Abstract
Patients with end-stage kidney disease (ESKD) on maintenance hemodialysis are subject to a high burden of inflammation and cardiovascular disease, driven at least in part by retention of uremic solutes. Existing dialysis technologies using high-flux membranes offer limited clearance of solutes >15 kDa. New approaches to improve the removal of large uremic toxins include the novel medium cut-off dialysis membranes with pores larger than those in high-flux membranes. These new membranes provide the potential to improve the clearance of large middle molecules up to 50 kDa. In this review, we discuss 18 uremic toxins with molecular weights between 15 and 60 kDa that are retained in ESKD, for which there is evidence of a link to inflammation and/or cardiovascular disease. These include inflammatory proteins, cytokines, adipokines and other signaling proteins. Improved clearance of this group of difficult to remove molecules has the potential to lead to improved outcomes in dialysis patients by reducing the burden of cardiovascular disease, which now needs to be assessed in robust clinical trials.
Keywords: cardiovascular disease; haemodialysis, inflammation; middle molecules; uremic toxins
INTRODUCTION
In the decades since the introduction and mainstream uptake of maintenance hemodialysis, outcomes in patients with end-stage kidney disease (ESKD) have improved. Patients on hemodialysis, however, continue to experience much higher rates of mortality than the general population, with particular burdens of cardiovascular disease and cardiovascular death. The nature of cardiovascular disease in uremia is generally recognized to be different from that in patients with normal kidney function, with a high frequency of ventricular remodeling and dysfunction, arrhythmia and sudden cardiac death, and calcific arterial and valve disease [1]. Risk factors for cardiovascular disease in ESKD are also different, with contributing factors such as phosphate, inflammatory markers and others. The pathogenesis and the drivers for this accelerated cardiovascular disease process are not completely understood, but the uremic milieu, with retention of numerous solutes normally cleared by the kidney or excreted in the urine, likely plays a large part. In this review, we will examine potential links between the large uremic toxins known as middle molecules and cardiovascular disease, and the gap that exists currently in our ability to remove these molecules.
LARGE UREMIC TOXINS
Hemodialysis effectively removes small water-soluble molecules that circulate without significant protein binding, exemplified by the use of urea clearance as a metric for dialysis dose and kinetic modeling. Molecules that are larger and/or protein-bound are, however, much more difficult to remove. The ‘middle molecules’, molecules from 500 to 60 000 Da, are a diverse group of molecules, many of which have known toxic effects. Dialysis technologies have been targeted to improve clearance of smaller middle molecules up to ∼15 kDa, such as beta-2 microglobulin, whilst avoiding albumin loss (66 kDa). Modern high-flux membranes are reasonably effective at removing proteins in this lower size range, but clearances are poor for molecules >15 kDa. Hemodiafiltration (HDF) improves the clearance of molecules up to ∼25 kDa and newer ‘medium cut-off’ membranes have the potential to more effectively remove larger molecules up to 50 kDa, with limited albumin loss [2]. Many of these larger middle molecules are linked directly, or in some cases indirectly, with cardiovascular disease and improved removal of such solutes is a generally accepted goal of improving dialysis technology.
A large number of molecules are known to be retained in uremia, and Table 1 describes 18 that fall into the category of large middle molecules with a size between 15 and 60 kDa, which are cleared poorly by conventional dialysis and for which there is evidence of their involvement in inflammation and or cardiovascular disease. The usual biological role of these molecules is diverse, and they include a number of cytokines, adipokines, growth factors and other signaling proteins. Levels of these proteins can be markedly elevated in uremia, with most cytokines and inflammatory proteins being 2- to 10-fold higher in uremia compared with subjects with normal renal function. Many other middle molecules are >10-fold higher and some proteins such as fibroblast growth factor (FGF)-23, which is directly involved in phosphate handling can be elevated >200-fold. Conventional dialysis is universally poor at removing these molecules.
Table 1.
Middle molecules in the range of 15–60 kDa that have evidence for involvement in inflammation and cardiovascular disease
| Molecule (alternative names) | Classification | Molecular size (kDa) | Usual biological role | Relative increase in dialysis or advanced CKD |
|---|---|---|---|---|
| Interleukin-18 | Cytokine | 18 | Pro-inflammatory | ∼2-fold higher |
| Interleukin-6 | Cytokine | 21–28 | Pro-inflammatory | 2- to 5-fold higher |
| Interleukin-1β | Cytokine | 17.5 | Pro-inflammatory | ∼2-fold higher |
| TNF-α | Cytokine | 17 | Pro-inflammatory | 4- to 5-fold higher |
| Soluble TNF receptor 1 (p75) | Protein | 27–30 | Limits TNF-α activity | 3- to 10-fold higher |
| Soluble TNF receptor 2 (p55) | Protein | 17 | Limits TNF-α activity | 3- to 10-fold higher |
| Pentraxin-3 | Protein | 40 | Opsonization and complement activation. Modulate macrophage activity | 2- to 7-fold higher |
| YKL-40 (CHI3L1) | Protein | 40 | Regulates local inflammatory markers. Other functions unclear | 2- to 5-fold higher |
| Adiponectin | Adipokine | 30 | Modulates glucose regulation and fatty acid oxidation | 2- to 3-fold higher |
| Visfatin (NAMPT) | Adipokine | 52 | Extracellularly stimulates angiogenesis and endothelial cell proliferation | 3- to 6-fold higher |
| Leptin | Adipokine | 16 | Regulates appetite and body energy stores | 3- to 4-fold higher |
| VEGF (vascular permeability factor) | Growth factor | 34 | Promotes endothelial cell proliferation, migration and differentiation | ∼2-fold higher |
| FGF-2 (basic fibroblast growth factor) | Growth factor | 18 | Angiogenic growth factor | 5- to 20-fold higher |
| FGF-23 | Growth factor | 32 | Regulates phosphate homeostasis | >200-fold higher |
| Complement factor D (C3 proactivator convertase) | Protein | 24 | Component of alternative complement pathway; humoral defense | 4- to 17-fold higher |
| Prolactin | Hormone | 23 | Diverse roles | 2- to 4-fold higher |
| β-trace protein (L-prostaglandin D2 synthase) | Protein | 26 | Catalyzes isomerization of precursor prostanoids to active forms | >35-fold higher |
| AGEs | Other | <1–70 | Unknown | 2- to 20-fold higher |
ROLE OF LARGE MIDDLE MOLECULES IN INFLAMMATION AND CARDIOVASCULAR DISEASE
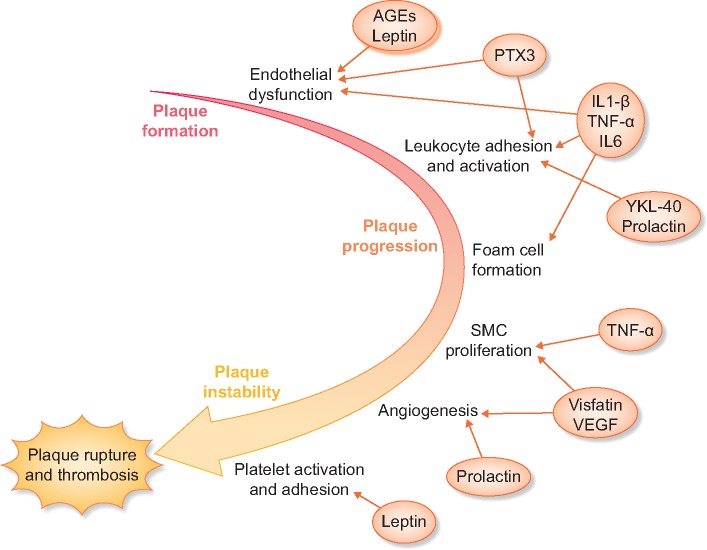
There is a strong body of evidence linking inflammation with cardiovascular disease. This relationship has been demonstrated in both the general population and in those with kidney disease. Many of the cytokines and associated inflammatory molecules that are elevated in uremia play a part in this inflammation–cardiovascular disease pathway (Figure 1).
FIGURE 1.
Middle molecules influence multiple steps in atherosclerosis progression. Elevated leptin and AGE levels are associated with endothelial dysfunction, which is also promoted by inflammatory molecules such as PTX3 and IL-1 beta. Elevated cytokines and other molecules such as prolactin and YKL-40 increase leukocyte adhesion and activation leading to foam cell formation. The progression to unstable plaque with migration and proliferation of smooth muscle cells and angiogenesis is also influenced by uremic toxins, eventually leading to plaque rupture and thrombosis.
Interleukin 1 beta (IL-1 beta) plays a key stimulatory role in the pro-inflammatory cytokine cascade and has been implicated in the process of atherosclerosis. Animal studies have demonstrated a causative role by examining both genetic or pharmacologic inhibition of IL-1 beta signaling and the progression of atherosclerotic disease [3]. In human studies, IL-1 beta is increased in diseased coronary arteries and correlates with plaque severity [4]. Gene polymorphism studies also indicate a relationship between interleukin-1 receptor antagonist protein (IL-1-RN) polymorphisms and coronary artery disease suggesting a causative role for IL-1 beta and atherosclerotic disease in humans [5]. IL-1 beta has also been associated with left ventricular hypertrophy in dialysis patients [6].
IL-18 is also typically elevated in uremia and in human studies, elevated levels have been associated with coronary artery calcification and cardiovascular mortality in chronic kidney disease (CKD) patients [7, 8]. In patients with coronary artery disease, high IL-18 levels are linked to unstable coronary plaque [9]. In animal models, IL-18 administration increases plaque formation in susceptible animal models, probably via an interferon (IFN)-gamma-dependent process [10].
Elevated levels of IL-6 are also associated with cardiovascular mortality [11] and left ventricular hypertrophy [6] in patients with CKD and those receiving hemodialysis. IL-6 is believed to play a central role in the development of atherosclerotic lesions, orchestrating the recruitment and ingress of inflammatory cells, as well as having local pro-coagulant effects in the vasculature that promote acute thrombosis [12]. The contribution of IL-6 to coronary heart disease is also supported by human Mendelian randomization studies [13].
Tumour necrosis factor alpha (TNF-alpha) is a cytokine that is produced by many cell types in response to inflammatory stimuli and also downstream of other cytokines such as IL-1. It has potent effects upregulating the immune response and produces a wide range of systemic effects including the suppression of appetite and induction of fever. TNF-alpha is typically elevated 4- to 5-fold in patients with ESKD and is associated with left ventricular hypertrophy in patients receiving dialysis [6]. High levels of circulating TNF-alpha are thought to contribute to myocardial dysfunction and fibrosis, as well as influencing vascular disease via multiple pathways, such as increasing coagulation activators, destabilizing endothelial structure and promoting proliferation and migration of vascular smooth muscle cells [14]. Intervention studies in humans demonstrated acute adverse vascular effects from TNF-alpha infusion and TNF-alpha is thus thought to contribute to the early development stages of atherosclerosis [15]. In ESKD, the soluble TNF receptors 1 and 2 (27 kDa and 17 kDa) are both also elevated significantly and may limit TNF-alpha activity in the acute setting. However, they also act to prolong the activity and half-life of circulating TNF-alpha, thus potentially contributing to the chronic inflammatory state and consequent cardiovascular disease.
Pentraxin-3 (PTX3), a 40-kDa member of the family of acute phase reactant pentraxins which include also C reactive protein (CRP), is considered to be part of the ‘long pentraxin’ subfamily. It is rapidly secreted primarily, but not exclusively, by leukocytes when stimulated by pro-inflammatory cytokines such as TNF-alpha and IL-1, or by other activators of the innate immune system, and has general functions including binding to pathogens for opsonization and complement activation. PTX3, similar to the prototypical short pentraxin CRP, has been observationally linked to increased risk of cardiovascular death and mortality in a number of studies, some including patients with CKD [16]. PTX3 may be a more specific marker than CRP for atherosclerosis however, as it is produced abundantly by cells within atherosclerotic lesions rather than by the liver, as is CRP. Several studies have linked PTX3 with unstable plaque in coronary and carotid arteries [17, 18]. It is not entirely clear, however, if PTX3 has a primary causative role in atherosclerosis, though PTX3 does have pro-thrombotic effects by interfering with nitric oxide (NO) production and augmenting the local expression of tissue factor in response to stimuli [19]. PTX3 can also be generated in vascular smooth muscle cells in response to atherogenic lipids, suggesting a possible place in the pathway of atherosclerosis progression [20].
ADIPOKINE DERANGEMENT IN UREMIA AND CARDIOVASCULAR DISEASE
Cell signaling proteins secreted by adipose tissue overlap in part with cytokines, but a number of these proteins secreted almost exclusively by adipose tissue have been termed adipokines. These molecules have a variety of roles and several are in the middle molecule size range and accumulate in kidney disease. These include adiponectin, which is exclusively generated by adipose tissue. Serum concentrations of adiponectin are in general inversely correlated with body fat quantities. Adiponectin circulates as trimers or larger conglomerates and the higher molecular weight forms are thought to be more biologically active with a protective role. Low adiponectin levels have been implicated in obesity-related insulin resistance, the metabolic syndrome and cardiovascular disease [21]. Although adiponectin is elevated in uremia, the relationship appears to be modified by amounts of fat mass and inflammation [22].
Adiponectin has variable relationships with atherosclerosis development in studies, possibly due to the variety of molecular sizes at which it exists, which may have different actions on different receptors. Most studies support an anti-inflammatory role, though whether this is true in CKD where generally high adiponectin levels coexist paradoxically with a high risk of cardiovascular disease is still unclear. Elevated adiponectin is associated with an increased risk for death in renal transplant recipients [23], and correlated with inflammatory cytokines such as IL-6 and TNF-alpha in dialysis population [24]. Other studies have found a U-shaped association between mortality and adiponectin [22], suggesting possible confounding by malnutrition and inflammation/wasting.
In contrast to adipokine, leptin has a clearer role in atherosclerosis, contributing to early-stage atheroma formation by impairing vasorelaxation via increasing endothelin and causing dysregulation of NO production [25]. Furthermore, leptin appears to potentiate Angiotensin II-related vascular dysfunction via increasing the expression of type-1 Angiotensin II receptors in vascular smooth muscle cells as well as promoting hypertrophy of these cells [26]. Leptin also increases local expression of inflammatory cytokines thus promoting inflammation-related damage [27]. In addition, leptin also enhances platelet activation leading to thrombus formation. Studies have demonstrated that high levels of circulating leptin are an independent risk factor for acute cardiovascular events [28].
Visfatin, also known as nicotinamide phosphoribosyltransferase (NAMPT) or historically as pre-B cell colony-enhancing factor, is an adipokine primarily but not exclusively secreted by visceral adipose tissue. At 52 kDa, it is one of the largest middle molecules to be elevated in uremia [29]. Intracellularly, it is involved in nicotinamide adenine dinucleotide biosynthesis but it is also released extracellularly, where it appears to have a wide range of effects including stimulating angiogenesis and endothelial cell proliferation. It also promotes the growth of vascular smooth muscle cells, has anti-apoptotic effects on macrophages and promotes vascular inflammation and endothelial damage [30]. High levels of visfatin expression have been found in atherosclerotic plaque in human studies. Furthermore, circulating levels of visfatin predict the presence of unstable plaque [31].
OTHER LARGE UREMIC TOXINS LINKED WITH CARDIOVASCULAR DISEASE
YKL-40, also known as chitinase-3-like protein 1 (CHI3L1), has a molecular weight of 40 kDa. It has precise role in inflammation and angiogenesis though its preciseness is unclear. It is secreted by activated macrophages and other cells and is induced by pro-inflammatory cytokines. YKL-40 is known to regulate other inflammatory markers, such as increasing the expression of monocyte chemoattractant protein-1 and activating cytoplasmic signaling pathways that increase cell proliferation and survival. Circulating YKL-40 levels have been observationally associated with stroke risk [32], and a possible causative role is supported by associations also with homozygosity for a single-nucleotide polymorphism in the CHI3L gene (which relates to YKL-40) [32]. In dialysis patients, YKL-40 correlates with the presence of known coronary artery disease, though studies in other populations do not support a clear link with coronary disease [33]. The study of YKL-40 has not yet progressed to delineate a clear mechanistic link with disease but given the appearance as a stroke marker this deserves further exploration.
The family of growth factors contains many arms that are involved broadly in stimulating proliferation and cellular differentiation, and several are increased in uremia, including vascular endothelial growth factor (VEGF) (elevated ∼2-fold), FGF-2 (elevated ∼5- to 20-fold) and FGF-23 (∼200-fold higher). The 34 kDa molecule VEGF is an essential regulator of angiogenesis both in normal physiology and in disease states such as cancer. VEGF promotes endothelial cell proliferation, migration and differentiation in endothelial cells and elsewhere. VEGF also appears to have an important role in cardiac adaptation to hypoxia and stretch, being upregulated by these stimuli and notably increased in cardiac hypertrophy [34]. VEGF expression is present in atherosclerotic plaque in human subjects and correlates with CKD grade in this setting [35].
FGF-2 is a signaling protein that is closely linked with the process of cardiac hypertrophy, providing paracrine mediation between cardiac fibroblasts and cardiomyocytes. In knockout mouse models, the lack of FGF-2 prevents compensatory hypertrophy in angiotensin II-dependent hypertension [36] and this has also been demonstrated in pressure/volume overload models, which are likely applicable to ESKD [37]. Interestingly, circulating FGF-2 has an inverse relationship with atheroma progression in CKD patients suggesting both deleterious and beneficial roles in cardiac disease [38]. FGF-23 has also been demonstrated to directly cause left ventricular hypertrophy when administered in animal models [39], and this can be attenuated with a FGF-23 blocker. Furthermore, FGF-23 levels correlate closely with Left Ventricular Hypertrophy in human observational studies [40], and this extends to patients with CKD [39].
Complement factor D is an integral component of the alternative complement pathway, being the rate-limiting step in the production of C3 convertase. It is elevated significantly (4- to 17-fold) in ESKD, and thus contributes to dysregulation of the complement system in these patients, priming them for excessive complement activation. Complement activation and deposition is known to play a role in endothelial dysfunction and cardiac ischemia-reperfusion injury. In animal models, blockade of complement components has been demonstrated to reduce tissue injury in this setting [41].
Prolactin levels in CKD are high due to reduced renal clearance and increased production. Prolactin is now recognized to have diverse roles, some of which are involved in cardiovascular disease at different levels. At the endothelial level, prolactin stimulates the adhesion of mononuclear cells to endothelial cells in response to inflammatory cytokines [42]. The main 23 kDa fragment is a driver of angiogenesis [43]. Excess prolactin in the setting of prolactinoma is also associated with impaired insulin sensitivity and adverse effects on lipids [44]. A role for prolactin in cardiovascular disease is supported by the association between prolactinoma and an elevated risk for incident cardiovascular disease in males compared with control subjects [45] and in CKD patients, prolactin levels have been positively associated with mortality and cardiovascular death [46].
Beta-trace protein is normally freely filtered and metabolized completely by the renal tubule, and it is usually elevated 20- to 30-fold in ESKD. Functionally it is involved in prostanoid synthesis and is heavily expressed in the heart and cardiac vessels. Beta-trace protein levels correlated strongly with cardiovascular disease and atheroma in hemodialysis patients [47], and prostaglandins from the pathway catalyzed by beta-trace protein have many cardiovascular effects. These are thought, however, to be cardioprotective with anti-thrombotic and anti-atherogenic potential [48], and in animal models gene knockout also causes accelerated atherosclerosis [49].
Advanced glycosylation end products (AGEs) refer to a large group of different molecules that result from the nonenzymatic glycation of proteins and lipids, and can be endogenously generated or from dietary sources, though the latter appears not to contribute to the high levels that accumulate in uremia [50]. They exist in a variety of sizes although in the hemodialysis population the main fractions are found at ∼70, ∼14 and <2 kDa [51]. AGE levels predict cardiovascular mortality in the dialysis population [52] and are thought to contribute structurally to vascular disease and inflammation [53]. The receptors for AGEs may also be involved in endothelial dysfunction in dialysis patients by enhancing adhesion molecule expression and interfering with NO synthesis in blood vessels [50].
CURRENT AND FUTURE STRATEGIES TO REMOVE LARGE MIDDLE MOLECULES
Conventional hemodialysis with high-flux membranes has very limited clearance of larger middle molecules, e.g. clearance of 1.8 mL/min for complement factor D (24 kDa) compared with 208 mL/min for urea [54]. Therapies utilizing convection such as HDF are one such strategy that has been promoted to improve clearance of middle molecules. Studies comparing low-flux hemodialysis and HDF have demonstrated better beta-2 microglobulin (<15 kDa) clearance with HDF [55] but comparisons between HDF and HD using high-flux membranes have been less impressive [56], and clearance of larger middle molecules is severely limited due to membrane pore size. Membranes with larger pores (‘high cut-off’ dialyzers) have been developed with initial interest primarily in use for acute kidney injury secondary to acute load of large middle molecules such as free light chains in myeloma [57]. The use of such membranes, however, results in a large amount of albumin loss along with the removal of the target proteins. Improvements in manufacturing technology have now allowed improvements in the uniformity of pore distribution, enabling the production of membranes that will allow greater clearance of larger molecules but without significant albumin loss (medium cut-off membranes). Studies to date show that these membranes improve clearance of larger molecules significantly compared with high-flux dialysis. For example, clearance of complement factor D (24 kDa) was improved from 1.8 mL/min to 26–35 mL/min using medium cut-off membranes compared with high flux, and the reduction ratio for YKL-40 (40 kDa) similarly increased from 19% to 61–71% [54]. This technology offers a leap in our ability to remove these large uremic toxins, and studies are now required to define the medium- and long-term safety of this approach as well as patient outcome data.
CONCLUSIONS
Large middle molecules are a diverse group of uremic toxins that contribute significantly to the high cardiovascular disease burden in ESKD. Dialysis technologies available to date offer limited clearance of these molecules. A new generation of membranes demonstrates improved clearance and now need to be tested prospectively to determine if improved clearance will lead to improved patient outcomes.
ACKNOWLEDGEMENTS
This article is published as part of a Supplement to NDT on ‘Translating Innovation to Clinical Outcomes’, financially supported by Baxter Healthcare Corporation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kahn MR, Robbins MJ, Kim MC. et al. Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol 2013; 10: 261–273 [DOI] [PubMed] [Google Scholar]
- 2. Wolley M, Jardine M, Hutchison CA.. Exploring the clinical relevance of providing increased removal of large middle molecules. Clin J Am Soc Nephrol 2018; 13: 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhaskar V, Yin J, Mirza AM. et al. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis 2011; 216: 313–320 [DOI] [PubMed] [Google Scholar]
- 4. Galea J, Armstrong J, Gadsdon P. et al. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol 1996; 16: 1000–1006 [DOI] [PubMed] [Google Scholar]
- 5. Francis SE, Camp NJ, Dewberry RM. et al. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation 1999; 99: 861–866 [DOI] [PubMed] [Google Scholar]
- 6. Gupta J, Dominic EA, Fink JC. et al. Association between inflammation and cardiac geometry in chronic kidney disease: findings from the CRIC Study. PLoS One 2015; 10: e0124772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Formanowicz D, Wanic-Kossowska M, Pawliczak E. et al. Usefulness of serum interleukin-18 in predicting cardiovascular mortality in patients with chronic kidney disease–systems and clinical approach. Sci Rep 2015; 5: 18332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiu Weber CI, Duchateau-Nguyen G, Solier C. et al. Cardiovascular risk markers associated with arterial calcification in patients with chronic kidney disease Stages 3 and 4. Clin Kidney J 2014; 7: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Qu P, Zhao J. et al. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch Med Sci 2014; 10: 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitman SC, Ravisankar P, Daugherty A.. Interleukin-18 enhances atherosclerosis in apolipoprotein E(-/-) mice through release of interferon-gamma. Circ Res 2002; 90: E34–E38 [DOI] [PubMed] [Google Scholar]
- 11. Rao M, Guo D, Perianayagam MC. et al. Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2005; 45: 324–333 [DOI] [PubMed] [Google Scholar]
- 12. Hartman J, Frishman WH.. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev 2014; 22: 147–151 [DOI] [PubMed] [Google Scholar]
- 13. Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012; 379: 1214–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kleinbongard P, Heusch G, Schulz R.. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 2010; 127: 295–314 [DOI] [PubMed] [Google Scholar]
- 15. Chia S, Qadan M, Newton R. et al. Intra-arterial tumor necrosis factor-alpha impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol 2003; 23: 695–701 [DOI] [PubMed] [Google Scholar]
- 16. Tong M, Carrero JJ, Qureshi AR. et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2007; 2: 889–897 [DOI] [PubMed] [Google Scholar]
- 17. Koga S, Ikeda S, Yoshida T. et al. Elevated levels of systemic pentraxin 3 are associated with thin-cap fibroatheroma in coronary culprit lesions: assessment by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv 2013; 6: 945–954 [DOI] [PubMed] [Google Scholar]
- 18. Shindo A, Tanemura H, Yata K. et al. Inflammatory biomarkers in atherosclerosis: pentraxin 3 can become a novel marker of plaque vulnerability. PLoS One 2014; 9: e100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carrizzo A, Lenzi P, Procaccini C. et al. Pentraxin 3 induces vascular endothelial dysfunction through a P-selectin/matrix metalloproteinase-1 pathway. Circulation 2015; 131: 1495–1505 [DOI] [PubMed] [Google Scholar]
- 20. Klouche M, Peri G, Knabbe C. et al. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis 2004; 175: 221–228 [DOI] [PubMed] [Google Scholar]
- 21. Kadowaki T, Yamauchi T, Kubota N. et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006; 116: 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rao M, Li L, Tighiouart H. et al. Plasma adiponectin levels and clinical outcomes among haemodialysis patients. Nephrol Dial Transplant 2008; 23: 2619–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alam A, Molnar MZ, Czira ME. et al. Serum adiponectin levels and mortality after kidney transplantation. Clin J Am Soc Nephrol 2013; 8: 460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayerden Ebinc F, Ebinc H, Derici U. et al. The relationship between adiponectin levels and proinflammatory cytokines and left ventricular mass in dialysis patients. J Nephrol 2009; 22: 216–223 [PubMed] [Google Scholar]
- 25. Freitas Lima LC, Braga VA, do Socorro de Franca Silva M. et al. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol 2015; 6: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeidan A, Purdham DM, Rajapurohitam V. et al. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J Pharmacol Exp Ther 2005; 315: 1075–1084 [DOI] [PubMed] [Google Scholar]
- 27. Yamagishi SI, Edelstein D, Du XL. et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 2001; 276: 25096–25100 [DOI] [PubMed] [Google Scholar]
- 28. Wallace AM, McMahon AD, Packard CJ. et al. Plasma leptin and the risk of cardiovascular disease in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 2001; 104: 3052–3056 [DOI] [PubMed] [Google Scholar]
- 29. Nusken KD, Petrasch M, Rauh M. et al. Active visfatin is elevated in serum of maintenance haemodialysis patients and correlates inversely with circulating HDL cholesterol. Nephrol Dial Transplant 2009; 24: 2832–2838 [DOI] [PubMed] [Google Scholar]
- 30. Peiro C, Romacho T, Carraro R. et al. Visfatin/PBEF/Nampt: a new cardiovascular target? Front Pharmacol 2010; 1: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dahl TB, Yndestad A, Skjelland M. et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 2007; 115: 972–980 [DOI] [PubMed] [Google Scholar]
- 32. Rathcke CN, Vestergaard H.. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res 2006; 55: 221–227 [DOI] [PubMed] [Google Scholar]
- 33. Pawlak K, Rozkiewicz D, Mysliwiec M. et al. YKL-40 in hemodialyzed patients with and without cardiovascular complications - the enhancement by the coexistence of the seropositivity against hepatitis C virus infection. Cytokine 2013; 62: 75–80 [DOI] [PubMed] [Google Scholar]
- 34. Mallamaci F, Benedetto FA, Tripepi G. et al. Vascular endothelial growth factor, left ventricular dysfunction and mortality in hemodialysis patients. J Hypertens 2008; 26: 1875–1882 [DOI] [PubMed] [Google Scholar]
- 35. Nakano T, Ninomiya T, Sumiyoshi S. et al. Chronic kidney disease is associated with neovascularization and intraplaque hemorrhage in coronary atherosclerosis in elders: results from the Hisayama Study. Kidney Int 2013; 84: 373–380 [DOI] [PubMed] [Google Scholar]
- 36. Pellieux C, Foletti A, Peduto G. et al. Dilated cardiomyopathy and impaired cardiac hypertrophic response to angiotensin II in mice lacking FGF-2. J Clin Invest 2001; 108: 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultz JE, Witt SA, Nieman ML. et al. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest 1999; 104: 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bozic M, Betriu A, Bermudez-Lopez M. et al. Association of FGF-2 concentrations with atheroma progression in chronic kidney disease patients. Clin J Am Soc Nephrol 2018; 13: 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ali FN, Falkner B, Gidding SS. et al. Fibroblast growth factor-23 in obese, normotensive adolescents is associated with adverse cardiac structure. J Pediatr 2014; 165: 738–743e731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bjerre M, Hansen TK, Flyvbjerg A.. Complement activation and cardiovascular disease. Horm Metab Res 2008; 40: 626–634 [DOI] [PubMed] [Google Scholar]
- 42. Montes de Oca P, Macotela Y, Nava G. et al. Prolactin stimulates integrin-mediated adhesion of circulating mononuclear cells to endothelial cells. Lab Invest 2005; 85: 633–642 [DOI] [PubMed] [Google Scholar]
- 43. Struman I, Bentzien F, Lee H. et al. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA 1999; 96: 1246–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Assuncao Alves Rodrigues LF, Campos SM, Miranda PA. et al. Prolactinoma: a condition associated with hypoadiponectinemia. Horm Metab Res 2012; 44: 832–838 [DOI] [PubMed] [Google Scholar]
- 45. Toulis KA, Robbins T, Reddy N. et al. Males with prolactinoma are at increased risk of incident cardiovascular disease. Clin Endocrinol (Oxf) 2018; 88: 71–76 [DOI] [PubMed] [Google Scholar]
- 46. Carrero JJ, Kyriazis J, Sonmez A. et al. Prolactin levels, endothelial dysfunction, and the risk of cardiovascular events and mortality in patients with CKD. Clin J Am Soc Nephrol 2012; 7: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shafi T, Parekh RS, Jaar BG. et al. Serum beta-trace protein and risk of mortality in incident hemodialysis patients. Clin J Am Soc Nephrol 2012; 7: 1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Orenes-Pinero E, Manzano-Fernandez S, Lopez-Cuenca A. et al. beta-Trace protein: from GFR marker to cardiovascular risk predictor. Clin J Am Soc Nephrol 2013; 8: 873–881 [DOI] [PubMed] [Google Scholar]
- 49. Ragolia L, Palaia T, Hall CE. et al. Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J Biol Chem 2005; 280: 29946–29955 [DOI] [PubMed] [Google Scholar]
- 50. Linden E, Cai W, He JC. et al. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol 2008; 3: 691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henle T, Deppisch R, Beck W. et al. Advanced glycated end-products (AGE) during haemodialysis treatment: discrepant results with different methodologies reflecting the heterogeneity of AGE compounds. Nephrol Dial Transplant 1999; 14: 1968–1975 [DOI] [PubMed] [Google Scholar]
- 52. Meerwaldt R, Hartog JW, Graaff R. et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3687–3693 [DOI] [PubMed] [Google Scholar]
- 53. Prasad A, Bekker P, Tsimikas S.. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev 2012; 20: 177–183 [DOI] [PubMed] [Google Scholar]
- 54. Kirsch AH, Lyko R, Nilsson LG. et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017; 32: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grooteman MP, van den Dorpel MA, Bots ML. et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 2012; 23: 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ok E, Asci G, Toz H. et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant 2013; 28: 192–202 [DOI] [PubMed] [Google Scholar]
- 57. Hutchison CA, Cockwell P, Reid S. et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol 2007; 18: 886–895 [DOI] [PubMed] [Google Scholar]