Abstract
Cardiovascular disease (CVD) is a highly common complication and the first cause of death in patients with end-stage renal disease (ESRD) on haemodialysis (HD). In this population, mortality due to CVD is 20 times higher than in the general population and the majority of maintenance HD patients have CVD. This is likely due to ventricular hypertrophy as well as non-traditional risk factors, such as chronic volume overload, anaemia, inflammation, oxidative stress, chronic kidney disease–mineral bone disorder and other aspects of the ‘uraemic milieu’. Better understanding the impact of these numerous factors on CVD would be an important step for prevention and treatment. In this review we focus non-traditional CVD risk factors in HD patients.
Keywords: cardiovascular disease, chronic kidney disease, haemodialysis
INTRODUCTION
Cardiovascular disease (CVD) is the major cause of morbidity and mortality in patients with end-stage renal disease (ESRD) on haemodialysis (HD) [1, 2]. Since ESRD frequently results from hypertension and diabetes mellitus, the increased CVD risk in these patients has been assumed to be the result of these underlying diseases. Nevertheless, it has been elucidated how ESRD represents per se a CVD risk factor independently by both hypertension and diabetes mellitus [1, 2].
CVD is present in >50% of patients undergoing dialysis and the relative risk of death due to CVD events in HD patients is reported to be 20 times higher than in the general population. In fact, in patients on renal replacement therapy (RRT) the prevalence of coronary heart disease and ventricular hypertrophy has been described to be 40% and 70%, respectively [3]. The prevalence of hypertension, a major risk factor for coronary artery disease (CAD) and left ventricular hypertrophy (LVH) is high in patients with chronic kidney disease (CKD) (87–90%). At least 35% of patients with CKD have evidence of an ischaemic event (myocardial infarction or angina) at the time of presentation to a nephrologist. The prevalence of LVH increases at each stage of CKD, reaching 75% at the time of dialysis initiation, and the modifiable risk factors for LVH include anaemia and systolic blood pressure, which are also worse at each stage of kidney disease.
Although there have been significant improvements in the management of CVD in the general population, it is not known if these interventions result in similar benefits for HD patients. In addition, differences in the types, distribution, mortality and pathophysiology of CVD in ESRD patients suggest that generalization of data from patients without kidney disease should be extrapolated with caution. CV risk factors among HD patients can be divided into those that are non-specific to kidney disease but are more prevalent and those that are specific to ESRD. There is higher prevalence of many traditional factors for CV risk (age, male gender, hypertension, diabetes, dyslipidaemia and physical inactivity). Furthermore, HD patients have disease-related risk factors such as anaemia, hyperhomocysteinaemia, chronic kidney disease–mineral bone disorder (CKD-MBD), oxidative stress, malnutrition and chronic inflammation. There is evidence that uraemic factors may be implicated in the pathogenesis of CV disease in HD patients, since CV survival improves after kidney transplantation even in high-risk patients [4, 5].
In this review we focus on non-traditional CV risk factors in HD patients.
URAEMIC TOXINS AND CVD
With the decline of renal function, a large group of substances are inadequately removed by the kidney: these are the uraemic toxins. They are biologically active and are both cause and consequence of CKD, with a detrimental effect on the CV system, possibly mediated by their impact on the functions of cells involved in myocardial and vessel functions [leucocytes, endothelial cells (ECs), smooth muscle cells, platelets] [6]. In fact, an inhibition of leucocyte activity observed in HD patients may compromise infection response and trigger a condition of micro-inflammation and consequently atherosclerosis. Moreover, uraemic toxins induce an enhancement of leucocyte oxidative activity, an up-regulation in leucocyte–endothelial interactions and an infiltration of macrophages and monocytes into vascular atherosclerotic lesions [3].
p-Cresol is a metabolic product of aromatic amino acids generated in the gut through the bacterial degradation of tyrosine and phenylalanine [7]. It is absorbed from the intestinal tract and almost completely converted in its main metabolites, p-cresylsulfate (pCS) and p-cresylglucuronide, two small molecules significantly bound to plasma proteins. While healthy kidneys efficiently remove p-cresol, it tends to accumulate in CKD patients, for two major reasons: because of the modifications of gut microbiome in CKD and the inability of the failing kidney to excrete it. High plasma concentrations of total p-cresol strongly associate to CV risk in CKD and are predictive of mortality [8, 9]. Since pCS remains difficult to remove by dialysis, the gut microbiota could be a future target to decrease pCS levels and its toxicity, even at earlier stages of CKD.
In vitro studies have demonstrated that p-cresol can cause EC dysfunction through a toxic mechanism mediated by Rho-kinase activity [10, 11]. Therefore therapeutic strategies to reduce plasma p-cresol levels are needed. In vitro studies have shown that sevelamer hydrochloride, a synthetic phosphate binder widely used in dialysis patients, can also bind p-cresol [12].
Another uraemic toxin is indoxyl sulphate (IS), which is greatly accumulated in CKD and its concentration can increase up to 50-fold compared with healthy people. IS results from tryptophan metabolism and it has a high-affinity of binding to albumins, a condition that hinders its removal by HD. It exerts pro-oxidative and pro-inflammatory activity, triggers immune response and stimulates the progression of CKD [13, 14].
The negative action of IS on the CV system seems to be concentration dependent. In CKD patients, IS induces a pro-oxidative action leading to increased CVD, while in normal renal function subjects, IS plays a potential protective role, eliminating hydroxyl radicals [3]. Several authors indicate a potential role of IS in the progression of vascular damage, haemostatic dysfunction and the progressive inflammatory process. According to Shivanna et al. [15], Chitalia et al. [16] and Kamiński et al. [17], IS is a potential CKD-related pro-thrombotic uraemic toxin that induces tissue factor (TF) in vascular smooth muscle cells (VSMCs) and increases post-vascular interventional pro-thrombotic risk in a TF-dependent manner.
Therefore both IS and p-cresol increased their concentrations in uraemic patients, inducing a reduction in endothelial proliferation and negatively influencing cell repair processes in vitro, both involved in atherosclerosis pathogenesis. Furthermore, both IS and p-cresol are able to inhibit nitric oxide (NO) production, which is decreased in CKD patients, and to reduce EC viability by in vitro reactive oxygen species (ROS) production.
Advanced glycation end products (AGEs), a group of compounds formed by the ‘Maillard reaction’ between reducing sugars and amino acids, lipids or DNA, are formed also in diseases with high levels of oxidative stress, such as ESRD. In these patients high levels of circulating AGEs result from increased formation and decreased renal clearance. Interactions between AGEs and their receptor AGEs (RAGEs) trigger various intracellular events, such as oxidative stress and inflammation, leading to CV complications. Today there is a large body of evidence showing that the interaction between AGEs and RAGEs plays a key role in vascular damage, with special concern for endothelial dysfunction and arterial stiffness [18].
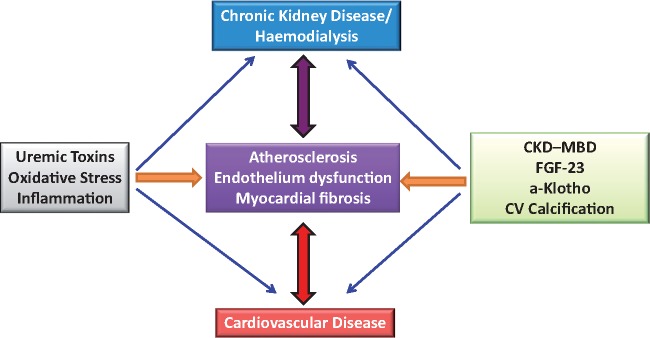
FIGURE 1.
Cardiovascular disease in dialysis.
OXIDATIVE STRESS, ENDOTHELIAL DYSFUNCTION AND CVD
Oxidative stress is considered an imbalance in the ROS production:degradation ratio. There is a lot of evidence that uraemia is a pro-oxidant condition. Since kidney is one of the most important sources of antioxidant enzymes, progressive loss of renal function increases the level of pro-oxidant substances [3]. Oxidative stress is quite common in ESRD and contributes to the progression of kidney damage by promoting renal ischaemia, by inciting glomerular injury, by inducing cell death and by stimulating inflammation [19]. In addition, oxidative stress gradually increases along with renal impairment and it is further exacerbated by HD. Known oxidative molecules are ROS and nitrogen species such as NO. The bioavailability of NO largely contributes to endothelial dysfunction in many diseases such as hypertension, atherosclerosis and diabetes. Asymmetric dimethylarginine (ADMA) is an endogenous amino acid similar to L-arginine and is able to inhibit endothelial nitric oxide synthase. ADMA is associated with impaired NO synthesis and is considered a predictor of CV events and death both in ESRD patients and in the general population [20]. Furthermore, EC damage is related to diseases such hypertension, renal failure and atherosclerosis and is considered responsible for accelerated atherosclerosis in ESRD patients. Increased levels of ADMA in ESRD due to a reduction in inactivation and elimination processes has been reported to be a novel risk factor for endothelial dysfunction. High ADMA levels are associated with endothelial dysfunction as well as with higher intima-media thickness and CV events in ESRD. Moreover, Ravani et al. [21] reported that high ADMA levels represent a strong independent risk factor for progression of CKD and patient death. Reduced NO synthesis by ECs due to accumulation of endogenous inhibitors of the NOS such as ADMA has been implicated in accelerating progression of endothelial dysfunction [22].
Recently Liakopoulos et al. [23] reported all conditions correlated to oxidative stress in HD patients. HD is characterized by excessive oxidative stress due to loss of antioxidants during procedures and to accumulation of oxidative products. It is thought that HD promotes the formation of pro-oxidant reactive oxygen molecules with a direct increase in plasma ROS levels after each HD session [24, 25]. Interestingly, Bayes et al. [26] found reduced serum vitamin E levels in HD patients, while Morena et al. [27] reported that, compared with healthy controls, patients undergoing chronic haemodiafiltration had significantly lower plasma levels of vitamin C.
The HD process promotes the formation and accumulation of oxidative products through activation of platelets, complement and polymorphonuclears. Two studies showed that plasma ROS levels were significantly increased in HD patients compared with healthy controls, a condition explained with the prominent dysfunction of the mitochondrial respiratory system in CKD and in HD patients [23].
CHRONIC INFLAMMATION AS A CV RISK FACTOR
Chronic inflammation in CKD patients may be detected by high levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6). In several studies it has been shown that plasma IL-6 levels are strong predictors of CV mortality in dialysis patients [28]. Furthermore, it has been claimed that endogenous methylarginines, ADMA and symmetric dimethylarginine (SDMA) and other methylarginines including L-NG monomethylarginine (LNMMA) are contributing factors to inflammation. In fact, high plasma ADMA levels are suggestive of endothelial dysfunction and atherosclerosis in the general population. ADMA is increased in ESRD and predicts mortality in this population. Moreover, ADMA increases IL-6 and tumour necrosis factor-alpha (TNF-α) expression, and ADMA represents the primary endogenous inhibitor of NO synthase, which leads to a reduction in the bioavailability of NO [29]. Interestingly, Emrich et al. [30] performed a study on blood samples from 528 patients with CKD Kidney Disease: Improving Global Outcomes (KDIGO) classification G2–G4, looking for an association of elevated plasma levels of ADMA, SDMA, acetylated ADMA, acetylated SDMA and LNMMA and atherosclerotic CVD. In particular, CKD patients in the highest tertile of plasma SDMA remained at highest risk for CKD progression and atherosclerotic CV events.
PROTEIN CARBAMYLATION
Blood levels of urea rise with a progressive decline in kidney function. Older studies suggested the non-toxicity of high blood urea concentration after acute infusion, but some evidence of direct and indirect toxicity of chronically high urea levels is emerging [31]. Chronically high levels of urea cause alterations in different target organs: (i) gastrointestinal epithelial barrier breakdown with bacterial toxins passage, causing systemic inflammation; (ii) activation of protein carbamylation reaction secondary to isocyanic acid dissociation, altering the structure and function of proteins; (iii) CV atherosclerosis by carbamylation of low-density lipoprotein; (d) renal fibrosis due to carbamylation of albumin and (e) anaemia by erythropoietin carbamylation. Carbamylation is linked to an increased CV risk in ESRD patients. Intensification of HD therapy in RRT patients may help in controlling low levels of plasma urea and reducing carbamylate protein concentrations by reducing CV risk [32].
CKD-MBD AND CARDIOVASCULAR CALCIFICATIONS
Cardiovascular calcifications (CVCs) are a well-known CV risk factor in HD patients. In vitro studies have shown that the process of vascular calcification does not consist of a simple deposition of calcium phosphate crystals, but appears to be an active regulated process resembling bone osteogenesis [33]. The vascular calcification process is complex and involves a transdifferentiation of VSMCs into cells resembling bone progenitor cells. These cells down-regulate the production of specific genes and up-regulate the growth of osteochondrogenesis markers, losing their contractile competence and acquiring collagen matrix production and forming calcium–phosphorus-rich vesicles able to start the mineralization process of the vessel internal surface [34]. In dialysis patients, CVC can increase the incidence of arrhythmias, sudden cardiac death, stroke and mortality. Calcified blood vessels lead to ischaemic CVD and increased pulse pressure and pulse wave velocity (PWV), contributing to the reduction of diastolic coronary perfusion and to LVH, which is a well-known CV risk factor. Aortic stenosis, a frequent consequence of calcification processes, increases cardiac afterload, thus further contributing to LVH [35]. In a recent meta-analysis, Wang et al. [36] confirmed that a higher degree of CVC was associated with an increased risk of mortality and that the number of calcified cardiac valves is correlated with the mortality risk. The prevalence of aortic arch calcification ranged from 23.27% to 57.60%, and in dialysis patients the presence of CVC increased the risk of CV mortality by 181% and by 73% for all-cause mortality. Moreover, patients in dialysis with two calcified cardiac valves had a greater mortality risk, as revealed by a subgroup analysis. This study also provides evidence that the detection of valve calcification is fundamental for risk stratification of dialysis.
Computed tomography (CT) is the gold standard for evaluation of vascular calcification, but plain radiographs could offer a less costly alternative with less radiation exposure, given also that the KDIGO guidelines suggest the use of lateral abdominal radiograph to detect the presence or absence of vascular calcification in CKD Stage 3–5 patients as a reasonable alternative to CT-based imaging [37]. In addition to lateral abdominal radiographs, plain radiographs of chest, pelvis, both hands and feet can also be utilized to evaluate calcification of the aortic arch, iliac arteries, femoral arteries and small arteries of the hands and feet.
Dimkovic et al. [38] conducted a longitudinal observational study, the aim of which was to assess CV mortality in HD patients with different baseline composite calcification scores, where composite calcification scores were determined by combining ultrasound (Adragao X-ray score) and X-ray analyses (X-ray score of the arteriovenous fistula). In patients dying from CVD, composite and Adragao calcification scores were significantly higher, as was PWV compared with survivors. Moreover, a post hoc analysis confirmed that deceased patients had significantly lower serum fetuin-A, osteoprotegerin (OPG), and haemoglobin, but higher C-reactive protein (CRP) levels as compared with surviving patients. The authors noted a relationship between PWV and the degree of vascular calcification. Although this relation is still a matter of debate, stiffened arteries may have a significant impact on CV remodelling, leading to LVH, which could contribute to heart failure and/or sudden cardiac death.
Since the Framingham risk score poorly predicts CV events in HD patients, several CKD-MBD biomarkers, such as fetuin-A, OPG and osteopontin, have been associated with vascular calcification [37, 39].
OPG is a soluble cytokine of the TNF-receptor superfamily expressed in osteoblasts, ECs, VSMCs or the myocardium. It is modulated by inflammatory cytokines and inhibits osteoclast differentiation and activity and for this reason it is called a ‘bone protector’. Osteogenic markers including OPG are suggested to play an important role in the mineralization of ectopic sites and are linked to vascular calcification. Although OPG is suggested to have an active role in vascular pathophysiology, it is not currently clear whether this role is beneficial or injurious to the vasculature. Fetuin-A is a circulating glycoprotein secreted by the hepatocytes. It acts as a potent systemic inhibitor of calcification and a negative acute phase reactant. In HD patients, plasma fetuin-A levels are lower than in healthy controls and associated with severe and extensive vascular calcification and arterial stiffness, as well as with increased all-cause and CV mortality [39].
Collado et al. [40] tried to assess whether serum OPG and/or fetuin-A predict mortality and CV morbidity and mortality in HD patients, as well as their association with atherosclerosis and/or vascular calcification and with cardiac structure and function. The presence of carotid atherosclerotic disease, defined by a pathological intima-media thickness and/or presence of carotid plaques with stenosis or calcified atherosclerotic plaques was associated with higher OPG levels but not with fetuin-A levels. These results provide additional evidence, as well as a recent meta-analysis that reported an increased mortality risk associated with higher OPG levels [41, 42]. It is still not clear whether OPG is causally related to atherosclerosis or is a marker of the atherosclerotic burden. Despite that higher OPG levels have also been associated with an increased CV risk in the general population, drugs targeting the receptor activator of nuclear factor κβ (RANK)–RANK ligand–OPG axis have not been associated with an effect on CV events in randomized controlled trials in the general population.
FIBROBLAST GROWTH FACTOR-23, KLOTHO AND CVD
Fibroblast growth factor-23 (FGF23) is a protein primarily secreted by bone tissue (osteoclasts and osteocytes) that regulates phosphate excretion together with parathyroid hormone (PTH) via interaction with FGF receptors. Physiologically FGF23 requires the presence of coreceptor α-Klotho to perform its activity. The Klotho gene encodes a single-pass transmembrane protein expressed in multiple tissues but is found at particularly high levels in the kidney. Clearly Klotho and FGF23 interplay to participate in mineral homeostasis [43, 44]. In fact, FGF23 exerts its effects at the level of proximal and distal kidney tubules, where it inhibits the sodium–phosphate co-transporter mechanism in a modality resembling PTH action, resulting in a phosphaturic effect and in sodium storage that leads to development of hypervolaemia and potentially hypertension.
FGF23 reduces phosphorus levels by inhibiting the synthesis of 1,25-hydroxyvitamin D and by suppressing PTH synthesis in the parathyroid glands, probably in a Klotho-dependent manner [3]. In CKD patients, levels of FGF23 increase early in the course of kidney disease already in Stage 2, earlier than both phosphorus and PTH increases [44]. Klotho and FGF23 might be novel diagnostic targets for the early diagnosis of renal dysfunction and prediction of chronic complications, including CVD in ESRD. In ESRD, FGF23 reaches levels 100 times the physiological values. According to some studies, an elevated plasma FGF23 concentration is independently associated with an increased risk of CKD progression, CV complications and mortality in ESRD. In addition, in the general population, FGF23 plasma levels in coronary patients were associated with a higher risk of death and CV, even after adjustment for confounding factors.
Elevated FGF23 levels were independently associated with LVH in a large CKD cohort. FGF23 caused pathological hypertrophy of isolated rat cardiomyocytes via FGF receptor–dependent activation of the calcineurin–nuclear factor of activated T cells signalling pathway, but this effect was independent of α-klotho [45].
As reported by Cozzolino and Mazzaferro [46], soluble Klotho is dramatically decreased in CKD and ESRD. Klotho deficiency and FGF23 elevation are associated with poor outcomes and complications in CKD/ESRD patients. They have also been proposed as sensitive biomarkers for adverse renal and extrarenal outcomes in patients with CKD and ESRD. Accumulating evidence from animal experiments has shown that Klotho deficiency causes vascular calcification, cardiac fibrosis and cardiac hypertrophy in CKD [47, 48]. A cross-sectional study of CKD patients with a modest decline in renal function revealed that serum Klotho is independently associated with arterial stiffness measured by ankle–brachial PWV [49]. Moreover, decreased Klotho production renders the kidney more susceptible to a variety of kidney insults and exacerbates uraemic cardiomyopathy and vascular calcification.
GUT MICROBIOME (GM) AS A POTENTIAL SOURCE OF URAEMIC TOXINS
The GM performs a multitude of functions and can be considered a metabolically active endogenous organ. Dysbiosis is the alteration of the human microbiota composition in a given site and can stimulate pro-inflammatory cytokine production and foam cells formation. This generates a ring reaction in which inflammation can induce oxidative stress and the latter in turn increases the inflammatory state [50]. The possible breaking of the intestinal barrier (epithelial tight junctions) can lead to the translocation of bacteria and endotoxins in the circulatory system, inducing inflammation. HD itself may induce intestinal ischaemia during hypotension with alteration of intestinal wall integrity. Evidence indicates a relationship between host and the altered microbiota in animals and humans with CKD. Many authors have described an important change in the structure and function of GM in humans and animals with advanced CKD; these studies showed a clear reduction in the Bifidobacterium spp.:Enterobacteriaceae ratio. Changes in the microbiota composition produce excessive quantities of uraemic toxins such as p-cresol sulphate, IS and trimetylamine-N-oxide, but fewer metabolites with a protective role in inflammation, oxidative stress, fibrosis and kidney diseases progression [50]. Gut-derived uraemic toxins contribute to the progression of CKD as well as CVD: the Eutox group showed that an elevated level of IS is associated with vascular stiffness, aortic calcification and higher CV mortality. Free serum levels of p-cresol are associated with CV mortality in dialysis patients [3].
ACIDOSIS AND CVD
Excretion of the acid produced by metabolism is mainly accomplished by two pathways: the lungs, responsible for eliminating volatile acids, and the kidneys, for the non-volatile acids. Metabolic acidosis is a common condition in ESRD patients, due to a decreased ability to excrete non-volatile acid and reduced renal synthesis of bicarbonate. It leads to malnutrition, inflammation, bone disease disorders and even a higher death risk [51].
Major acid–base variations during dialysis and the imbalances in serum calcium levels intensified by them play a role in CVD in HD patients. There is a significant positive association of metabolic acidosis status with high-sensitivity CRP serum concentrations. The aim of the study by Raikou and Kyriaki [52] was the association between low serum bicarbonate concentrations and CVD in patients on intermittent dialysis. They found that metabolic acidosis status was significantly associated with the existence of both peripheral vascular disease and diastolic dysfunction. However, they did not find that the low bicarbonates were an independent risk factor for diastolic dysfunction after adjustment for covariates and they found no significant association between low bicarbonate and systolic cardiac disorder or coronary artery disease. Another prospective study of Voiculeț et al. [53] highlighted the repercussions of low levels and variation in serum bicarbonate on bone mineral metabolism parameters, as well as on vascular stiffness and the vascular calcification process in chronic HD patients. Although HD is the most important method of correction for the metabolic acidosis of ESRD patients, the changes in serum bicarbonate levels produced by a dialysis session are sometimes too abrupt and tempestuous, producing adverse consequences. A statistically significant association has been found between dialysis bicarbonate, average serum calcium levels and serum phosphorus, as well as PWV mean values and the number of vascular calcifications. It is important to avoid large variations in serum bicarbonate levels in HD patients because these variations can increase vascular stiffness, vascular calcification and, in general, CV risk.
CONCLUSIONS AND PERSPECTIVES
Numerous other risk factors involved in the pathogenesis of CVD in dialysis patients are not included in this review. In particular, there is evidence that intradialytic volume overload can affect the development of LVH and cardiac failure. Fluctuations in plasma levels of sodium, potassium, calcium and magnesium during HD treatment may trigger life-threatening cardiac arrhythmias. Moreover, hypotension during dialysis can induce ischaemic events in different organs and tissues, such as the myocardium, gut and central nervous system, even if clinically undetectable. Today, other factors are under investigation in ESRD patients, such as cardiotonic steroids (CTS), a new class of hormones with the ability to bind and inhibit the ubiquitous Na+/K+ transport enzyme pump. Serum levels of ouabain, telocinobufagin and marinobufagenin are substantially elevated in patients with ESRD. There is some evidence that chronic exposure to CTS can contribute to CVD development characterized by LVH, fibrosis, diastolic dysfunction, arrhythmias and a reduction in ejection fraction [54].
Considering the high burden that CVD has in HD patients, additional studies are needed to improve the treatment of this condition. CVD is very common in HD patients and causes a high risk of mortality. There are a lot of nontraditional factors that contribute to worsen this condition. A better understanding of these through clinical and experimental studies is necessary to try to improve life in HD patients and to reduce their elevated risk of CV morbidity and mortality.
ACKNOWLEDGEMENTS
This article is published as part of a Supplement to NDT on ‘Translating Innovation to Clinical Outcomes’, financially supported by Baxter Healthcare Corporation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Fox CS, Matsushita K, Woodward M. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis of 1 024 977 individuals. Lancet 2012; 380: 1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahmoodi BK, Matsushita K, Woodward M. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012; 380: 1649–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cozzolino M, Galassi A, Pivari F. et al. The cardiovascular burden in end-stage renal disease. Contrib Nephrol 2017; 191: 44–57 [DOI] [PubMed] [Google Scholar]
- 4. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 5. Herzog CA, Asinger RW, Berger AK. et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011; 80: 572–586 [DOI] [PubMed] [Google Scholar]
- 6. Moradi H, Sica DA, Kalantar-Zadeh K.. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol 2013; 38: 136–148 [DOI] [PubMed] [Google Scholar]
- 7. Evenepoel P, Meijers BKI, Bammens BRM. et al. Uremic toxins originating from colonic microbial metabolism. Kidney Int 2009; 76: S12–S19 [DOI] [PubMed] [Google Scholar]
- 8. Meijers BK, Bammens B, De Moor B. et al. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008; 73: 1174–1180 [DOI] [PubMed] [Google Scholar]
- 9. Meijers BK, Claes K, Bammens B. et al. P-cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 2010; 5: 1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerini C, Dou L, Anfosso F. et al. P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost 2004; 92: 140–150 [DOI] [PubMed] [Google Scholar]
- 11. Ying Y, Yang K, Liu Y. et al. A uremic solute, p-cresol, inhibits the proliferation of endothelial progenitor cells via the p38 pathway. Circ J 2011; 75: 2252–2259 [DOI] [PubMed] [Google Scholar]
- 12. De Smet R, Thermote F, Lameire N. et al. Sevelamer hydrochloride (Renagel) adsorbs the uremic compound indoxyl sulfate, indole and p-cresol. J Am Soc Nephrol 2004; 15: 505A [Google Scholar]
- 13. Niwa T. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci 2010; 72: 1–11 [PMC free article] [PubMed] [Google Scholar]
- 14. Barisione C, Ghigliotti G, Canepa M. et al. Indoxyl sulfate: a candidate target for the prevention and treatment of cardiovascular disease in chronic kidney disease. Curr Drug Targets 2015; 16: 366–372 [DOI] [PubMed] [Google Scholar]
- 15. Shivanna S, Kolandaivelu K, Shashar M. et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J Am Soc Nephrol 2016; 27: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chitalia VC, Shivanna S, Martorell J. et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 2013; 127: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamiński TW, Pawlak K, Karbowska M. et al. Indoxyl sulfate—the uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol 2017; 18: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stinghen AEM, Massy ZA, Vlassara H. et al. Uremic toxicity of advanced glycation end products. J Am Soc Nephrol 2016; 27: 354–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haugen E, Nath KA.. The involvement of oxidative stress in the progression of renal injury. Blood Purif 1999; 17: 58–65 [DOI] [PubMed] [Google Scholar]
- 20. Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension 2012; 59: 375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravani P, Tripepi G, Malberti F. et al. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol 2005; 16: 2449–2455 [DOI] [PubMed] [Google Scholar]
- 22. Małyszko J, Matuszkiewicz J.. Rowińska endothelium, asymmetric dimethylarginine, and atherosclerosis in chronic kidney disease. Pol Arch Intern Med 2018; 128: 145–147 [DOI] [PubMed] [Google Scholar]
- 23. Liakopoulos V, Roumeliotis S, Gorny X. et al. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev 2017; 2017: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen MF, Chang CL, Liou SY.. Increase in resting levels of superoxide anion in the whole blood of uremic patients on chronic hemodialysis. Blood Purif 1998; 16: 290–300 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen T, Lethias C, Zingraff J. et al. Hemodialysis membrane-induced activation of phagocyte oxidative metabolism detected in vivo and in vitro within microamounts of whole blood. Kidney International 1985; 28: 158–167 [DOI] [PubMed] [Google Scholar]
- 26. Bayes B, Pastor MC, Bonal J. et al. Homocysteine and lipid peroxidation in haemodialysis: role of folinic acid and vitamin E. Nephrol Dial Transplant 2001; 16: 2172–2175 [DOI] [PubMed] [Google Scholar]
- 27. Morena M, Cristol JP, Bosc JY. et al. Convective and diffusive losses of vitamin C during haemodiafiltration session: a contributive factor to oxidative stress in haemodialysis patients. Nephrol Dial Transplant 2002; 17: 422–427 [DOI] [PubMed] [Google Scholar]
- 28. Sun J, Axelsson J, Machowska A. et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol 2016; 11: 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pedraza-Chaverri J, Sánchez-Lozada LG, Osorio-Alonso H. et al. New pathogenic concepts and therapeutic approaches to oxidative stress in chronic kidney disease. Oxid Med Cell Longev 2016; 2016: 6043601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emrich IE, Zawada AM, Martens-Lobenhoffer J. et al. Symmetric dimethylarginine (SDMA) outperforms asymmetric dimethylarginine (ADMA) and other methylarginines as predictor of renal and cardiovascular outcome in non-dialysis chronic kidney disease. Clin Res Cardiol 2018; 107: 201–213 [DOI] [PubMed] [Google Scholar]
- 31. Lau WL, Waziri ND.. Urea, a true uremic toxin: the empire strikes back. Clin Sci 2016; 131: 3–12 [DOI] [PubMed] [Google Scholar]
- 32. Nesrallah GE, Mustafa RA, MacRae J. et al. Canadian Society of Nephrology guidelines for the management of patients with ESRD treated with intensive hemodialysis. Am J Kidney Dis 2013; 62: 187–198 [DOI] [PubMed] [Google Scholar]
- 33. Vervloet MG, Sezer S, Massy ZA. et al. The role of phosphate in kidney disease. Nat Rev Nephrol 2017; 13: 27–38 [DOI] [PubMed] [Google Scholar]
- 34. Vervloet M, Cozzolino M.. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int 2017; 91: 808–817 [DOI] [PubMed] [Google Scholar]
- 35. Bover J, Evenepoel P, Ureña-Torres P. et al. Pro: cardiovascular calcifications are clinically relevant. Nephrol Dial Transplant 2015; 30: 345–351 [DOI] [PubMed] [Google Scholar]
- 36. Wang Z, Jiang A, Wei F. et al. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: a meta-analysis. BMC Cardiovasc Disord 2018; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ketteler M, Block GA, Evenepoel P. et al. Executive summary of the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD–MBD) guideline update: what’s changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 38. Dimkovic N, Schlieper G, Jankovic A. et al. Prognostic value of cardiovascular calcifications in hemodialysis patients: a longitudinal study. Int Urol Nephrol 2018; 50: 939–946 [DOI] [PubMed] [Google Scholar]
- 39. Liabeuf S, Okazaki H, Desjardins L. et al. Vascular calcification in chronic kidney disease: are biomarkers useful for probing the pathobiology and the health risks of this process in the clinical scenario? Nephrol Dial Transplant 2014; 29: 1275–1284 [DOI] [PubMed] [Google Scholar]
- 40. Collado S, Coll E, Nicolau C. et al. Serum osteoprotegerin in prevalent hemodialysis patients: associations with mortality, atherosclerosis and cardiac function. BMC Nephrol 2017; 18: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuźniewski M, Fedak D, Dumnicka P. et al. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv Med Sci 2016; 61: 269–275 [DOI] [PubMed] [Google Scholar]
- 42. Pichler G, Haller MC, Kainz A. et al. Prognostic value of bone- and vascular-derived molecular biomarkers in hemodialysis and renal transplant patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2017; 32: 1566–1578 [DOI] [PubMed] [Google Scholar]
- 43. Huang CL, Moe OW.. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch 2011; 462: 185–193 [DOI] [PubMed] [Google Scholar]
- 44. Desjardins L, Liabeuf S, Renard C. et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int 2012; 23: 2017–2025 [DOI] [PubMed] [Google Scholar]
- 45. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cozzolino M, Mazzaferro S.. The fibroblast growth factor 23: a new player in the field of cardiovascular, bone and renal disease. Curr Vasc Pharmacol 2010; 8: 404–411 [DOI] [PubMed] [Google Scholar]
- 47. Xie J, Yoon J, An SW. et al. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 2015; 26: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu MC, Shi M, Zhang J. et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 2010; 78: 1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kitagawa M, Sugiyama H, Morinaga H. et al. A decreased level of serum soluble klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 2013; 8: e56695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sircana A, De Michieli F, Parente R. et al. Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol Res 2018: S1043–6618: 31208–31202 [DOI] [PubMed] [Google Scholar]
- 51. Vashistha T, Kalantar-Zadeh K, Molnar MZ. et al. Dialysis modality and correction of uremic metabolic acidosis: relationship with all-cause and cause-specific mortality. Clin J Am Soc Nephrol 2013; 8: 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raikou VD, Kyriaki D.. Association between low serum bicarbonate concentrations and cardiovascular disease in patients in the end-stage of renal disease. Diseases 2016; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Voiculeț C, Zară O, Bogeanu C. et al. The role of oral sodium bicarbonate supplementation in maintaining acid-base balance and its influence on the cardiovascular system in chronic hemodialysis patients - results of a prospective study. J Med Life 2016; 9: 449–454 [PMC free article] [PubMed] [Google Scholar]
- 54. Pavlovic D. The role of cardiotonic steroids in the pathogenesis of cardiomyopathy in chronic kidney disease. Nephron Clin Pract 2014; 128: 11–21 [DOI] [PubMed] [Google Scholar]