic acidosis. Mol Genet Metab Rep 2015;4:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rana M, de Coo I, Diaz F, Smeets H, Moraes CT. An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Ann Neurol 2000;48:774–781. [PubMed] [Google Scholar]
- 6.Rizzo G, Pizza F, Scaglione C, et al. A case of fragile X premutation tremor/ataxia syndrome with evidence of mitochondrial dysfunction. Mov Disord 2006;21:1541–1542. [DOI] [PubMed] [Google Scholar]
- 7.Acín-Pérez R, Bayona-Bafaluy MP, Fernández-Silva P, et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell 2004;13:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]