Abstract
Haemodiafiltration (HDF) combines diffusive and convective solute removal in a single treatment session. HDF provides a greater removal of higher molecular weight uraemic retention solutes than conventional high-flux haemodialysis (HD). Recently completed randomized clinical trials suggest better patient survival with online HDF. The treatment is mainly used in Europe and Japan. This review gives a brief overview of the presently available evidence of the effects of HDF on clinical end points, it speculates on possible mechanisms of a beneficial effect of HDF as compared with standard HD and ends with some perspectives for the future.
Keywords: chronic hemodialysis, clinical outcome, hemodiafiltration, online hemodiafiltration, review
INTRODUCTION
The concept of haemodiafiltration (HDF), i.e. combining diffusive and convective transport within a single treatment session, was introduced decades ago. Clinical research on HDF has been focussed on assessing the effects on biomarkers, on the function of various organs and on clinical end points, such as mortality. The assessment of its benefits and disadvantages, however, is complicated since in many of these studies different HDF techniques were used [e.g. offline HDF, pre- and post-dilution online HDF, acetate free biofiltration and haemofiltration (HF)]. Moreover, most comparative studies have differed markedly in size, follow-up period, design and methodology [e.g. observational versus randomized controlled trials (RCTs)]. Studies addressing clinically relevant endpoints were predominantly done during the past decade and were mainly obtained comparing online post-dilution HDF with other dialysis techniques. This article gives a brief overview of the presently available evidence of the effects of online HDF on clinical endpoints, it speculates on possible mechanisms of a beneficial effect of HDF as compared with standard HD and ends with some perspectives for the future.
HAEMODIAFILTRATION AND SURVIVAL
Observational studies
Several large observational studies on convective techniques have been published in recent decades. Most, but not all, investigations showed a reduction in the mortality risk of patients treated with haemodialysis (HD). However, since the decision to treat patients with HDF in observational studies is generally based on clinical grounds and not on selection by chance, residual confounding can never be ruled out, even though extensive corrections are made to minimize selection bias.
RCTs
Over the past years, four large RCTs comparing HDF with HD have been published [1–4]. All studies aimed to address the question whether online HDF is superior to standard HD in terms of effect on relevant clinical endpoints, although the design of the studies showed some differences. For instance, in the CONvective TRAnsport STudy (CONTRAST), a low-flux membrane was used, while in other studies high-flux membranes were used in the control group. An important characteristic of all four studies was that the actual delivered convection volume showed a considerable range. This was not an a priori aim of the respective studies, it just happened in everyday clinical practice. None of the four studies gave an undisputable answer to the basic objective, i.e. finding out whether HDF is superior or not. While in CONTRAST and the Turkish HDF study (THDFS) a modest, non-significant effect of treatment with HDF on all-cause mortality was shown {hazard rate (HR) 0.95 [95% confidence interval (CI) 0.75–1.20] and HR 0.79 (95% CI 0.55–1.14), respectively} [1, 2], in the Spanish Estudio de Supervivencia de Hemodiafiltración On-Line (ESHOL) study a markedly reduced mortality risk was observed [HR 0.70 (95% CI 0.53–0.92)] [3]. Whereas the achieved convection volume was 20.7 L/session in CONTRAST and 19.6 L/session in THDFS, in ESHOL it was 22.9–23.9 L/session. Post hoc analysis of all three studies showed a significantly lower mortality in the group of patients treated with the highest convection volumes, even after extensive adjustments were made [1–3].
Several meta-analyses on the effects of convective therapies, based on pooling aggregate published data, have been made available but produced conflicting results [5–8]. A major limitation is that these meta-analyses were done on published rather than on individual data of the various trials. Further, they differed considerably in the selection of the studies in the convective treatment arm (offline HDF, online HDF, acetate-free biofiltration, HF and high-flux HD). Since the magnitude of the convection volume is currently considered as a key parameter for the efficacy of HDF, comparison of various low-dose convective techniques is no longer clinically relevant. So these meta-analyses should be considered with this limitation in mind and are no longer very useful.
Therefore we did an individual participant data (IPD) analysis on the results of the four big studies—CONTRAST, THDFS, ESHOL and the French HDF study—allowing a detailed analysis in a large group of patients. After collecting the mortality data for patients who were censored alive in the individual studies, 352 of a total of 355 censored patients were traced, so the IPD encompassed 2793 patients [9, 10]. From this IPD meta-analysis, which includes only RCTs with a mean convection volume of ∼19 L/session, it appeared that the risk of all-cause and cardiovascular disease (CVD) mortality was significantly reduced in the HDF group [HR 0.86 (95% CI 0.75–0.99) and HR 0.77 (95% CI 0.61–0.97)]. Post hoc analysis in tertiles of the achieved convection volume [<19, 19–23 and >23 L, body surface area (BSA) adjusted] suggested a minimum necessary volume of 23 L/1.73 m2 BSA/session. To date, this is the best available evidence on the question of whether HDF is superior to standard HD or not. Recently the French Renal Epidemiology and Information Network registry also suggested the superiority for HDF, and in a large observational study the suggestion that a minimum dosage of ∼70 L/week (which equals ∼3 × 23 L/session) is necessary to fully obtain the benefit was confirmed [11, 12] (Table 1).
Table 1.
HR and 95% CI for all-cause mortality and cause-specific mortality by delivered BSA-standardized convection volume with standard HD as reference. Adjusted for age, sex, albumin, history of CVD and history of diabetes [10]
| Online HDF convection volume, delivered BSA-standardized in L/1.73 m2 per treatment |
||||
|---|---|---|---|---|
| HD | <19 | 19–23 | >23 | |
| All-cause mortality | ||||
| Crude | 1 | 0.91 (0.74–1.13) | 0.88 (0.72–1.09) | 0.73 (0.59–0.91) |
| Adjusted | 1 | 0.83 (0.66–1.03) | 0.93 (0.75–1.16) | 0.78 (0.62–0.98) |
| CVD mortality | ||||
| Crude | 1 | 1.00 (0.71–1.40) | 0.71 (0.50–1.01) | 0.69 (0.48–0.98) |
| Adjusted | 1 | 0.92 (0.65–1.30) | 0.71 (0.49–1.03) | 0.69 (0.47–1.00) |
| Infections | ||||
| Crude | 1 | 1.50 (0.93–2.41) | 0.96 (0.56–1.65) | 0.56 (0.30–1.08) |
| Adjusted | 1 | 1.50 (0.92–2.46) | 0.97 (0.54–1.74) | 0.62 (0.32–1.19) |
| Sudden death | ||||
| Crude | 1 | 1.24 (0.80–1.91) | 0.91 (0.57–1.47) | 0.60 (0.35–1.03) |
| Adjusted | 1 | 1.09 (0.69–1.74) | 1.04 (0.63–1.70) | 0.69 (0.39–1.20) |
Mechanisms of beneficial effects
Cause-specific benefits of HDF
To answer the question ‘why’ all-cause and CVD mortality is reduced by HDF, we must first ascertain that other causes of death, such as fatal infections and malignancies (the most common non-cardiac fatalities), sudden deaths and withdrawal from treatment [13] are equally distributed between the HD and HDF groups. If so, the question arises whether the reduction in CVD mortality is mainly due to a decrease in heart diseases only or also due to a decline in fatal vascular events, such as stroke and ruptured aneurysms. If not, it is useful to know whether all potential causes of cardiac death are reduced, including heart failure, ischaemic heart disease and arrhythmias, or just one specific diagnosis, such as sudden cardiac death.
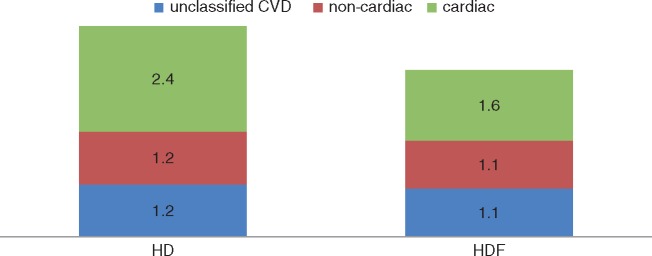
With respect to the first question, recent findings from the IPD analysis indicate that treatment with HDF is neither associated with beneficial effects on fatal infections, nor with a lower incidence of fatal malignancies, withdrawal from treatment or sudden death. With respect to the second question, observations from the IPD analysis also show that the beneficial effect of HDF is restricted to ‘cardiac’ causes only: splitting fatal CVD into cardiac and non-cardiac sources did not show any beneficial effect on stroke or peripheral vascular disease (Figure 1). Finally, subdivision of ‘cardiac’ fatalities into congestion, arrhythmias and myocardial infarction did not suggest that one particular type of heart disease is prevented by treatment with HDF [14].
FIGURE 1.
Annualized CVD mortality per 100 patient-years in the HD and online HDF groups. The numbers in the boxes represent fatal CVD events/100 patient-years. The difference in fatal cardiac events between HD and HDF is significant (P = 0.01). Reprinted from Nubé et al. [14].
Yet, as the absolute number of fatalities in the latter subgroups was rather small, caution is warranted. Moreover, although overall not significant, stratification of the convection volume in thirds showed a distinct trend for sudden death: the larger the convection volume, the lower the mortality risk. Hence it cannot be completely ruled out that unusual large convection volumes lower the incidence of sudden death.
Why is cardiac mortality reduced by HDF?
Removal of uraemic toxins
HDF is capable of removing retained small and middle molecular weight (MMW) substances, which accumulate in patients with end-stage kidney disease (ESKD) who are treated with HD. Uraemic solutes are generally subdivided into three major classes: (i) small water-soluble compounds (WSCs; <500 Da), (ii) MMW substances (0.5–40 kDa) and (iii) protein-bound toxins (PBTs) [15]. While WSCs, such as urea and creatinine, are mainly removed by diffusion, convection is the driving force for the removal of larger MMW solutes, which cross the dialysis membrane by solute drag effectuated by the transmembrane pressure gradient. While WSCs can be removed with any membrane, MMW substances can only be eliminated through high-flux dialysers. PBTs are difficult to remove because only the free fraction, consisting mostly of low molecular weight (MW) substances, can cross the dialysis membrane. For this purpose, again, any membrane is appropriate [16].
Small WSCs. Urea, a small solute (60 Da) that accumulates in chronic kidney disease (CKD), is most frequently used as a measure for dialysis adequacy by the formula Kt/Vurea. Although Kt/Vurea is increased by HDF [17], previously it was found that an increase in urea clearance did not improve survival. With respect to creatinine (113 Da), which is most frequently used for the estimation of kidney function by measuring creatinine clearance, similar findings have been found. Elevated phosphorus (95 Da) levels are associated with vascular calcifications and CVD death. Although phosphorus itself is a small molecule, in the living body it behaves more or less like a middle molecule due to the surrounding water mantle. While both phosphorus levels and the prescription of oral binders were lower in HDF compared with low-flux HD [1, 18], in two recent RCTs [2, 3] blood levels did not differ between high-flux HD and HDF. It should be realized, however, that an elevated phosphate value is just one component of the multifaceted CKD–mineral and bone disease (MBD), which includes also derangements in calcium, vitamin D status and resistance, levels of parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). Since in these patients phosphate is also dependent on the degree of residual kidney function (RKF) and the use of CKD-MBD-specific medication, including phosphate binders, vitamin D analogues and calcimimetics, the dialysis mode is just one aspect in the complex interplay between these components. Currently it is questionable whether the lowering of serum phosphorus itself is associated with improved clinical outcome in patients with ESKD [19].
MMW substances. The group of MMW substances that accumulates in ESKD consists mainly of small peptides, many of which have been implied in inflammation, endothelial damage, smooth muscle cell proliferation and oxidative stress and interference in the coagulation cascade. As most of these processes may contribute to CVD, removal of these substances—by convection—may improve clinical outcome. Although their elimination is considerably enhanced by application of HDF [20], especially in patients with only marginal RKF, neither the lowering of β2-microglobulin (MW 11.8 kDa), the pro-inflammatory cytokines interleukin 6 (MW 21 kDa) and tumor necrosis factor-α (TNF-α) (MW 25.6 kDa) or complement factor D (MW 24 kDa) have been convincingly shown to underlie the beneficial clinical effects of convective therapies.
Considering CKD-MBD, high PTH (MW 9.4 kDa) levels have been associated with various manifestations of CVD. Reduction by medication, however, did not improve clinical outcome. In studies comparing high-flux HD with HDF, differences were not observed. In contrast, promising results have been obtained for FGF23 (MW 32 kDa), which is the earliest detectable biochemical alteration in CKD-MBD [21]. Levels of this phosphatonin are 100–1000-fold higher in ESKD than in healthy individuals. Removal of FGF23 was markedly higher during HDF than during high-flux HD [22]. Since FGF23 has been related to left ventricular hypertrophy and CVD events [23], especially congestive heart failure in patients with CKD Stages 2–4 [24], a reduction by HDF may lower CVD mortality in ESKD.
PBCs. Multiple toxic effects have been attributed to retained PBCs, which are largely intestinally generated. Retention of PBCs may contribute to inflammatory processes, oxidative stress, endothelial dysfunction, cardiac cell proliferation and mesenchymal transition, which all may have an adverse influence on the cardiovascular system. p-Cresol is generated by intestinal bacteria and conjugated to p-cresylsulfate and p-cresylglucuronide. Both p-cresol derivatives (pCs; MW 188 Da), indoxylsulphate (IS; MW 212 Da) and indole acetic acid (IAA; MW 175 Da) have been shown to contribute to uraemic syndrome. In addition, in CKD, increased levels have been described for a variety of hippurates, including glucuronide conjugates of hydroxyhippuric acid (HA; MW 179 Da), which originates especially from polyphenolic compounds in the diet such as fruit, tea and coffee. Increased HA levels appear especially toxic for renal tubular and glomerular functions.
Advanced glycation endproducts (AGEs), such as N-carboxymethyl-lysine and pentosidine, are another category of PBCs, as these compounds have a heterogeneous MW (<10 kDa) and originate partly from AGE-rich food products. AGEs have a profibrotic action, contribute to the release of pro-inflammatory cytokines and promote oxidative stress.
The PBCs pCS, IS and IAA are difficult to eliminate because of the large distribution volume, their high binding coefficient and the fact that only the free fraction can be removed by diffusion. Whereas high-flux HD did not augment the reduction of the unbound fraction as obtained by low-flux HD, the addition of convective transport by post-dilution HDF yielded conflicting results [25, 26]. Considering AGEs, HDF resulted in considerably enhanced reduction ratios, most likely due to the fact that the MW of several of these peptides is substantially greater than that of other PBCs [27]. Whether these manoeuvres contribute to the improved clinical outcome of HDF, however, is uncertain.
Haemodynamic factors
Intradialytic haemodynamic (in)stability
The most important acute complication of dialysis is intradialytic hypotension (IDH), which has been defined as a decline in systolic blood pressure (BP) >20 mmHg or a decline of mean arterial pressure of 10 mmHg, associated with clinical events and the need for nursing intervention. Other definitions have been used as well. Depending on the definition used and the population investigated, the reported incidence varies between 10 and 30%. Notably, IDH has been related to end-organ ischaemia and mortality [28]. Recent sophisticated studies demonstrated that IDH is associated with hypoperfusion of vital organs, including the gut, brain and heart [29–31]. As a result, not only translocation of bacterial products from the intestinal cavity to the blood may occur, but also brain and cardiac dysfunction. In fact, the dialysis procedure itself may worsen the prevalent micro-circulatory dysfunction of many organs that is a common feature in these patients.
Treatment with cool dialysate reduced IDH, mitigated HD-induced brain injury and improved CVD survival [32]. In two large RCTs, BP stability during HDF was superior to HD [3, 33]. When cool dialysate was used both in HDF and HD, intradialytic haemodynamic changes, as measured by BP, blood volume, cardiac output and microcirculation, did not differ [34]. Likewise, solute movements between the intra- and extracellular compartments during HDF and cool dialysate HD were similar [35]. Hence it appears that intradialytic haemodynamic stability is better preserved during HDF than during standard HD, but analogous to cool dialysate HD. Unfortunately, none of the recent RCTs comparing HDF with HD reported the temperature of the dialysis fluid. In a recent study, cardiac magnetic resonance imaging was done during HD and HDF. Although profound deleterious effects were seen on various cardiac variables, there were no differences between the two treatment arms [36, 37]. The latter study, however, was small (n = 12) and included only stable patients. Haemodynamic effects of HDF and possible differences with standard HD should be further studied.
Interestingly, from a recent echocardiographic study it appeared that neither the variations in blood pressure nor ultrafiltration rate was related to the HD-induced changes in the perfusion of the heart [38]. Hence non-haemodynamic factors may contribute substantially to the HD-induced perfusion defects in vital organs.
Long-term haemodynamics
Apart from repetitive and recurring intradialytic haemodynamic changes, the chronic hypertensive BP burden in ESKD, with an estimated prevalence of 85% in chronic HD patient, may also affect the structure and function of vital organs in the long term. In the IPD analysis from four RCTs comparing HDF with HD [5], the reduction in the all-cause and CVD mortality risks in HDF patients was independent from pre-dialysis BP values. Echocardiographic analysis of a large subset of the CONTRAST cohort (n = 342) revealed that left ventricular mass and ejection fraction tended to worsen over time in the HD group, but remained stable in HDF patients. The difference between HD and HDF was borderline significant at 1 year (P = 0.06) [39]. Pulse wave velocity did not differ in this study. Independent of treatment allocation, however, mean arterial pressure decreased over time, mainly due to a reduction in peripheral resistance (Mostovaya IM et al., unpublished data).
Perspectives and conclusions
Based on the above considerations, we have to conclude that the precise mechanisms of a beneficial effect on the risk of cardiac mortality are not fully elucidated. Indeed, the initial hypothesis was that better correction of the uraemic milieu would result in less cardiovascular organ damage, resulting in an improvement in clinical cardiovascular outcome. So far, changes in biochemical variables, such as the ones mentioned above, failed to show a consistent relation with outcome variables. So basically the present evidence does not convincingly support this initial hypothesis. It is tempting to speculate that the effect on haemodynamic stability, i.e. the lower risk of hypotensive periods during HDF, is of considerable relevance in this respect. In the meta-analysis of the presently available studies on clinical outcome, the median follow-up was 2.5 years [5]. It is difficult to imagine how in such a relatively short period the switch to HDF could have altered the cardiovascular structure in such a way that outcome improves. One may argue that this favours the thinking of a favourable effect on ‘cardiovascular function’ rather than ‘cardiovascular structure’. Japanese investigators presented data of their nationwide registry in which they analysed the effect of a switch to HDF (in Japan in pre-dilution mode) on survival [40]. Their data seem to indicate that survival curves start to diverge within months after the switch. These considerations could be interpreted as favouring the ‘haemodynamic hypothesis’ mentioned above. However, it is important to realize that HDF in Japan is delivered in pre-dilution mode. A head-to-head comparison of the effects of various types of HDF on meaningful clinical endpoints has never been done.
When a centre decides to start an HDF programme, the lack of a full understanding of these mechanisms may not be considered as a drawback. On the other hand, when designing alternative treatment strategies, such as new membranes, providing identical or even better clearance profiles of various substances than HDF, this lack of a detailed understanding of the relation between the removal of substances and outcome may be considered an important drawback.
For the clinicians in daily clinical practice, it is important to know that online HDF is generally considered to be safe, on the condition that it is performed and monitored according to the manufacturer’s instructions and (inter)national guidelines on this subject [41]. The key requirements for an HDF programme and protocols for how to implement HDF at a sufficient dose are logical and not terribly difficult to implement and are described in detail elsewhere [42–44]. In a recent paper, dosing adjustments of anti-infectious agents were summarized [45]. Indeed, starting up an HDF programme may mean extra investments in machinery and in the training of personnel. But after that, the costs of the maintenance phase appear more or less identical to a regular HD programme using high-flux membranes. Online HDF is probably cost effective. Analyses so far have not identified specific subgroups more likely to benefit than others. This suggests that it could be applied to large groups of patients. It is important to mention that all studies so far were done on the standard thrice weekly schedule. There are virtually no data on the effects of HDF in more intensified schedules.
So what are the real barriers to the spread of online HDF as the new standard of care [46–48]? HDF is mostly accepted in Europe, whereas acceptance in Japan is clearly increasing. Presently in the US, acceptance is virtually zero, but this may be changing in the (near) future [46, 49]. Within Europe there are enormous differences between regions, cities and hospitals/centres. The main explanation seems to be that there is considerable variability in the acceptance of the idea that HDF is superior to standard HD. Indeed, some argue that the presently available evidence cannot be considered as representing ‘solid proof’. Therefore it is very fortunate that on 1 January 2018, the CONVINCE study officially started. This project is supported by the European Commission (Horizon programme grant agreement 754803). The study will be done in ∼1800 patients in multiple European countries. The main objectives are to compare standard high-flux HD and online HDF when consistently delivered in high volume (post-dilution >23 L/4 h session) in terms of effects on all-cause mortality. Secondary endpoints include cause-specific mortality (cardiovascular and non-cardiovascular disease), non-fatal and fatal cardiovascular events, all-cause hospitalizations and cost-effectiveness. Further, an important secondary outcome will be the patient-reported outcomes. It is increasingly acknowledged that the objectives of a treatment as defined by patients are not necessarily identical to those identified by care providers. As a consequence, even in the absence of a difference in ‘traditional’ clinical endpoints, any meaningful difference in patient-reported outcome(s), or for instance in nutritional status as was recently reported, could already be considered as reason enough to choose HDF as the standard of care [50]. A rationale and design paper of CONVINCE is in preparation. It seems reasonable to assume that this study will finally deliver the undisputable answer on the question of superiority.
ACKNOWLEDGEMENTS
This article is published as part of a Supplement to NDT on ‘Translating Innovation to Clinical Outcomes’, financially supported by Baxter Healthcare Corporation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Grooteman MP, van den Dorpel MA, Bots ML. et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 2012; 23: 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ok E, Asci G, Toz H. et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant 2013; 28: 192–202 [DOI] [PubMed] [Google Scholar]
- 3. Maduell F, Moreso F, Pons M. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morena M, Jaussent A, Chalabi L. et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int 2017; 91: 1495–1509 [DOI] [PubMed] [Google Scholar]
- 5. Mostovaya IM, Blankestijn PJ, Bots ML. et al. Clinical evidence on hemodiafiltration: a systematic review and a meta-analysis. Semin Dial 2014; 27: 119–127 [DOI] [PubMed] [Google Scholar]
- 6. Nistor I, Palmer SC, Craig JC. et al. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic review of randomized controlled trials. Am J Kidney Dis 2014; 63: 954–967 [DOI] [PubMed] [Google Scholar]
- 7. Susantitaphong P, Siribamrungwong M, Jaber BL.. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2013; 28: 2859–2874 [DOI] [PubMed] [Google Scholar]
- 8. Wang AY, Ninomiya T, Al-Kahwa A. et al. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: a systematic review and meta-analysis of randomized trials. Am J Kidney Dis 2014; 63: 968–978 [DOI] [PubMed] [Google Scholar]
- 9. Davenport A, Peters SA, Bots ML. et al. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int 2016; 89: 193–199 [DOI] [PubMed] [Google Scholar]
- 10. Peters SA, Bots ML, Canaud B. et al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant 2016; 31: 978–984 [DOI] [PubMed] [Google Scholar]
- 11. Canaud B, Barbieri C, Marcelli D. et al. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int 2015; 88: 1108–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mercadal L, Franck JE, Metzger M. et al. Hemodiafiltration versus hemodialysis and survival in patients with ESRD: the French Renal Epidemiology and Information Network (REIN) registry. Am J Kidney Dis 2016; 68: 247–255 [DOI] [PubMed] [Google Scholar]
- 13. den Hoedt CH, Bots ML, Grooteman MP. et al. Should we still focus that much on cardiovascular mortality in end stage renal disease patients? The CONvective TRAnsport STudy. PLoS One 2013; 8: e61155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nubé MJ, Peters SA, Blankestijn PJ. et al. Mortality reduction by online-hemodiafiltration: a cause-specific analysis. Nephrol Dial Transplant 2017; 32: 548–555 [DOI] [PubMed] [Google Scholar]
- 15. Neirynck N, Vanholder R, Schepers E. et al. An update on uremic toxins. Int Urol Nephrol 2013; 45: 139–150 [DOI] [PubMed] [Google Scholar]
- 16. Glorieux GL, Krieter DH.. Effects on the removal of uremic toxins In: Nubé MJ, Grooteman MPC, Blankestijn PJ (eds). Hemodiafiltration: Theory, Technology and Clinical Practice, 1st edn. Basel: Springer International, 2016: 165–182 [Google Scholar]
- 17. Nubé MJ. Why is high volume online post-dilution hemodiafiltration associated with improved survival? In: Nubé MJ, Grooteman MPC, Blankestijn PJ (eds). Hemodiafiltration: Theory, Technology and Clinical Practice, 1st edn. Basel: Springer International, 2016: 239–254 [Google Scholar]
- 18. Penne EL, van der Weerd NC, van den Dorpel MA. et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis 2010; 55: 77–87 [DOI] [PubMed] [Google Scholar]
- 19. Cannata-Andia JB, Naves-Diaz M.. Phosphorus and survival: key questions that need answers. J Am Soc Nephrol 2009; 20: 234–236 [DOI] [PubMed] [Google Scholar]
- 20. Maduell F, Navarro V, Cruz MC. et al. Osteocalcin and myoglobin removal in on-line hemodiafiltration versus low- and high-flux hemodialysis. Am J Kidney Dis 2002; 40: 582–589 [DOI] [PubMed] [Google Scholar]
- 21. Isakova T, Wahl P, Vargas GS. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79: 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patrier L, Dupuy AM, Granger VA. et al. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J Nephrol 2013. Mar; 26: 342–349 [DOI] [PubMed] [Google Scholar]
- 23. Scialla JJ, Wolf M.. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014; 10: 268–278 [DOI] [PubMed] [Google Scholar]
- 24. Seiler S, Rogacev KS, Roth HJ. et al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2–4. Clin J Am Soc Nephrol 2014; 9: 1049–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krieter DH, Hackl A, Rodriguez A. et al. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol Dial Transplant 2010; 25: 212–218 [DOI] [PubMed] [Google Scholar]
- 26. Meert N, Eloot S, Waterloos MA. et al. Effective removal of protein-bound uraemic solutes by different convective strategies: a prospective trial. Nephrol Dial Transplant 2008; 24: 562–570 [DOI] [PubMed] [Google Scholar]
- 27. Lin CL, Huang CC, Yu CC. et al. Reduction of advanced glycation end product levels by on-line hemodiafiltration in long-term hemodialysis patients. Am J Kidney Dis 2003; 42: 524–531 [DOI] [PubMed] [Google Scholar]
- 28. van der Sande FM, Kooman JP, Konings CJ. et al. Thermal effects and blood pressure response during postdilution hemodiafiltration and hemodialysis: the effect of amount of replacement fluid and dialysate temperature. J Am Soc Nephrol 2001. Sep; 12: 1916–1920 [DOI] [PubMed] [Google Scholar]
- 29. Burton JO, Jefferies HJ, Selby NM. et al. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 2009; 4: 1925–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eldehni MT, Odudu A, McIntyre CW.. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 2015; 26: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polinder-Bos HA, García DV, Kuipers J. et al. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol 2018; 29: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu HJ, Yen CH, Hsu KH. et al. Association between cold dialysis and cardiovascular survival in hemodialysis patients. Nephrol Dial Transplant 2012; 27: 2457–2464 [DOI] [PubMed] [Google Scholar]
- 33. Locatelli F, Altieri P, Andrulli S. et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol 2010; 21: 1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cornelis T, van der Sande FM, Eloot S. et al. Acute hemodynamic response and uremic toxin removal in conventional and extended hemodialysis and hemodiafiltration: a randomized crossover study. Am J Kidney Dis 2014; 64: 247–256 [DOI] [PubMed] [Google Scholar]
- 35. Kumar S, Khosravi M, Massart A. et al. Haemodiafiltration results in similar changes in intracellular water and extracellular water compared to cooled haemodialysis. Am J Nephrol 2013; 37: 320–324 [DOI] [PubMed] [Google Scholar]
- 36. Buchanan C, Mohammed A, Cox E. et al. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol 2017; 28: 1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blankestijn PJ, Davenport A.. Changes in cardiac output and perfusion during hemodialysis and hemodiafiltration treatments determined by cardiac magnetic resonance imaging. J Am Soc Nephrol 2017; 28: 1013–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Assa S, Hummel YM, Voors AA. et al. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol 2012; 7: 1615–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mostovaya IM, Bots ML, van den Dorpel MA. et al. A randomized trial of hemodiafiltration and change in cardiovascular parameters. Clin J Am Soc Nephrol 2014; 9: 520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masakane I, Kikuchi K, Kawanishi H.. Evidence for the clinical advantages of predilution on-line hemodiafiltration. Contrib Nephrol 2017; 189: 17–23 [DOI] [PubMed] [Google Scholar]
- 41. Tattersall JE, Ward RA.. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant 2013; 28: 542–550 [DOI] [PubMed] [Google Scholar]
- 42. Penne EL, van der Weerd NC, Bots ML. et al. Patient- and treatment-related determinants of convective volume in post-dilution haemodiafiltration in clinical practice. Nephrol Dial Transplant 2009; 24: 3493–3499 [DOI] [PubMed] [Google Scholar]
- 43. Roij van Zuijdewijn de CLM, Chapdelaine I. et al. Achieving high convection volumes in postdilution online hemodiafiltration: a prospective multicenter study. Clin Kidney J 2017; 10: 804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chapdelaine I, de Roij van Zuijdewijn CL, Mostovaya IM. et al. Optimization of the convection volume in online post-dilution haemodiafiltration: practical and technical issues. Clin Kidney J 2015; 8: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jager NG, Zandvliet AS, Touw DJ. et al. Optimization of anti-infective dosing regimens during online haemodiafiltration. Clin Kidney J 2017; 10: 282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blankestijn PJ. Has the time now come to more widely accept hemodiafiltration in the United States? J Am Soc Nephrol 2013; 24: 332–334 [DOI] [PubMed] [Google Scholar]
- 47. Canaud B, Blankestijn PJ, Davenport A. et al. Reconciling and closing the loop between evidence-based and practice-based medicine: the case for hemodiafiltration. Am J Kidney Dis 2016; 68: 176–179 [DOI] [PubMed] [Google Scholar]
- 48. Canaud B, Vienken J, Ash S. et al. Hemodiafiltration to address unmet medical needs ESKD patients. Clin J Am Soc Nephrol 2018. pii: CJN.12631117. doi: 10.2215/CJN.12631117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ward RA, Vienken J, Silverstein DM. et al. Regulatory considerations for hemodiafiltration in the United States. Clin J Am Soc Nephrol 2018. pii: CJN.12641117. doi: 10.2215/CJN.12641117. [Epub ahead of print] PubMed PMID: 29511058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Molina P, Vizcaíno B, Molina MD. et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: the ProtEin Stores prEservaTion (PESET) study. Nephrol Dial Transplant 2018; 33: 1223–1235 [DOI] [PubMed] [Google Scholar]