Abstract
Recent advances in chemical composition and new production techniques resulted in improved biocompatibility and permeability of dialysis membranes. Among these, the creation of a new class of membranes called medium cut-off (MCO) represents an important step towards improvement of clinical outcomes. Such membranes have been developed to improve the clearance of medium to high molecular weight (MW) solutes (i.e. uraemic toxins in the range of 5–50 kDa). MCO membranes have peculiar retention onset and cut-off characteristics. Due to a modified sieving profile, MCO membranes have also been described as high-retention onset. The significant internal filtration achieved in MCO haemodialysers provides a remarkable convective clearance of medium to high MW solutes. The marginal loss of albumin observed in MCO membranes compared with high cut-off membranes is considered acceptable, if not beneficial, producing a certain clearance of protein-bound solutes. The application of MCO membranes in a classic dialysis modality characterizes a new technique called expanded haemodialysis. This therapy does not need specific software or dedicated hardware, making its application possible in every setting where the quality of dialysis fluid meets current standards.
Keywords: dialysis membranes, expanded haemodialysis, internal filtration, medium cut-off, uraemic toxins
INTRODUCTION
In spite of remarkable advances, high rates of hospitalization and mortality still characterize haemodialysis (HD) therapy. Although survival and quality of life have improved compared with the past, results are still unsatisfactory. New strategies and solutions are definitely required to respond to unmet clinical needs [1–3]. Poor clinical outcomes, however, are not only due to the increased age and comorbidity of the population, but also due to intrinsic limitations of current techniques. These are due to the inability of current dialysis membranes to remove the full spectrum of uraemic toxins accumulated in the body [4–6].
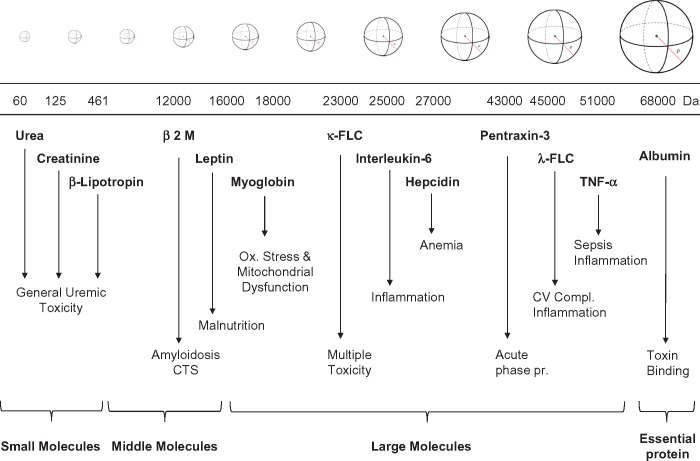
Complications such as anaemia, skeleton abnormalities, neuropathies and dialysis-related amyloidosis in patients with adequate urea kinetics and Kt/V have been correlated with uraemic toxins in the molecular range of 5000–50 000 Da not being adequately cleared by current dialysis techniques [5, 7, 8]. Classic and new uraemia retention molecules have been identified [7, 9, 10]. In particular, high levels of parathyroid hormone, fibroblast growth factor 23, osteoclastin, osteoprotegerin and other molecules involved in bone and calcium–phosphate metabolism have been associated with osteodystrophy. Hepcidin and proteins inhibiting bone marrow and erythropoiesis are responsible of uraemic anaemia. Homocysteine and mediators of inflammation have been correlated with accelerated atherosclerosis and cardiovascular complications. Increased leptin levels result in a significant reduction of appetite. Recently, κ free light chains (κFLCs; 22 000 Da) and λ free light chains (λFLCs; 42 000 Da) have also been identified as toxic molecules in uraemic patients [5]. Several of these molecules have a molecular weight (MW) well beyond the removal capacity of classic high-flux (HF) membranes and have molecular radii larger than the pores of dialysis membranes [11, 12]. Figure 1 summarizes the spectrum of uraemic toxins with specific reference to the MW of different retained molecules. Classic HF membranes are effective in removing small and some middle molecules, but other molecules are retained. Using different HF membranes, we demonstrated that convection (flux) is the most important determinant of beta-2 microglobulin (β2M) removal [7, 8]. Such studies paved the way for wide use of haemodiafiltration (HDF) as a technique to exploit the maximum removal capacity of HF membranes [7, 13, 14]. HDF is now applied, especially in Europe and Asia, with online production of replacement fluid (online HDF), allowing achievement of convective volumes up to 25 L/session [14, 15]. A convective clearance >23 L/session in online HDF showed survival benefits over standard HD in a clinical study [15]. In spite of simplified procedures and automatic equipment, online HDF requires a complex setup and dedicated technology, which are not universally approved or available. In spite of results achieved by onine HDF, clinical outcomes in dialysis are still suboptimal and unsatisfactory. Mortality is still high and so is the rate of hospitalization and cardiovascular complications [16].
FIGURE 1.
Schematic representation of different classes of uraemic toxins with their molecular size and relevant clinical effects.
INNOVATION IN HD IS MANDATORY
Because of the unsolved challenges in HD, there is high demand for innovative therapeutic solutions, new strategies, new materials and devices potentially capable of achieving improved clinical outcomes. This requires not only advanced research in the area of biomaterials and membranes, but also the design of specific techniques to exploit the maximum benefits from new membranes and materials. The evolution in biomaterials and membrane technology represents a good opportunity to find solutions for unanswered questions [17, 18]. New sciences such as microfluidics and nanotechnology may further contribute to significant progress in this direction [11, 19–22]. We can anticipate improvements in patient care thanks to treatment personalization and precision medicine. In fact, we might improve our results by refining therapeutic targets for specific patients, analysing their individual characteristics.
NEW DEVELOPMENTS IN DIALYSIS MEMBRANES
The advent of machines with ultrafiltration (UF) control systems ∼25 years ago led to the wide application of HF membranes. Thanks to their higher hydraulic permeability and larger pore dimensions, such membranes improved middle-molecule clearance and permitted the development of new techniques such as haemofiltration and HDF [14, 23]. Further evolution in membrane technology led to the development of protein-leaking membranes, also called super-flux or high cut-off membranes [17]. They are more permeable than conventional HF membranes and allow removal of larger solutes, with the drawback of some albumin loss. These membranes are used to remove toxins in the high MW range that are elevated in blood during sepsis, rhabdomyolysis and haematological disorders. In such circumstances, high MW solutes such as cytokines, myoglobin or FLCs are the main targets for removal. The limitation of these membranes however is the albumin leak due to the pore size opening, and their use is therefore indicated for only a few sessions in diffusive mode. Wider or prolonged application is not advised until more information is available on clinical benefits, side effects and threats.
MEDIUM CUT-OFF MEMBRANES
A new class of membranes called medium cut-off (MCO) has been recently introduced [18, 24, 25]. Despite the simplified definition indicating an intermediate value of cut-off, a more complex set of features characterizes these membranes [12].
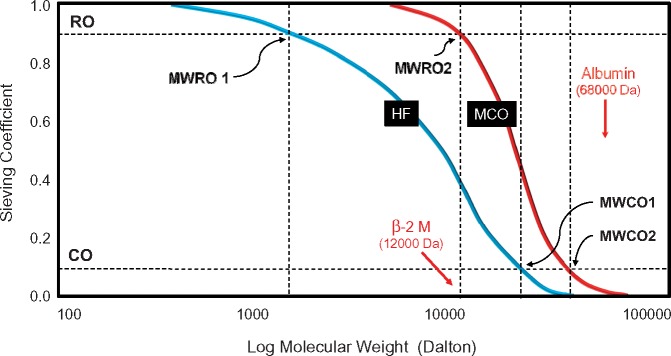
Evaluation of a dialysis membrane includes the identification of permeability characteristics in terms of sieving capacity. The sieving curve shows a progressive reduction of the observed values for solute sieving as the solute MW increases. When 90% of the solute is retained in the filtration process (sieving = 0.1), the corresponding MW of that solute defines the cut-off value of the membrane (MWCO). On the other side of the sieving curve, the MW at which 10% of the solute is retained (sieving = 0.9) defines the retention onset of the membrane (MWRO). The peculiar permeability profile of MCO membranes is displayed in Figure 2. The pore size distribution of the two membranes is the key factor in determining the different sieving properties and the remarkable separation of the two curves. In the multidimensional evaluation of dialysis membranes recently proposed [12], the MWRO value can be used to differentiate membranes independently depending on the cut-off value. As shown in the figure, the MCO membrane presents an MWRO in the range of β 2M, while at that MW classic HF membranes display a sieving value of 0.45. At the same time, the MWCO is very close to the value of HF membranes, limiting the loss of albumin. For this reason, the MCO membranes have also been defined as high retention onset membranes [11, 26]. MCO membranes are designed to improve clearances of medium to large MW solutes while avoiding significant albumin loss. This may represent a new approach to the treatment of uraemia, with potential effects on clinical outcomes.
FIGURE 2.
Sieving curves of classic HF and MCO membranes. The MWCO and MWRO characterize the shape of the sieving curve for each membrane and ultimately define the permeability properties.
EXPANDED HD
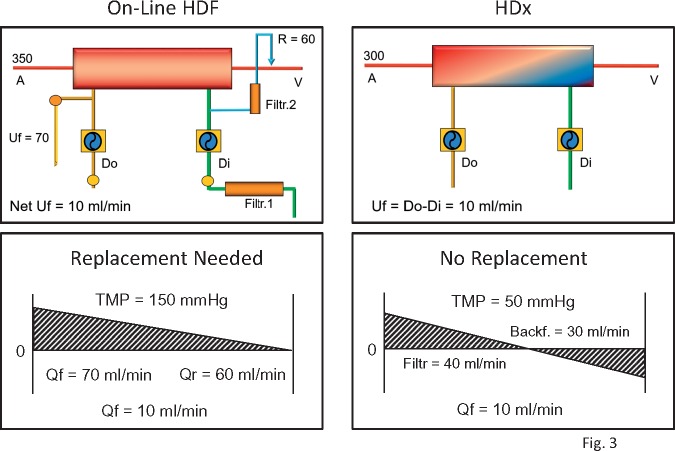
The clinical application of MCO may require updating the nomenclature of dialysis techniques. The term expanded HD (HDx) has been proposed to define a treatment where diffusion and convection are conveniently combined inside a hollow-fibre dialyser equipped with an MCO membrane [26, 27]. A standard dialysis machine can easily perform this technique without specific software or additional complex technology (Figure 3). Because convective clearance (K) results from the product of the UF rate (Qf) and sieving (S) of the selected molecule (K = Qf × S), when the sieving is low, the only way to increase K is to increase Qf. In the past, this was difficult due to limited hardware capabilities, membrane fouling, low routine blood flows and the requirement of expensive bags of substitution fluid. With the advent of online HDF, the problem of fluid procurement has been solved and high convection rates have been made possible thanks to the combined pre- and post-dilution configuration. HDx with MCO membranes represents a step forward in terms of efficiency and simplification (Figure 3). In HDx, the convective transport required to remove medium to large MW solutes is the result of a complex mechanism hidden inside the MCO haemodialyser [28, 29]. Reduction of the fibre inner diameter increases the wall shear rate, with a cleaning effect at the blood membrane interface and improved solute transport [30]. The increase of the end-to-end pressure drop produces significant implications on the cross-filtration profile along the length of the fibres. The combination of hydraulic permeability and geometric structure of the fibres enhances the process of internal filtration and backfiltration [31]. This mechanism, although invisible, allows a significant amount of convection inside the dialyser, where filtration takes place in the proximal part and backfiltration compensates for the excessive filtration rate in the distal part [32]. The UF control system of the dialysis machine regulates the process and provides the exact amount of net filtration required for the scheduled weight loss of the patient. A blood flow of 300 mL/min is sufficient to achieve optimal clearance in the system.
FIGURE 3.
HDx versus online HDF. The layout of the HDx circuit is simpler and there is no need for reinfusion (Qr) while net UF is set by the fluid balance control of the machine. The mechanism inside the filters is depicted in the lower panels for both techniques. In online HDF, large amounts of UF are achieved with high TMP and then replaced in the venous line after multiple steps of filtration of fresh dialysate. In HDx, the convection flow is maintained by internal filtration but it is compensated by the mechanism of backfiltration inside the filter. The special configuration of the MCO membrane with reduced inner diameter allows for high rates of internal filtration and backfiltration.
THEORETICAL MODELS AND EXPERIMENTAL DATA
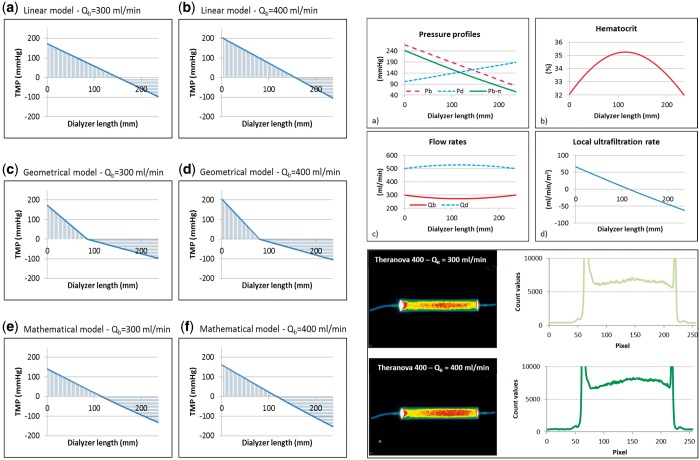
We have recently provided a theoretical model to characterize internal filtration in MCO haemodialysers [32]. Among different theoretical models, linear equations seem to represent the best fit for empirical results achieved in experimental conditions (Figure 4). This model allows one to estimate haematocrit variations along the length of the haemodialysers and to derive local cross-filtration at different points of the fibre bundle. In particular, for the Theranova 400 (1.7 m2) filter (Theranova, San Francisco, CA, USA) at blood flows of 300 and 400 mL/min, internal filtration was estimated to be 30 and 40 mL/min, respectively. Those values obtained from the linear model described in Lorenzin et al. [32] were then compared with the results achieved in a real experimental setting using a technique that was previously validated [33, 34]. Blood and dialysate compartment pressure along with transmembrane pressure (TMP) were measured in a closed in vitro circuit with human blood [blood flow (QB) = 300 and 400 mL/min; dialysate flow (QD) = 500 mL/min; net UF rate (net UF = 0 mL/min)]. A non-diffusible marker molecule (albumin macro-aggregates) labelled with metastable 99Tc was used to evaluate local cross-filtration at different points along the length of the filter. Relative variations in the concentration of the marker molecule along the length of the filter reported in the lower right panel of Figure 4 were used to calculate local cross-filtration. Based on the marker concentration profiles, internal filtration (IF) was estimated. For the Theranova 400, internal filtration was 29.7 and 41.6 mL/min for QB of 300 and 400 mL/min, respectively, confirming the validity of the linear theoretical model.
FIGURE 4.
In the left panel, the three theoretical models utilized to estimate internal filtration are reported (reprinted with permission from Lorenzin et al. [32]). The top right panel describes the haematocrit curve and the local filtration in different points along the length of the filter according to the linear theoretical model (reprinted with permission from Lorenzin et al. [32]). In the lower right panel, the scintigraphic images experimentally achieved using a non-diffusible marker molecule in a closed-loop circulation are reported. The curves describe the concentration of the marker molecule along the length of the haemodialysers in a condition of zero net filtration. Variations from inlet to peak concentration allow measurement of direct filtration while the variation from peak concentration to filter outlet allows measurement of backfiltration. These curves were achieved at steady state in conditions of 300 and 400 mL/min of blood flow in a 1.7 MCO haemodialyser. Data confirmed the linear theoretical model prediction.
PRACTICAL IMPLICATIONS OF INTERNAL FILTRATION
We can make a simulation using the sieving curves in Figure 2 and the internal filtration values obtained for a 2.0 m2 Theranova haemodialyser. Recent studies on HDF have suggested achieving at least 23 L/session of UF [15]. Considering the MW of β 2M and the sieving properties of a standard HF membrane, total β 2M clearance per session will be 23 × 0.5 = 11.5 L. To achieve the same result with an MCO membrane where S = 0.9, one will only need 12.7 L. According to our studies [32–34], a 2.0 m2 MCO haemodialyser generates an average internal filtration of 40 mL/min at zero net filtration in a 240-min dialysis session (Qb = 300 and Qd = 500, net UF = 16 mL/min), the internal filtration will be 56 mL/min with a backfiltration of 40 mL/min. An internal filtration of 56 mL/min will provide a total of 13 440 mL of filtration that, when multiplied by 0.9 (sieving), will produce an overall β 2M clearance of 12.96 L. The result is comparable and even superior to the numbers achievable with HDF, with a simpler treatment and fewer technical requirements. With this mechanism and the improved sieving characteristics, HDx may obtain additional benefits in terms of clearances for solutes such as FLCs, whose clearance in HDF is marginal.
HDx AND MCO MEMBRANES: THE DYNAMIC DUO
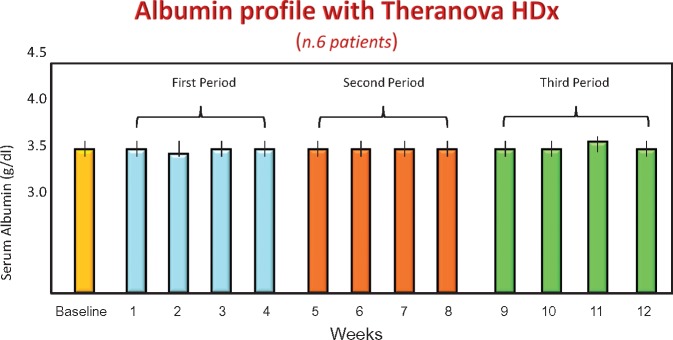
The shape of the sieving curve of the MCO membrane is peculiar and optimized to perform HDx. Using a simple UF-controlled HD technique, solute clearances in the spectrum of MWs traditionally retained with other techniques and membranes appear enhanced. In HDF, large amounts of UF require the replacement of volume by commercially prepared or online-produced fluids. In HDx this is not needed. A significant amount of internal convection is present, but it is masked and balanced by an adequate amount of internal backfiltration. Thus, in the presence of enhanced sieving values for large molecules such as β 2M or FLCs, relatively high clearances are achieved without the fluid exchange volumes normally required in HDF. The fibre length and inner diameter are essential elements to optimize internal filtration and the mechanism of filtration–backfiltration. There is no need for complicated equipment and a blood flow of ≥300 mL/min and a dialysate flow of ≥500 mL/min are adequate. Due to the significant amount of backfiltration occurring during treatment, water purity is important to avoid back transport of contaminants. One issue that remains open is the effect of marginal leakage of albumin during HDx. This will be clarified in long-term studies, as the effects of uraemic toxins are usually retained by classic membranes. We explored the behaviour of albumin on six patients treated for >6 months and the results are reported in Figure 5. We measured albumin loss once per week and found an amount between 2 and 4 g/session. This resulted in absolutely no variation in albumin concentration after 6 months of HDx therapy with Theranova (Figure 5).
FIGURE 5.
Albumin concentrations in six patients treated for 6 months with Theranova filters and HDx.
CONCLUSIONS
The need to improve outcomes in clinical dialysis requires a continuous process of innovation and research [10, 35, 36]. The case of MCO membranes and HDx is a typical example of progress and innovation in dialysis. Of course, more evidence will be required to support the hypothesis that this dynamic duo may produce a significant effect in dialysis outcomes. However, the rationale is solid and the initial results are encouraging [37, 38]. The execution of a large randomized controlled trial is unlikely due to a lack of resources, but also due to a better understanding of the difference between the statistical and clinical meaning of an intervention. Several years of observation and an enormous sample would be required because of high dropout rates in long-term studies and the relatively low frequency of events considered as solid endpoints. Furthermore, the heterogeneity of the studied population and the different referral patterns will make it difficult to decide who should be the candidate for the trial and what the exclusion criteria should be. It will be easier to perform simple pragmatic trials utilizing registries and big data analysis derived from electronic medical records once HDx is sufficiently adopted in the clinical routine.
The required evidence may result from group analysis or even single-patient evaluation according to precision medicine criteria. A new emerging question concerns the indications for HDx and the identification of the population or the individuals who are likely to benefit from the application of this technique. We must determine if HDx should be best used as a rescue therapy in patients with uraemic toxin–related complications (erythropoietin-resistant anaemia, malnutrition-inflammation complex syndrome, bone and cardiovascular abnormalities) or as an elective therapy for incident patients before severe complications develop. HDx could be an ideal transition therapy for patients moving from peritoneal dialysis and waiting for a transplant. This is an area where the application of precision medicine and treatment personalization will be highly useful [38–41]. The response to these questions may come from personal experience and pragmatic trials, as has happened for previous innovative therapies such as bicarbonate dialysis and HDF. The application and rapid diffusion of these techniques in the past were the result of personal experience and self-evidence. We could never demonstrate statistically the superiority of bicarbonate over acetate, of HF membranes over cuprophan and of UF control systems over manual TMP regulation, but the adoption of such innovations has been rapid and extensive. If HDx maintains the promises coming from its rationale, we may imagine seeing a similar phenomenon in the years to come.
ACKNOWLEDGEMENTS
This article is published as part of a Supplement to NDT on ‘Translating Innovation to Clinical Outcomes’, financially supported by Baxter Healthcare Corporation.
CONFLICT OF INTEREST STATEMENT
C.R. received speakers honoraria from FMC, ESTOR, GE, Medtronic, Toray Medical, Biomerieux and B. Braun. C.R. is a consultant for Astute Medical, Asahi Medical, Baxter, OCD, Jaffron and Cytosorbents. The other authors have no conflicts of interest to declare.
REFERENCES
- 1. Vanholder R, De Smet R, Glorieux G. et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 2003; 63: 1934–1943 [DOI] [PubMed] [Google Scholar]
- 2. Neirynck N, Vanholder R, Schepers E. et al. An update on uremic toxins. Int Urol Nephrol 2013; 45: 139–150 [DOI] [PubMed] [Google Scholar]
- 3. Miyata T, Jadoul M, Kurokawa K. et al. Beta-2 microglobulin in renal disease. J Am Soc Nephrol 1998; 9: 1723–1735 [DOI] [PubMed] [Google Scholar]
- 4. Cianciolo G, Coli L, La Manna G. et al. Is beta2-microglobulin-related amyloidosis of hemodialysis patients a multifactorial disease? A new pathogenetic approach. Int J Artif Organs 2007; 30: 864–878 [DOI] [PubMed] [Google Scholar]
- 5. Desjardins L, Liabeuf S, Lenglet A. et al. Association between free light chain levels, and disease progression and mortality in chronic kidney disease. Toxins (Basel) 2013; 5: 2058–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ronco C, Brendolan A, Bragantini L. et al. Technical and clinical evaluation of different short, highly efficient dialysis techniques. Contrib Nephrol 1988; 61: 46–68 [DOI] [PubMed] [Google Scholar]
- 7. Locatelli F, Mastrangelo F, Redaelli B. et al. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. Kidney Int 1996; 50: 1293–1302 [DOI] [PubMed] [Google Scholar]
- 8. Locatelli F, Gauly A, Czekalski S. et al. The MPO Study: just a European HEMO Study or something very different? Blood Purif 2008; 26: 100–104 [DOI] [PubMed] [Google Scholar]
- 9. Babb AL, Popovich RP, Christopher TG. et al. The genesis of the square meter-hour hypothesis. Trans Am Soc Artif Intern Organs 1971; 17: 81–91 [PubMed] [Google Scholar]
- 10. Yu X. The evolving patterns of uremia: unmet clinical needs in dialysis. Contrib Nephrol 2017; 191: 1–7 [DOI] [PubMed] [Google Scholar]
- 11. Clark WR, Gao D, Neri M. et al. Solute transport in hemodialysis: advances and limitations of current membrane technology. Contrib Nephrol 2017; 191: 84–99 [DOI] [PubMed] [Google Scholar]
- 12. Ronco C, Neri M, Lorenzin A. et al. Multidimensional classification of dialysis membranes. Contrib Nephrol 2017; 191: 115–126 [DOI] [PubMed] [Google Scholar]
- 13. Ronco C. Hemodiafiltration: technical and clinical issues. Blood Purif 2015; 40(Suppl 1): 2–11 [DOI] [PubMed] [Google Scholar]
- 14. Ronco C. Hemodiafiltration: evolution of a technique towards better dialysis care. Contrib Nephrol 2011; 168: 19–27 [DOI] [PubMed] [Google Scholar]
- 15. Maduell F, Moreso F, Pons M. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barreto FC, Stinghen AE, de Oliveira RB. et al. The quest for a better understanding of chronic kidney disease complications: an update on uremic toxins. J Bras Nefrol 2014; 36: 221–235 [DOI] [PubMed] [Google Scholar]
- 17. Ward RA. Protein-leaking membranes for hemodialysis: a new class of membranes in search of an application? J Am Soc Nephrol 2005; 16: 2421–2430 [DOI] [PubMed] [Google Scholar]
- 18. Boschetti-de-Fierro A, Voigt M, Storr M. et al. MCO membranes: enhanced selectivity in high-flux class. Sci Rep 2015; 5: 18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellomo R, Ronco C.. Blood purification in the intensive care unit: evolving concepts. World J Surg 2001; 25: 677–683 [DOI] [PubMed] [Google Scholar]
- 20. Ronco C, Brendolan A, Dan M. et al. Adsorption in sepsis. Kidney Int 2000; 58(Suppl 76): S148–S155 [DOI] [PubMed] [Google Scholar]
- 21. Haase M, Kellum JA, Ronco C.. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol 2012; 8: 735–739 [DOI] [PubMed] [Google Scholar]
- 22. Neri M, Villa G, Garzotto F. et al. Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care 2016; 20: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronco C, Crepaldi C, Brendolan A. et al. Evolution of synthetic membranes for blood purification: the case of the Polyflux family. Nephrol Dial Transplant 2003; 18(Suppl 7): vii10–vii20 [DOI] [PubMed] [Google Scholar]
- 24. Boschetti-de-Fierro A, Voigt M, Storr M. et al. Extended characterization of a new class of membranes for blood purification: the high cut-off membranes. Int J Artif Organs 2013; 36: 455–463 [DOI] [PubMed] [Google Scholar]
- 25. Kirsch AH, Lyko R, Nilsson LG. et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017; 32: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ronco C. The rise of expanded hemodialysis. Blood Purif 2017; 44: I–VIII [DOI] [PubMed] [Google Scholar]
- 27. Ronco C, La Manna G.. Expanded hemodialysis: a new therapy for a new class of membranes. Contrib Nephrol 2017; 190: 124–133 [DOI] [PubMed] [Google Scholar]
- 28. Ronco C. Fluid mechanics and crossfiltration in hollow-fiber hemodialyzers. Contrib Nephrol 2007; 158: 34–49 [DOI] [PubMed] [Google Scholar]
- 29. Rangel AV, Kim JC, Kaushik M. et al. Backfiltration: past, present and future. Contrib Nephrol 2011; 175: 35–45 [DOI] [PubMed] [Google Scholar]
- 30. Ronco C, Brendolan A, Lupi A. et al. Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int 2000; 58: 809–817 [DOI] [PubMed] [Google Scholar]
- 31. Fiore GB, Ronco C.. Principles and practice of internal hemodiafiltration. Contrib Nephrol 2007; 158: 177–184 [DOI] [PubMed] [Google Scholar]
- 32. Lorenzin A, Neri M, Clark WR. et al. Modeling of internal filtration in theranova hemodialyzers. Contrib Nephrol 2017; 191: 127–141 [DOI] [PubMed] [Google Scholar]
- 33. Fiore GB, Guadagni G, Lupi A. et al. A new semiempirical mathematical model for prediction of internal filtration in hollow fiber hemodialyzers. Blood Purif 2006; 24: 555–568 [DOI] [PubMed] [Google Scholar]
- 34. Ronco C, Brendolan A, Feriani M. et al. A new scintigraphic method to characterize ultrafiltration in hollow fiber dialyzers. Kidney Int 1992; 41: 1383–1393 [DOI] [PubMed] [Google Scholar]
- 35. Jankowska M, Cobo G, Lindholm B. et al. Inflammation and protein-energy wasting in the uremic milieu. Contrib Nephrol 2017; 191: 58–71 [DOI] [PubMed] [Google Scholar]
- 36. Foley RN, Parfrey PS, Harnett JD. et al. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol 1996; 7: 728–736 [DOI] [PubMed] [Google Scholar]
- 37. Mitra S, Kharbanda K.. Effects of expanded hemodialysis therapy on clinical outcomes. Contrib Nephrol 2017; 191: 188–199 [DOI] [PubMed] [Google Scholar]
- 38. Zickler D, Schindler R, Willy K. et al. Medium cut-off (MCO) membranes reduce inflammation in chronic dialysis patients-a randomized controlled clinical trial. PLoS One 2017; 12: e0169024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hutchison CA, Wolley M.. The rationale for expanded hemodialysis therapy (HDx). Contrib Nephrol 2017; 191: 142–152 [DOI] [PubMed] [Google Scholar]
- 40. Heyne N. Expanded hemodialysis therapy: prescription and delivery. Contrib Nephrol 2017; 191: 153–157 [DOI] [PubMed] [Google Scholar]
- 41. Willy K, Girndt M, Voelkl J. et al. Expanded haemodialysis therapy of chronic haemodialysis patients prevents calcification and apoptosis of vascular smooth muscle cells in vitro. Blood Purif 2018; 45: 131–138 [DOI] [PubMed] [Google Scholar]